Abstract
Advancements in biomaterial science and available cell sources have spurred the translation of tissue-engineering technology to the bedside, addressing the pressing clinical demands for replacement cardiovascular tissues. Here, the in vivo status of tissue-engineered blood vessels, heart valves, and myocardium is briefly reviewed, illustrating progress toward a tissue-engineered heart for clinical use.
The goal of tissue engineering is to create an implantable construct that eventually transforms into autologous tissue, indistinguishable in form and function from its native counterpart. The cardiovascular system has been identified as a target for tissue engineering since the inception of the field, and the potential of tissue engineering to benefit patients with cardiovascular disease is even more relevant in the present day. Currently, cardiovascular disease accounts for 20% of global mortality and is the most common cause of death in adults within the U.S. (47). While significant strides have been made in medical management, surgical intervention requiring the use of prosthetic implants continues to be critical in many adult patients, and heart transplant remains the only definitive treatment for end-stage heart failure. In addition, congenital cardiac anomalies are the leading cause of death within the neonatal period, and surgical intervention is often unavoidable in this patient population (55). Currently used prosthetic materials are subject to calcific degradation, infection, rejection, poor durability, and somatic overgrowth (the process by which a child outgrows their graft, often necessitating reoperative procedures).
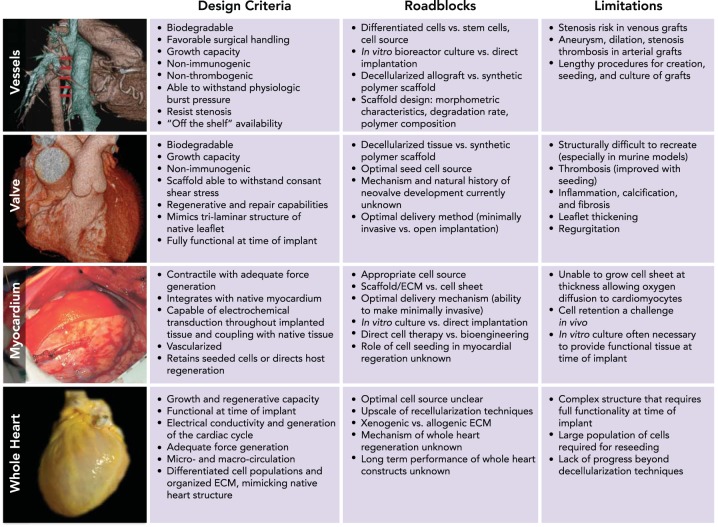
Cardiovascular tissue engineering promises an unlimited source of non-immunogenic functional tissue with growth capacity and the ability to remodel over the course of a patient's life. The clear advantages over currently used prostheses have driven advancements in tissue-engineered valves, vessels, and myocardial patches for the repair of congenital cardiac anomalies and treatment of valvular or ischemic heart disease, demonstrating significant progress toward the holy grail of a tissue-engineered human heart (73). Successful engineered tissue relies on design criteria, which are briefly reviewed in Table 1 and include three primary components (7, 36): 1) a scaffold that provides a three-dimensional template for tissue growth, 2) cells seeded or cultured on the scaffold, and 3) humoral and mechanical signaling that directs neotissue formation and host remodeling.
Table 1.
Design considerations for cardiovascular tissue engineering
| Strategy | Description | Pros | Cons |
|---|---|---|---|
| Decellularization-recellularization | Decellularization of desired structure from cadaveric or animal source with eventual reseeding | Preserved architecture and mechanical properties | Availability of tissues |
| Size mismatch | |||
| Possible antigenicity | |||
| Possible pathogen transmission | |||
| Cell sheets | Culturing of individual cell lines into sheets, which are assembled into the desired structure | Versatile design | Time and resource intensive |
| Unlimited source | Can require multiple layers to create thick tissues | ||
| Not readily available for implantation | |||
| Bioengineering | Degradable, synthetic scaffold generated using a variety of mechanisms that are seeded with cells and either incubated in a bioreactor or immediately implanted | Readily available | Time and resource intensive |
| Versatile design parameters | |||
| Unlimited source | |||
| Minimally invasive approach possible (valves) | |||
| Hydrogels | Gels composed of extracellular matrix elements that can be used alone, in combination with synthetic matrices, or in combination with seeding | Easily miniaturized and automated | Lower mechanical stability |
| Injectable (minimally invasive) | Limited scope of use without combination with synthetic matrices |
| Cell Source | |||
|---|---|---|---|
| Embryonic stem cells (ESCs) | Derived from embryonic cells | Can be differentiated into any cell line | Association with teratoma formation |
| Can provide a large number of cells | Possible immunogenicity requiring immunosuppression | ||
| · | Ethical concerns over initial cell source | ||
| Induced pluripotent stem cells (iPSCs) | Reprogramming of terminally differentiated cells using transcription factors linked to pluripotency, allowing behavior similar to ESCs | Reduces some immunological concerns | Requires use of retroviral agents |
| Can obtain theoretically unlimited cell numbers | Concern over karyotypic abnormalities | ||
| Efficacy of process is low | |||
| Adult stem cells | Cells within various tissues that retain the ability to terminally differentate into a limited number of tissues | Eliminates immunological concerns | Provides only a limited number of cells |
| Relatively easily obtained | |||
| No reports of tumorigenesis |
The design strategies to create autologous tissue are as diverse as the potential applications of tissue-engineered implants and are therefore difficult to encompass in a single discussion. This review will focus on the preclinical models and clinical trials that have bridged the divide between bench-top discovery and bedside application of cardiovascular tissue engineering with respect to blood vessels, heart valves, myocardium, and the whole heart.
Tissue-Engineered Blood Vessels and Vascular Grafts
Design requirements for an engineered vascular conduit include a functional intimal endothelial cell monolayer, a medial smooth muscle layer, and an adventitial layer of fibroblasts all supported by a robust extracellular matrix that can withstand physiological circulatory pressures. Weinberg and Bell were one of the first to attempt tissue engineering of a cardiovascular structure by creating a tubular vessel-like construct of cultured endothelial cells, smooth muscle cells, and fibroblasts on a collagen scaffold (70), prompting research to optimize a tissue-engineered blood vessel.
Shinoka et al. advanced the field in the late 1990s by using ovine and canine models to evaluate the feasibility of culturing autologous fibroblasts, endothelial cells, and smooth muscle cells on porous polymeric scaffolds for pulmonary artery, abdominal aorta, and inferior vena cava conduits (60, 61, 68). Clinical application of tissue-engineered vascular grafts created by culture of autologous cells on a polycaprolactone-polylactic acid copolymer reinforced with woven polyglycolic acid were subsequently reported with promising results (49). To overcome the time, cost, safety, and primary cell availability limitations of in vitro culture, use of bone marrow cells for seeding the synthetic scaffold directly before implantation was validated using a canine model (25, 41). This approach was then successfully translated to create vascular conduits for extra-cardiac cavopulmonary connections (modified Fontan circulation) and vascular patches in a cohort of 42 pediatric patients with congenital cardiac anomalies (FIGURE 1) (59). Long-term follow-up identified stenosis as the primary cause of graft failure (24% of patients). An FDA-approved clinical trial is currently evaluating the use of these biodegradable scaffold-based cell-seeded TEVGs for treatment of single ventricle cardiac anomalies, and initial results are encouraging.
FIGURE 1.
Translation of tissue-engineered heart components: criteria, roadblocks, and limitations
Independent progress in the translation of tissue engineered vascular conduits (23), myocardium (6), and heart valves (9) encourages integrated approaches to developing whole heart constructs. Recent advancements in whole organ decellularization provide an alternative approach to generating an off-the-shelf human heart (19). Figures adapted with permission from Refs. 6, 9, 23, 40.
An alternative approach to the use of biodegradable polymeric scaffold materials that has also been applied clinically is known as “tissue engineering by self-assembly” (TESA). L'Heureux et al. pioneered TESA technology by sequential layering of cultured human smooth muscle and fibroblast cell sheets and subsequent bioreactor culture followed by endothelial cell seeding to create a trilayered blood vessel that closely mimicked native human artery and could withstand supraphysiological burst pressures (34). Long-term study of age- and risk-matched human TESA blood vessels in nude rat and immune-compromised macaques demonstrated the clinical potential of this technology for the construction of viable autologous small-diameter arteries (32). In 2007, these sheet-based tissue-engineered blood vessels were successfully translated to the clinic as arteriovenous shunts in 10 patients with end-stage renal disease (ESRD) (43). Graft failure (3/10) was attributed to dilatation, aneuryism, and thrombosis (43). Since the lengthy culture (6–9 mo) and autologous cell source required for the TESA approach limits its relevance for urgent clinical use, Niklason et al. cultured human cadaveric smooth muscle cells on tubular polyglycolic acid fiber scaffolds in a pulsatile bioreactor followed by decellularization after polymer degradation and preservation at 4°C to provide a “readily available” tissue-engineered blood vessel for arteriovenous conduits that was evaluated in a baboon model (8). Recent application of a similar approach to a TESA graft (the “Lifeline” graft) has been applied clinically in three patients requiring arteriovenous shunts for hemodialysis access, but 2/3 of grafts experienced stenosis and thrombosis requiring intervention to maintain secondary patency (73). Long-term efficacy of the Lifeline and decellularized grafts are currently under investigation in FDA-approved clinical trials.
The clinical experience with tissue-engineered vascular grafts has laid the foundation for extensive research toward the development of the ideal tissue-engineered vascular conduit. Thrombosis and stenosis due to intimal hyperplasia are the primary modes of failure common to both venous and arterial circulations, whereas the arterial circulation is further challenged by risk of dilatation and aneurysm (24, 74). Recent research in rodent, ovine, and nonhuman primate models has begun to elucidate the mechanisms of TEVG stenosis and to identify the roles of the host inflammatory response and mechano-biological pathways during neovessel growth and remodeling (12, 29, 53). Similarly, current efforts to generate novel scaffold polymers and designs optimized for the arterial circulation that obviate the need for cell seeding or in vitro culture indicate that a completely “off-the-shelf” tissue-engineered vascular conduit is rapidly approaching clinical translation (26, 31, 72).
Tissue-Engineered Heart Valves
Despite decades of refinement, current strategies for heart valve replacement continue to be less than optimal for long-term use. While mechanical valves are capable of lasting for more than 15 years, they are prone to infection and thrombosis (46). Bioprosthetic valves, on the other hand, are generally free from thrombotic complications, yet deteriorate and calcify over a relatively short time period (10–15 years) (46). In addition, lack of growth potential is a significant limitation in pediatric patients. The Ross procedure, in which the pulmonic valve is transplanted into an aortic position and replaced with a cadaveric graft, successfully bypasses many of the concerns regarding bioprosthetic and mechanical valves, but is often complicated by preexisting disease in both the pulmonic and aortic valves. Tissue engineering offers a tool that could limit the morbidity of valve replacements, especially in children.
Heart valves are uniquely exposed to an environment of constant shear stress, presenting challenges in biomaterial and cell source selection. An ideal tissue-engineered heart valve would withstand its hemodynamic and mechanical environment while also promoting the cell signaling, proliferation, differentiation, organization, and ECM production mediated by these dynamic forces that guide valvular neotissue formation. Equally important, but more difficult to achieve, is the tri-laminar structure of the leaflet: the ventricularis layer (predominantly interstitial cells, collagen, and elastin), the spongiosa layer (proteoglycans and collagen), and the fibrosa layer (valvular interstitial cells, collagen).
Scaffold design in heart valves has two primary approaches: decellularized tissue-based and synthetic polymer-based grafts. Decellularized scaffolds offer the significant advantage of preserving the complex leaflet's extracellular matrix, providing the optimal environment for valve formation (4). One of the initial groups to explore this approach was Elkins et al., who decellularized human cadaveric valves, patented as the SynerGraft (13). After successful implantation of unseeded grafts into an ovine model, these valves were translated to the clinic for implantation in the pulmonary position. Initial results showed lower levels of alloantibodies than those of historic control patients receiving traditional allografts (23). Addition of endothelial cell seeding by Dohmen et al. improved results in terms of thrombotic complications and immunogenic reactions against collagen (10). Ten-year follow-up demonstrated that flow velocities remained within physiological parameters, unlike cryopreserved allografts (FIGURE 1) (9). To increase tissue-engineered heart valve availability, the natural transition was to decellularize xenografts. While initially promising, long-term results with the Matrix P, a decellularized porcine graft, showed a high frequency of failure due to chronic inflammation and fibrosis (53, 66).
Synthetic polymer grafts bypass the limitation of tissue availability and xenograft-related rejection, and can be created by a variety of methodologies including 3D printing, electrospinning, stereolithography, fused deposition modeling, and selective laser sintering (46). One of the early studies in this field used a scaffold of polyglycolic acid and polylactic acid seeded with autologous myofibroblasts to create a single-valve leaflet (5). In an ovine model, these constructs demonstrated appropriate extracellular matrix and cellular organization, but Doppler imaging revealed leaflet thickening. Early studies by Nakayama et al. in goat models developed cell-seeded 3D printed molds and demonstrate a particularly promising area of research that would allow physicians to create size-appropriate, patient-specific valve constructs (50). Recently, the Hoerstrup group also made significant strides using bone-marrow-derived mononuclear cell- or fibroblast-seeded polyglycolic acid scaffolds implanted into sheep and non-human primates that demonstrate progression toward normal valve architecture (69). Regurgitation was observed in these models, and further research is therefore required to optimize scaffold design. The Hoerstrup group has innovated the use of a device allowing minimally invasive transapical implantation of this tissue-engineered heart valve (69), an approach that has previously been used with decellularized grafts in preclinical models (11, 15).
While the majority of tissue-engineered heart valve research has been conducted in large animals such as non-human primates, sheep, pigs, and goats, rodent models can provide a wealth of information due to the advent of transgenic technology and the abundance of available reagents optimized for these species. Recently, several groups have published data from both mouse and rat models. For example, implantation of a valved conduit directly into the intra-abdominal aorta (2) or after heterotopic heart transplantation (27) provides the opportunity for rigorous examination of the cellular mechanisms underlying both neo-valve development and tissue-engineered heart valve failure, which will be invaluable in further translation of second-generation tissue-engineered heart valves.
Tissue-Engineered Myocardium
The ideal tissue-engineered myocardial construct possesses electrical conductivity and the ability to generate adequate contractile force for the cardiac cycle, all while coupling with surrounding native tissue (64). Here, we focus primarily on two categories of myocardial tissue engineering: scaffold-free constructs made of layered sheets of cardiomyocytes or cardiomyocyte progenitors, and the application of synthetic scaffolds, which are either acellular or seeded with cells and/or signaling factors. Another promising approach is the direct introduction of specific cell populations, biomaterials, or combinations thereof via intramural or coronary injection, and we point interested readers to comprehensive works regarding these approaches for more information (20, 42, 48, 54, 64, 77).
Scaffold-free engineered myocardium has been explored extensively in rat models of MI following the work of the Zimmermann group. Neonatal rat cardiomyocytes were cultured in a mixture of collagen I, Matrigel, and serum for 12 days, then implanted as a monolayer sheet on uninjured hearts. Successful vascularization of the engineered heart tissue (EHT) and preservation of cardiomyocyte structure served as an important proof-of-concept study (78). Since then, the cell sheet approach pioneered by the innovative temperature-dependent culture techniques of the Shimizu laboratory has successfully created functional myocardium, demonstrating electrically coupled graft and host tissues (19, 44, 57), improved ejection fraction, and decreased ventricular dilation (19, 57, 62). These findings have been primarily validated in small animal models; however, Sawa et al. did describe the use of an autologous myoblast cell sheet that resulted in a nearly twofold increase in ejection fraction (26% to 46%) in a 56-yr-old patient with dilated cardiomyopathy (55).
Bioengineered scaffolds, or “cardiac patches,” are designed to provide physical support to injured myocardium with a mechanical profile matching that of the normal adjacent myocardium. Natural bio-materials such as collagen, fibrin, or alginante are popular source materials for myocardial scaffolds, whereas common synthetics include polycaprolactone, polylactic acid, or polyethylene glycol (16). In an important proof-of-concept study, porcine models of myocardial infarction (MI) demonstrated improved myocardial neovascularization and improved LV contractility after implantation of fibrin patches seeded with human embryonic stem cell-derived smooth muscle and endothelial cells (75). Similarly, use of cell-free degradable synthetic elastomers has shown decreased ventricular dilation and improved contractile function in both small and large animal models (22). Cross-linking scaffolds with growth factors such as VEGF may improve neovascularization of implanted constructs and increase cell retention, which is a primary challenge in engineering myocardium of suitable thickness for human implantation (45). Initial clinical trials demonstrate improved ventricular regeneration and diastolic function with application of a collagen scaffold seeded with BM-MNCs, accompanied by BM-MNC injection upon implantation (FIGURE 1) (6, 64). In addition, decellularized porcine small intestine submucosa (SIS) patches (CorMatrix) are FDA approved for pericardial closure. Further clinical support is garnered by a case series with no reported implant failure in 11 patients receiving ventricular patches after MI, and 4 ongoing FDA-approved clinical trials evaluating the use of decellularized porcine SIS in cardiac tissue repair should further elucidate the validity of CorMatrix ECM (64, 76).
Hydrogel technology has allowed the design of a minimally invasive, catheter-based approach to myocardial tissue engineering, which is an attractive alternative to open implantation. Alginate hydrogel administration in both rat and porcine MI models has demonstrated attenuation of left ventricular enlargement, ECM synthesis, and myofibroblast proliferation (35, 38). Early Phase I FDA-approved trials have demonstrated similar efficacy of transcoronary alginate infusion for treatment of MI within humans, and Phase II trials are currently underway (54). Further work seeks to expand the indications of this therapy to patients with congestive heart failure (37).
Progress Toward the Tissue-Engineered Heart
A common challenge facing many myocardial tissue-engineering approaches is lack of adequate vascularization to support the metabolic needs of the neo-myocardium and to thereby prevent thinning, dilatation, and subsequent failure (28). These challenges are even more daunting in the context of whole-heart tissue engineering. As a result, much of the work in whole-heart tissue engineering has focused on using decellularized cadaveric hearts, retaining native macro- and microvasculature and ECM, which best promote seeded cell differentiation into site-specific cell populations (63, 65). Several decellularization methodologies have been described, ranging from freeze/thaw cycles, sonication, and enzymatic and chemical digestion, with the most promising approach being introduction of these agents by coronary perfusion (1, 3). In a seminal study, Ott et al. employed antegrade coronary perfusion with SDS to produce acellular constructs while preserving ECM constituents (52). Hearts were recellularized via intramural injection of neonatal cardiomyoctes and coronary perfusion of aortic endothelial cells in a bioreactor, and after 8 days of culture these cardiac constructs could generate pump function equivalent to ∼2% of adult or 25% of a 16-wk fetal heart (52).
Successful decellularization of pig hearts (67, 71) may provide a solution to the shortage of allogenic cardiac grafts. In 2013, the Ott group decelullarized 10 cadaveric human hearts (21, 40), further anticipating the upscale of recellularization methods. However, a significant challenge to whole-heart tissue engineering is that the number of cells needed to recellularize a porcine or human heart construct is orders of magnitude larger than that needed for a murine heart (3). Induced pluripotent stem cells (iPS) are an attractive cell source for whole-heart tissue engineering since they are theoretically unlimited in number and easily obtained from somatic cell sources; however, further refinement in differentiation protocols is necessary to generate the heterogeneous cardiomyocyte phenotypes present in mature myocardium (16). Recently, Lu et al. used human iPS-derived cardiovascular progenitor cells to recellularize a mouse heart via coronary perfusion and demonstrated cell migration, proliferation, and differentiation into functional cardiomyocytes and endothelial cells in situ, indicating the promise of this approach (39). Human embryonic stem cell (hESC)-derived cardiomyocytes are an alternative source for seed cells and are currently the most advanced toward clinical utilization as demonstrated by an ongoing Phase I trial for treatment of severe heart failure (clinicaltrials.gov identifier: NCT02057900). While application of hESC-derived cardiovascular progenitor cells may provide a solution to the current challenges facing whole-heart recellularization, this approach is limited by risks of malignant degeneration, immunological rejection, and potential ethical pitfalls. Whole-heart recellularization may be a nascent technology and the least clinically feasible approach at present; however, recent advances toward preclinical validation support its continued pursuit.
Conclusions and Future Directions
In a 2007 review, Zimmerman et al. suggested that tissue engineering whole-heart constructs for clinical application would not be realized in the near future (79). Instead, the proposal was to focus efforts on independent translation of tissue-engineered vascular constructs, valves, and myocardium for the greatest immediate clinical impact. While recent advances in tissue engineering of individual cardiovascular structures indicate progress toward incorporation of these strategies into a prototypical whole-heart construct (Table 2), many pressing questions persist. The optimal strategy for fashioning one heart component may not be optimal for another, and we predict that the tissue-engineered heart will necessarily be a dynamic integration of both structure and strategy. Specifically, it is unclear whether cell seeding is requisite if an optimal scaffold is used; however, the ideal biomaterial for “cell-free” cardiovascular tissue engineering has yet to be elucidated. If cell seeding is required, the optimal cell source (BM-MNCs, iPS cells, ESCs, differentiated cardiomyocytes, etc.) is still unclear and will likely vary depending on the target heart component. Along with the necessity of cell seeding, the debate surrounding direct implantation vs. in vitro culture has not been definitively resolved. Advancements in bioreactor culture have demonstrated promise on the bench top, but data supporting the long-term efficacy of these constructs once up-scaled to the size of a human heart is lacking. Ultimately, the burning question remains: Is it possible to create a readily available three-dimensional myocardial construct for clinical application that retains the structural and functional complexity of the human heart?
Table 2.
Translational spectrum of cardiovascular tissue engineering
| Notable Preclinical Studies (Ref.) | First in Human (Ref.) | Clinical Trials (Ref.) | |
|---|---|---|---|
| Whole heart | |||
| Myocardium |
|
|
|
| Valve |
|
|
|
| Blood vessel |
|
|
|
iPS, induced pluripotent stem cell; LV, left ventricle; PEUU, poly(etherurethane urea); MI, myocardial infarction; VEGF, vascular endothelial growth factor; hESC, human embryonic stem cell; EC, endothelial cell; SMC, smooth muscle cell; EHT, engineered heart tissue; TEHV, tissue-engineered heart valve; BM-MNC, bone marrow mononuclear cell; TEVG, tissue-engineered vascular graft; TESA, tissue engineering by self-assembly; PGS, poly(glycerol sebacate); CABG, coronary artery bypass graft; LVAS, left ventricular assist system; ECM, extracellular matrix; RVOT, right ventricular outflow tract; ESRD, end-stage renal disease; STEMI, ST segment elevation myocardial infarction; PV, pulmonary valve; hHV, human heart valve; TV, tricuspid valve; EC TCPC, extra cardiac total cavopulmonary connection; HAVG, human acellular vascular graft; RA, right atrium; PA, pulmonary arteries. Where applicable, the unique clinicaltrials.gov identifier for each trial is included in parentheses.
Footnotes
Funding for this work was provided by National Institutes of Health Grant R01-098228.
C. Breuer receives grant support from Gunze Limited. C. Breuer is on the Scientific Advisory Board for Cook Biomedical.
Author contributions: C. Best, E.O., and C. Breuer conception and design of research; C. Best and J.B. prepared figures; C. Best, E.O., V.P., and M.S. drafted manuscript; C. Best, E.O., V.P., M.S., J.B., and C. Breuer edited and revised manuscript; C. Breuer approved final version of manuscript.
References
- 1.Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, Preuss K, Lichtenberg A. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods 17: 915–926, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Assmann A, Zwirnmann K, Heidelberg F, Schiffer F, Horstkötter K, Munakata H, Gremse F, Barth M, Lichtenberg A, Akhyari P. The degeneration of biological cardiovascular prostheses under pro-calcific metabolic conditons in a small animal model. Biomaterials 35: 7416–7428, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13: 27–53, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth C, Korossis SA, Wilcox HE, Watterson KG, Kearney JN, Fisher J, Ingham E. Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J Heart Valve Dis 11: 457–462, 2002. [PubMed] [Google Scholar]
- 5.Breuer CK, Shinoka T, Tanel RE, Zünd G, Mooney DJ, Ma PX, Miura T, Colan S, Langer R, Mayer JE, Vacanti JP. Tissue engineering lamb heart valve leaflets. Biotechnol Bioeng 50: 562–567, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Chachques JC, Trainini JC, Lago N, Cortes-Morichetti M, Schussler O, Carpentier A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM Trial): clinical feasibility study. Ann Thorac Surg 85: 901–908, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Cleary MA, Geiger E, Grady C, Best C, Naito Y, Breuer C. Vascular tissue engineering:the next generation. Trends Mol Med 18: 395–405, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Dahl SLM, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begelman KG, Niklason LE. Readily available tissue-engineered vascular grafts. Sci Transl Med 3: 68ra9-68ra9, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg 92: 1308–1314, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Dohmen PM, Lembcke A, Hotz H, Kivelitz D, Konertz WF. Ross operation with a tissue-engineered heart valve. Ann Thorac Surg 74: 1438–1442, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Driessen-Mol A, Emmert MY, Dijkman PE, Frese L, Sanders B, Weber B, Cesarovic N, Sidler M, Leenders J, Jenni R, Grünenfelder J, Falk V, Baaijens FPT, Hoerstrup SP. Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity. J Am Coll Cardiol 63: 1320–1329, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Duncan DR, Chen PY, Patterson JT, Lee YU, Hibino N, Cleary M, Naito Y, Yi T, Gilliland T, Kurobe H, Church SN, Shinoka T, Fahmy TM, Simons M, Breuer CK. TGFbetaR1 inhibition blocks the formation of stenosis in tissue-engineered vascular grafts. J Am Coll Cardiol 65: 512–514, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkins RC, Dawson PE, Goldstein S, Walsh SP, Black KS. Decellularized human valve allografts. Ann Thorac Surg 71: S428–32, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Emmert MY, Weber B, Behr L, Frauenfelder T, Brokopp CE, Grünenfelder J, Falk V, Hoerstrup SP. Transapical aortic implantation of autologous marrow stromal cell-based tissue-engineered heart valves: first experiences in the systemic circulation. JACC Cardiovasc Interv 4: 822–823, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Emmert MY, Weber B, Behr L, Sammut S, Frauenfelder T, Wolint P, Scherman J, Bettex D, Grünenfelder J, Falk V, Hoerstrup SP. Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: technical considerations and implications for translational cell-based heart valve concepts. Eur J Cardiothorac Surg 45: 61–68, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Eng G, Lee BW, Radisic M, Vunjak-Novakovic G. Cardiac tissue engineering. In: Principles of Tissue Engineering (4th ed.) Vacanti RLL. Boston, MA: Academic, 2014, p. 771–792. [Google Scholar]
- 17.Frey N, Linke A, Suselbeck T, Muller-Ehmsen J, Vermeersch P, Schoors D, Rosenberg M, Bea F, Tuvia S, Leor J. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ Cardiovasc Interv 7: 806–812, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, Sacks MS, Keller BB, Wagner WR. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol 49: 2292–2300, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuta A. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res 98: 705–712, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Georgiadis V, Knight RA, Jayasinghe SN, Stephanou A. Cardiac tissue engineering: renewing the arsenal for the battle against heart disease. Integr Biol (Camb) 6: 111–126, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Guyette JP, Song JJ, Chuang W, Ng R, Charest JM, Gaudette GR, Vacanti JP, Ott HC. Decellularizing human hearts: characterizing native cardiac matrix for clinical translation. J Heart Lung Transplant 32: S45, 2015. [Google Scholar]
- 22.Hashizume R, Fujimoto KL, Hong Y, Guan J, Toma C, Tobita K, Wagner WR. Biodegradable elastic patch plasty ameliorates left ventricular adverse remodeling after ischemia-reperfusion injury: a preclinical study of a porous polyurethane material in a porcine model. J Thorac Cardiovasc Surg 146: 391–399. e1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins JA, Hillman ND, Lambert LM, Jones J, Di Russo GB, Profaizer T, Fuller TC, Minich LL, Williams RV, Shaddy RE. Immunogenicity of decellularized cryopreserved allografts in pediatric cardiac surgery: comparison with standard cryopreserved allografts. J Thorac Cardiovasc Surg 126: 247–253, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139: 431–436, e1–e2, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Hibino N, Shinoka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg 129: 1064–1070, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Ichihara Y, Shinoka T, Matsumura G, Ikada Y, Yamazaki K. A new tissue-engineered biodegradable surgical patch for high-pressure systems. Interact Cardiovasc Thorac Surg 20: 768–776, 2015. [DOI] [PubMed] [Google Scholar]
- 27.James I, Yi T, Tara S, Best C, Stuber A, Shah K, Austin B, Sugiura T, Lee YU, Lincoln J, Trask A, Shinoka T, Breuer C. Hemodynamic characterization of a mouse model for investigating the cellular and molecular mechanisms of neotissue formation in tissue engineered heart valves. Tissue Eng Part C Methods 21: 987–994, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126: S29—S37, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Khosravi R, Miller KS, Best CA, Shih YC, Lee YU, Yi T, Shinoka T, Breuer CK, Humphrey JD. Biomechanical diversity despite mechanobiological stability in tissue engineered vascular grafts two years post-implantation. Tissue Eng Part A 21: 1529–1538, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konertz W, Dohmen PM, Liu J, Beholz S, Dushe S, Posner S, Lembcke A, Erdbrugger W. Hemodynamic characteristics of the Matrix P decellularized xenograft for pulmonary valve replacement during the Ross operation. J Heart Valve Dis 14: 78–81, 2005. [PubMed] [Google Scholar]
- 31.Kurobe H, Maxfield MW, Tara S, Rocco KA, Bagi PS, Yi T, Udelsman B, Zhuang ZW, Cleary M, Iwakiri Y, Breuer CK, Shinoka T. Development of small diameter nanofiber tissue engineered arterial grafts. PLos One 10: e0120328, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.L'Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NAF, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 12: 361–365, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.L'Heureux N, McAllister TN, la Fuente de LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med 357: 1451–1453, 2007. [DOI] [PubMed] [Google Scholar]
- 34.L'Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J 12: 47–56, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 117: 1388–1396, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Langer R, Vacanti JP. Tissue engineering. Science 260: 920–926, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Lee RJ, Hinson A, Helgerson S, Bauernschmitt R, Sabbah HN. Polymer-based restoration of left ventricular mechanics. Cell Transplant 22: 529–533, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petnehazy O, Landa N, Feinberg MS, Konen E, Goitein O, Tsur-Gang O, Shaul M, Klapper L, Cohen S. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol 54: 1014–1023, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun 4: 2307, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Maher B. Tissue engineering: how to build a heart. Nature 499: 20–22, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Matsumura G, Ishihara Y, Miyagawa-Tomita S, Ikada Y, Matsuda S, Kurosawa H, Shinoka T. Evaluation of tissue-engineered vascular autografts. Tissue Eng 12: 3075–3083, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Matsuura K, Masuda S, Shimizu T. Cell sheet-based cardiac tissue engineering. Anat Rec (Hoboken) 297: 65–72, 2014. [DOI] [PubMed] [Google Scholar]
- 43.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, la Fuente de LM, L'Heureux N. Effectiveness of haemodialysis access with an autologoustissue-engineered vascular graft: a multicentre cohort study. Lancet 373: 1440–1446, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Miyagawa S, Sawa Y, Sakakida S, Taketani S, Kondoh H, Memon IA, Imanishi Y, Shimizu T, Okano T, Matsuda H. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardium. Transplantation 80: 1586–1595, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Miyagi Y, Chiu LLY, Cimini M, Weisel RD, Radisic M, Li RK. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 32: 1280–1290, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Morsi YS. Bioengineering strategies for polymeric scaffold for tissue engineering an aortic heart valve: an update. Int J Artif Organs 37: 651–667, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, and Stroke Statistics Subcommittee . Heart disease and stroke statistics: 2015 update: a report from the American Heart Association. Circulation 131: e29–322, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Murry CE, Field LJ, Menasché P. Cell-based cardiac repair: reflections at the 10-year point. Circulation 112: 3174–3183, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Naito Y, Imai Y, Shinoka T, Kashiwagi J, Aoki M, Watanabe M, Matsumura G, Kosaka Y, Konuma T, Hibino N, Murata A, Miyake T, Kurosawa H. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J Thorac Cardiovasc Surg 125: 419–420, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Nakayama Y, Takewa Y, Sumikura H, Yamanami M, Matsui Y, Oie T, Kishimoto Y, Arakawa M, Ohmuma K, Tajikawa T, Kanda K, Tatsumi E. In-body tissue-engineered aortic valve (Biovalve type VII) architecture based on 3D printer molding. J Biomed Mater Res B Appl Biomater 103: 1–11, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science 284: 489–493, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14: 213–221, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Prichard HL, Manson RJ, DiBernardo L, Niklason LE, Lawson JH, Dahl SLM. An early study on the mechanisms that allow tissue-engineered vascular grafts to resist intimal hyperplasia. J Cardiovasc Trans Res 4: 674–682, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radisic M, Christman KL. Materials science and tissue engineering: repairing the heart. Mayo Clin Proc 88: 884–898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A, Shimizu T, Okano T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today 42: 181–184, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Scholl FG, Boucek MM, Chan KC, Valdes-Cruz L, Perryman R. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: human applications in congenital heart disease. World J Pediatr Congenit Heart Surg 1: 132–136, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, Kurosawa H, Kobayashi E, Okano T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation 118: S145–S152, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Shinoka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 344: 532–533, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Shinoka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 129: 1330–1338, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, Vacanti JP, Mayer JE. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg 115: 536–546, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, Stamp A, Taylor G, Moran AM, Landis W. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg 68: 2298–2304, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE, Catterall WA. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci USA 106: 16568–16573, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki S, Narita Y, Yamawaki A, Murase Y, Satake M, Mutsuga M, Okamoto H, Kagami H, Ueda M, Ueda Y. Effects of extracellular matrix on differentiation of human bone marrow-derived mesenchymal stem cells into smooth muscle cell lineage: utility for cardiovascular tissue engineering. Cells Tissues Organs 191: 269–280, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Taylor DA, Sampaio LC, Gobin A. Building new hearts: a review of trends in cardiac tissue engineering. Am J Transplant 14: 2448–2459, 2014. [DOI] [PubMed] [Google Scholar]
- 65.van Dijk A, Niessen HWM, Zandieh Doulabi B, Visser FC, van Milligen FJ. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell Tissue Res 334: 457–467, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Voges I, Brasen JH, Entenmann A, Scheid M, Scheewe J, Fischer G, Hart C, Andrade A, Pham HM, Kramer HH, Rickers C. Adverse results of a decellularized tissue-engineered pulmonary valve in humans assessed with magnetic resonance imaging. Eur J Cardiothorac Surg 44: e272–e279, 2013. [DOI] [PubMed] [Google Scholar]
- 67.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods 16: 525–532, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe M, Shinoka T, Tohyama S, Hibino N, Konuma T, Matsumura G, Kosaka Y, Ishida T, Imai Y, Yamakawa M, Ikada Y, Morita S. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng 7: 429–439, 2001. [DOI] [PubMed] [Google Scholar]
- 69.Weber B, Scherman J, Emmert MY, Gruenenfelder J, Verbeek R, Bracher M, Black M, Kortsmit J, Franz T, Schoenauer R, Baumgartner L, Brokopp C, Agarkova I, Wolint P, Zünd G, Falk V, Zilla P, Hoerstrup SP. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J 32: 2830–2840, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science 231: 397–400, 1986. [DOI] [PubMed] [Google Scholar]
- 71.Weymann A, Patil NP, Sabashnikov A, Jungebluth P, Korkmaz S, Li S, Veres G, Soós P, Ishtok R, Chaimow N, Pätzold I, Czerny N, Schies C, Schmack B, Popov AF, Simon AR, Karck M, Szabó G. Bioartificial heart: a human-sized porcine model–the way ahead. PLos One 9: e111591, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu W, Allen RA, Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med 18: 1148–1153, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wystrychowski W, Cierpka L, Zagalski K, Garrido S, Dusserre N, Radochonski S, McAllister TN, L'Heureux N. Case study: first implantation of a frozen, devitalized tissue-engineered vascular graft for urgent hemodialysis access. J Vasc Access 12: 67–70, 2011. [DOI] [PubMed] [Google Scholar]
- 74.Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L, L'Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg 60: 1353–1357, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Xiong Q, Hill KL, Li Q, Suntharalingam P, Mansoor A, Wang X, Jameel MN, Zhang P, Swingen C, Kaufman DS, Zhang J. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells 29: 367–375, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanagawa B, Rao V, Yau TM, Cusimano RJ. Initial experience with intraventricular repair using CorMatrix extracellular matrix. Innovations (Phila) 8: 348–352, 2013. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Y, Feric NT, Thavandiran N, Nunes SS, Radisic M. The role of tissue engineering and biomaterialsin cardiac regenerative medicine. Can J Cardiol 30: 1307–1322, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zimmermann WH, Didié M, Wasmeier GH, Nixdorff U, Hess A, Melnychenko I, Boy O, Neuhuber WL, Weyand M, Eschenhagen T. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation 106: I151–I157, 2002. [PubMed] [Google Scholar]
- 79.Zimmermann WH, Tiburcy M, Eschenhagen T. Cardiac tissue engineering: a clinical perspective. Future Cardiol 3: 435–445, 2007. [DOI] [PubMed] [Google Scholar]