Abstract Abstract
Background
A series of databases is being prepared to list the valid species of Opiliones worldwide. This paper containing nomenclatural acts is meant to accompany Part 2, which includes the members of the infraorder Grassatores of the superfamilies Samooidea and Zalmoxoidea plus the Grassatores currently not allocated to any family (i.e. Grassatores incertae sedis).
New information
The following 32 taxonomic changes are proposed here:
(1-3) The Afrotropical genera Hovanoceros Lawrence, 1959, Malgaceros Lawrence, 1959 and Tetebius Roewer, 1949 (all currently in Samoidae) are all newly transferred to Biantidae.
(4-5) Microminua soerenseni Soares & Soares, 1954, from Brazil is newly transferred to Tibangara (Gonyleptoidea: Cryptogeobiidae), newly combined as Tibangara soerenseni new comb., new familial allocation for the species.
(6-7) The new genus Llaguenia Gen. nov is erected for the South American species Zamora peruviana Roewer, 1956, newly combined as Llaguenia peruviana new comb., and newly placed in Gonyleptoidea: Cranaidae (Prostygninae).
(8) Bebedoura Roewer, 1949, known from a single Brazilian species, is transferred from Tricommatinae to Grassatores incertae sedis.
(9) Microconomma Roewer, 1915, known from a single Cameroonian species, is transferred from Samoidae to Grassatores incertae sedis.
(10) Stygnomimus Roewer, 1927, with two Indomalayan species and hitherto included in the Stygnommatidae, is here formally considered Grassatores incertae sedis.
(11) Bichito González-Sponga, 1998, known from a single Venezuelan species, originally described in Phalangodidae: Phalangodinae, and currently in Grassatores incertae sedis is transferred to Samoidae.
(12) The Neotropical genus Microminua Sørensen, 1932, currently with two species, is newly transferred from Kimulidae to Samoidae.
(13-14) Cornigera González-Sponga, 1987 (currently in Samoidae), is newly considered a junior subjective synonym of Microminua, and its single species is combined under Microminua as Microminua flava (González-Sponga, 1987) new comb.
(15) Niquitaia González-Sponga, 1999 (originally in Phalangodidae: Phalangodinae, currently in Zalmoxidae), monotypic from Venezuela, is newly transferred to Samoidae.
(16) Heteroscotolemon Roewer, 1912 originally described in Phalangodidae: Phalangodinae, and currently in Grassatores incertae sedis is transferred to Zalmoxidae.
(17) While the Australasian genus Zalmoxista Roewer, 1949 is currently in Samoidae and some of its former species have been transferred to Zalmoxis Sørensen, 1886, Zalmoxista americana Roewer, 1952 from Peru, is here newly transferred to Zalmoxidae into Minuides Sørensen, 1932, forming the combination Minuides americanus (Roewer, 1952) new comb. (specific name inflected to match the masculine gender).
(18) Neobabrius Roewer, 1949 (currently in Phalangodidae), monotypic from Indonesia, is newly transferred to Zalmoxidae.
(19) While Crosbyella Roewer, 1927, belongs to Phalangodidae, Crosbyella roraima Goodnight & Goodnight, 1943 (originally Phalangodinae, but currently Zalmoxidae without generic assignment) is here transferred to Soledadiella González-Sponga, 1987, as Soledadiella roraima new comb. (Zalmoxoidea: Zalmoxidae).
(20) Zalmoxissus Roewer, 1949 is newly synonymized with Zalmoxis Sørensen, 1886 (Zalmoxidae).
(21) The original spelling Zalmoxis sorenseni Simon, 1892 is restored from the unjustified emendation soerenseni.
(22) The Neotropical genus Phalangodella Roewer, 1912 (originally in Phalangodidae: Tricommatinae, but currently in Grassatores incertae sedis) is newly transferred to Zalmoxoidea incertae sedis and (23-26) four other genera are newly synonymized with it: Phalangodella Roewer, 1912 = Exlineia Mello-Leitão, 1942 = Langodinus Mello-Leitão, 1949 = Cochirapha Roewer, 1949 = Phalpuna Roewer, 1949, generating the following new combinations (27-32): Phalangodella fulvescens (Mello-Leitão, 1943) new comb., Phalangodella milagroi (Mello-Leitão, 1942) new comb., Phalangodella rhinoceros (Mello-Leitão, 1945) new comb., Phalangodella flavipes (Mello-Leitão, 1949) new comb., Phalangodella rugipes (Roewer, 1949) new comb. and Phalangodella urarmata (Roewer, 1949) new comb.
Keywords: New genus, new familial assignments, new synonymies, genital morphology, Gonyleptoidea , Phalangodoidea , Brazil, Ecuador, Indonesia, Madagascar, Mozambique, Peru, Venezuela
Introduction
This work is a companion to the 2nd data paper in a series containing a checklist of the valid species of harvestmen in the World: "World Checklist of Opiliones species (Arachnida). Part 2: Laniatores – Samooidea, Zalmoxoidea and Grassatores incertae sedis" (Kury et al. 2015). Herein, reasoning for taxonomic decisions is given associated with nomenclatural acts to clarify new taxonomic changes in the list presented in that work.
Harvestmen taxonomy, founded in the classic and typological Roewerian system (e.g. Roewer 1912) has been criticized by several authors. Discarding of the hierarchical weighting of the typical Roewerian morphological diagnostic characters and the use of alternative sources of morphological and molecular information and the biogenetic framework allowed the appearance of a new distinct classification system that caused a revolution in Opiliones systematics.
We are currently in the middle of this systematic reorganization. Modern approaches are very different to the classical ones, and we now typically have a critical amount of new information about the arrangement of the major Opiliones lineages that is highly incongruent with the Roewerian system. But there is a huge impediment – a considerable fraction of harvestmen species are known only from their original and often insufficient descriptions, and their names remain tied to an obsolete taxonomic system. Huge efforts were made by modern opilionologists (e.g. Kury 2003) to utilize the poor information available for an enormous amount of taxa, and to accommodate them into a more natural system. However, this is not enough and many harvestmen still remain as incertae sedis in several taxonomical categories.
For that reason it is not rare for newly written compilations in Opiliones, such as catalogues, checklists, etc., to typically include several taxonomic and nomenclatural changes (e.g. Kury 2003). With critical reading of the published bibliography (mainly the old works), re-examination of each type specimen, examination of male genital morphology drawings, sequencing of DNA, etc. comes important hints to make some of the immeasurable changes needed in this new harvestmen taxonomic system. The current work deals with one of the most challenging and frustrating segments of Opiliones systematics,the superfamilies Samooidea and Zalmoxoidea, which have been extensively worked on in recent years including huge changes in their taxonomy and systematics (e.g. Kury 2003, Sharma and Giribet 2011, Sharma et al. 2011a, Pérez-González and Kury 2007b, Sharma et al. 2011b). Increasing amounts of phylogenetic evidence (e.g. Sharma and Giribet 2011) support both lineages as natural, corroborating their excision from the nonsense Roewerian Phalangodidae – one of the most resilient bastions of the ever crumbling Roewerian system. Likewise, the Samooidea and Zalmoxoidea include the highest number of incertae sedis taxa which still often challenge the work of the harvestmen taxonomists today.
Material and methods
The large collections of our own musems, MNRJ (Museu Nacional/ UFRJ, Rio de Janeiro) and MACN (Museo Argentino de Ciencias Naturales "Bernardino Rivadavia", Buenos Aires), have been complemented along the years by several visits to major repositories in the world. Re-study of type specimens and search for homologies in genital morphology associated with a cladistic framework allowed us to dismantle Roewer's system. The advent of the internet and wide digitalization of literature allowed access to countless rare works, allowing spelling checks, and solidly anchoring checklists.
Optical photograph images were taken along the years with a variety of hardware. Most recent photos have been integrated with the stacker software CombineZP Suite (Hadley 2009) based on preliminary images at different focal planes.
Male genitalia preparation follows Acosta et al. (2007), and were temporarily mounted in glycerol. Penis were drawn using a camera lucida attached to different kinds of compound microscopes. Genitalia ink drawings were digitalized and vectorized using Corel Draw X7. All figures were edited using Photoshop CS5 and Corel Draw X7 softwares.
Abbreviations: AK = Adriano Kury reference number; AMNH = American Museum of Natural History, New York, USA; FNMH = Field Natural History Museum, Chicago, USA; MCNC = Museo de Ciencias Naturales de Caracas, Caracas, Venezuela; MNHN = Muséum national d'Histoire naturelle, Paris, France; MSNG = Museo Civico di Storia Naturale "Giacomo Doria", Genoa, Italy; MZSP = Museu de Zoologia da Universidade de São Paulo, Brazil; SMF = Naturmuseum Senckenberg, Frankfurt am Main, Germany; ZMUC = Universitetets Zoologiske Museum Copenhagen, Denmark.
Results
Biantidae
There are two closely related genera from Madagascar and a third from Eastern Continental Africa which could all be closely related. They are here included in Biantidae.
Hovanoceros Lawrence, 1959, new familial allocation
Hovanoceros Lawrence 1959: 71 [type species: Hovanoceros bison Lawrence, 1959, by original designation].
Hovanoceros bison Lawrence, 1959
Hovanoceros bison Lawrence 1959: 71.
Type data. 2 ♂ syntypes (MNHN, not examined) MADAGASCAR, Ambodivoangi, Maroantsetra.
Historical systematic background. Lawrence (1959) described the monotypic Hovanoceros from Madagascar, including in it in the Phalangodidae: Samoinae, along with Malgaceros and Anaceros. Staręga (1992) included only Anaceros in Biantidae (probably because of the lack of a common ocularium), leaving the other two in Samoidae. There are a number of undescribed species currently under study.
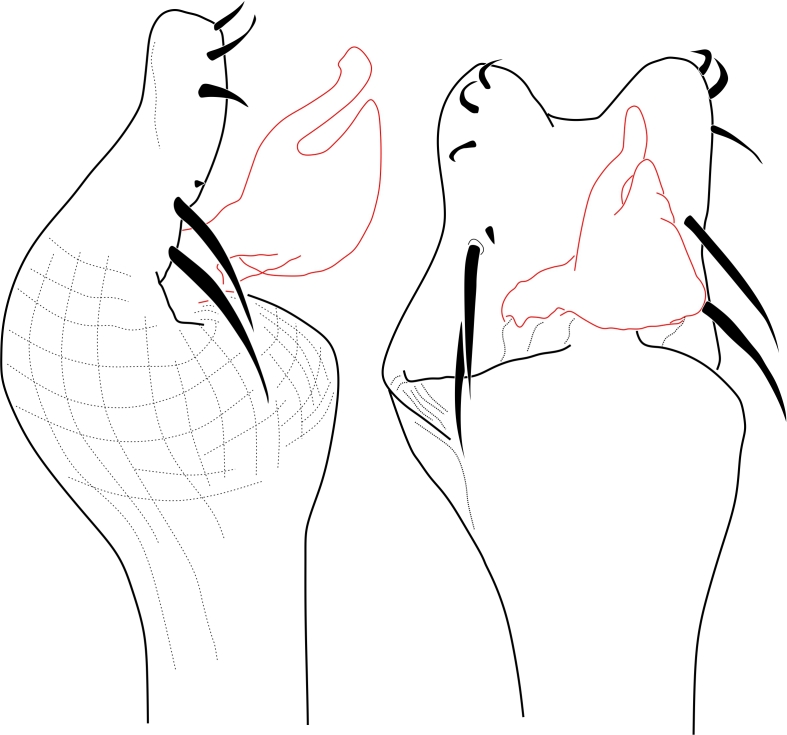
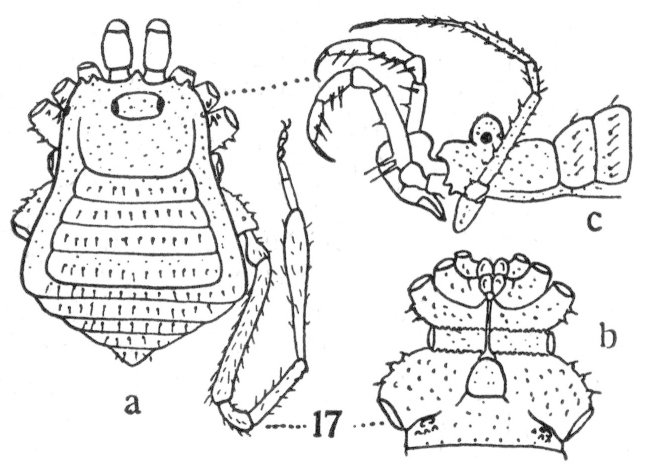
Rationale for the new familial allocation. External features include a huge protuberant ocularium and an unusual scutal armature, but they do not point conclusively to any specific family (See Fig. 1. Male genitalia presents an eversible stylus, flanked by a pair of rigid laminar conductors. The capsula externa consists of a bifid pair of soft titillators, with multiple digitiform apical lobules. This pattern doesn't present any known condition in Samoidae and perfectly matches that of Biantidae (Fig. 2, and is herein transferred into the family Biantidae.
Figure 1.
Biantidae, Hovanoceros sp., from Madagascar (FMNH AK 093), habitus (without chelicerae and pedipalps), lateral view. Photo by A.B. Kury (ABK). Scale bar = 0.5 mm.
Figure 2.
Biantidae, Hovanoceros sp. from Madagascar (FMNH AK 093) penis, distal portion, dorsal view. Drawing by ABK. Blue = titillators; red = conductors. Scale bar = 0.02 mm.
Malgaceros Lawrence, 1959, new familial allocation
Malgaceros Lawrence 1959: 79 [type species: Malgaceros boviceps Lawrence, 1959, by original designation].
Malgaceros boviceps Lawrence, 1959
Malgaceros boviceps Lawrence 1959: 80.
Type data. 3 ♂ 1 ♀ syntypes (MNHN, not examined), MADAGASCAR, Nosy Be.
Background and allocation. Malgaceros shares the same history in the literature as Hovanoceros.
Rationale for the new familial allocation. It is clearly closely related to Hovanoceros as mentioned in the original description, and is here transferred into the same family Biantidae.
Tetebius Roewer, 1949, new familial allocation
Tetebius Roewer 1949a: 288 [type species: Tetebius latibunus Roewer, 1949, by original designation].
Historical systematic background. Tetebius is a monotypic genus with a species from Mozambique, which was originally placed in Phalangodidae: Phalangodinae. It was removed from the Phalangodidae but not placed anywhere else by Staręga (1989). Then it was transferred to Samoidae by Staręga (1992).
Rationale for the new familial allocation. Tetebius latibunus has not been examined by us. External features are inconclusive, but the sketch illustration of the penis as drawn by Roewer (1942a) in ventral view appears close to that of Hovanoceros (See Fig. 3), and so Tetebius is here transferred into the same family Biantidae.
Figure 3.
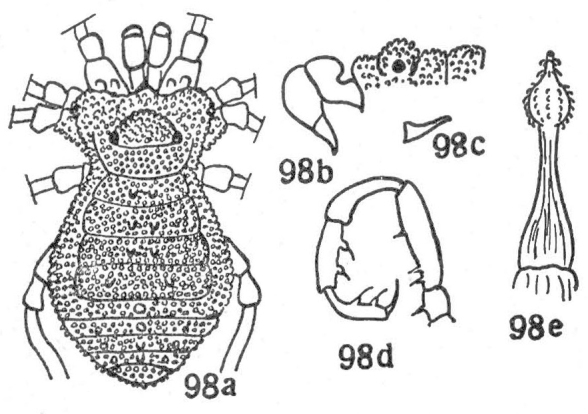
Biantidae, Tetebius latibunus Roewer, 1949, from Mozambique. From original description.
Cryptogeobiidae
As Cryptogeobiidae are members of the Gonyleptoidea, they are not treated in the Part 2 of this series, but rather in Part 5 (Lesser Gonyleptoidea).
Tibangara Mello-Leitão, 1940
Tibangara Mello-Leitão 1940: 100 [type species: Tibangara nephelina Mello-Leitão, 1940, by original designation].
Taxonomic background. Tibangara was recently transferred from Phalangodidae to Cryptogeobiidae (Kury 2014).
Tibangara soerenseni (Soares & Soares, 1954) new comb., new familial allocation
Microminua soerenseni Soares and Soares 1954: 503, fig. 13.
Gen. sp. W: Kury 2014: 11.
Type data. ♀ holotype (MZSP 842, examined), ♀ paratypes (MZSP 833, examined), from BRAZIL, Rio de Janeiro, Rio de Janeiro, Corcovado.
Rationale for the new familial and generic allocation of the species. Males and females of this species have been examined from the type locality (as well as the types) and based both on external features as in male genitalia they are close relatives of Tibangara nephelina. Their similarity with Kimulidae is only superficial (Fig. 4).
Figure 4.
Cryptogeobiidae, Tibangara soerenseni (Soares & Soares, 1954) comb. nov. Non-type male (MNRJ 8184), from Rio de Janeiro, Brazil. Habitus, dorsal view. Photo by Daniele R. Souza (with permission).
Cranaidae
Cranaidae: Prostygninae
Llaguenia Gen. nov. /new genus
Type species. Zamora peruviana Roewer, 1956.
Etymology. Generic name derives from Hacienda Llaguén in Peru, the collection locality of the type species.
Diagnosis. In both sexes, dorsal scutum outline alpha with coda notably elongate and slightly divergent. Mesotergum divided into four areas. Area I divided into left and right halves. Scutum and free tergites smooth and unarmed. Ocularium extremely narrow. Scutal groove U-shaped, notably elongate. Cheliceral hand of male swollen. Pedipalpal femur cylindrical, unarmed ventrally. Coxa IV outline widely surpasses dorsal scutum in dorsal view, and parallel to main body axis. Femur IV of male incrassate and armed with row of eight proventral spines. Penis ventral plate subrectangular, with lateral and apical wide concavities, three pairs of small macrosetae (MS) C, one pair of small MS D, and two pairs of very long MS A. Stylus sinuous, with slightly squared head, and well developed thumb-like dorsal process. Cutervolus and Prostygnus have a sexually dimorphic ocularium sexually dimorphic, hugely developed in males, scutal area III armed with powerful erect acuminate spines, free tergite III with paired spines, cheliceral hand immensely swollen, pedipalpal femur with ventral row of spines (Prostygnus only), MS A short, distal border of ventral plate straight (Prostygnus) or strongly convex (Cutervolus).
Llaguenia peruviana (Roewer, 1956), new comb.
Zamora peruviana Roewer 1956: 436, figs. 8–9.
Type data. ♂ holotype (SMF RII 9701, examined), 1 ♀ paratype (SMF RII 9702, examined), PERU, La Libertad, Hacienda Llaguén, forest of Rejo Cargaruay, 2650 m.
Rationale for the new familial allocation. The subfamily Prostygninae is currently in Cranaidae (Kury 2012). The holotype of Zamora peruviana has been examined, and although it has an apparent metasarcid facies (Fig. 5), male genitalia strongly resembles the ones depicted for Prostygninae in Kury (2012) (Compare Fig. 6). Zamora is no longer considered to be a cranaid (see Kury and Villarreal M. 2015), and L. peruviana does not have any special similarity to grant its permanence in that genus. Consequently, this species does not belong in any of the superfamilies so far treated in this planned series of papers, nor should remain incertae sedis anymore, and hereby formally transferred to Gonyleptoidea: Cranaidae (Prostygninae).
Figure 5.
Cranaidae: Prostygninae, Llaguenia peruviana (Roewer, 1956), male holotype (SMF RII 9701) from Peru. Habitus, lateral view. Photo by A. Pérez-González (APG).
Figure 6.
Cranaidae Prostygninae, Llaguenia peruviana Roewer, 1956, male holotype (SMF RII 9701), from Peru. Pars distalis of penis, lateral and dorso-lateral views. Drawing by APG/ABK. Red = glans.
Assorted Grassatores
Phalangodidae was once a great repository of diverse species (e.g. Roewer 1912), with no clear monophyly. Revision is increasingly showing that many genera originally described in Phalangodidae: Phalangodinae the mid-20th century should be allocated elsewhere (e.g. Staręga 1989), diminishing this family to a natural Holarctic natural core. Of these "false phalangodines", Beloniscus and closely allied genera are currently under study for inclusion into another family, and so are not treated here.
Grassatoresincertae sedis
Bebedoura Roewer, 1949
Bebedoura Roewer 1949c: 56 [type species: Bebedoura rugosa Roewer, 1949, by original designation].
Bebedoura rugosa Roewer, 1949
Bebedoura rugosa Roewer 1949c: 56, figs 108a-d; Kury 2003: 201.
Type data. ♂ holotype (SMF RII 6897/17, examined), from BRAZIL, Pernambuco, Bebedouro (wrongly cited as in "São Paulo" state in).
Historical systematic background. Bebedoura was originally included in Phalangodidae: Tricommatinae. A recent cladistic analysis of this group (Kury 2014) detected that Tricommatinae as then recognised consisted of two divergent groups (Gonyleptidae: Tricommatinae versus Cryptogeobiidae) plus some extraneous genera of gonyleptids of uncertain placement, such as Bebedoura. However, Kury was inconsistent because he listed Bebedoura among the Gonyleptidae incertae sedis, and at the same time commented "probably Escadabiidae", which are mutually excluve placements.
Rationale for the removal from Tricommatinae. The female holotype of Bebedoura rugosa has been studied by ABK (see Figs 7, 8), but it failed to provide any evidence for a sure familial placement. The ocularium is seamlessly prolonged into a huge forward bent cone with a secondary branch found only in Podoctidae. Leg IV vaguely resembles some Zalmoxidae because of its curved femur armed with a retrodistal apophysis (females usually do not possess those in many families). The Cryptogeobiidae which possess similar ocularia (e.g., Pseudopachylus, Tibangara) do not share any other special similarity with Bebedoura. There are some Gonyleptidae (Pachylinae) with females also known to bearmed on leg IV, while a divided area I occurs in many Gonyleptoidea. Consequently, Bebedoura is here formally transfered to Grassatores incertae sedis (See Figs 7, 8).
Figure 7.
Grassatores, Bebedoura rugosa Roewer, 1949, female holotype (SMF RII 6897/17), from Pernambuco, Brazil. Habitus, dorsal view. Photo by Tiago N. Bernabé. Scale bar = 1 mm.
Figure 8.
Grassatores, Bebedoura rugosa Roewer, 1949, female holotype (SMF RII 6897/17), from Pernambuco, Brazil. Habitus, lateral view. Photo by Tiago N. Bernabé (with permission). Scale bar = 1 mm.
Microconomma Roewer, 1915
Microconomma Roewer 1915: 12; Roewer 1949c: 58 [type species: Microconomma armatipes Roewer, 1915, by monotypy].
Microconomma armatipes Roewer, 1915
Microconomma armatipes Roewer 1915: 12; Roewer 1927: 84, fig. 81; Roewer 1949c: 58.
Type data. ♂ holotype (SMF RI 1131, not examined), from CAMEROON, Kamerun Mountains, Bakossu, 400 m.
Historical systematic background. Monotypic genus, with one species from Cameroon. Microconomma was originally placed in Phalangodinae. It was transferred by Roewer (1949c) to the subfamily Samoinae (since revised as Samoidae). Staręga (1989) listed it in an as then undescribed family, which only much later would be described as Pyramidopidae (Sharma et al. 2011b). But Sharma et al. (2011a) kept this genus in Samoidae.
Rationale for the removal from Samoidae. At the moment we have no evidence to support either previously suggested assignment and so transfer this genus to Grassatores incertae sedis. The original description is too superficial to allow any judgement, but the species in question, as illustrated, doesn't fit the typical Samoidae habitus as defined by Pérez-González and Kury (2007b). On the other hand, the well-defined cheliceral bulla points away from the Samoidae, and more to the Zalmoxoidea. Therefore, it is more sensible for now to list this taxon as Grassatores incertae sedis.
Stygnomimus Roewer, 1927
Stygnomimus Roewer 1927: 305 [type species: Stignomimus conopygus Roewer, 1927 by monotypy].
Stygnomimus conopygus Roewer, 1927
Stignomimus conopygus Roewer 1927a: 305.
Type data. ♂ holotype (SMF RII/63/1, examined), [Indonesia, Riau Islands], Riouw Archipelago.
Stygnomimus malayensis Suzuki, 1969
Stignomimus malayensis Suzuki 1969: 32.
Type data. ♀ holotype (not examinined)Templer Park, Malaysia.
Historical systematic background The original assignment of this Indomalayan genus to Stygnommatidae by Roewer (1927) was made based on apparently trivial characters such as the absence of a common ocularium. Suzuki (1969) provided a beautiful description of a second species, unfortunately known only from a female.
Rationale for the removal from Stygnommatidae. There is no positive evidence yet to assign the genus to Stygnommatidae or even to Samooidea. Pérez-González (2006) suggested it should be set apart until further study, and this opinion is here formalized, so transferred to Grassatores insertae sedis.
Samoidae
Bichito González-Sponga, 1998, new familial assignment
Bichito González-Sponga 1998: 29 [type species: Bichito pijiguaoensis González-Sponga, 1998, by original designation].
Bichito pijiguaoensis González-Sponga, 1998
Bichito pijiguaoensis González-Sponga 1998: 29, figs 8–14; Kury 2003a: 23.
Type data. ♂ holotype (MAGS 1222a, not examined); 1 ♀ paratype (MAGS 1222b, not examined); 5 ♂ 5 ♀ 3 juv. paratypes (MAGS, not examined), from VENEZUELA, Bolívar, Cedeño: Near bauxite mines of Los Pijiguaos, 06°35’20’’N, 66°45’12’’W, 80 m.
Historical systematic background. Originally described in Phalangodinae, removed to Grassatores incertae sedis by Kury (2003).
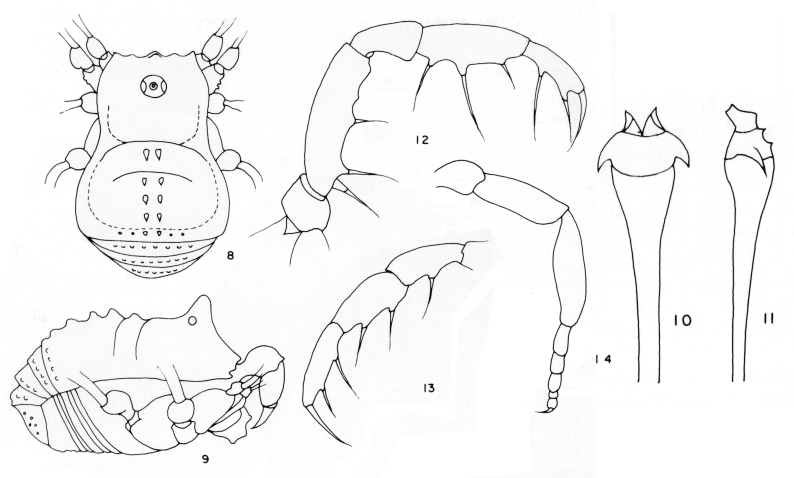
Rationale for the new placement. The original drawings of the male genitalia are poorly detailed, but they allow recognition of an everted penis with two conductors, capsula interna and glans without stragulum or modified follis (Fig. 9). This genital morphology, combined with the remarkable sexual dimorphism of enlarged metatarsus III in males supports the transferrence of this species to Samoidae (Fig. 9).
Figure 9.
Samoidae, Bichito pijiguaoensis González-Sponga, 1998, from Venezuela. From original description.
Microminua Sørensen, 1932, new familial assignment
Microminua Sørensen in Henriksen 1932: 245 [type species: Microminua parvula Sørensen, 1932, by monotypy].
Cornigera Gonzalez-Sponga 1987: 86 [type species: Cornigera flava González-Sponga, 1987, by original designation]. NEW SYNONYMY
Rationale for the new placement. The new family placement is fully supported by the male genital groundplan where the truncus is cylindrical, without a well-defined ventral plate as in Gonyleptoidea, with pars distalis, compressed dorsoventrally, not differentiated from pars basalis by any remarkable groove or constriction and laterally armed with strong spatulate (foliar) spines. The capsula interna is eversible and formed by a pair of conductors completely fused. The follis is not modified into a stragulum and not observed externally. This genital morphology matches the penial groundplan described for Samoidae by Pérez-González and Kury (2007b). The absence of the Kimulidae penial morphology where pars distalis has a very peculiar form with the lamina ventralis surrounding the capsula interna (conductors + stylus) avoids keeping this genus in Kimulidae.
Microminua flava (González-Sponga, 1987), new comb.
Cornigera flava Gonzalez-Sponga 1987: 86, figs. 58-63.
Type data. ♂ holotype (MCNC, not examined), VENEZUELA, Miranda, Zamora: Salmerón, 250 m.
Microminua parvula Sørensen, 1932
Microminua parvula Sørensen in Henriksen 1932: 245, fig. 8.
Type data. 30 ♂ ♀ syntypes (ZMUC; subsample at SMF RII/6237/165-4, examined), from VENEZUELA, Distrito Federal, Hacienda La Moka, right margin of Siquire River, 10 km NE Santa Lucia on road Caracas-Santa Lucia.
Historical systematic background. Microminua was originally established in Minuidae by Sørensen (Henriksen 1932) along with the type species Microminua parvula Sørensen, 1932 from Venezuela. It was removed to Phalangodidae: Phalangodinae by Mello-Leitão (1938). Soares and Soares (1954) described a second species Microminua soerenseni Soares & Soares, 1954 from Rio de Janeiro, Brazil, based on a single female. Gonzalez-Sponga (1987) described in Phalangodinae the monotypic genus Cornigera, along with the species Cornigera flava, from Miranda, Venezuela. Kury (2003) transferred Cornigera from Phalangodinae to Samoidae, and Microminua from Phalangodinae back to Minuidae.
Rationale for the generic synonymy. The male genitalia of the type material of Microminua parvula (syntypes in SMF and ZMUC (See Fig. 10) have been examined for the first time, and they match closely to those of Cornigera flava (Fig. 12. Also the external features (Fig. 11 of the type species of both alleged genera are a near perfect match, not to mention their geographic distribution, as both species are from Venezuelan coastal range.Consequently, Cornigera is considered a junior synonym of Microminua, with two valid species in the family Samoidae.
Figure 10.
Samoidae, Microminua parvula Sørensen, 1932, genitalia of male syntype (SMF RII 6237), from Venezuela. Drawing by APG/ABK. Blue = capsula externa, red = capsula interna.
Figure 12.
Samoidae, Cornigera flava González-Sponga 1987, from original description.
Figure 11.

Samoidae, Microminua parvula Sørensen, 1932, male syntype (SMF RII 6237), from Venezuela. Photo by APG.
Niquitaia González-Sponga, 1999, new familial assignment
Niquitaia González-Sponga 1999: 64 [type species: Niquitaia convexa González-Sponga, 1999, by original designation].
Niquitaia convexa González-Sponga, 1999
Niquitaia convexa González-Sponga 1999: 64, figs 19–24; Kury 2003: 246.
Type data. ♂ holotype (MAGS 1254a, not examined); 1 ♀ paratype (MAGS 1254b, not examined); 1 ♂ 3 ♀ paratypes (MAGS, not examined), VENEZUELA, Trujillo, Boconó: km 4 road Boconó-Niquitao, 1400 m.
Historical systematic background. Niquitaia was originally established in Phalangodidae: Phalangodinae along with the type species Niquitaia convexa from Venezuela. It was removed to Zalmoxidae by Kury (2003).
Rationale for the new placement. The external morphology is very similar to Kalominua Sørensen, 1932 (Samoidae): body as an asymmetrical hourglass, anterior half of scutum much shorter, posterior half rounded, laterally convex appearance, male genitalia without stragulum, with pars distalis flattened dorso-ventrally with ventral region wide, undivided and armed with strong macrosetae (Fig. 13). All of the above characters justify the inclusion of Niquitaia in Samoidae.
Figure 13.
Samoidae, Niquitaia convexa González-Sponga, 1999, from Venezuela. From original description.
Zalmoxidae
The Zalmoxidae are here augmented by the inclusion of two genera originally described in Phalangodidae and one species, originally included in a genus of Samoidae.
Heteroscotolemon Roewer, 1912 new familial assignment
Heteroscotolemon Roewer 1912: 150; Kury 2003: 24 [type species: Heteroscotolemon australis Roewer, 1912, by monotypy].
Heteroscotolemon australis Roewer, 1912
Heteroscotolemon australis Roewer 1912: 151, fig. 34; Kury 2003: 24.
Type data. ♀ [originally reported as ♂] holotype (SMF RI/207, examined), from “Guayana: Nieder-Oyopock” [FRENCH GUYANA, Lower Oyapock River, which marks the fro ntier with Brazil].
Historical systematic background. Heteroscotolemon was originally included in Phalangodinae, then transferred to Grassatores incertae sedis by Kury (2003).
Rationale for the new placement. Evidence for the new placement as a large zalmoxid comes from the backward pointed scutal grooves, bimerous distitarsus I, strongly armed pedipalpal trochanter and femur. All other Laniatores recorded from French Guyana are either Gonyleptoidea, which typically have distitarsus I trimerous, or Stenostygninae, which do not have a common ocularium. Heteroscotolemon australis is very different from Parascotolemon ornatum Roewer, 1912, a typical local zalmoxid (See Figs 14, 15). However, here Heteroscotolemon is formally transferred to Zalmoxidae, pending its further study.
Figure 14.
Zalmoxidae, Heteroscotolemon australis Roewer, 1912, female holotype (SMF), habitus, dorsal view. Photo by APG.
Figure 15.
Zalmoxidae, Heteroscotolemon australis Roewer, 1912, female holotype (SMF), habitus, lateral view. Photo by APG.
Minuides Sørensen, 1932
Minuides Sørensen in Henriksen 1932: 237 [type species: Minuides setosa Sørensen, 1932, by monotypy].
Minuides americanus (Roewer 1952), new comb.
Zalmoxista americana Roewer 1952: 40.
Type data. ♀ holotype (SMF RII 10226/240, not examined), PERU, Pasco, Laguna Punrun, 4400 m, near Cerro do Pasco in drainage of Junin Lake.
Historical systematic background. Roewer (1949a) originally created Zalmoxista in Phalangodinae to include two species from Australia and one from New Caledonia. Later he added a fourth species from Peru (Zalmoxista americana Roewer, 1952), in a very brief unillustrated description. Kury (2003) treated this Peruvian “Zalmoxista” americana as Grassatores incertae sedis. The type species, Phalangodes australis Sørensen, 1886, was transferrred to Samoidae by Pérez-González and Kury 2007a, but the other two Australasian species were included in Zalmoxis by Sharma (2012).
Rationale for the new placement. The identity of "Zalmoxista" americana is highly doubtful, but it may be recognized as a Zalmoxidae. Another zalmoxid genus which has the same tarsal counts and the same conformation of scutal areas is Minuides, which currently includes several Neotropical species of doubtful monophyly. Therefore this species is here included in Minuides (Zalmoxidae) pending further study.
Neobabrius Roewer, 1949, new familial assignment
Neobabrius Roewer 1949c: 19 [type species: Neobabrius parvulus Roewer, 1949, by original designation].
Neobabrius parvulus Roewer, 1949
Neobabrius parvulus Roewer 1949c: 19, figs 17a-c.
Type data. ♂ holotype, 1 ♂ 1 ♀ paratypes (SMF RII 3138/79, not examined), from INDONESIA, Jawa Timur, Bawean Island.
Historical systematic background. This monotypic genus was originally in Phalangodinae and represents a neglected taxon. Goodnight and Goodnight (1957) synonymized a great number of genera with Zalmoxis, but overlooked Neobabrius. Staręga (1989) when resurrecting Zalmoxidae, gave a list of Phalangodinae of obscure systematic position, but Neobabrius was again not included. It was neither mentioned in Sharma et al. (2011a).
Rationale for the new placement. Roewer's description of Neobabrius parvulus (Fig. 16) allows us to recognize a body structure extremely similar to Zalmoxis heynemani Suzuki, 1977 (Fig. 17), and as a typical zalmoxid due the genitalic features, Fig. 17 outline of dorsal scutum, shapes of areas and ocularium, proportion of coxae and stigmatic areas, spination of pedipalps, and metatarsus IV incrassate. Therefore Neobabrius is here transferred to Zalmoxidae. Old World Zalmoxidae are currently included in only one genus Zalmoxis, but we do not wish to propose any additional changes yet, leaving the Indonesian Neobabrius as a separate valid genus of Zalmoxidae for now.
Figure 16.
Zalmoxidae, Neobabrius parvulus Roewer, 1949, from Indonesia, Bawean Island. From original description.
Figure 17.
Zalmoxidae, Zalmoxis heynemani Suzuki, 1977, from Philippines, Mindanao. From original description.
Soledadiella González -Sponga, 1987
Soledadiella Gonzalez-Sponga 1987: 304 [type species: Soledadiella barinensis González-Sponga, 1987, by original designation].
Soledadiella roraima (Goodnight and Goodnight, 1943), new comb.
Crosbyella roraima Goodnight and Goodnight 1943: 1, figs. 1–4.
Pellobunus roraima:Goodnight and Goodnight 1947: 5.
“Crosbyella” roraima: Kury 2003: 241.
Type data. ♀ holotype (AMNH, examined only by photograph), from [VENEZUELA, Bolívar], “Rondon Camp, Mt. Roraima, 6900 feet.”
Historical systematic background. This species from the border of Brazil and Venezuela has originally been assigned to Crosbyella (Phalangodinae) and then Pellobunus (Samoidae). Kury (2003) recognized it as a zalmoxid, but refrained from indicating a particular genus. There are a great number of described Venezuelan species of Zalmoxidae, mostly placed in monotypic genera of no meaning.
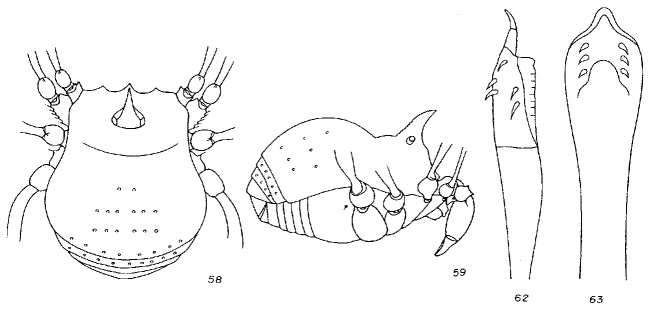
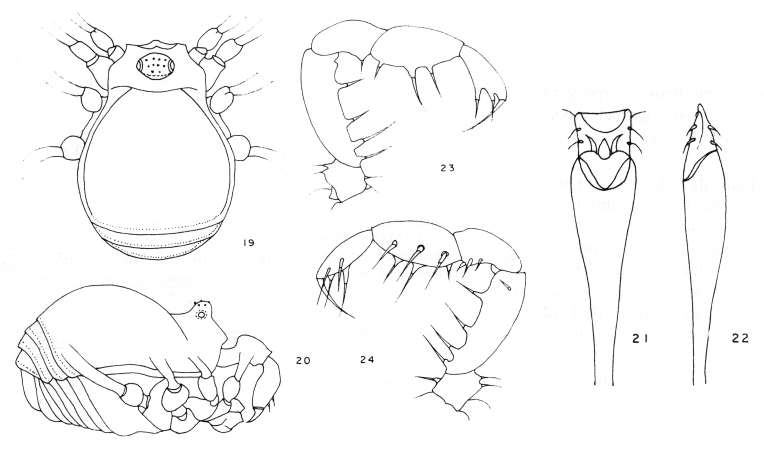
Rationale for the placement. Examining two pictures of the female holotype of C. roraima (in AMNH, photos courtesy of R. Pinto-da-Rocha), suggests placement in the genus Soledadiella: Ocularium not greatly developed and situated far from margin of carapace; abdominal scutum much larger than carapace, widening posteriorly, with convex sides without constriction, its posterior border straight; scutal area I slightly longer than each of the others; scutal grooves gently curved, pointing backwards; scutal areas and free tergites densely covered by coarse rounded granules, while carapace is smooth. (See Figs 18, 19). The tarsal counts of Soledadiella roraima nov. comb. are 4/6/5/6, while the formula for typical Soledadiella is 5/6/5/6. However, here S. roraima is treated as Soledadiella (Zalmoxidae).
Figure 18.

Zalmoxidae, Soledadiella roraima (Goodnight & Goodnight, 1943), female holotype (AMNH), habitus, dorsal view. Photo by Ricardo Pinto-da-Rocha (with permission).
Figure 19.

Zalmoxidae, Soledadiella roraima (Goodnight & Goodnight, 1943), female holotype (AMNH), habitus, dorso-lateral view. Photo by Ricardo Pinto-da-Rocha (with permission).
Zalmoxis Sørensen, 1886
Zalmoxis Sørensen 1886: 64 [type species: Zalmoxis robusta Sørensen, 1886 by subsequent designation of Roewer (1949c)].
Zalmoxissus Roewer 1949b: 143 [type species: Zalmoxis tristis Thorell, 1891, by original designation]. NEW SYNONYMY
Historical systematic background. Roewer (Roewer 1949c, Roewer 1949a) founded innumerous Palaeotropic genera close to Zalmoxis based on minimal variations of scutal armature and tarsal segmentation.
Fundamentation of the synonymy. All of those but the monotypic Zalmoxissus were synonymized under Zalmoxis by Sharma et al. (2011a), or Sharma (2012). We believe it was an oversight (especially because the name was published in a short, obscure paper), as Zalmoxissus tristis (Thorell, 1891) matches the current diagnosis of Zalmoxis and therefore we also propose Zalmoxissus as a further junior synonym of Zalmoxis (Zalmoxidae).
Zalmoxis sorenseni Simon, 1892 original spelling restored
Zalmoxis sorenseni Simon 1892: 44, pl. 2, figs. 7–8.
Zalmoxis soerenseni [unjustified emended spelling]: Roewer 1912: 129; Sharma et al. 2011a: 53.
Comment. Zalmoxis sorenseni Simon, 1892 appears in the literature wrongly as Zalmoxis soerenseni Simon, 1892. The change of spelling proposed by Roewer 1912 to better reflect the Latin rendering of the Danish name Sørensen, is an incorrect subsequent spelling. ICZN (article 32.5.1.) says: "If there is in the original publication itself, without recourse to any external source of information, clear evidence of an inadvertent error, such as a lapsus calami or a copyist's or printer's error, it must be corrected. Incorrect transliteration or latinization, or use of an inappropriate connecting vowel, are not to be considered inadvertent errors."
Zalmoxissus tristis (Thorell, 1891)
Zalmoxis tristis Thorell 1891: 750.
Zalmoxida tristis: Roewer 1912: 134.
Type data. ♂(?) holotype (MCSN) from PAPUA NEW GUINEA, National Capital District, Yule Island (“Yule-Roro”).
Zalmoxoidea (no familial inclusion)
Phalangodella Roewer, 1912, new superfamilial assignment
Phalangodella Roewer 1912: 160 [type species: Phalangodella aequatorialis Roewer, 1912, by monotypy].
Erxlineia Mello-Leitão 1942: 315 [original incorrect spelling; lapsus calami].
Exlineia Mello-Leitão 1943: 2 [type species: Exlineia milagroi Mello-Leitão, 1942, by original designation]. Syn. nov.
Langodinus Mello-Leitão 1949: 7 [type species: Langodinus flavipes Mello-Leitão, 1949, by original designation]. Syn. nov.
Cochirapha Roewer 1949c: 40 [type species: Cochirapha rugipes Roewer, 1949, by original designation]. Syn. nov.
Phalpuna Roewer 1949c: 41 [type species: Phalpuna urarmata Roewer, 1949, by original designation]. Syn. nov.
Historical systematic background. The monotypic genus Phalangodella was originally placed in Phalangodidae Tricommatinae. It was transferred to Grassatores incertae sedis by Kury (2003). A number of species have been subsequently described under Phalangodella, but they are all currently included in Cryptogeobiidae (Kury 2014). Exlineia Mello-Leitão, 1942 and Cochirapha Roewer, 1949c were originally placed in Phalangodidae: Phalangodinae. Both were transferred to Zalmoxidae by Kury (2003). Langodinus Mello-Leitão, 1949 and Phalpuna Roewer, 1949c were originally placed in Phalangodidae: Phalangodinae. Both were removed to Grassatores incertae sedis by Kury (2003).
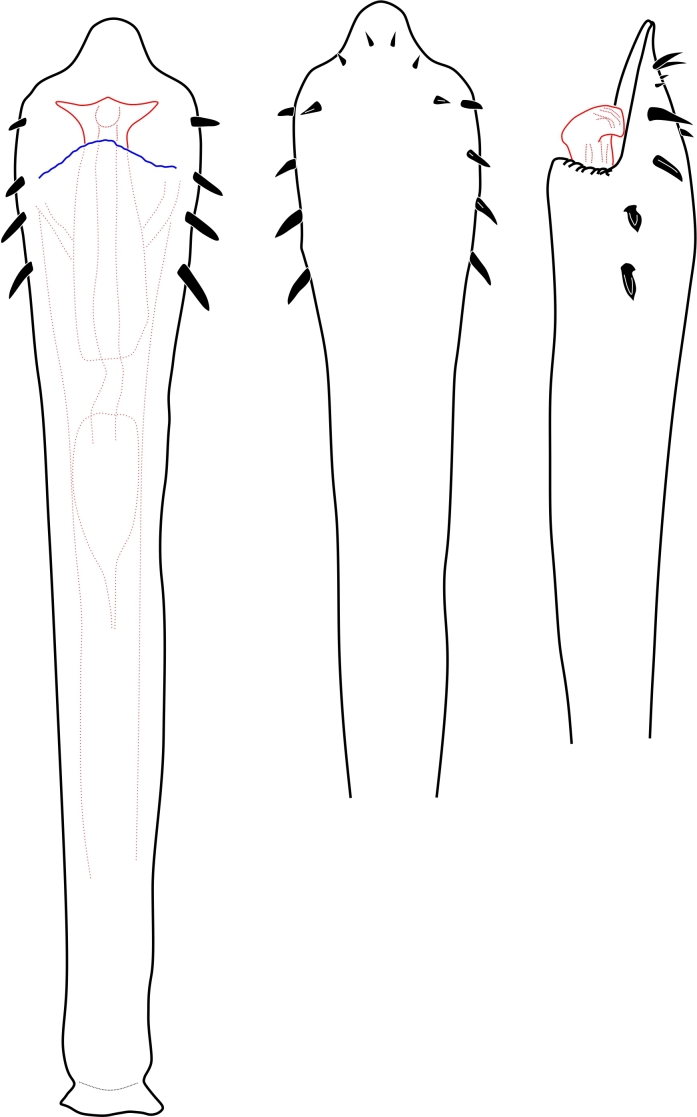
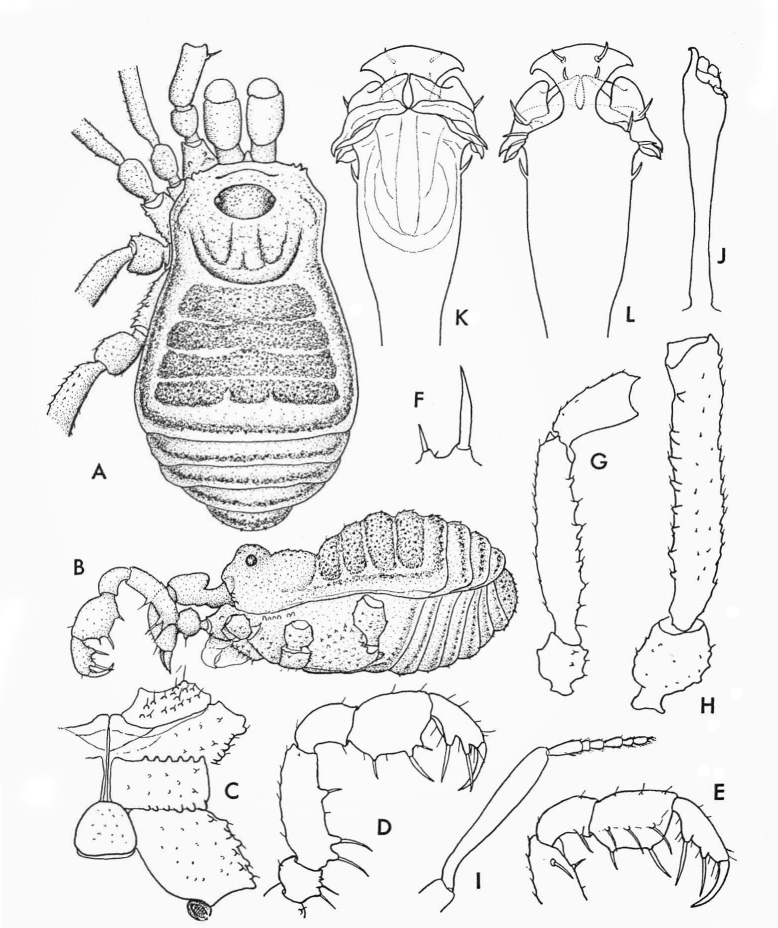
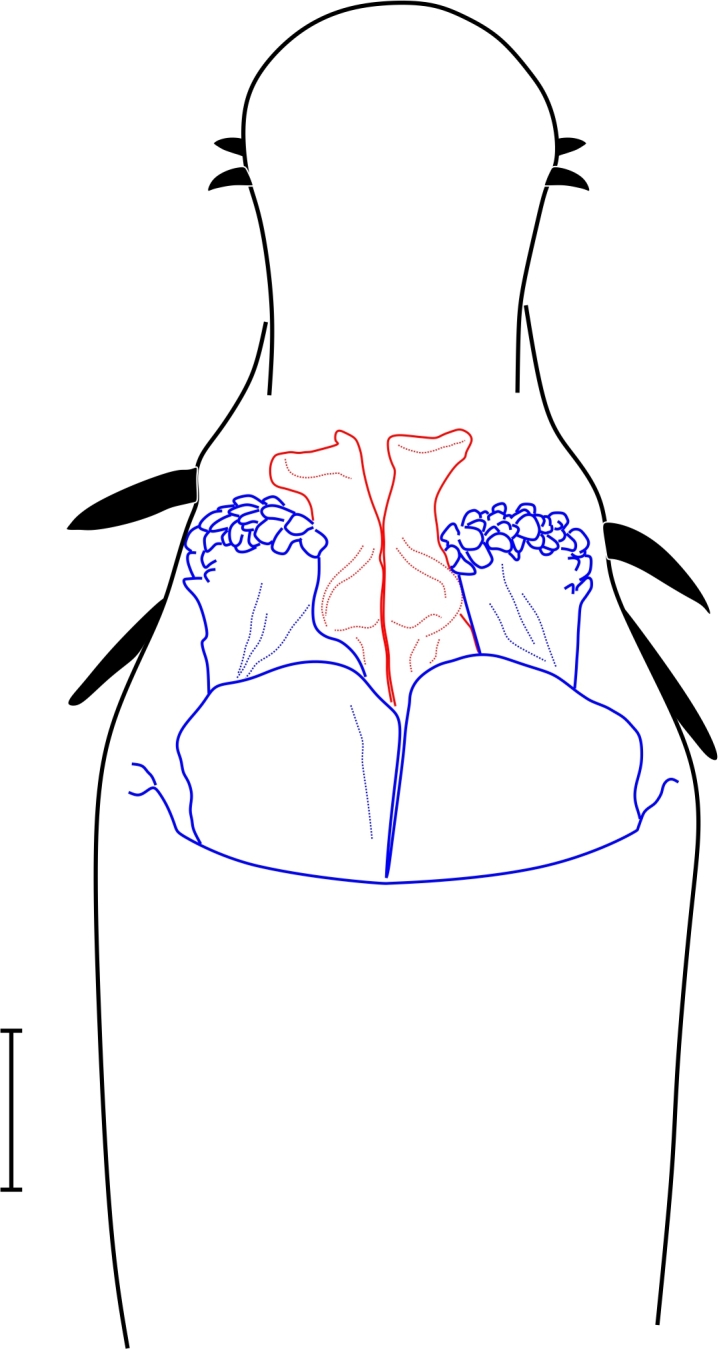
Rationale for the placement. The male genitalia of Phalangodella sp. has a jackknife structure exclusive to Zalmoxoidea with a foldable capsula externa (stragulum). The presence of a rudimentary pergula and rutrum justifies its inclusion in Zalmoxoidea, closest to Zalmoxidae and Icaleptidae, although it is not a perfect match with either (Figs 20, 21). The five genera here included in synonymy all share the same body plan (judging by the information available in the literature), and only have been separated in the past due to the oversplitting nature of the Roewerian system. Fig. 22 The extremely narrow marginal ocularium is easily recognizable (e.g. Fig. 22), even in the sketch drawings of the previous literature. The revised genus Phalangodella is here transferred to Zalmoxidae.
Figure 20.
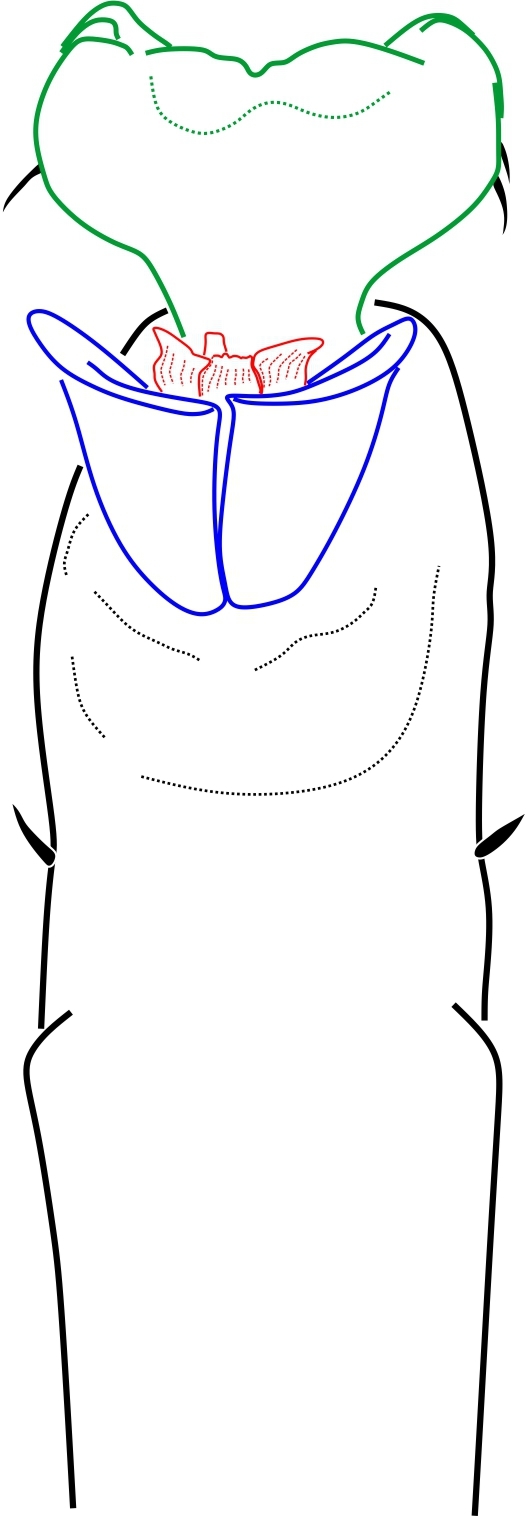
Zalmoxoidea, Phalangodella sp., male (MNRJ 2438) from Ecuador. Penis, expanded, dorsal view. Drawing by APG/ABK. Blue = stragulum; green = rutrum; red = capsula interna.
Figure 21.
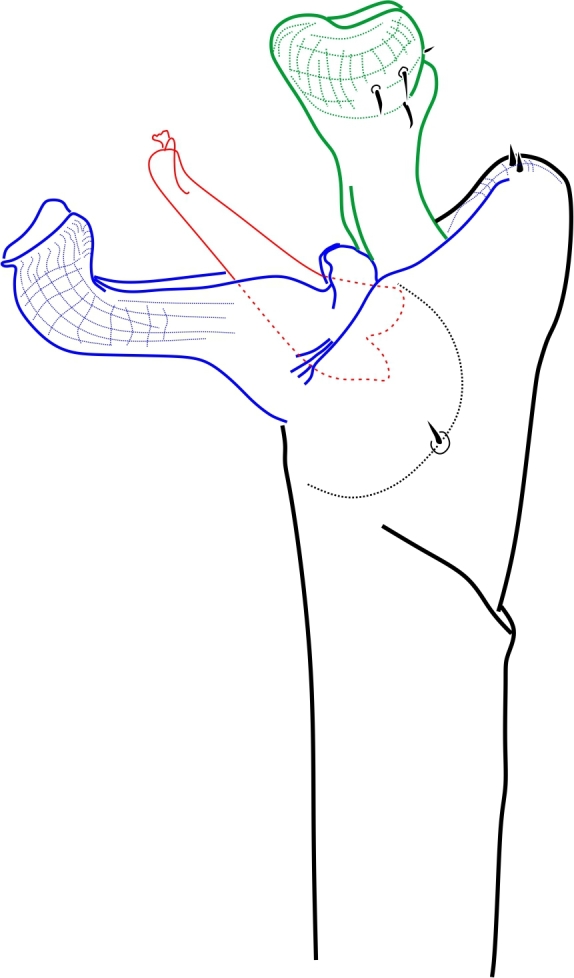
Zalmoxoidea, Phalangodella sp., male (MNRJ 2438) from Ecuador. Penis, expanded, lateral view. Drawing by APG/ABK. Blue = stragulum; green = rutrum; red = capsula interna.
Figure 22.
Zalmoxoidea, Phalangodella sp., from Manabi, Ecuador, male, habitus, dorsal view (MNRJ 19336). Photo by Daniele R. Souza (with permission). Scale bar = 1 mm.
Included species.
Phalangodella aequatorialis Roewer, 1912 (type species)
Phalangodella flavipes (Mello-Leitão, 1949) new comb. for Langodinus flavipes Mello-Leitão 1949: 7.
Phalangodella fulvescens (Mello-Leitão, 1943) new comb. for Exlineia fulvescens Mello-Leitão 1943: 2.
Phalangodella milagroi (Mello-Leitão, 1942) new comb. for Erxlineia milagroi Mello-Leitão 1942: 315, fig. 1.
Phalangodella rhinoceros (Mello-Leitão, 1945) new comb. for Exlineia rhinoceros Mello-Leitão 1945: 149, figs. 1–2.
Phalangodella rugipes (Roewer, 1949) new comb. for Cochirapha rugipes Roewer 1949c: 40, fig. 69.
Phalangodella urarmata (Roewer, 1949) new comb. for Phalpuna urarmata Roewer 1949c: 41, figs. 71a-e.
Generic diagnosis. Dorsal scutum campaniform elongate, densely covered by long-haired setiferous tubercles. Carapace elongate, with ocularium very narrow, marginal as a blunt protuberance. Mesotergum divided into 4 areas by substraight grooves, last groove curved backwards. Both male and female possess femur IV thickened, curved and with distal prolateral spine and tibia IV moderately incrassate. Penis with short scattered macrosetae; rudimentary proto-pergula (projected distal ring) and proto-rutrum (thick mushroom-shaped apical process). Capsula externa jackknife-like unfolding by means of a long stragulum, divided into left and right halves. Capsula interna without lateral sclerites, and with apical expansion as a parastyllar collar. Truncus cylindrical with subdistal fold encircling lateral and ventral parts.
Acknowledgements
We thank Petra Sierwald (FMNH) for the loan of Malagasy Biantidae. APG wants to thank Peter Jäger and Nikolaj Scharff for hospitiality in his visit to their museums. All illustrations from the literature are used here under written permission of the respective copyright holders. This study has been supported by grant # 562149/2010-4 (PROTAX – OPESC project) and scholarship # 302116/2010-9 (PQ - AMMA project) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to ABK. The SEM micrographs were taken in the SEM Lab of Marine Diversity of the MNRJ (financed by PETROBRAS), with the kind assistance of Elivaldo de Lima / Amanda Veiga.
Author contributions
ABK surveyed the taxonomy and nomenclatural acts on the literature, visited museums, revised material, type or otherwise, and drafted the manuscript.
APG studied the complex male genitalia of non-gonyleptoid Laniatores, tried to justify systematic arrangements, proposed homologies for morphological strucutures and studied alternative placements for extraneous genera.
Both authors have worked decades on the systematics of Laniatores, doing all parts of this research, including fieldwork, and imaging to slide/stub preparations, and organization of morphological matrices.
References
- Acosta L. E., Pérez-González A., Tourinho A. L. Methods and Techniques of Study: Methods for taxonomic study. In: Acosta L. E., Pérez-González A., Tourinho A. L., editors. Harvestmen, the biology of Opiliones. Harvard University Press; Cambridge: 2007. 597 [Google Scholar]
- Gonzalez-Sponga M. A. Aracnidos de Venezuela. OpilionesLaniatores I. Familias Phalangodidae y Agoristenidae. Academia de Ciencias Fisicas, Matematicas y Naturales; Caracas: 1987. 562 [Google Scholar]
- González-Sponga M. A. Aracnidos de Venezuela. Cinco nuevos generos y cinco nuevas especies de microopiliones en la hojarasca del bosque tropical (Opiliones: Laniatores: Phalangodidae) Acta Biologica Venezuelica. 1998;18(4):27–41. [Google Scholar]
- González-Sponga M. A. Aracnidos de Venezuela. Cinco nuevos géneros y cinco nuevas especies de microopiliones hemiedaficos (Opiliones Laniatores Phalangodidae) Acta Biologica Venezuelica. 1999;19(2):55–69. [Google Scholar]
- Goodnight C. J., Goodnight M. L. Phalangida from South America. American Museum Novitates. 1943;1234:1–19. [Google Scholar]
- Goodnight C. J., Goodnight M. L. Phalangida from Tropical America. 1947;32(1):1–58. [Google Scholar]
- Goodnight C. J., Goodnight M. L. Opiliones . Insects of Micronesia, Bernice P. Bishop Museum, Honolulu. 1957;3(2):71–83. [Google Scholar]
- Hadley A. CombineZP: GNU public license software. http://www.hadleyweb.pwp.blueyonder.co.uk. 2009 32-bit.
- Henriksen K. L. Descriptiones Laniatorum (Arachnidorum Opilionum Subordinis) fecit William Sørensen. Opus posthumum recognovit et edidit Kai L. Henriksen. Det Kongelige Danske Videnskabernes Selskabs skrifter, Naturvidenskabelig og Mathematisk Afdeling (ser. 9) 1932;3(4):197–422. [Google Scholar]
- Kury Adriano B., Villarreal M. Osvaldo. The prickly blade mapped: establishing homologies and a chaetotaxy for macrosetae of penis ventral plate in Gonyleptoidea (Arachnida, Opiliones, Laniatores) http://dx.doi.org/10.1111/zoj.12225. Zool J Linn Soc. 2015;174(1):1–46. doi: 10.1111/zoj.12225. [DOI] [Google Scholar]
- Kury A. B. Annotated catalogue of the Laniatores of the New World (Arachnida, Opiliones) Revista Ibérica de Aracnología, vol especial monográfico. 2003;1:1–337. [Google Scholar]
- Kury A. B. First report of the male of Zamora granulata Roewer 1928, with implications on the higher taxonomy of the Zamorinae (Opiliones, Laniatores, Cranaidae) Zootaxa. 2012;3546:29–42. [Google Scholar]
- Kury A. B. Why does the Tricommatinae position bounce so much within Laniatores? A cladistic analysis, with description of a new family of Gonyleptoidea (Opiliones, Laniatores) Zoological Journal of the Linnean Society. 2014;172:1–48. doi: 10.1111/zoj.12165. [DOI] [Google Scholar]
- Kury A. B., Souza D. R., Pérez-González A. World Checklist of Opiliones species (Arachnida). Part 2: Laniatores – Samooidea, Zalmoxoidea and Grassatores incertae sedis. Biodiversity Data Journal. 2015;3:e6482. doi: 10.3897/BDJ.3.e6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. F. Arachnides-Opilions. Faune de Madagascar. Publications de L'Institut de Recherche Scientifique Tananarive Tsimbazaza. 1959;9:1–121. [Google Scholar]
- Mello-Leitão C. F. Considerações sobre os Phalangodoidea Soer. com descrição de novas formas. Annaes da Academia Brasileira de Sciencias. 1938;10(2):135–145. [Google Scholar]
- Mello-Leitão C. F. Mais alguns novos Opiliões Sul-Americanos. Annaes da Academia Brasileira de Sciencias. 1940;12(2):93–107. [Google Scholar]
- Mello-Leitão C. F. Oito novos Laniatores do Equador. Anais da Academia Brasileira de Ciências. 1942;14(4):315–325. [Google Scholar]
- Mello-Leitão C. F. Arácnidos recogidos en el Ecuador y el Perú por la Señora H. E. Frizell Don. Comunicaciones zoologicas del Museo de Historia natural de Montevideo. 1943;1(5):1–8. [Google Scholar]
- Mello-Leitão C. F. de. Considerações sôbre o genero Eusarcus Perty e descrição de quatro novos Laniatores. Anais da Academia Brasileira de Ciências. 1945;17(2):149–162. [Google Scholar]
- Mello-Leitão C. F. de. Famílias, subfamília, espécies generos novos de opiliões e notas de sinonimia. Boletim do Museu Nacional, (Nova Série Zoologia) 1949;94:1–33. [Google Scholar]
- Pérez-González Abel. Revisão sistemática e análise filogenética de Stygnommatidae (Arachnida, Opiliones) Unpublished Ph.D. Thesis. Programa de Pós-Graduação em Zoologia, UFRJ; Rio de Janeiro: 2006. 308 [Google Scholar]
- Pérez-González A., Kury A. B. Kimulidae Pérez González, Kury and Alonso-Zarazaga, new name, pp 207–209. In: Pinto-da-Rocha R., Machado G., Giribet G., editors. Harvestmen: the biology of the Opiliones. Harvard University Press; Cambridge and London: 2007. 597 [Google Scholar]
- Pérez-González A., Kury A. B. Samoidae Sørensen, 1886. In: Pinto-da-Rocha R., Machado G., Giribet G., editors. Harvestmen: the biology of the Opiliones. Harvard University Press; Cambridge and London: 2007. 597 [Google Scholar]
- Roewer C. F. Die Familien der Assamiiden und Phalangodiden der Opiliones-Laniatores. (= Assamiden, Dampetriden, Phalangodiden, Epedaniden, Biantiden, Zalmoxiden, Samoiden, Palpipediden anderer Autoren) Archiv für Naturgeschichte, AbtA, Original-Arbeiten. 1912;78(3):1–242. [Google Scholar]
- Roewer C. F. 106 neue Opilioniden. Archiv für Naturgeschichte, Abt A, Original-Arbeiten. 1915;81(3):1–152. [Google Scholar]
- Roewer C. F. Weitere Weberknechte I. (1. Ergänzung der: "Weberknechte der Erde," 1923) Abhandlungen der Naturwissenschaftlichen Verein zu Bremen. 1927;26(2):261–402. [Google Scholar]
- Roewer C. F. Über Phalangodidae II. Weitere Weberknechte XIV. Senckenbergiana. 1949;30(4):247–289. [Google Scholar]
- Roewer C. F. Einige neue Gattungen der Phalangodidae (Opiliones) Veröffentlichungen aus dem Museum für Natur-, Völker- u. Handelskunde in Bremen, Bremen, Reihe A Naturwissenschaften. 1949;1:143–144. [Google Scholar]
- Roewer C. F. Über Phalangodiden I. (Subfam. Phalangodinae, Tricommatinae, Samoinae.) Weitere Weberknechte XIII. Senckenbergiana. 1949;30(1):11–61. [Google Scholar]
- Roewer C. F. Neotropische Arachnida Arthrogastra, zumeist aus Peru [I] Senckenbergiana. 1952;33(1):37–58. [Google Scholar]
- Roewer C. F. Arachnida Arthrogastra aus Peru, II. Senckenbergiana Biologica. 1956;37(5):429–445. [Google Scholar]
- Sharma P. New Australasian Zalmoxidae (Opiliones: Laniatores) and a new case of male polymorphism in Opiliones. Zootaxa. 2012;3236:1–35. [Google Scholar]
- Sharma P., Giribet G. The evolutionary and biogeographic history of the armoured harvestmen – Laniatores phylogeny based on ten molecular markers, with the description of two new families of Opiliones (Arachnida) Invertebrate Systematics. 2011;25:106–142. doi: 10.1071/is11002. [DOI] [Google Scholar]
- Sharma P., Kury A. B., Giribet G. The Zalmoxidae (Arachnida: Opiliones: Laniatores) of the Paleotropics: a catalogue of Southeast Asian and Indo-Pacific species. Zootaxa. 2011;2972:37–58. [Google Scholar]
- Sharma P., Prieto C. E., Giribet G. A new family of Laniatores (Arachnida: Opiliones) from the Afrotropics. Invertebrate Systematics. 2011;25:143–154. doi: 10.1071/IS11003. [DOI] [Google Scholar]
- Simon E. Arachnides des îles Philippines. Annales de la Société Entomologique de France, Paris (séries 6) 1892;61:35. [Google Scholar]
- Soares B. A.M., Soares H. E.M. Algumas notas sobre opiliões com descrição de novas formas. Papéis avulsos do Departamento de Zoologia. 1954;11(25):401–507. [Google Scholar]
- Sørensen W. E. Opiliones, pp. 53–86. In: Koch L., Keyserling E. von, editors. Die Arachniden Australiens nach der Natur beschrieben und abgebildet. Vol. 2. Bauer & Raspe; Nürnberg: 1886. [Google Scholar]
- Staręga W. Harvestmen (Opiliones) from the Mascarene Islands and resurrection of the family Zalmoxidae. Annals of the Natal Museum. 1989;30:1–8. [Google Scholar]
- Staręga W. An annotated check-list of harvestmen, excluding Phalangiidae, of the Afrotropical Region (Opiliones) Annals of the Natal Museum. 1992;33(2):271–336. [Google Scholar]
- Suzuki S. On a collection of opilionids from Southeast Asia. Journal of Science of the Hiroshima University, Series B, Division 1 (Zoology), 1969;22(2):11–77. [Google Scholar]
- Thorell T. T.T. Opilioni nuovi o poco conosciuti dell´Arcipelago Malese. Annali del Museo Civico di Storia Naturale di Genova. 1891;10:669–770. [Google Scholar]