Abstract
Chronic hepatitis B (CHB) is a global health issue that increases the risk of liver cirrhosis and hepatocellular carcinoma in infected patients. Metabolic syndrome (MetS) is a disease endemic mostly to the developed countries. It is associated with high cardiovascular mortality and morbidity, diabetes mellitus as well as cancer. In this manuscript, we systematically review the published data on the relationship between MetS and CHB infection. Multiple studies have described highly variable correlations between CHB on one hand and MetS, non-alcoholic fatty liver disease and dyslipidemia on the other. No association between CHB and diabetes mellitus or atherosclerosis has been described as of now. The presence of MetS in patients infected with hepatitis B virus increases the risk of fibrosis, cirrhosis and hepatocellular carcinoma. Appropriate lifestyle, but also pharmacological interventions are needed to prevent the development of these complications.
Keywords: Hepatitis B, Nonalcoholic fatty liver disease, Fibrosis, Cirrhosis, Metabolic syndrome, Hepatocellular carcinoma
Core tip: Currently, no clear relationship between chronic hepatitis B (CHB) and the prevalence of metabolic syndrome (MetS) could be established, but observations on large patient cohorts reveal some interesting patterns. Surprisingly, male patients with CHB may have lower prevalence of MetS than patients without CHB, but this has not been observed in females. Furthermore, CHB is probably not associated with higher risk of type 2 diabetes mellitus or atherosclerosis. Regarding the clinical outcomes, available data do not sufficiently reveal all of the possible interactions between MetS, its individual components and CHB.
INTRODUCTION
Approximately 2 billion people are infected with hepatitis B virus (HBV) during their lifetime. Around 350-400 million people are infected at any given moment. One hundred twenty-five million of these come from China. Acute hepatitis B infection progresses in a proportion of patients into chronicity. Chronic hepatitis B (CHB) subsequently increases the risk of liver cirrhosis and hepatocellular carcinoma (HCC). In CHB patients HCC could occur even without the presence of cirrhosis[1]. More than one million patients with CHB die due to liver failure or HCC annually[2].
Metabolic syndrome (MetS) has various definitions, however all these definitions stress the presence of abdominal obesity in conjunction with other parameters. Widely used criteria from the International Diabetes Federation define central obesity as waist circumference ≥ 94 cm for males and ≥ 80 cm for females in western population; ≥ 90 cm for males ≥ 80 cm for females for Asian population excluding Japanese, or BMI > 30 kg/m2). Two or more of the following criteria also need to be fulfilled: (1) “Raised triglycerides ≥ 150 mg/dL (1.7 mmol/L) or specific treatment for this lipid abnormality; (2) reduced HDL cholesterol < 40 mg/dL (1.03 mmol/L) in males < 50 mg/dL (1.29 mmol/L) in females or specific treatment for this lipid abnormality; (3) raised blood pressure systolic ≥ 130 or diastolic ≥ 85 mmHg or treatment of previously diagnosed hypertension; and (4) raised fasting plasma glucose ≥ 100 mg/dL (5.6 mmol/L), or previously diagnosed type 2 diabetes If above 5.6 mmol/L or 100 mg/dL, oral glucose tolerance test is strongly recommended but is not necessary[3].”
Patients suffering from MetS have higher risks of cardiovascular morbidity and mortality, diabetes mellitus and cancer[4,5]. Nonalcoholic fatty liver disease (NAFLD) is currently considered a hepatic manifestation of MetS.
ASSOCIATION BETWEEN THE PREVALENCE OF MetS AND CHB
The presence of MetS in patients with CHB (HBsAg positive) was evaluated in 10 published studies[6-15]. The results are summarized in Table 1. The majority were done in Asia, one study analyzed a large population database in the United States and two papers report the data from central Europe. The data presented in these studies are very heterogeneous. In three out of seven Asian studies, authors reported an inverse correlation between the prevalence of MetS and CHB in the whole cohort[6-8]. In the next two Asian studies, this inverse correlation between CHB and MetS was present, after adjustment, only in males, while no correlation was observed in females[10,15]. One study did not found any association between the prevalence of these two diseases[9], and in the retrospective cohort from Shanghai authors found that patients with CHB had a higher prevalence of MetS compared to the uninfected controls[12]. The study from United States that evaluated the proposed relationship in the large population database (NHANES III) found previously documented inverse correlation in the whole cohort and males, however this correlation was not present in women. The results were adjusted for race and other confounders, however it is prudent to mention that roughly 80% of controls were non-Hispanic whites, but the prevalence of hepatitis B was higher in other races[11]. Although not mentioned specifically in this study, it is known that a significant proportion of CHB patients in the United States is of Asian descent[16]. No relationship between CHB and MetS was found in both studies from Europe, however, the study by Jarčuška et al[13] and Janicko et al[14] included only very specific young Roma population, but the second study expanded this population with also majority Caucasian population. A meta-analysis of four, previously mentioned studies from Asia[6,8-10] was performed by Wang et al[17]. Altogether, authors included 10015 patients with CHB and 79475 controls. Despite the findings of the original studies, no difference in the prevalence of MetS in the CHB patients and controls was found (OR = 0.82; 95%CI: 0.66-1.02 by random effect model; heterogeneity: I-squared 84.8%)[17].
Table 1.
Association between chronic hepatitis B virus infection and metabolic syndrome
| Ref. (Region) | Race | Study design | HBV patients/controls | Prevalence MS in HBV patients/controls | Result/statistical significance |
| Jan et al[6] (Taiwan) | Asian | Population-based | 5995 HBV patients/ 47533 controls | 8.0%/10.9% | Inverse correlation between MS and HBV infection |
| Cross-sectional study | OR = 0.72 (0.65-0.79); P < 0.001 | ||||
| aOR = 0.84 (0.76-0.93); P < 0.0011 | |||||
| Luo et al[7] (China) | Asian | Cross-sectional study | 858 HBV patients/ 6579 controls | 5.9%/8.8% | Inverse correlation between MS and HBV infection |
| OR = 0.65 (0.48-0.88); P = 0.003 | |||||
| Wong et al[8] (Hong Kong, China) | Asian | Case series | 91 HBV patients/ 922 controls | 11.0%/20.2% | Inverse correlation between MS and HBV infection; P = 0.034 |
| Li et al[9] (Taiwan) | Asian | Case series | 3408 HBV patients/ 22897 controls | 13.4%/14.0% | No correlation between MS and HBV infection |
| Chung et al[10] (South Korea) | Asian | Cross-sectional study | 521 HBV patients/ 8953 controls | 19.5%/20.8% in men | Inverse correlation between MS and HBV infection in men only after adjustation |
| 14.3%/13.7% in women | |||||
| OR = 0.92 (0.72-1.17); P = 0.492; NS | |||||
| aOR = 0.75 (0.57-0.98); P = 0.033b | |||||
| No correlation between MS and HBV infection in women | |||||
| OR = 1.05 (0.56-1.96) NS | |||||
| aOR = 0.80 (0.38-1.66); P = 0.545; NS2 | |||||
| Jinjuvadia et al[11] (United States) | Caucasian (80%) | Large population database | 593 594 HBV patients/7280620 patients with past exposure to hepatitis B/138283905 controls | 10.4%/25.6% total | Inverse correlation between MS and HBV infection in all patients |
| OR = 0.34 (0.13-0.87) | |||||
| aOR = 0.32 (0.12-0-82); P = 0.019c | |||||
| Inverse correlation between MS and HBV infection in men | |||||
| OR = 0.13 (0.04-0.44) | |||||
| aOR = 0.14 (0.04-0.55)3 | |||||
| No correlation between MS and HBV infection in women | |||||
| OR = 0.89 (0.30-2.65) | |||||
| aOR = 0.73 (0.22-2.46)3 | |||||
| Zhou et al[12] (China) | Asian | Retrospective cohort study | 480 HBV patients/ 496 controls | 24.5%/10.5% | Correlation between MS and HBV infection |
| OR = 2.46 (1.77-3.41) | |||||
| aOR = 2.27 (1.52-3.38)4 | |||||
| Jarčuška et al[13] (Slovakia) | Caucasian + Roma | Cross-sectional study | 66 HBV patients/ 789 controls | 24.6%/24.7% | No correlation between MS and HBV infection; P = 0.561; NS |
| Janicko et al[14] (Slovakia) | Roma | Cross-sectional study | 55 HBV patients/ 387 controls | 27.8%/29.6% | No correlation between MS and HBV infection; P = 0.785; NS |
| Choi et al[15] (South Korea) | Asian | Population database | 209 HBV patients/ 4899 controls | 23.4%/31.5% in men | Inverse correlation between MS and HBV infection in men only after adjustation |
| 18.6%/23.7% in women | |||||
| OR = 0.66 (0.42-1.05); P = 0.079; NS | |||||
| OR = 0.61 (0.375-0.998); P = 0.0495 | |||||
| No correlation between MS and HBV infection in women | |||||
| OR = 0.74 (0.44-1.22); P = 0.235; NS | |||||
| aOR = 0.70 (0.40-1.21); P = 0.197; NS5 |
Adjusted for age and sex;
Adjusted for age, body mass index, alaninaminotranferase, alcohol intake, smoking, exercise, family income and educational status;
Adjusted for age, sex, race, smoking and alcohol status;
Adjusted for age, gender, smoking, passive smoking, alcohol consumption, high-energy food intake, fresh fruit and vegetable intake, physical activity;
Adjusted for age, location, smoking habits, alcohol consumption, exercise habits, income status, and education levels. MS: Metabolic syndrome; NS: Not significant; HBV: Hepatitis B virus; OR: Odds ration; aOR: Adjusted odds ratio.
As is evident from the conflicting results of published studies, currently no clear relationship between CHB and MetS prevalence could be established. However, it is necessary to note that male population with CHB may have lower prevalence of MetS and the prevalence of MetS increases with age not only in uninfected patients but also in patients with CHB[13].
The presence of antibodies against HBV core antigen (antiHBc) without HBsAg positivity signifies previous contact with hepatitis B. Four studies examined the relationship between antiHBc positivity and the presence of MetS. In the NHANES III cohort no difference in the MetS prevalence between antiHBc positive patients and controls was found (OR = 0.87, 95%CI: 0.69-1.08, adjusted for age, sex, race, smoking and alcohol status)[11]. Another cross-sectional study from Taiwan included 8226 subjects with mean age 19.2 ± 2.3 years. AntiHBc positive patients in this study had 58% higher risk of having MetS (P < 0.05)[18]. Similarly, antiHBc positive subjects had higher prevalence of MetS compared to antiHBc negative controls (29.8% vs 22%, P = 0.008) also in the study from central Europe. However, antiHBc positive patients were also significantly older compared to antiHBc negative patients[13]. In the specific population of young Roma people, no significant difference in the MetS prevalence was found (31.9% vs 26.7%, not significant)[14].
Very limited data suggest lower prevalence of MetS in subjects vaccinated against hepatitis B. In the above mentioned study from Taiwan, antiHBs positive, antiHBc negative subjects had lower prevalence of MetS compared to antiHBs negative controls (OR = 0.76, 95%CI: 0.6-0.96, adjusted for age, gender and BMI). Due to the design of the study, it is difficult to determine if this association is only arbitrary or has a clinical foundation[18].
LIPOPROTEIN METABOLISM IN THE PATIENTS WITH CHB
The lipid profile in the serum of patients with CHB has recently drawn significant attention[6-11,13-15,19-22]. An overview of the published studies is in the Table 2. The levels of total cholesterol were significantly lower in most of the CHB patients compared to controls in practically all of the published studies. Two of the studies also reported lower levels of apolipoprotein B100, which is the principal protein component of low and very low-density lipoprotein particles[13,14]. The data on individual lipoprotein classes are more conflicting. Currently published studies mostly did not find any difference in the levels of low-density lipoproteins (LDL) in patients with CHB and controls. Nevertheless, three studies did report significant differences in LDL values[9,13,22], however the direction and magnitude of these differences differed greatly between studies and subgroups within individual studies. Same conclusions can be drawn from the published data about triglycerides and high-density lipoproteins (Table 2).
Table 2.
Levels of lipoproteins in chronic hepatitis B virus patients and controls
| Ref. | Laboratory parameter | HBV patients vs controls statistical significance | HBV patients vs controls |
| Su et al[19] | Total cholesterol | P < 0.05 | 181.7 ± 29.8 mg/dL vs 186.8 ± 33.3 mg/dL |
| LDL-C | NS | 108.7 ± 25.9 mg/dL vs 109.4 ± 28.6 mg/dL | |
| HDL-C | P < 0.01 | 53.4 ± 11.6 mg/dL vs 56.5 ± 13.5 mg/dL | |
| TG | NS | 99.2 ± 54.0 mg/dL vs 102.7 ± 57.6 mg/dL | |
| Jan et al[6] | TG | OR = 0.64 (0.60-0.69) | |
| HDL-C | OR = 0.89 (0.80-0.99) | ||
| Luo et al[7] | TG | OR = 0.62 (0.53-0.72); P = 0.002 | |
| HDL-C | NS | ||
| Chen et al[20] | Cholesterol | P < 0.001 | |
| TG | P < 0.001 | ||
| Wong et al[8] | Total cholesterol | P = 0.004 | 4.9 ± 0.8 mmol/L vs 5.2 ± 1.0 mmol/L |
| LDL-C | NS | 2.9 ± 0.8 mmol/L vs 3.0 ± 0.9 mmol/L | |
| HDL-C | NS | 1.5 ± 0.4 mmol/L vs 1.5 ± 0.4 mmol/L | |
| TG | P = 0.027 | 1.0 (0.1-2.9) mmol/L vs 1.1 (0.3-21.3) mmol/L | |
| Hsu et al[21] | LDL-C | NS | |
| HDL-C | aOR = 0.004 (0.001-0.017); P < 0.0011 | ||
| TG | aOR = 0.107 (0.054-0.213); P < 0.0011 | ||
| Li et al[9] | Total cholesterol ≤ 45 yr in women | P < 0.001 | 178 mg/dL vs 174 mg/dL |
| Total cholesterol > 45 yr in women | P = 0.040 | 201 mg/dL vs 205 mg/dL | |
| LDL-C ≤ 45 yr in women | P = 0.040 | 103.5 mg/dL vs 101.2 mg/dL | |
| LDL-C > 45 yr in women | NS | 123.6 mg/dL vs 126.8 mg/dL | |
| HDL-C ≤ 45 yr in women | P < 0.001 | 63.3 mg/dL vs 61.5 mg/dL | |
| HDL-C > 45 yr in women | NS | 60.1 mg/dL vs 59.4 mg/dL | |
| TG ≤ 45 yr in women | NS | 67 mg/dL vs 67 mg/dL | |
| TG > 45 yr in women | P < 0.001 | 85 mg/dL vs 93 mg/dL | |
| Total cholesterol ≤ 45 yr in men | NS | 183 mg/dL vs 182 mg/dL | |
| Total cholesterol > 45 yr in men | P < 0.001 | 188 mg/dL vs 197 mg/dL | |
| LDL-C ≤ 45 yr in men | NS | 49.8 mg/dL vs 49.7 mg/dL | |
| LDL-C > 45 yr in men | P < 0.001 | 117.6 mg/dL vs 123 mg/dL | |
| HDL-C ≤ 45 yr in men | NS | 51 mg/dL vs 51 mg/dL | |
| HDL-C > 45 yr in men | NS | 49.8 mg/dL vs 49.7 mg/dL | |
| TG ≤ 45 yr in men | P = 0.017 | 100 mg/dL vs 104 mg/dL | |
| TG > 45 yr in men | P < 0.001 | 102 mg/dL vs 116 mg/dL | |
| Liu et al[22] | Total cholesterol | P < 0.05 | 193 ± 36 mg/dL vs 197 ± 36 mg/dL |
| LDL-C | P < 0.05 | 124 ± 31 mg/dL vs 126 ± 36 mg/dL | |
| HDL-C | NS | 53 ± 16 mg/dL vs 53 ± 16 mg/L | |
| TG | NS | 126 ± 129 mg/dL vs 131 ± 87 mg/dL | |
| Chung et al[10] | TG in men | P < 0.001 | 4.59 ± 0.48 mg/dL vs 4.75 ± 0.52 mg/dL |
| HDL-C in men | P = 0.039 | 3.81 ± 0.26 mg/dL vs 3.84 ± 0.25 mg/dL | |
| TG in women | NS | 4.45 ± 0.30 mg/dL vs 4.50 ± 0.50 mg/dL | |
| HDL-C in women | NS | 4.01 ± 0.20 mg/dL vs 3.97 ± 0.24 mg/dL | |
| Jinjuvadia et al[11] | TG | NS (total, in men, in women) | |
| HDL-C (total) | OR = 0.37 (0.15-0.91) | ||
| HDL-C in men | NS | ||
| HDL C in women | OR = 0.26 (0.07-0.93) | ||
| Jarčuška et al[13] | Total cholesterol | P = 0.001 | 4.54 ± 0.84 mmol/L vs 5.00 ± 0.99 mmol/L |
| LDL –C | P = 0.001 | 2.29 ± 0.58 mmol/L vs 2.60 ± 0.68 mmol/L | |
| HDL-C | NS | 1.19 ± 0.35 mmol/L vs 1.19 ± 0.41mmol/L | |
| TG | NS | 1.11 ± 0.59 mmol/L vs 1.31 ± 0.91 mmol/L | |
| ApoB100 | P = 0.013 | 0.71 ± 0.21 g/L vs 0.77 ± 0.23 g/L | |
| Janicko et al[14] | Total cholesterol | P = 0.035 | 4.45 ± 1.21 mmol/L vs 4.71 ± 1.23 mmol/L |
| LDL –C | NS | 2.20 ± 0.88 mmol/L vs 2.50 ± 0.90 mmol/L | |
| HDL-C | NS | 1.10 ± 0.53 mmol/L vs 1.10 ± 0.36 mmol/L | |
| TG | NS | 1.02 ± 1.56 mmol/L vs 1.15 ± 1.75 mmol/L | |
| ApoB100 | P = 0.025 | 0.66 ± 0.26 g/L vs 0.74 ± 0.29 g/L | |
| Choi et al[15] | TG in men | OR = 0.63 (0.40-0.99); P = 0.043 | |
| HDL-C in men | NS | ||
| TG in women | OR = 0.34 (0.17-0.69); P = 0.003 | ||
| HDL-C in women | NS |
Multivariate analyses using logistic regression, the status of HBsAg positivity as the dependent variable, age, sex, body mass index, serum TG, LDL-C, HDL-C and alaninamonotransferase level as independent variables were performed. NS: Not significant; HBV: Hepatitis B virus; OR: Odds ration; TG: triglycerides; HDL-C: High density lipoproteins; LDL-C: Lowe density lipoproteins; ApoB100: Apolipoprotein B100; aOR: Adjusted odds ratio.
The risk of atherosclerosis related outcomes has been evaluated in only one large study from Taiwan that included 3931 CHB patients and 18541 controls followed for 17 years. The HBsAg seropositivity did not increase the risk of coronary heart disease, cerebrovascular disease and atherosclerosis in general[23].
No simple reason for these changes in the lipoprotein metabolism in CHB patients has been confirmed in the literature. However, multiple proposed explanations exist. It has been shown that total cholesterol correlates with liver function and prognosis in patients with advanced liver disease. Therefore, at least in a proportion of patients, the low total cholesterol could be associated with incipient liver failure. Hepatitis B infection also interferes with the hepatocyte metabolism. It has been known for some time that HBV modifies the expression of host genes. Particularly the genes for enzymes of lipid biosynthesis pathways were the largest upregulated category in one published murine model[24]. On the other hand, data from hepatoma cell cultures suggest that hepatocytes infected with HBV have lower concentrations of apolipoprotein mRNA[25]. The binding of apolipoprotein H to the HBsAg could also result in the lower plasma apolipoprotein levels[26]. Therefore, despite the lack of strong cytotoxic effect, HBV infection profoundly alters the metabolism of infected hepatocytes.
CHB, INSULIN RESISTANCE AND DIABETES MELLITUS
Chronic hepatitis C is an important risk factor for insulin resistance that accelerates fibrogenesis in the liver[27]. Moreover, patients with hepatitis C and insulin resistance have poorer response to antiviral treatment[28]. This relationship in CHB patients is not so straightforward. The insulin resistance was not associated with HBsAg positive patients in a study by Wang et al[29] from Taiwan. However, another study from Korea reported that patients with CHB had higher levels of fasting insulin, HOMA-IR index and lower QUICKI index[30]. In a recent meta-analysis of 15 studies, no increase in the risk of type 2 diabetes mellitus (T2DM) attributable to CHB without cirrhosis was reported. However, increased risk of T2DM was reported in CHB patients with liver cirrhosis compared to CHB patients without cirrhosis (OR = 1.74, 95%CI: 1.43-2.13). Authors of this meta-analysis concluded that HBV itself might not be pro-diabetic[31]. Shen et al[32] tried to identify the risk factors for T2DM in patients infected with hepatitis B. Multivariate analysis revealed that besides general risk factors (family history, low education level, elevated triglycerides, gamma-glutamyl transferase, and alcoholic steatosis), three hepatitis B related risk factors (high viral load, long duration of infection and presence of cirrhosis) contributed independently to the risk of T2DM.
CHB AND NAFLD
Insulin resistance is the principal pathophysiological mechanism behind the MetS. Although not officially included in the definition of MetS, NAFLD is very prevalent is patients with MetS and its clinical prevalence is estimated around 20% in the general population of developed countries. Histological prevalence could be even higher. In a series of consecutive liver biopsies from potential liver donors, the prevalence of histological changes associated with NAFLD was 50%[33-35]. Non-alcoholic fatty liver disease could progress to non-alcoholic steatohepatitis (NASH) and liver cirrhosis. The prevalence of NASH is estimated around 2%-3% of general population. It progresses to cirrhosis in 20%-25% of the cases. The mortality of NASH related cirrhosis is estimated around 40% mostly due to liver failure or HCC[36]. Besides liver related mortality, the presence of NASH also increases the risk of total and cardiovascular mortality[37]. The definitive diagnosis of NAFLD is confirmed by quantification of the fat in the liver biopsy specimen, which should be more than 5%[38]. The liver biopsy is not feasible for the routine diagnosis of NAFLD and ultrasound is commonly used instead. The correlation between ultrasound and biopsy is very good (Spearman’s coefficient: 0.80; 95%CI: 0.71-0.88, P < 0.001)[39]. Another relatively sensitive and specific noninvasive test for NAFLD is proton-magnetic resonance spectroscopy (1H-MRS) that determines intrahepatic triglyceride content[40]. The NAFLD-associated fibrosis could also be evaluated noninvasively by transient elastography, serum biomarkers or the combination of both[41].
In the large study that included 33439 subjects who received health check-up at single center in Taiwan, NAFLD was found in 43.9% in general uninfected population compared to only 38.9% in patients with CHB. This result was also significant in lean and overweight subgroups separately[42]. However, two other, smaller studies did not find significant difference in the prevalence of NAFLD between CHB and uninfected patients[29,43]. Study by Wong et al[8] determined the NAFLD prevalence in 91 patients with CHB and 922 controls by proton magnetic spectroscopy. The intrahepatic triglyceride content was significantly lower in infected patients compared to the controls (1.3% vs 2.1%, P < 0.001). When the presence of NAFLD was determined using 5% cut-off for triglyceride content, patients with CHB had markedly lower prevalence compared to uninfected patients (13.5%; 95%CI: 6.4%-20.6% vs 28.3%; 95%CI: 25.3%-31.2%).
Some histological data on the prevalence of NAFLD in CHB patients are available, however bioptic studies naturally did not include the control group. Machado et al[44] published a meta-analysis of 17 studies that assessed hepatic steatosis prevalence by histology and compared the results to hepatitis C patients. Overall, NAFLD prevalence was 29.6%, patients with CHB had significantly lower prevalence of hepatic steatosis compared to patients with hepatitis C (OR = 0.55, 95%CI 0.45-0.67, P < 0.001) and the prevalence was comparable to uninfected patients. In CHB patients, the risk factors for hepatic steatosis were male sex, higher BMI, diabetes mellitus, higher levels of serum glucose, triglycerides, total cholesterol and higher alcohol consumption. On the other hand, the steatosis was negatively associated with HBV DNA. Other virus related factors, such as HBeAg, genotype or histology did not have any association with hepatic steatosis. In the study by Zheng et al[45], that included only CHB patients, hepatic steatosis also correlated well with fasting insulin levels, however only BMI and total cholesterol were predictors of hepatic fibrosis.
The combination of CHB and NAFLD could potentially accelerate the development of fibrosis. This was assessed in the study by Peng et al[46] who found that in patients with CHB and hepatic steatosis the stages of fibrosis were independently associated only with histology activity index and HBV DNA. No significant association between hepatic steatosis and the stages of cirrhosis was reported in this study. Above-mentioned meta-analysis confirmed that the presence of NAFLD does not worsen the necroinflammation or the degree of fibrosis in patients with CHB[44].
In conclusion, the prevalence of NAFLD is comparable, but may even be lower in patients with CHB than in general population. Surprisingly, the presence of NAFLD is not associated with the severity of liver fibrosis.
HEPATITIS B VIRAL LOAD AND MetS
Multiple studies on animal models have shown that HBV influences metabolism, most likely by changes in the gene expression. Some authors therefore use the term “metabolovirus”, because the regulation of gene expression of HBV and key metabolic genes in hepatocytes is very similar[47]. It is known that HBV DNA load increases the risk of liver cirrhosis and HCC[48,49]. The presence of a relationship between MetS and HBV DNA viral load has not been confirmed to date. Some studies did not find any relationship[50,51], while another proposed an inverse correlation between HBV DNA and liver steatosis[52] that has been shown also in the already mentioned meta-analysis. Pooled data from seven studies in this meta-analysis showed significant negative effect of HBV load on the prevalence of hepatic steatosis[44]. On the other hand, in a study from our group CHB patients with MetS had significantly higher HBV DNA load compared to infected patients without MetS[13]. These results are not directly comparable as the our study used clinically diagnosed MetS compared to ultrasonographically diagnosed liver steatosis.
The association between HBV viral load and components of MetS is also interesting. Body weight, glycemia and triglycerides have typical distribution with pathological values on both ends of the spectrum. Authors of a cross-sectional study from Taiwan reported that the patients with BMI from 23 to 24.9 had the lowest levels of HBV DNA[53]. Analogous results have been produced by our study regarding total cholesterol levels and apolipoprotein B100 levels. Both parameters had quadratic relationship with HBV DNA load. Patients with subnormal but also higher than normal levels of total cholesterol and also apolipoprotein B100 had higher levels of HBV DNA compared to patients with both parameters in the normal range(Figure 1)[13]. Patients with low levels of total cholesterol often have advanced fibrosis that already impacts liver function. It is known that the risk of advanced fibrosis increases with the viral load[48]. On the other end of the spectrum, patients with high total cholesterol often have MetS and, as shown in our study[13], also the higher risk of increased HBV DNA. Other studies most commonly described linear inverse relationship of HBV DNA and other parameters of lipoprotein metabolism, such as high density lipoprotein[54] or triglycerides[21] that was not present in our data[13,14]. No relationship was revealed between HBV DNA and glycemia, HOMA-IR in any of the studies[21]. In the REVEAL study, HBV DNA load showed inverse correlation with extreme (P = 0.004) and central obesity (P = 0.004) even after adjustment for triglycerides, hyperuricemia, gender and history of hypertension in HBe positive patients (i.e., patients with high viral replication). In HBe negative patients inverse correlation with triglycerides was demonstrated[55].
Figure 1.
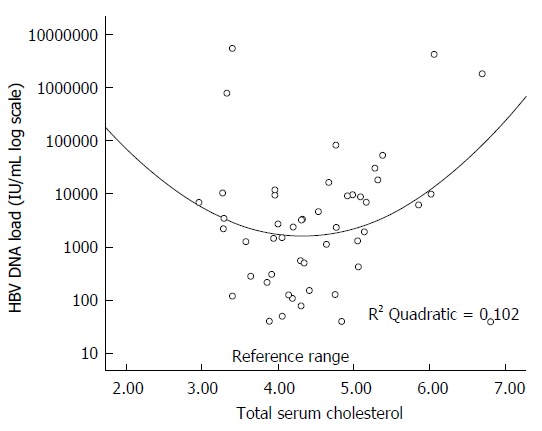
Association between total cholesterol and hepatitis B virus DNA load. Adapted from Janicko et al[14] (2014).
CHB AND THE RISK OF ATHEROSCLEROSIS
CHB is an inflammatory condition. Other diseases with chronic low grade inflammation have been shown to increase the risk of atherosclerosis[56]. Nevertheless, the additional risk for atherosclerosis that could be attributed to CHB has been explored in surprisingly few studies. One study from Japan did not find significant differences between CHB patients and controls in systolic blood pressure, bilateral ankle brachial index, heart-ankle pulse wave velocity and the heart-carotid pulse wave velocity[57]. Also no significant difference was found when carotis intima-media thickness, maximal common carotid artery intima media thickness or extracranial carotid artery atherosclerotic score were evaluated in CHB patients[58]. Patients with HBsAg, but also antiHBc positivity have similar coronarography findings as uninfected, otherwise healthy patients[59,60]. No differences between these two groups of patients were found in the levels of high sensitivity C-reactive protein[60]. Despite these findings, it is necessary to note that CHB patients had significantly lower carotis intima-media thickness compared to the patients with NASH in one study[61]. Regarding clinical outcomes, patients with CHB had comparable cardiovascular mortality risk as uninfected patients[23].
It may seem that CHB patients do not have increased risk of atherosclerosis compared to uninfected patients, however several observations and hypotheses about the possible pro-atherogenic influence of CHB do exist. One paper from Turkey showed that inactive HBsAg carriers have greater mean platelet volume, considered to be an emerging risk factor for atherothrombosis, although clinical relevance of this observation is unknown[62]. Furthermore, a case report of 34 years old, hepatitis B infected man with multiple cerebral arterial stenoses without any risk factors for atherosclerosis was described by Korean authors[63].
MetS AND THE RISK OF FIBROSIS AND HCC IN PATIENTS WITH CHB
Both CHB as well as NAFLD associated with MetS led to the development of liver fibrosis, cirrhosis and HCC. Larger waist circumference, dyslipidemia and arterial hypertension in patients with CHB were associated with superimposed NASH in multivariate analysis[64]. A group of Spanish authors evaluated the severity of liver fibrosis by transient elastography in chronic HBV carriers, that were thought to be inactive. Central obesity, elevated fasting glucose, elevated TG, and lower HDL-C were associated with liver fibrosis[65]. Simultaneous presence of MetS and CHB increases the risk of liver fibrosis independently of biochemical activity and HBV DNA load[66]. CHB patients with MetS have higher prevalence of cirrhosis compared to CHB patients without MetS (38% vs 11%, P < 0.001)[67]. Kaplan-Meier analysis showed significantly more frequent development of cirrhosis and cirrhosis decompensation in CHB patients with diabetes mellitus compared to nondiabetic patients during 12-year follow-up[68]. Analysis of NHANES III cohort showed that the presence of T2DM or insulin resistance is an independent predictor of mortality in CHB patients[69]. Another prospective study from Taiwan included 2903 HBsAg positive men followed for the median of 14.7 years. Higher BMI at baseline correlated with the incidence of NAFLD, liver cirrhosis and HCC. Higher BMI was also a significant risk factor for liver related mortality[70]. Body mass index, levels of insulin, glycated albumin and HOMA-IR correlated directly with the incidence of HCC, but triglycerides and LDL showed an inverse correlation[71]. CHB and MetS increased the risk of HCC, but also intrahepatic cholangiocarcinoma in United States population as well[72].
Because of the adverse influence of MetS and increased total cholesterol on the HBV DNA viral load and clinical outcomes of these patients, intervention with statin therapy has been proposed. Statins decrease HCV RNA load in patients with chronic hepatitis C[73,74]. Limited data are available on the pleiotropic effects of statins in patients with hepatitis B. In one study, the inhibition of HBsAg secretion into culture medium of Hep3B cells by lovastatin was observed[75]. Simvastatin has been shown to potentiate the anti-HBV activity of several nucleot(s)ide analogues (lamivudine, adefovir, tenofovir and entecavir) in vitro[76]. Data on clinical outcomes of statin therapy in CHB patients are even more limited. Recent study from Taiwan showed that this therapy reduces the risk of HCC in CHB patients in dose dependent manner[77]. Another observational study showed that CHB patients taking metformin or statins had lower risk of HCC as well[78]. Unfortunately, both trials were observational and no randomized controlled trials are available.
CONCLUSION
Multiple, but not all, studies showed that patients with CHB have lower risk of MetS, NAFLD and dyslipidemia. Patients with CHB without cirrhosis do not have increased risk of T2DM. CHB is probably not associated with higher risk of atherosclerosis as well. Regarding the clinical outcomes, available data do not sufficiently reveal all of the possible interactions between MetS, its individual components and CHB. Although more studies on the topic are needed, we can be reasonably sure that the simultaneous presence of both diseases accelerates fibrogenesis, increases the risk of liver cirrhosis and HCC. Therefore, it is necessary to influence the MetS by lifestyle interventions as well as pharmacotherapy. Preliminary observational studies suggested the beneficial effect of statins and insulin sensitizers on the risk of HCC.
Footnotes
Conflict-of-interest statement: Authors report no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 6, 2015
First decision: July 14, 2015
Article in press: October 13, 2015
P- Reviewer: Nakamoto S S- Editor: Yu J L- Editor: Filipodia E- Editor: Ma S
References
- 1.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 2.Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13 Suppl 1:S47–S49. doi: 10.1016/0264-410x(95)80050-n. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 4.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:385–391. [PubMed] [Google Scholar]
- 5.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 6.Jan CF, Chen CJ, Chiu YH, Chen LS, Wu HM, Huang CC, Yen MF, Chen TH. A population-based study investigating the association between metabolic syndrome and hepatitis B/C infection (Keelung Community-based Integrated Screening study No. 10) Int J Obes (Lond) 2006;30:794–799. doi: 10.1038/sj.ijo.0803204. [DOI] [PubMed] [Google Scholar]
- 7.Luo B, Wang Y, Wang K. Association of metabolic syndrome and hepatitis B infection in a Chinese population. Clin Chim Acta. 2007;380:238–240. doi: 10.1016/j.cca.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Chan HY, Woo J, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533–540. doi: 10.1016/j.jhep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Li WC, Lee YY, Chen IC, Sun C, Chiu FH, Chuang CH. Association between the hepatitis B and C viruses and metabolic diseases in patients stratified by age. Liver Int. 2013;33:1194–1202. doi: 10.1111/liv.12224. [DOI] [PubMed] [Google Scholar]
- 10.Chung TH, Kim MC, Kim CS. Association between Hepatitis B Surface Antigen Seropositivity and Metabolic Syndrome. Korean J Fam Med. 2014;35:81–89. doi: 10.4082/kjfm.2014.35.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinjuvadia R, Liangpunsakul S. Association between metabolic syndrome and its individual components with viral hepatitis B. Am J Med Sci. 2014;347:23–27. doi: 10.1097/MAJ.0b013e31828b25a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Cui Y, Deng H, Yu J. Association between hepatitis B virus infection and metabolic syndrome: a retrospective cohort study in Shanghai, China. BMC Public Health. 2014;14:516. doi: 10.1186/1471-2458-14-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarčuška P, Janičko M, Kružliak P, Novák M, Veselíny E, Fedačko J, Senajová G, Dražilová S, Madarasová-Gecková A, Mareková M, et al. Hepatitis B virus infection in patients with metabolic syndrome: a complicated relationship. Results of a population based study. Eur J Intern Med. 2014;25:286–291. doi: 10.1016/j.ejim.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Janicko M, Senajová G, Drazilová S, Veselíny E, Fedacko J, Siegfried L, Kristian P, Virág L, Pella D, Mareková M, et al. Association between metabolic syndrome and hepatitis B virus infection in the Roma population in eastern Slovakia: a population-based study. Cent Eur J Public Health. 2014;22 Suppl:S37–S42. doi: 10.21101/cejph.a3900. [DOI] [PubMed] [Google Scholar]
- 15.Choi JS, Han KJ, Lee S, Chun SW, Kim DJ, Kim HC, Kim HM. Serum HBV surface antigen positivity is associated with low prevalence of metabolic syndrome in Korean adult men. J Epidemiol. 2015;25:74–79. doi: 10.2188/jea.JE20140053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do S. The natural history of hepatitis B in Asian Americans. Asian Am Pac Isl J Health. 2001;9:141–153. [PubMed] [Google Scholar]
- 17.Wang CC, Tseng TC, Kao JH. Hepatitis B virus infection and metabolic syndrome: fact or fiction? J Gastroenterol Hepatol. 2015;30:14–20. doi: 10.1111/jgh.12700. [DOI] [PubMed] [Google Scholar]
- 18.Yen SL, Chiu TY, Lin YC, Lee YC, Lee LT, Huang KC. Obesity and hepatitis B infection are associated with increased risk of metabolic syndrome in university freshmen. Int J Obes (Lond) 2008;32:474–480. doi: 10.1038/sj.ijo.0803753. [DOI] [PubMed] [Google Scholar]
- 19.Su TC, Lee YT, Cheng TJ, Chien HP, Wang JD. Chronic hepatitis B virus infection and dyslipidemia. J Formos Med Assoc. 2004;103:286–291. [PubMed] [Google Scholar]
- 20.Chen JY, Wang JH, Lin CY, Chen PF, Tseng PL, Chen CH, Chang KC, Tsai LS, Chen SC, Lu SN. Lower prevalence of hypercholesterolemia and hyperglyceridemia found in subjects with seropositivity for both hepatitis B and C strains independently. J Gastroenterol Hepatol. 2010;25:1763–1768. doi: 10.1111/j.1440-1746.2010.06300.x. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CS, Liu CH, Wang CC, Tseng TC, Liu CJ, Chen CL, Chen PJ, Chen DS, Kao JH. Impact of hepatitis B virus infection on metabolic profiles and modifying factors. J Viral Hepat. 2012;19:e48–e57. doi: 10.1111/j.1365-2893.2011.01535.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu PT, Hwang AC, Chen JD. Combined effects of hepatitis B virus infection and elevated alanine aminotransferase levels on dyslipidemia. Metabolism. 2013;62:220–225. doi: 10.1016/j.metabol.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Wang CH, Chen CJ, Lee MH, Yang HI, Hsiao CK. Chronic hepatitis B infection and risk of atherosclerosis-related mortality: A 17-year follow-up study based on 22,472 residents in Taiwan. Atherosclerosis. 2010;211:624–629. doi: 10.1016/j.atherosclerosis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Hajjou M, Norel R, Carver R, Marion P, Cullen J, Rogler LE, Rogler CE. cDNA microarray analysis of HBV transgenic mouse liver identifies genes in lipid biosynthetic and growth control pathways affected by HBV. J Med Virol. 2005;77:57–65. doi: 10.1002/jmv.20427. [DOI] [PubMed] [Google Scholar]
- 25.Norton PA, Gong Q, Mehta AS, Lu X, Block TM. Hepatitis B virus-mediated changes of apolipoprotein mRNA abundance in cultured hepatoma cells. J Virol. 2003;77:5503–5506. doi: 10.1128/JVI.77.9.5503-5506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neurath AR, Strick N. The putative cell receptors for hepatitis B virus (HBV), annexin V, and apolipoprotein H, bind to lipid components of HBV. Virology. 1994;204:475–477. doi: 10.1006/viro.1994.1558. [DOI] [PubMed] [Google Scholar]
- 27.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 29.Wang CC, Hsu CS, Liu CJ, Kao JH, Chen DS. Association of chronic hepatitis B virus infection with insulin resistance and hepatic steatosis. J Gastroenterol Hepatol. 2008;23:779–782. doi: 10.1111/j.1440-1746.2007.05216.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee JG, Lee S, Kim YJ, Cho BM, Park JS, Kim HH, Cheong J, Jeong DW, Lee YH, Cho YH, et al. Association of chronic viral hepatitis B with insulin resistance. World J Gastroenterol. 2012;18:6120–6126. doi: 10.3748/wjg.v18.i42.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Shen Y, Cai H, Liu YM, Qin G. Hepatitis B virus infection status and risk of type 2 diabetes mellitus: A meta-analysis. Hepatol Res. 2015:Epub ahead of print. doi: 10.1111/hepr.12481. [DOI] [PubMed] [Google Scholar]
- 32.Shen Y, Zhang J, Cai H, Shao JG, Zhang YY, Liu YM, Qin G, Qin Y. Identifying patients with chronic hepatitis B at high risk of type 2 diabetes mellitus: a cross-sectional study with pair-matched controls. BMC Gastroenterol. 2015;15:32. doi: 10.1186/s12876-015-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogdanova K, Poczatkova H, Uherkova L, Riegrova D, Rypka M, Feher J, Marchesini G, Vesely J. Non-alcoholic fatty liver disease (NAFLD)--a novel common aspect of the metabolic syndrome. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:101–104. doi: 10.5507/bp.2006.014. [DOI] [PubMed] [Google Scholar]
- 34.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47:239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 36.McCullogh AJ. Natural history of nonalcoholic steatohepatitis. In: Arroyo V, Forns X, Garcia-Pagán JC, Rodés , editors. Progress in the Treatment of Liver Diseases. Barcelona: Ars medica; 2003. pp. 219–225. [Google Scholar]
- 37.Jarcuska P, Janicko M, Drazilová S, Senajová G, Veselíny E, Fedacko J, Siegfried L, Kristian P, Tkác M, Pella D, et al. Gamma-glutamyl transpeptidase level associated with metabolic syndrome and proinflammatory parameters in the young Roma population in eastern Slovakia: a population-based study. Cent Eur J Public Health. 2014;22 Suppl:S43–S50. doi: 10.21101/cejph.a3901. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto E, Tokushige K, Farrell GC. Histological features of non-alcoholic fatty liver disease: what is important? J Gastroenterol Hepatol. 2012;27:5–7. doi: 10.1111/j.1440-1746.2011.06957.x. [DOI] [PubMed] [Google Scholar]
- 39.Shannon A, Alkhouri N, Carter-Kent C, Monti L, Devito R, Lopez R, Feldstein AE, Nobili V. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J Pediatr Gastroenterol Nutr. 2011;53:190–195. doi: 10.1097/MPG.0b013e31821b4b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson NA, Walton DW, Sachinwalla T, Thompson CH, Smith K, Ruell PA, Stannard SR, George J. Noninvasive assessment of hepatic lipid composition: Advancing understanding and management of fatty liver disorders. Hepatology. 2008;47:1513–1523. doi: 10.1002/hep.22220. [DOI] [PubMed] [Google Scholar]
- 41.Jarcuska P, Janicko M, Veselíny E, Jarcuska P, Skladaný L. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411:1009–1017. doi: 10.1016/j.cca.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Cheng YL, Wang YJ, Kao WY, Chen PH, Huo TI, Huang YH, Lan KH, Su CW, Chan WL, Lin HC, et al. Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up. PLoS One. 2013;8:e72049. doi: 10.1371/journal.pone.0072049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu QH, Jie YS, Shu X, Chen LB, Cao H, Li G. Relationship of fatty liver with HBV infection, hyperlipidemia and abnormal alanine aminotransferase. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2009;23:141–143. [PubMed] [Google Scholar]
- 44.Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361–1367. doi: 10.1111/j.1440-1746.2011.06801.x. [DOI] [PubMed] [Google Scholar]
- 45.Zheng RD, Xu CR, Jiang L, Dou AX, Zhou K, Lu LG. Predictors of hepatic steatosis in HBeAg-negative chronic hepatitis B patients and their diagnostic values in hepatic fibrosis. Int J Med Sci. 2010;7:272–277. doi: 10.7150/ijms.7.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng D, Han Y, Ding H, Wei L. Hepatic steatosis in chronic hepatitis B patients is associated with metabolic factors more than viral factors. J Gastroenterol Hepatol. 2008;23:1082–1088. doi: 10.1111/j.1440-1746.2008.05478.x. [DOI] [PubMed] [Google Scholar]
- 47.Chiang CH, Huang KC. Association between metabolic factors and chronic hepatitis B virus infection. World J Gastroenterol. 2014;20:7213–7216. doi: 10.3748/wjg.v20.i23.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 50.Minakari M, Molaei M, Shalmani HM, Mohammad Alizadeh AH, Jazi AH, Naderi N, Shavakhi A, Mashayekhi R, Zali MR. Liver steatosis in patients with chronic hepatitis B infection: host and viral risk factors. Eur J Gastroenterol Hepatol. 2009;21:512–516. doi: 10.1097/MEG.0b013e328326792e. [DOI] [PubMed] [Google Scholar]
- 51.Shi JP, Fan JG, Wu R, Gao XQ, Zhang L, Wang H, Farrell GC. Prevalence and risk factors of hepatic steatosis and its impact on liver injury in Chinese patients with chronic hepatitis B infection. J Gastroenterol Hepatol. 2008;23:1419–1425. doi: 10.1111/j.1440-1746.2008.05531.x. [DOI] [PubMed] [Google Scholar]
- 52.Rastogi A, Sakhuja P, Kumar A, Hissar S, Jain A, Gondal R, Sarin SK. Steatosis in chronic hepatitis B: prevalence and correlation with biochemical, histologic, viral, and metabolic parameters. Indian J Pathol Microbiol. 2011;54:454–459. doi: 10.4103/0377-4929.85074. [DOI] [PubMed] [Google Scholar]
- 53.Chiang CH, Lai JS, Sheu JC, Yen LL, Liu CJ, Huang KC. The risky body mass index ranges for significant hepatitis B viral load: A campus-based study. Obes Res Clin Pract. 2012;6:e1–e90. doi: 10.1016/j.orcp.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Mohamadkhani A, Sayemiri K, Ghanbari R, Elahi E, Poustchi H, Montazeri G. The inverse association of serum HBV DNA level with HDL and adiponectin in chronic hepatitis B infection. Virol J. 2010;7:228. doi: 10.1186/1743-422X-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiang CH, Yang HI, Jen CL, Lu SN, Wang LY, You SL, Su J, Iloeje UH, Chen CJ. Association between obesity, hypertriglyceridemia and low hepatitis B viral load. Int J Obes (Lond) 2013;37:410–415. doi: 10.1038/ijo.2012.63. [DOI] [PubMed] [Google Scholar]
- 56.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 57.Moritani M, Adachi K, Arima N, Takashima T, Miyaoka Y, Niigaki M, Furuta K, Sato S, Kinoshita Y. A study of arteriosclerosis in healthy subjects with HBV and HCV infection. J Gastroenterol. 2005;40:1049–1053. doi: 10.1007/s00535-005-1655-3. [DOI] [PubMed] [Google Scholar]
- 58.Yang KC, Chen MF, Su TC, Jeng JS, Hwang BS, Lin LY, Liau CS, Lee YT. Hepatitis B virus seropositivity is not associated with increased risk of carotid atherosclerosis in Taiwanese. Atherosclerosis. 2007;195:392–397. doi: 10.1016/j.atherosclerosis.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 59.Tong DY, Wang XH, Xu CF, Yang YZ, Xiong SD. Hepatitis B virus infection and coronary atherosclerosis: results from a population with relatively high prevalence of hepatitis B virus. World J Gastroenterol. 2005;11:1292–1296. doi: 10.3748/wjg.v11.i9.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amirzadegan A, Davoodi G, Boroumand MA, Darabyan S, Dehkordi MR, Goodarzynejad H. Association between hepatitis B surface antibody seropositivity and coronary artery disease. Indian J Med Sci. 2007;61:648–655. [PubMed] [Google Scholar]
- 61.Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126–1132. doi: 10.1016/j.jhep.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 62.Turhan O, Coban E, Inan D, Yalcin AN. Increased mean platelet volume in chronic hepatitis B patients with inactive disease. Med Sci Monit. 2010;16:CR202–CR205. [PubMed] [Google Scholar]
- 63.Kim JT, Park MS, Nam TS, Choi SM, Lee SH, Kim BC, Kim MK, Cho KH. Multiple cerebral arterial stenosis associated with hepatitis B virus infection. J Clin Neurol. 2011;7:40–42. doi: 10.3988/jcn.2011.7.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bondini S, Kallman J, Wheeler A, Prakash S, Gramlich T, Jondle DM, Younossi ZM. Impact of non-alcoholic fatty liver disease on chronic hepatitis B. Liver Int. 2007;27:607–611. doi: 10.1111/j.1478-3231.2007.01482.x. [DOI] [PubMed] [Google Scholar]
- 65.Mena Á, Pedreira JD, Castro Á, López S, Vázquez P, Poveda E. Metabolic syndrome association with fibrosis development in chronic hepatitis B virus inactive carriers. J Gastroenterol Hepatol. 2014;29:173–178. doi: 10.1111/jgh.12432. [DOI] [PubMed] [Google Scholar]
- 66.Wong GL, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM, Chan HY, Tse CH, Wong VW. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B--a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39:883–893. doi: 10.1111/apt.12658. [DOI] [PubMed] [Google Scholar]
- 67.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58:111–117. doi: 10.1136/gut.2008.157735. [DOI] [PubMed] [Google Scholar]
- 68.Huang YW, Wang TC, Lin SC, Chang HY, Chen DS, Hu JT, Yang SS, Kao JH. Increased risk of cirrhosis and its decompensation in chronic hepatitis B patients with newly diagnosed diabetes: a nationwide cohort study. Clin Infect Dis. 2013;57:1695–1702. doi: 10.1093/cid/cit603. [DOI] [PubMed] [Google Scholar]
- 69.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410–1415. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- 70.Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, Tsai KS, Chen CJ. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol. 2008;26:5576–5582. doi: 10.1200/JCO.2008.16.1075. [DOI] [PubMed] [Google Scholar]
- 71.Zhao J, Zhao Y, Wang H, Gu X, Ji J, Gao C. Association between metabolic abnormalities and HBV related hepatocelluar carcinoma in Chinese: a cross-sectional study. Nutr J. 2011;10:49. doi: 10.1186/1475-2891-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mihaila RG, Nedelcu L, Fratila O, Rezi EC, Domnariu C, Deac M. Effects of lovastatin and pentoxyphyllin in nonalcoholic steatohepatitis. Hepatogastroenterology. 2009;56:1117–1121. [PubMed] [Google Scholar]
- 74.Rao GA, Pandya PK. Statin therapy improves sustained virologic response among diabetic patients with chronic hepatitis C. Gastroenterology. 2011;140:144–152. doi: 10.1053/j.gastro.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 75.Lin YL, Shiao MS, Mettling C, Chou CK. Cholesterol requirement of hepatitis B surface antigen (HBsAg) secretion. Virology. 2003;314:253–260. doi: 10.1016/s0042-6822(03)00403-3. [DOI] [PubMed] [Google Scholar]
- 76.Bader T, Korba B. Simvastatin potentiates the anti-hepatitis B virus activity of FDA-approved nucleoside analogue inhibitors in vitro. Antiviral Res. 2010;86:241–245. doi: 10.1016/j.antiviral.2010.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 78.Chen CI, Kuan CF, Fang YA, Liu SH, Liu JC, Wu LL, Chang CJ, Yang HC, Hwang J, Miser JS, et al. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine (Baltimore) 2015;94:e462. doi: 10.1097/MD.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]


