Abstract
The prevalence of hepatocellular carcinoma (HCC) worldwide parallels that of persistent infection with the hepatitis B virus (HBV) and/or hepatitis C virus (HCV). According to recommendations by the World Health Organization guidelines for HBV/HCV, alpha-fetoprotein (AFP) testing and abdominal ultrasound should be performed in routine surveillance of HCC every 6 mo for high-risk patients. These examinations have also been recommended worldwide by many other HCC guidelines over the past few decades. In recent years, however, the role of AFP in HCC surveillance and diagnosis has diminished due to advances in imaging modalities. AFP was excluded from the surveillance and/or diagnostic criteria in the HCC guidelines published by the American Association for the Study of Liver Diseases in 2010, the European Association for the Study of the Liver in 2012, and the National Comprehensive Cancer Network in 2014. Other biomarkers, including the Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), des-γ-carboxyprothrombin, Dickkopf-1, midkine, and microRNA, are being studied in this regard. Furthermore, increasing attention has focused on the clinical utility of biomarkers as pre-treatment predictors for tumor recurrence and as post-treatment monitors. Serum and tissue-based biomarkers and genomics may aid in the diagnosis of HCC, determination of patient prognosis, and selection of appropriate treatment. However, further studies are needed to better characterize the accuracy and potential role of these approaches in clinical practice.
Keywords: Hepatocellular carcinoma, Biomarker, Guideline, Surveillance, Diagnosis, Prognosis
Core tip: Hepatocellular carcinoma (HCC) is a major global health problem due to the high prevalence of the risk factors hepatitis B virus and hepatitis C virus infection. Thus, a good surveillance program and diagnostic strategy for the early detection of HCC should be available. This review summarizes the controversies regarding and perspectives on clinical utility of biomarkers in HCC, especially the current role of alpha-fetoprotein and des-γ-carboxyprothrombin. In addition, research frontiers and prospects for novel biomarkers to evaluate the prognosis for HCC and to facilitate post-treatment monitoring are reviewed.
INTRODUCTION
In 2012, liver cancer was the fifth most common cancer (782000 new cases) and the second leading cause of cancer-related death (746000 cases) worldwide[1]. Hepatocellular carcinoma (HCC) accounts for more than 90% of primary liver cancers and is a major global health problem because of the high prevalence of the risk factors hepatitis B virus (HBV) and hepatitis C virus (HCV) infection[2]. Worldwide, approximately 54% of HCC cases can be attributed to HBV infection, while 31% can be attributed to HCV infection, with the remaining 15% associated with other causes[3-5]. In accordance with the recommendations of the World Health Organization (WHO), vaccination against hepatitis B has been implemented in many countries since 1991[6]. In addition, an enhanced understanding of the pathophysiology of HBV and HCV infection has led to developments in diagnostic procedures and improvements in therapy and prevention, so the clinical care for patients with HBV- or HCV-related liver disease has advanced considerably over the past two decades[7-10]. However, the incidence of HCC worldwide continues to rise, likely due to the often prolonged period between viral infection and manifestation of HCC[2,11,12]. Some studies estimate that up to 20%-30% of patients infected with HBV and/or HCV will develop a progressive liver disease leading to cirrhosis and HCC[13,14]. Cirrhosis rates begin to become significant after 20 years of infection, and HCC rates begin to become significant after 30 years of infection[15,16]. Thus, a good surveillance program and diagnostic strategy for the early detection of HCC should be available.
Serum biomarkers are striking potential tools for surveillance and early diagnosis of HCC thanks to the non-invasive, objective, and reproducible assessments they potentially enable. Worldwide, alpha-fetoprotein (AFP) testing and abdominal ultrasound (US) every 6 mo are recommended for routine surveillance of HCC in high-risk patients according to many HCC guidelines[17]. AFP has also been used as a diagnostic test for HCC and to evaluate prognosis and monitor recurrence following treatment[18]. However, controversy regarding the clinical utility of AFP has arisen in recent years. AFP was excluded from the surveillance and diagnostic criteria in the HCC guidelines published by the American Association for the Study of Liver Diseases (AASLD) in 2010[19], and AFP was not recommended as a sensitive or specific diagnostic test in the HCC guidelines published by the European Association for the Study of the Liver (EASL) in 2012[20] and in the HCC guidelines published by the National Comprehensive Cancer Network (NCCN) in 2014[21]. In Asian countries, AFP was still recommended for HCC surveillance in combination with US and was recommended as an adjunctive diagnostic tool by the HCC guidelines published by the Asian Pacific Association for the Study of the Liver (APASL) in 2010[22], by the current guidelines published in China in 2011[23], and by the current guidelines published in Japan in 2013[24]. Other biomarkers, including the Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), des-γ-carboxyprothrombin (DCP), Dickkopf-1 (DKK1), midkine (MDK), and microRNA (miRNA), are being studied in this regard. Furthermore, increasing attention has focused on the clinical utility of biomarkers as pre-treatment predictors for tumor recurrence and as post-treatment monitors.
This article provides an overview of current biomarkers in HCC with respect to their clinical utility in surveillance, early diagnosis, prediction of prognosis, and monitoring of response to therapy. The controversy of using biomarkers in these settings is discussed in light of typical HCC guidelines worldwide, and the prospects for novel HCC biomarkers are also discussed.
HBV/HCV GUIDELINES PROMOTING THE MANAGEMENT OF HCC IN HIGH-RISK PATIENTS
The prevalence of HCC worldwide parallels that of viral hepatitis. Chronic HBV infection is a leading cause of HCC in most African and Asian countries, except Japan, and chronic HCV infection predominantly contributes to HCC in Europe, Japan, and North America[25,26]. An estimated 2 billion people worldwide have signs of past or present infection with HBV, and 240 million people have a chronic infection[7]. More than 185 million people have been infected with HCV, and one third of those will develop a chronic infection[27]. Longitudinal studies of untreated individuals with chronic HBV infection indicate that they have an 8%-20% cumulative risk of developing cirrhosis over 5 years[28,29]. In people with cirrhosis, there is an approximately 20% annual risk of hepatic decompensation and an annual incidence of HCC of < 1% to 5%[2,30]. In persons with chronic HCV infection, the risk of liver cirrhosis is 15%-30% within 20 years[31,32], and the risk of HCC in persons with cirrhosis is approximately 2%-4% per year[33].
Universal hepatitis B immunization programs that target infants, with the first dose at birth, have been highly effective in reducing the incidence and prevalence of hepatitis B in many countries where infection is endemic[34-37]. However, these programs will not affect HBV-related deaths until several decades after their introduction[38]. Many guidelines focusing on the management of HBV/HCV infection have been published worldwide, such as guidelines published by the AASLD[39,40], the APASL[41,42], the EASL[43,44], the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN)[45], the Canadian Association for the Study of the Liver (CASL)[46], and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN)[47]. The WHO also published its first guidelines for the prevention, care, and treatment of people living with chronic HCV infection in April 2014[48], and the WHO published similar guidelines for chronic HBV infection in March 2015[38] to help low- and middle-income countries, in particular, to plan for the development and expanded scale of hepatitis B/C prevention, care, and treatment.
According to recommendations in HBV/HCV guidelines from the WHO[38,48], AFP testing and US were suggested to be performed for routine surveillance of HCC every 6 mo in individuals with cirrhosis. In Japan, where HCV is the most significant etiological factor for developing HCC, there is a more detailed definition for high risk patients of HCC - the “very-high-risk group” that includes patients with HBV- or HCV-related liver cirrhosis, and the “high-risk group” that includes patients with HBV- or HCV-related chronic liver disease or liver cirrhosis due to other causes[49].
BIOMARKERS IN HCC SURVEILLANCE: CONTROVERSIES AND PROSPECTS
Surveillance of patients at increased risk for HCC has been shown to result in the detection of early-stage tumors and an increased likelihood of undergoing potentially curative therapies[50-54]. The overall 5-year survival rate for patients with HCC is about 40%, but liver resection of early HCC could result in a 5-year survival rate of 60%-70%[55-57]. Surveillance is therefore required to detect HCC at an early stage and increase the chances of effective treatment.
AFP as a traditional biomarker for HCC surveillance: Its current role and controversy
AFP testing and US are the most widely used methods of HCC surveillance[58,59]. Data have indicated that AFP testing and US every 6 mo affect disease-specific mortality compared to no intervention [odds ratio: 0.57, 95% confidence interval (CI): 0.37-0.89][38]. In addition, surveillance every 6 mo using both AFP and US has been found to be the most cost-effective strategy[60-62].
AFP has been widely used in clinical practice as a traditional biomarker for HCC surveillance over the past two decades[63-65]. However, there is increasing debate regarding the utility of AFP as a surveillance test[66-68]. Analysis of recent studies has indicated that AFP testing lacks adequate sensitivity and specificity for effective surveillance[69-71]. AFP levels are normal in up to 40% of patients with HCC, particularly during the early stage of the disease (low sensitivity)[72-74]. Elevated AFP levels may be seen in patients with cirrhosis or exacerbation of chronic hepatitis or cholangiocarcinoma (low specificity)[75,76]. In addition, some studies have indicated that AFP has substantially limited diagnostic accuracy in detecting small HCC[77].
Given these findings, US is regarded as a more appropriate test for surveillance with an acceptable diagnostic accuracy (sensitivity ranging from 58% to 89%, specificity greater than 90%)[69,78]. Currently, US is recommended as the only tool for HCC surveillance in some Western countries. AFP was excluded from the surveillance criteria in the HCC guidelines published by the AASLD in 2010[19], and AFP is regarded as a suboptimal tool for surveillance according to the HCC guidelines published by the EASL in 2012[20]. Nevertheless, the performance of US in early detection of HCC is highly dependent on the expertise of the examiner and the quality of the equipment. A randomized controlled trial found that surveillance with AFP in conjunction with US reduced the mortality of HCC[79], and the position that AFP should be included in the HCC surveillance guidelines of the AASLD is gaining support[80]. Currently, the combination of AFP and US at approximately 6 mo intervals is still recommended by many HCC guidelines in Asia, such as guidelines in Japan[24], China[23], and guidelines published by the APASL[22] (Figure 1). Thus, whether AFP should be excluded from surveillance criteria needs to be investigated in more large, randomized controlled trials.
Figure 1.
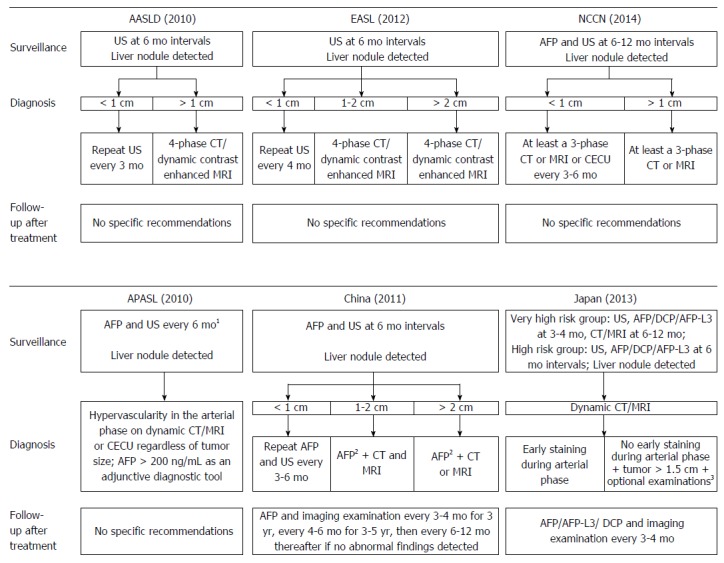
The clinical utility of biomarkers according to typical hepatocellular carcinoma guidelines worldwide. A: Typical hepatocellular carcinoma (HCC) guidelines in Western countries; B: Typical HCC guidelines in Asian countries. 1Alpha-fetoprotein (AFP) alone is not recommended for diagnosis of HCC; the measurement of both AFP and des-γ-carboxyprothrombin (DCP) provides a higher level of sensitivity without decreasing specificity; 2AFP ≥ 400 ng/mL over 1 mo or AFP ≥ 200 ng/mL over 2 mo; 3Optional examinations include computed tomography (CT)-angiography, liver-specific contrast-enhanced magnetic resonance imaging (MRI), contrast ultrasound (US), or liver tumor biopsy.
The combined testing of AFP, AFP-L3, and DCP for HCC surveillance
The effectiveness of surveillance depends on various factors. Inclusion of new diagnostic tests in surveillance programs may allow the detection of additional small HCC. Two other serum biomarkers besides AFP - the Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) and des-γ-carboxyprothrombin (DCP, also known as prothrombin-induced by vitamin K absence-II, PIVKA-II) - have been studied around the world to explore their clinical usefulness in determining the risk of HCC in high-risk populations.
The clinical utility of highly sensitive AFP-L3 in early prediction of HCC developing in patients with chronic HBV or HCV infection was recently evaluated in a large Japanese study, and results indicated that elevated AFP-L3 was an early predictor of HCC development even if AFP levels were low and suspicious US findings were absent. Elevated AFP-L3 was noted in 34.3% of patients 1 year prior to diagnosis of HCC[81]. Numerous studies have found that the combined testing of DCP and AFP has a sensitivity of 47.5%-94.0% and a specificity of 53.3%-98.5% in early detection of HCC, and these figures are higher than those for either marker alone[82-84]. In some countries such as Japan, combined measurement of DCP and AFP-L3 reportedly increased the detectability of small HCC[85,86].
There are also several biomarkers in addition to AFP, AFP-L3, and DCP, such as Golgi protein 73 (GP73)[87,88], interleukin-6 (IL-6)[89,90], and squamous cell carcinoma antigen (SCCA)[91], that are currently being studied. However, these biomarkers have usually been evaluated, alone or in combination, in a diagnostic rather than surveillance setting. Moreover, their diagnostic performance has often been assessed with a markedly higher prevalence of HCC than would be expected in the context of surveillance.
Current expert opinion from Western countries has been rather critical of the clinical value of biomarkers[92]. Imaging-based surveillance criteria were recommended in guidelines from Western countries, such as the updated HCC guidelines published by the AASLD in 2000[19] and similar guidelines published by the EASL in 2012[20]. In Asian countries, as typified by HCC guidelines in Japan, US and measurement of AFP, AFP-L3, or DCP are recommended to be performed at intervals of 3-4 mo for the very-high-risk group (patients with HBV- or HCV-related liver cirrhosis) and at 6 mo intervals for the high-risk group (patients with HBV- or HCV-related chronic liver disease or liver cirrhosis due to other causes)[24,93].
BIOMARKERS FOR DIAGNOSIS OF HCC: THEIR EVOLUTION AND PROSPECTS
Accurate diagnosis of small liver nodules is of paramount importance. In general, the tests used to diagnose HCC around the world include diagnostic imaging, serological diagnosis, and histological diagnosis. Prior to 2000, a definite diagnosis was based on a biopsy, but this approach had several limitations related to feasibility due to location and risk of complications, such as bleeding or needle-track seeding[94]. With the development of imaging techniques, a unique dynamic radiological behavior - contrast uptake in the arterial phase of computed tomography (CT), magnetic resonance imaging (MRI), angiography, or US - represented the backbone of radiological diagnosis of early HCC.
The role of AFP in diagnosis of HCC: No longer in use or used as an adjunctive diagnostic tool?
AFP has served as a diagnostic test for HCC since the 1970s, when most patients with HCC were diagnosed at an advanced stage and with clinical symptoms. At that time, a level of 500 ng/mL AFP was considered diagnostic[95]. However, the usefulness of AFP as a diagnostic test in small HCC is limited. According to a systematic review, AFP with a cut-off value of 20 ng/mL had a sensitivity, specificity, and a positive likelihood ratio (LR+) of diagnosing HCC smaller than 5 cm in diameter of 49%-71%, 49%-86%, and 1.28-4.03, respectively; AFP with a cut-off value of 200 ng/mL had a sensitivity, specificity, and an LR+ of 4%-31%, 76%-100%, and 1.13-54.25, respectively[77].
Although the sensitivity and specificity of serum AFP as a biomarker is being challenged, a high level or a steadily increasing level of serum AFP strongly suggest development of HCC[96,97]. Elevated serum AFP and a typical enhancement pattern in dynamic imaging have provided critical clues for the diagnosis of HCC over the past few decades. Nevertheless, the importance in AFP has diminished in recent guidelines for diagnosis of HCC, and the importance of imaging has increased based on the high accuracy of up-to-date radiologic modalities[98].
According to the diagnostic criteria in the HCC guidelines published by the EASL in 2000[99] and similar guidelines published by the AASLD in 2005[25] the NCCN in 2009[26], HCC diagnosis is based on the tumor size, AFP, and imaging examination. Guidelines from the Korean Liver Cancer Study Group (KLCSG) published in 2003[100] also featured algorithms similar to those in the aforementioned guidelines, with the exception that HCC was diagnosed based on imaging and AFP, regardless of tumor size. However, the updated HCC guidelines published by the KLCSG in 2009 suggested that a tumor of 2 cm or larger in patients with liver cirrhosis that has characteristics typical of HCC in dynamic contrast enhancement CT or MRI could be diagnosed as HCC regardless of the serum AFP level[101]. According to updated HCC guidelines published by the AASLD in 2010, nodules larger than 1 cm found during US surveillance of a cirrhotic liver should be investigated further with either a four-phase multidetector CT scan or dynamic contrast enhanced MRI. If the appearance of the nodule is typical of HCC, the lesion should be treated as HCC; if the findings are not characteristic or the vascular profile is not typical, a second contrast enhanced study involving another imaging modality should be performed, or the lesion should be biopsied[19]. In agreement with updated guidelines from the AASLD, the panel that drafted the HCC guidelines of the NCCN in 2014 also considered an imaging finding of classic enhancement to be more definitive in this instance, since the level of serum AFP may be elevated in persons with certain nonmalignant conditions, or it may be within normal limits in a substantial percentage of patients with HCC[21]. According to the HCC guidelines published by the APASL in 2010[22], typical HCC can be diagnosed based on imaging regardless of tumor size if a typical vascular pattern (i.e., arterial enhancement with portal venous washout) is obtained on dynamic CT/MRI or contrast-enhanced US. According to the same guidelines, AFP was recommended as an adjunctive diagnostic tool, and AFP alone was not recommended for diagnosis of HCC. Similar recommendations were made by HCC guidelines published in China[23] and Japan[24].
Perspectives on the combined testing of AFP and other biomarkers for diagnosis of HCC
Advances in technology and an increased understanding of HCC biology have led to the discovery of novel biomarkers. To date, many biomarkers have been proposed as a complement or substitute for AFP in the diagnosis of HCC. AFP-L3 can differentiate an increase in AFP due to HCC from that in patients with benign liver disease[102,103]. AFP-L3 with a cut-off value of 10% had a sensitivity, specificity, and LR+ in diagnosing HCC smaller than 5 cm in diameter of 22%-33%, 93%-94%, and 4.6-0.8, respectively; AFP-L3 with a cut-off value of 15% had a sensitivity, specificity, and LR+ of 21%-49%, 94%-100%, and 8.1-45.1 respectively[77]. DCP has also been recognized as a highly specific marker for HCC[104]. DCP with a cut-off value of 40 mAU/mL had a sensitivity, specificity, and LR+ in diagnosing HCC smaller than 5 cm in diameter of 14%-54%, 95%-99%, and 6.9-29.7, respectively; DCP with a cut-off value of 100 mAU/mL had a sensitivity, specificity, and LR+ of 7%-56%, 72%-100%, and 3.6-13.0, respectively[77].
Data have indicated that the combined testing of DCP and AFP or AFP-L3 could help to increase the sensitivity of HCC diagnosis[105-107], but this approach is used in only a few countries[17], such as Japan[108,109]. In 2014, a large-scale, multi-center study investigated the measurement of both AFP and DCP in differentiating Chinese patients with HCC (71.18% with HBV infection) from patients without HCC and normal subjects. Results showed that the combined testing of DCP with a cut-off value of 86 mAU/mL and AFP with a cut-off value of 21 ng/mL resulted in a sensitivity of approximately 90% in diagnosis of HCC, which was significantly higher than that for DCP or AFP alone. This finding held even for a tumor smaller than 2.0 cm[110]. These results suggest that the measurement of both AFP and DCP may facilitate the diagnosis of patients with a broad range of HCC. However, the clinical utility of DCP in China has not been noted by HCC guidelines in China[111,112], and more large-scale prospective studies should be performed to provide sufficient evidence.
In recent years, numerous studies have investigated the clinical usefulness of other biomarkers in the early diagnosis of HCC, including GP73[87,88], glypican-3 (GPC3)[113,114], osteopontin[115,116], and vascular endothelial growth factor (VEGF)[117]. Most recently, research on DKK1 and MDK as diagnostic serum biomarkers has garnered interest. In 2012, Shen et al[118] published a retrospective, cross-sectional study involving 424 patients with HCC and 407 controls without HCC, and they found that DKK1 was highly accurate at diagnosing AFP-negative patients with HCC, including patients with early-stage HCC. They also found that the measurement of DKK1 and AFP together improved the accuracy with which HCC was diagnosed in comparison to any test alone. In 2013, Zhu et al[119] published a study involving 388 patients with HCC and 545 different controls, and they found that serum MDK had a markedly higher level of sensitivity than AFP (86.9% vs 51.9%) even when diagnosing very early-stage HCC (80% vs 40%). Zhu et al[119] also found that MDK could have a sensitivity as high as 89.2% when diagnosing cases of AFP-negative HCC.
Noncoding RNA and miRNA in particular have received considerable attention as novel potential biomarkers over the past few years[120]. Li et al[121] found that three miRNAs (miR-25, miR-375, and let-7f) had a sensitivity and specificity as high as 97.9% and 99.1%, respectively, in diagnosing HCC. Zhou et al[122] found that a panel of seven microRNAs (miR-122, miR-192, miR-21, miR-223, miR-26a, miR-27a, and miR-801) could provide a high level of diagnostic accuracy for identification of HBV-related HCC. Tomimaru et al[123] found that the combination of miRNA-21 with AFP improved the power of differentiation between HCC and chronic hepatitis, with a sensitivity of 81.0% and a specificity of 80%. However, the potential for miRNA to serve as a biomarker has not been equally analyzed in all conditions potentially leading to HCC. Systemic analyses of alcoholism, non-alcoholic steatohepatitis (NASH), and HCV-related conditions are pending.
BIOMARKERS: PREDICTION OF PROGNOSIS AND MONITORING OF THE RESPONSE TO THERAPY
Tumor invasiveness, metastasis, and recurrence often result in poor clinical outcomes for patients with HCC[124,125]. Currently, the measurement of biomarker levels both before and after HCC treatment is clinically valuable as a simple way to monitor treatment outcomes (usually in combination with radiological analysis) and to predict prognosis, recurrence, and survival.
AFP, AFP-L3, and DCP: Diagnostic biomarkers could also be used to determine the prognosis for HCC and to facilitate post-treatment monitoring
Several biomarkers that have been evaluated for their power in diagnosing HCC have also been studied for their prognostic significance. A high level of AFP expression in serum correlates with a profound cell proliferation, profound angiogenesis, and limited apoptosis and is associated with a poor prognosis[126,127]. AFP was one of the most robust predictors of death in patients with cirrhosis and HCC[128], and it also has significance at predicting survival after liver transplantation[129]. Changes in AFP while on the waitlist also predicted post-transplant survival, and identifying these changes could facilitate better patient selection to optimize organ allocation and post-transplant outcomes[18]. A change in AFP levels has been found to correlate with radiologic response and overall survival after locoregional therapy. For example, a 50% decrease in AFP levels resulted in a better time-to-progression [hazard ratio (HR): 2.8, 95%CI: 1.5-5.1)] and overall survival (HR: 2.7, 95%CI: 1.6-4.6) in comparison to patients whose AFP levels failed to respond to treatment with transarterial chemoembolization (TACE) or transarterial radioembolization (TARE)[130]. Whether AFP is useful at predicting the response to sorafenib is controversial[127,131], and several studies have indicated that AFP response was correlated with time-to-progression (7.9 mo vs 2.4 mo, P = 0.004) and overall survival (13.3 mo vs 8.2 mo, P = 0.022)[132].
AFP-L3[133,134] and DCP[135,136] were also identified as prognostic biomarkers for survival after resection of HCC. Patients that have undergone resection of HCC and who had elevated levels of AFP, AFP-L3, and DCP at the baseline had a worse prognosis than patients who tested positive for just one or two of the markers before surgery[137-140].
Among the current guidelines for HCC management worldwide, the guidelines of the NCCN[21] published in 2014 recommend high-sectional imaging every 3-6 mo for 2 years and then every 6-12 mo for post-treatment monitoring. If AFP levels are initially elevated, the guidelines recommend that monitoring be performed every 3 mo for 2 years and then every 6-12 mo. The Indian National Association for Study of the Liver (INASL) published the first guidelines in India in 2014[141], and these guidelines make similar recommendations. The guidelines recommend that post-treatment monitoring be performed with dynamic CT or MRI studies every 3 mo for the first 2 years and then routine surveillance every 6 mo thereafter. The guidelines also note that the serum tumor markers AFP and DCP may help to evaluate the response to treatment or evaluate follow-up when AFP or DCP is elevated at diagnosis and when AFP or DCP decreases after treatment but rises again. The guidelines do note, however, that tumor markers cannot replace imaging modalities. According to the HCC guidelines published in Japan in 2013[24], follow-up using the serum biomarkers AFP, AFP-L3, and DCP and imaging should be performed every 3-4 mo after treatment. According to HCC guidelines published in China in 2011[23], post-treatment monitoring with AFP and imaging should be performed every 3-4 mo for 3 years, every 4-6 mo for 3-5 years, and then every 6-12 mo thereafter if no abnormal findings are detected.
Research frontiers and prospects for novel biomarkers to evaluate the prognosis for HCC and to facilitate post-treatment monitoring
Biomarkers, including DKK1[142], GPC3[143], and indocyanine green 15 min after administration (ICG-R15)[144], reflect current knowledge of the pathways involved in hepatocarcinogenesis and appear to have prognostic value. However, prospective validation studies still need to be performed. In patients with advanced HCC who are treated with sorafenib, serum VEGF and angiopoietin 2 (Ang2) levels were identified as independent prognostic factors for overall survival[127].
Moreover, gamma-glutamyl transpeptidase (GGT) was identified as a prognostic maker by studies of different subgroups of patients published over the past 5 years[145]. Sheen et al[146] found that patients who had HCC with type B GGT mRNA had worse outcomes, earlier recurrence, and more post-recurrence deaths. Several studies of patients with HCC undergoing hepatic resection have revealed a correlation between elevated levels of GGT and worse survival for patients with HBV-related HCC, Child-Pugh A liver function, or multi-nodular tumors[147-149]. In addition, several studies have also revealed the predictive value of GGT in patients with unresectable HCC who were treated with TACE or chemotherapy[150-153].
In addition to their diagnostic potential, miRNAs may help to predict prognosis for HCC. Tomimaru et al[123] found that the level of miR-21 expression was high in Asian patients with HCC and that the level declined after surgery. They also found that a high level of miR-21 expression in plasma correlated with a shorter cumulative survival following treatment. Köberle et al[154] found in European patients with HCC that higher levels of miR-1 and miR-122 expression were associated with longer overall survival compared to lower levels of expression of those miRNAs. They concluded that miR-1 may be a predictive biomarker of HCC independent of liver function. A 31-miRNA signature correlates with the stage of disease, and a distinct 20-miRNA signature that is associated with metastasis of HCC has also been identified[155]. These findings constitute mounting evidence that miRNA signature profiling can be of use in prognostic stratification. Despite their promising potential, miRNA-based biomarkers pose several problems in terms of their use in clinical practice[156].
CONCLUSION
Current HCC guidelines in Western countries have been rather critical of the clinical value of biomarkers. Over the past few decades, a simple approach in the form of measuring AFP levels has been widely used for routine surveillance and noninvasive diagnosis of HCC and to evaluate prognosis and monitor recurrence after treatment. AFP was excluded from the surveillance and/or diagnostic criteria in HCC guidelines published by the AASLD in 2010, the HCC guidelines published by the EASL in 2012, and the HCC guidelines published by the NCCN in 2014. Nonetheless, AFP is still regarded as a useful surveillance tool and an adjunctive tool by many HCC guidelines in Asia, such as guidelines from Japan, guidelines from China, and guidelines published by the APASL.
Advances in technology and an increased understanding of HCC biology have led to the discovery of novel biomarkers. Data have indicated that the combined testing of AFP, AFP-L3, and DCP could help to increase the sensitivity of diagnosis of HCC, but this approach is currently used in only a few countries, such as Japan. In recent years, numerous studies have investigated the clinical usefulness of some novel biomarkers in early diagnosis of HCC, including GP73, GPC3, osteopontin, VEGF, DKK1, MDK, and miRNA. Moreover, the prognostic significance of these biomarkers has also been evaluated. Serum and tissue-based biomarkers and genomics may aid in diagnosis of HCC, determination of patient prognosis, and selection of appropriate treatment. However, further studies are needed to better characterize the accuracy and potential role of these approaches in clinical practice. The prevailing hope is that novel biomarkers can support clinicians in their daily practice and improve care for patients with HCC.
Footnotes
Supported by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 19, 2015
First decision: July 14, 2015
Article in press: October 13, 2015
P- Reviewer: Sanchez-Yague J S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
References
- 1.World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Accessed March 2, 2015. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405–419. [PubMed] [Google Scholar]
- 7.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen MA, Idrees M. Evidence-based consensus on the diagnosis, prevention and management of hepatitis C virus disease. World J Hepatol. 2015;7:616–627. doi: 10.4254/wjh.v7.i3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Zhong Y, Guo L. Strategies to prevent hepatitis B virus infection in China: immunization, screening, and standard medical practices. Biosci Trends. 2013;7:7–12. [PubMed] [Google Scholar]
- 11.Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis. 1997;1:559–68, vi-vii. doi: 10.1016/s1089-3261(05)70321-4. [DOI] [PubMed] [Google Scholar]
- 12.Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53:451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 14.Poh Z, Goh BB, Chang PE, Tan CK. Rates of cirrhosis and hepatocellular carcinoma in chronic hepatitis B and the role of surveillance: a 10-year follow-up of 673 patients. Eur J Gastroenterol Hepatol. 2015;27:638–643. doi: 10.1097/MEG.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaughan GW, Omata M, Amarapurkar D, Bowden S, Chow WC, Chutaputti A, Dore G, Gane E, Guan R, Hamid SS, et al. Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:615–633. doi: 10.1111/j.1440-1746.2007.04883.x. [DOI] [PubMed] [Google Scholar]
- 16.Busch K, Thimme R. Natural history of chronic hepatitis B virus infection. Med Microbiol Immunol. 2015;204:5–10. doi: 10.1007/s00430-014-0369-7. [DOI] [PubMed] [Google Scholar]
- 17.Song P, Tobe RG, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, Tang W. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int. 2012;32:1053–1063. doi: 10.1111/j.1478-3231.2012.02792.x. [DOI] [PubMed] [Google Scholar]
- 18.Rich N, Singal AG. Hepatocellular carcinoma tumour markers: current role and expectations. Best Pract Res Clin Gastroenterol. 2014;28:843–853. doi: 10.1016/j.bpg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Benson AB, D’Angelica MI, Abrams TA, Are C, Bloomston PM, Chang DT, Clary BM, Covey AM, Ensminger WD, Iyer R, et al. Hepatobiliary cancers, version 2.2014. J Natl Compr Canc Netw. 2014;12:1152–1182. doi: 10.6004/jnccn.2014.0112. [DOI] [PubMed] [Google Scholar]
- 22.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NHaFP Commission. The Guideline on Diagnosis and Treatment for Primary Liver Cancer (2011 version, in Chinese). Accessed 16 March, 2015. Available from: http://www.moh.gov.cn/mohyzs/s3586/201110/53153.shtml.
- 24.Clinical practice guidelines for hepatocellular carcinoma (2013 version) Kanehara, Tokyo, Japan: Hepatology. JSo; 2013 (in Japanese). [Google Scholar]
- 25.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 26.Benson AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D’Angelica MI, Davila R, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 28.Pan CQ, Zhang JX. Natural History and Clinical Consequences of Hepatitis B Virus Infection. Int J Med Sci. 2005;2:36–40. doi: 10.7150/ijms.2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 30.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 31.Guo F, Gao Y, Wang QX, Sun DG, Ji Y, Cong X, Sun Y, Wang H, Wei L. [Clinical outcomes of women with transfusion-associated hepatitis C after 10-15 years follow-up] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2004;18:132–136. [PubMed] [Google Scholar]
- 32.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 33.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 34.Luo Z, Li L, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis. 2012;16:e82–e88. doi: 10.1016/j.ijid.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–6557. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 36.Spradling PR, Xing J, Williams R, Masunu-Faleafaga Y, Dulski T, Mahamud A, Drobeniuc J, Teshale EH. Immunity to hepatitis B virus (HBV) infection two decades after implementation of universal infant HBV vaccination: association of detectable residual antibodies and response to a single HBV challenge dose. Clin Vaccine Immunol. 2013;20:559–561. doi: 10.1128/CVI.00694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, Kao JH, Lin YC, Chen HL, Hsu HY, et al. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology. 2007;132:1287–1293. doi: 10.1053/j.gastro.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Accessed April 5, 2015. Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ [PubMed]
- 39.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 40.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, et al. Erratum to: Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:809–810. doi: 10.1007/s12072-012-9386-z. [DOI] [PubMed] [Google Scholar]
- 42.Omata M, Kanda T, Yu ML, Yokosuka O, Lim SG, Jafri W, Tateishi R, Hamid SS, Chuang WL, Chutaputti A, et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409–435. doi: 10.1007/s12072-012-9342-y. [DOI] [PubMed] [Google Scholar]
- 43.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 44.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Sokal EM, Paganelli M, Wirth S, Socha P, Vajro P, Lacaille F, Kelly D, Mieli-Vergani G. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Hepatol. 2013;59:814–829. doi: 10.1016/j.jhep.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Myers RP, Ramji A, Bilodeau M, Wong S, Feld JJ. An update on the management of hepatitis C: consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol. 2012;26:359–375. doi: 10.1155/2012/947676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, Rosenthal P, Schwarz KB. NASPGHAN practice guidelines: Diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr. 2012;54:838–855. doi: 10.1097/MPG.0b013e318258328d. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection. Accessed April 5, 2015. Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/ [PubMed]
- 49.Clinical Practice Guidelines for Hepatocellular Carcinoma - The Japan Society of Hepatology 2009 update. Hepatol Res. 2010;40 Suppl 1:2–144. doi: 10.1111/j.1872-034X.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 50.Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, Orsini L, Kim WR, Roberts LR. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617–623.e1. doi: 10.1016/j.cgh.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 51.Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119–126. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 52.Jou JH, Chen PH, Jazwinski A, Bouneva I, Smith AD, Muir AJ. Rates of surveillance and management of hepatocellular carcinoma in patients evaluated at a liver transplant center. Dig Dis Sci. 2010;55:3591–3596. doi: 10.1007/s10620-010-1366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramachandran J. Surveillance for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S50–S56. doi: 10.1016/j.jceh.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song P, Feng X, Zhang K, Song T, Ma K, Kokudo N, Dong J, Yao L, Tang W. Screening for and surveillance of high-risk patients with HBV-related chronic liver disease: promoting the early detection of hepatocellular carcinoma in China. Biosci Trends. 2013;7:1–6. [PubMed] [Google Scholar]
- 56.Sasaki K, Matsuda M, Ohkura Y, Kawamura Y, Inoue M, Hashimoto M, Ikeda K, Kumada H, Watanabe G. The influence of histological differentiation grade on the outcome of liver resection for hepatocellular carcinomas 2 cm or smaller in size. World J Surg. 2015;39:1134–1141. doi: 10.1007/s00268-014-2806-6. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Z, Lei J, Li B, Yan L, Wang W, Wei Y, Cheng K. Liver resection and radiofrequency ablation of very early hepatocellular carcinoma cases (single nodule & lt; 2 cm): a single-center study. Eur J Gastroenterol Hepatol. 2014;26:339–344. doi: 10.1097/MEG.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 58.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 59.Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, Huang HC, Chen WM, Shen CH, Lu CH, Wu CS, et al. Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Am J Gastroenterol. 2015;110:836–844; quiz 845. doi: 10.1038/ajg.2015.100. [DOI] [PubMed] [Google Scholar]
- 60.Cho HJ, Seo YS, Lee KG, Hyun JJ, An H, Keum B, Kim JH, Yim HJ, Jeen YT, Lee HS, et al. Serum aminotransferase levels instead of etiology affects the accuracy of transient elastography in chronic viral hepatitis patients. J Gastroenterol Hepatol. 2011;26:492–500. doi: 10.1111/j.1440-1746.2010.06419.x. [DOI] [PubMed] [Google Scholar]
- 61.Chrysanthos NV, Papatheodoridis GV, Savvas S, Kafiri G, Petraki K, Manesis EK, Archimandritis AJ. Aspartate aminotransferase to platelet ratio index for fibrosis evaluation in chronic viral hepatitis. Eur J Gastroenterol Hepatol. 2006;18:389–396. doi: 10.1097/00042737-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 63.McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, Dunaway E, Williams J. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842–846. doi: 10.1053/jhep.2000.17914. [DOI] [PubMed] [Google Scholar]
- 64.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 65.Lok AS, Lai CL. alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9:110–115. doi: 10.1002/hep.1840090119. [DOI] [PubMed] [Google Scholar]
- 66.Lee E, Edward S, Singal AG, Lavieri MS, Volk M. Improving screening for hepatocellular carcinoma by incorporating data on levels of α-fetoprotein, over time. Clin Gastroenterol Hepatol. 2013;11:437–440. doi: 10.1016/j.cgh.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 67.Singal AG, Conjeevaram HS, Volk ML, Fu S, Fontana RJ, Askari F, Su GL, Lok AS, Marrero JA. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21:793–799. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherman M. Serological surveillance for hepatocellular carcinoma: time to quit. J Hepatol. 2010;52:614–615. doi: 10.1016/j.jhep.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 69.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paul SB, Gulati MS, Sreenivas V, Madan K, Gupta AK, Mukhopadhyay S, Acharya SK. Evaluating patients with cirrhosis for hepatocellular carcinoma: value of clinical symptomatology, imaging and alpha-fetoprotein. Oncology. 2007;72 Suppl 1:117–123. doi: 10.1159/000111717. [DOI] [PubMed] [Google Scholar]
- 72.Barletta E, Tinessa V, Daniele B. Screening of hepatocellular carcinoma: role of the alpha-fetoprotein (AFP) and ultrasonography. Recenti Prog Med. 2005;96:295–299; quiz 328. [PubMed] [Google Scholar]
- 73.Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127:S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 74.Marrero JA. Screening tests for hepatocellular carcinoma. Clin Liver Dis. 2005;9:235–251, vi. doi: 10.1016/j.cld.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Di Bisceglie AM, Hoofnagle JH. Elevations in serum alpha-fetoprotein levels in patients with chronic hepatitis B. Cancer. 1989;64:2117–2120. doi: 10.1002/1097-0142(19891115)64:10<2117::aid-cncr2820641024>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 76.Zhou XD, Tang ZY, Fan J, Zhou J, Wu ZQ, Qin LX, Ma ZC, Sun HC, Qiu SJ, Yu Y, et al. Intrahepatic cholangiocarcinoma: report of 272 patients compared with 5,829 patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1073–1080. doi: 10.1007/s00432-009-0547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17–30. doi: 10.1007/s12072-007-9038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol. 2003;39:1076–1084. doi: 10.1016/s0168-8278(03)00349-0. [DOI] [PubMed] [Google Scholar]
- 79.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marrero JA, El-Serag HB. Alpha-fetoprotein should be included in the hepatocellular carcinoma surveillance guidelines of the American Association for the Study of Liver Diseases. Hepatology. 2011;53:1060–1061; author reply 1061-1062. doi: 10.1002/hep.24033. [DOI] [PubMed] [Google Scholar]
- 81.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Tanaka J, Kagebayashi C, Satomura S. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol. 2014;49:555–563. doi: 10.1007/s00535-013-0883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimauchi Y, Tanaka M, Kuromatsu R, Ogata R, Tateishi Y, Itano S, Ono N, Yutani S, Nagamatsu H, Matsugaki S, et al. A simultaneous monitoring of Lens culinaris agglutinin A-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin as an early diagnosis of hepatocellular carcinoma in the follow-up of cirrhotic patients. Oncol Rep. 2000;7:249–256. doi: 10.3892/or.7.2.249. [DOI] [PubMed] [Google Scholar]
- 84.Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, Busuttil RW, Tong MJ. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–1548. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]
- 85.Kasahara A, Hayashi N, Fusamoto H, Kawada Y, Imai Y, Yamamoto H, Hayashi E, Ogihara T, Kamada T. Clinical evaluation of plasma des-gamma-carboxy prothrombin as a marker protein of hepatocellular carcinoma in patients with tumors of various sizes. Dig Dis Sci. 1993;38:2170–2176. doi: 10.1007/BF01299891. [DOI] [PubMed] [Google Scholar]
- 86.Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, Ohnishi K. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. Am J Gastroenterol. 1999;94:650–654. doi: 10.1111/j.1572-0241.1999.00930.x. [DOI] [PubMed] [Google Scholar]
- 87.Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687–1693. doi: 10.1136/gut.2010.214916. [DOI] [PubMed] [Google Scholar]
- 88.Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D’Amelio A, Lok AS, Block TM. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–1012. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 89.Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, Boncimino A, Moratti R, Corazza GR. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353–358. doi: 10.1093/annonc/mdm448. [DOI] [PubMed] [Google Scholar]
- 90.Hsia CY, Huo TI, Chiang SY, Lu MF, Sun CL, Wu JC, Lee PC, Chi CW, Lui WY, Lee SD. Evaluation of interleukin-6, interleukin-10 and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur J Surg Oncol. 2007;33:208–212. doi: 10.1016/j.ejso.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 91.Giannelli G, Fransvea E, Trerotoli P, Beaugrand M, Marinosci F, Lupo L, Nkontchou G, Dentico P, Antonaci S. Clinical validation of combined serological biomarkers for improved hepatocellular carcinoma diagnosis in 961 patients. Clin Chim Acta. 2007;383:147–152. doi: 10.1016/j.cca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 92.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 94.Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 95.Kew MC. Alpha-fetoprotein. In: Read AE eMTiG., editor. London: Butterworths; 1975. p. 91. [Google Scholar]
- 96.Chan SL, Mo F, Johnson PJ, Siu DY, Chan MH, Lau WY, Lai PB, Lam CW, Yeo W, Yu SC. Performance of serum α-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB (Oxford) 2014;16:366–372. doi: 10.1111/hpb.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford) 2005;7:26–34. doi: 10.1080/13651820410024049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song do S, Bae SH. Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period. Clin Mol Hepatol. 2012;18:258–267. doi: 10.3350/cmh.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 100.Park JW. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004;10:88–98. [PubMed] [Google Scholar]
- 101.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 102.Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, Taketa K, Endo Y, Nagataki S. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328:1802–1806. doi: 10.1056/NEJM199306243282502. [DOI] [PubMed] [Google Scholar]
- 103.Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, Satomura S, Matsuura S, Kawai T, Hirai H. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–5423. [PubMed] [Google Scholar]
- 104.Gao J, Feng X, Inagaki Y, Song P, Kokudo N, Hasegawa K, Sugawara Y, Tang W. Des-γ-carboxy prothrombin and c-Met were concurrently and extensively expressed in hepatocellular carcinoma and associated with tumor recurrence. Biosci Trends. 2012;6:153–159. doi: 10.5582/bst.2012.v6.4.153. [DOI] [PubMed] [Google Scholar]
- 105.Hu B, Tian X, Sun J, Meng X. Evaluation of individual and combined applications of serum biomarkers for diagnosis of hepatocellular carcinoma: a meta-analysis. Int J Mol Sci. 2013;14:23559–23580. doi: 10.3390/ijms141223559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hadziyannis E, Sialevris K, Georgiou A, Koskinas J. Analysis of serum α-fetoprotein-L3% and des-γ carboxyprothrombin markers in cases with misleading hepatocellular carcinoma total α-fetoprotein levels. Oncol Rep. 2013;29:835–839. doi: 10.3892/or.2012.2147. [DOI] [PubMed] [Google Scholar]
- 107.Song P, Feng X, Zhang K, Song T, Ma K, Kokudo N, Dong J, Tang W. Perspectives on using des-γ-carboxyprothrombin (DCP) as a serum biomarker: facilitating early detection of hepatocellular carcinoma in China. Hepatobiliary Surg Nutr. 2013;2:227–231. doi: 10.3978/j.issn.2304-3881.2013.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song P, Tang W, Hasegawa K, Kokudo N. Systematic evidence-based clinical practice guidelines are ushering in a new stage of standardized management of hepatocellular carcinoma in Japan. Drug Discov Ther. 2014;8:64–70. doi: 10.5582/ddt.8.64. [DOI] [PubMed] [Google Scholar]
- 109.Zhang K, Song P, Gao J, Li G, Zhao X, Zhang S. Perspectives on a combined test of multi serum biomarkers in China: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Drug Discov Ther. 2014;8:102–109. doi: 10.5582/ddt.2014.01026. [DOI] [PubMed] [Google Scholar]
- 110.Song P, Feng X, Inagaki Y, Song T, Zhang K, Wang Z, Zheng S, Ma K, Li Q, Kong D, et al. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-γ-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: A multi-center case-controlled study of 1,153 subjects. Biosci Trends. 2014;8:266–273. doi: 10.5582/bst.2014.01116. [DOI] [PubMed] [Google Scholar]
- 111.Song P. Standardizing management of hepatocellular carcinoma in China: devising evidence-based clinical practice guidelines. Biosci Trends. 2013;7:250–252. [PubMed] [Google Scholar]
- 112.Song PP, Gao JJ, Kokudo N, Dong JH, Tang W. “Knowledge into action” Exploration of an appropriate approach for constructing evidence-based clinical practice guidelines for hepatocellular carcinoma. Biosci Trends. 2012;6:147–152. doi: 10.5582/bst.2012.v6.3.147. [DOI] [PubMed] [Google Scholar]
- 113.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 114.Tangkijvanich P, Chanmee T, Komtong S, Mahachai V, Wisedopas N, Pothacharoen P, Kongtawelert P. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol. 2010;25:129–137. doi: 10.1111/j.1440-1746.2009.05988.x. [DOI] [PubMed] [Google Scholar]
- 115.Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, Cho SY, Hong YJ, Park HY, Lee M, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101:2051–2059. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 116.Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, Hainaut P, Marrero JA, Beretta L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483–490. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mukozu T, Nagai H, Matsui D, Kanekawa T, Sumino Y. Serum VEGF as a tumor marker in patients with HCV-related liver cirrhosis and hepatocellular carcinoma. Anticancer Res. 2013;33:1013–1021. [PubMed] [Google Scholar]
- 118.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 119.Zhu WW, Guo JJ, Guo L, Jia HL, Zhu M, Zhang JB, Loffredo CA, Forgues M, Huang H, Xing XJ, et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res. 2013;19:3944–3954. doi: 10.1158/1078-0432.CCR-12-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2000;64:311–336. doi: 10.3322/caac.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798–9807. doi: 10.1158/0008-5472.CAN-10-1001. [DOI] [PubMed] [Google Scholar]
- 122.Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, Wang JF, Zhang Z, Lu S, Huang X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–4788. doi: 10.1200/JCO.2011.38.2697. [DOI] [PubMed] [Google Scholar]
- 123.Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, Tanemura M, Tomokuni A, Takemasa I, Umeshita K, et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56:167–175. doi: 10.1016/j.jhep.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 124.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abdel-Wahab M, El-Husseiny TS, El Hanafy E, El Shobary M, Hamdy E. Prognostic factors affecting survival and recurrence after hepatic resection for hepatocellular carcinoma in cirrhotic liver. Langenbecks Arch Surg. 2010;395:625–632. doi: 10.1007/s00423-010-0643-0. [DOI] [PubMed] [Google Scholar]
- 126.Mitsuhashi N, Kobayashi S, Doki T, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, et al. Clinical significance of alpha-fetoprotein: involvement in proliferation, angiogenesis, and apoptosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:e189–e197. doi: 10.1111/j.1440-1746.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 127.Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mailey B, Artinyan A, Khalili J, Denitz J, Sanchez-Luege N, Sun CL, Bhatia S, Nissen N, Colquhoun SD, Kim J. Evaluation of absolute serum α-fetoprotein levels in liver transplant for hepatocellular cancer. Arch Surg. 2011;146:26–33. doi: 10.1001/archsurg.2010.295. [DOI] [PubMed] [Google Scholar]
- 130.Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, Ibrahim SM, Sato KT, Baker T, Miller FH, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734–5742. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 131.Nakazawa T, Hidaka H, Takada J, Okuwaki Y, Tanaka Y, Watanabe M, Shibuya A, Minamino T, Kokubu S, Koizumi W. Early increase in α-fetoprotein for predicting unfavorable clinical outcomes in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol. 2013;25:683–689. doi: 10.1097/MEG.0b013e32835d913b. [DOI] [PubMed] [Google Scholar]
- 132.Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V, Giordano L, Santoro A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101–107. doi: 10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 133.Matsuda M, Asakawa M, Amemiya H, Fujii H. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol. 2011;26:731–738. doi: 10.1111/j.1440-1746.2010.06532.x. [DOI] [PubMed] [Google Scholar]
- 134.Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Yamada S, Asanoma M. Prediction of recurrence of hepatocellular carcinoma after curative hepatectomy using preoperative Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein. Hepatol Res. 2012;42:887–894. doi: 10.1111/j.1872-034X.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- 135.Fujiyama S, Tanaka M, Maeda S, Ashihara H, Hirata R, Tomita K. Tumor markers in early diagnosis, follow-up and management of patients with hepatocellular carcinoma. Oncology. 2002;62 Suppl 1:57–63. doi: 10.1159/000048277. [DOI] [PubMed] [Google Scholar]
- 136.Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Yamaguchi A, Isogai M, Kaneoka Y, Washizu J. Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2006;4:111–117. doi: 10.1016/s1542-3565(05)00855-4. [DOI] [PubMed] [Google Scholar]
- 137.Kiriyama S, Uchiyama K, Ueno M, Ozawa S, Hayami S, Tani M, Yamaue H. Triple positive tumor markers for hepatocellular carcinoma are useful predictors of poor survival. Ann Surg. 2011;254:984–991. doi: 10.1097/SLA.0b013e3182215016. [DOI] [PubMed] [Google Scholar]
- 138.Nakagawa S, Beppu T, Okabe H, Sakamoto K, Kuroki H, Mima K, Nitta H, Imai K, Hayashi H, Sakamoto Y, et al. Triple positive tumor markers predict recurrence and survival in early stage hepatocellular carcinoma. Hepatol Res. 2014;44:964–974. doi: 10.1111/hepr.12277. [DOI] [PubMed] [Google Scholar]
- 139.Park H, Park JY. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. Biomed Res Int. 2013;2013:310427. doi: 10.1155/2013/310427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng J, Wang W, Zhang Y, Liu X, Li M, Wu Z, Liu Z, Lv Y, Wang B. Prognostic role of pre-treatment serum AFP-L3% in hepatocellular carcinoma: systematic review and meta-analysis. PLoS One. 2014;9:e87011. doi: 10.1371/journal.pone.0087011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar A, Acharya SK, Singh SP, Saraswat VA, Arora A, Duseja A, Goenka MK, Jain D, Kar P, Kumar M, et al. The Indian National Association for Study of the Liver (INASL) Consensus on Prevention, Diagnosis and Management of Hepatocellular Carcinoma in India: The Puri Recommendations. J Clin Exp Hepatol. 2014;4:S3–S26. doi: 10.1016/j.jceh.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang Y, Yang X, Zhao F, Shen Q, Wang Z, Lv X, Hu B, Yu B, Fan J, Qin W. Overexpression of Dickkopf-1 predicts poor prognosis for patients with hepatocellular carcinoma after orthotopic liver transplantation by promoting cancer metastasis and recurrence. Med Oncol. 2014;31:966. doi: 10.1007/s12032-014-0966-8. [DOI] [PubMed] [Google Scholar]
- 143.Cui X, Li Z, Gao PJ, Gao J, Zhu JY. Prognostic value of glypican-3 in patients with HBV-associated hepatocellular carcinoma after liver transplantation. Hepatobiliary Pancreat Dis Int. 2015;14:157–163. doi: 10.1016/s1499-3872(15)60349-6. [DOI] [PubMed] [Google Scholar]
- 144.Fung J, Poon RT, Yu WC, Chan SC, Chan AC, Chok KS, Cheung TT, Seto WK, Lo CM, Lai CL, et al. Use of liver stiffness measurement for liver resection surgery: correlation with indocyanine green clearance testing and post-operative outcome. PLoS One. 2013;8:e72306. doi: 10.1371/journal.pone.0072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Z, Song P, Xia J, Inagaki Y, Tang W, Kokudo N. Can gamma-glutamyl transferase levels contribute to a better prognosis for patients with hepatocellular carcinoma? Drug Discov Ther. 2014;8:134–138. doi: 10.5582/ddt.2014.01025. [DOI] [PubMed] [Google Scholar]
- 146.Sheen IS, Jeng KS, Tsai YC. Is the expression of gamma-glutamyl transpeptidase messenger RNA an indicator of biological behavior in recurrent hepatocellular carcinoma? World J Gastroenterol. 2003;9:468–473. doi: 10.3748/wjg.v9.i3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ju MJ, Qiu SJ, Fan J, Zhou J, Gao Q, Cai MY, Li YW, Tang ZY. Preoperative serum gamma-glutamyl transferase to alanine aminotransferase ratio is a convenient prognostic marker for Child-Pugh A hepatocellular carcinoma after operation. J Gastroenterol. 2009;44:635–642. doi: 10.1007/s00535-009-0050-x. [DOI] [PubMed] [Google Scholar]
- 148.Zhao WC, Fan LF, Yang N, Zhang HB, Chen BD, Yang GS. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:858–864. doi: 10.1016/j.ejso.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 149.Zhao WC, Zhang HB, Yang N, Fu Y, Qian W, Chen BD, Fan LF, Yang GS. Preoperative predictors of short-term survival after hepatectomy for multinodular hepatocellular carcinoma. World J Gastroenterol. 2012;18:3272–3281. doi: 10.3748/wjg.v18.i25.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carr BI, Pancoska P, Branch RA. Low alpha-fetoprotein hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1543–1549. doi: 10.1111/j.1440-1746.2010.06303.x. [DOI] [PubMed] [Google Scholar]
- 151.Guiu B, Deschamps F, Boulin M, Boige V, Malka D, Ducreux M, Hillon P, de Baère T. Serum gamma-glutamyl-transferase independently predicts outcome after transarterial chemoembolization of hepatocellular carcinoma: external validation. Cardiovasc Intervent Radiol. 2012;35:1102–1108. doi: 10.1007/s00270-011-0293-9. [DOI] [PubMed] [Google Scholar]
- 152.Nishikawa H, Nishijima N, Arimoto A, Inuzuka T, Kita R, Kimura T, Osaki Y. Prognostic factors in patients with hepatitis B virus-related hepatocellular carcinoma undergoing nucleoside analog antiviral therapy. Oncol Lett. 2013;6:1213–1218. doi: 10.3892/ol.2013.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang JB, Chen Y, Zhang B, Xie X, Zhang L, Ge N, Ren Z, Ye SL. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2011;23:787–793. doi: 10.1097/MEG.0b013e32834902dd. [DOI] [PubMed] [Google Scholar]
- 154.Köberle V, Kronenberger B, Pleli T, Trojan J, Imelmann E, Peveling-Oberhag J, Welker MW, Elhendawy M, Zeuzem S, Piiper A, et al. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur J Cancer. 2013;49:3442–3449. doi: 10.1016/j.ejca.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 155.Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 156.Schütte K, Schulz C, Link A, Malfertheiner P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis. World J Hepatol. 2015;7:139–149. doi: 10.4254/wjh.v7.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]


