Abstract
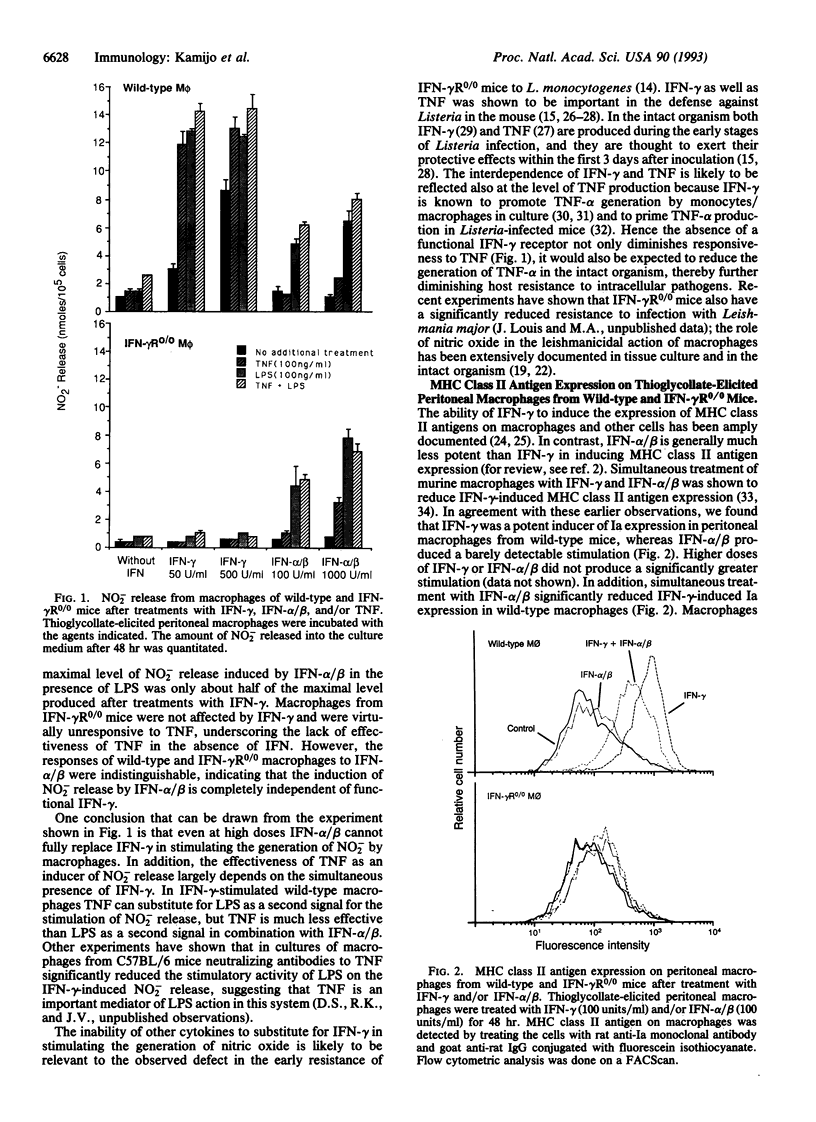
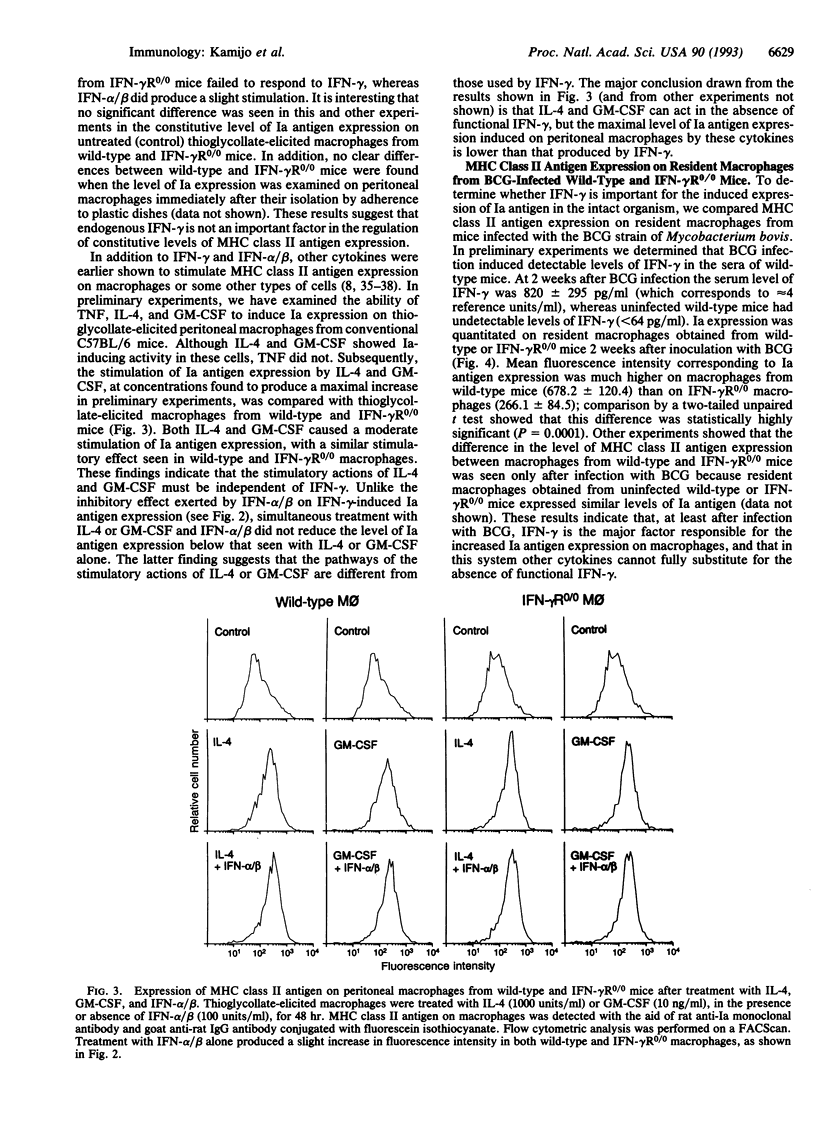
Availability of mice with a targeted disruption of the interferon gamma (IFN-gamma) receptor gene (IFN-gamma R0/0 mice) made it possible to examine parameters of macrophage activation in the absence of a functional IFN-gamma receptor. We asked to what extent other cytokines could replace IFN-gamma in the induction of nitric oxide or major histocompatibility complex class II antigen (Ia) expression in peritoneal macrophages. In thioglycollate-elicited macrophages from wild-type mice, tumor necrosis factor (TNF) alone was virtually ineffective in inducing release of NO2- (the endproduct of nitric oxide generation), but TNF enhanced NO2- release in the presence of IFN-gamma. In macrophages from IFN-gamma R0/0 mice, which were unresponsive to IFN-gamma, TNF completely failed to stimulate NO2- release. The stimulatory actions of IFN-alpha/beta on NO2- release were indistinguishable in wild-type and IFN-gamma R0/0 macrophages: IFN-alpha/beta was ineffective on its own, showed marginal stimulation of NO2- release in combination with TNF, and was moderately effective in the presence of lipopolysaccharide. The level of constitutive Ia antigen expression was not significantly different in peritoneal macrophages from wild-type and IFN-gamma R0/0 mice. An increased Ia expression was induced by IL-4 and granulocyte-macrophage colony-stimulating factor in both wild-type and IFN-gamma R0/0 macrophages, but the magnitude of this induction was less than with optimal concentrations of IFN-gamma in macrophages from wild-type mice. IFN-alpha/beta showed only a minor stimulatory effect on Ia expression in both wild-type and IFN-gamma R0/0 macrophages. Simultaneous treatment of wild-type macrophages with IFN-alpha/beta and IFN-gamma reduced the IFN-gamma-induced Ia expression in wild-type macrophages, but IFN-alpha/beta did not show an inhibitory effect on IL-4- or granulocyte-macrophage-colony-stimulating factor-induced Ia expression in either wild-type or IFN-gamma R0/0 macrophages. The important role of IFN-gamma in the regulation of the induced expression of major histocompatibility complex class II antigen was confirmed by showing that after systemic infection with the BCG strain of Mycobacterium bovis resident peritoneal macrophages from IFN-gamma R0/0 mice had a lower level of Ia expression than macrophages from wild-type mice. The inability of other cytokines to substitute fully for IFN-gamma in macrophage activation helps to explain the earlier observed decreased resistance of IFN-gamma R0/0 mice to some infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenzana-Seisdedos F., Mogensen S. C., Vuillier F., Fiers W., Virelizier J. L. Autocrine secretion of tumor necrosis factor under the influence of interferon-gamma amplifies HLA-DR gene induction in human monocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6087–6091. doi: 10.1073/pnas.85.16.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Beutler B., Tkacenko V., Milsark I., Krochin N., Cerami A. Effect of gamma interferon on cachectin expression by mononuclear phagocytes. Reversal of the lpsd (endotoxin resistance) phenotype. J Exp Med. 1986 Nov 1;164(5):1791–1796. doi: 10.1084/jem.164.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E., Herberman R. B., Varesio L. Requirement for protein synthesis for induction of macrophage tumoricidal activity by IFN-alpha and IFN-beta but not by IFN-gamma. J Immunol. 1984 Jun;132(6):3226–3228. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza I., Männel D., Ruppel A., Falk W., Krammer P. H. Interferon gamma and lymphotoxin or tumor necrosis factor act synergistically to induce macrophage killing of tumor cells and schistosomula of Schistosoma mansoni. J Exp Med. 1987 Aug 1;166(2):589–594. doi: 10.1084/jem.166.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L. A., Wahl L. M., Vogel S. N. Analysis of Ia antigen expression in macrophages derived from bone marrow cells cultured in granulocyte-macrophage colony-stimulating factor or macrophage colony-stimulating factor. J Immunol. 1988 Apr 15;140(8):2652–2660. [PubMed] [Google Scholar]
- Fischer H. G., Frosch S., Reske K., Reske-Kunz A. B. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988 Dec 1;141(11):3882–3888. [PubMed] [Google Scholar]
- Fung-Leung W. P., Mak T. W. Embryonic stem cells and homologous recombination. Curr Opin Immunol. 1992 Apr;4(2):189–194. doi: 10.1016/0952-7915(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Havell E. A. Listeria monocytogenes-induced interferon-gamma primes the host for production of tumor necrosis factor and interferon-alpha/beta. J Infect Dis. 1993 Jun;167(6):1364–1371. doi: 10.1093/infdis/167.6.1364. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993 Mar 19;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Inaba K., Kitaura M., Kato T., Watanabe Y., Kawade Y., Muramatsu S. Contrasting effect of alpha/beta- and gamma-interferons on expression of macrophage Ia antigens. J Exp Med. 1986 Apr 1;163(4):1030–1035. doi: 10.1084/jem.163.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Cox F. E. Nonspecific defence mechanism: the role of nitric oxide. Immunol Today. 1991 Mar;12(3):A17–A21. doi: 10.1016/S0167-5699(05)80006-4. [DOI] [PubMed] [Google Scholar]
- Ling P. D., Warren M. K., Vogel S. N. Antagonistic effect of interferon-beta on the interferon-gamma-induced expression of Ia antigen in murine macrophages. J Immunol. 1985 Sep;135(3):1857–1863. [PubMed] [Google Scholar]
- Mogensen S. C., Virelizier J. L. The interferon-macrophage alliance. Interferon. 1987;8:55–84. [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A. Innate immunity to a facultative intracellular bacterial pathogen. Curr Opin Immunol. 1992 Feb;4(1):20–24. doi: 10.1016/0952-7915(92)90118-x. [DOI] [PubMed] [Google Scholar]
- Schorle H., Holtschke T., Hünig T., Schimpl A., Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991 Aug 15;352(6336):621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Clark-Lewis I., McKimm-Breschkin L., Harris A. W., Schrader J. W. Interferon-gamma induces enhanced expression of Ia and H-2 antigens on B lymphoid, macrophage, and myeloid cell lines. J Immunol. 1983 Aug;131(2):788–793. [PubMed] [Google Scholar]
- Zlotnik A., Fischer M., Roehm N., Zipori D. Evidence for effects of interleukin 4 (B cell stimulatory factor 1) on macrophages: enhancement of antigen presenting ability of bone marrow-derived macrophages. J Immunol. 1987 Jun 15;138(12):4275–4279. [PubMed] [Google Scholar]



