Abstract
The natural history of hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) is dismal (approximately 2-4 mo), and PVTT is reportedly found in 10%-40% of HCC patients at diagnosis. According to the Barcelona Clinic Liver Cancer (BCLC) Staging System (which is the most widely adopted HCC management guideline), sorafenib is the standard of care for advanced HCC (i.e., BCLC stage C) and the presence of PVTT is included in this category. However, sorafenib treatment only marginally prolongs patient survival and, notably, its therapeutic efficacy is reduced in patients with PVTT. In this context, there have been diverse efforts to develop alternatives to current standard systemic chemotherapies or combination treatment options. To date, many studies on transarterial chemoembolization, 3-dimensional conformal radiotherapy, hepatic arterial chemotherapy, and transarterial radioembolization report better overall survival than sorafenib therapy alone, but their outcomes need to be verified in future prospective, randomized controlled studies in order to be incorporated into current treatment guidelines. Additionally, combination strategies have been applied to treat HCC patients with PVTT, with the hope that the possible synergistic actions among different treatment modalities would provide promising results. This narrative review describes the current status of the management options for HCC with PVTT, with a focus on overall survival.
Keywords: Hepatocellular carcinoma, Portal vein tumor thrombosis, Sorafenib, Transarterial chemoembolization, Transarterial radioembolization, Hepatic arterial chemotherapy, Radiotherapy
Core tip: Hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) is associated with a grave prognosis if left untreated. Sorafenib is the only treatment modality recommended for treating HCC patients with PVTT according to most international HCC treatment guidelines. However, the survival benefits observed following systemic sorafenib treatment are only marginal. Under these circumstances, the need for better treatment options remains unfulfilled. In this comprehensive review, various treatment options are presented-including transarterial chemoembolization, transarterial radioembolization, hepatic arterial infusion, chemotherapy, and radiotherapy-and their outcomes, along with combination strategies.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the world’s fifth most common cancer, and predominantly occurs in patients with liver cirrhosis[1]. Portal vein tumor thrombosis (PVTT) is reportedly found in 10%-40% of HCC patients at diagnosis[2-4]. PVTT is associated with a dismal prognosis, as it is closely related to intrahepatic metastasis and tumor recurrence, and patients only demonstrate 2-4 mo of overall survival with the best supportive care[3-5].
In HCC patients with PVTT, the management options are limited and the optimal treatments remain largely controversial. Curative-intent surgery is often technically challenging, and liver transplant is mostly contraindicated due to high tumor recurrence rates[6,7]. Transarterial chemoembolization (TACE) is generally contraindicated as it can subsequently induce hepatic necrosis and worsen liver function. Radiofrequency ablation is not effective or safe due to the proximity of the hepatic vasculature. External beam radiation plays a limited role in PVTT due to the sensitivity of the liver to radiation and potential for liver failure.
The Barcelona Clinic Liver Cancer (BCLC) Staging System is the most widely adopted HCC management guideline, which classifies HCC with PVTT as advanced HCC (BCLC stage C)[8]. According to the BCLC guidelines, sorafenib is the standard of care for patients with advanced HCC (Figure 1). However, the survival benefits observed following sorafenib treatment are limited, and this underscores the need for better treatment strategies[9]. In recent years, there have been attempts to develop alternative or combination treatments in order to improve the overall survival of patients with HCC and PVTT. In this narrative article, these diverse treatment modalities are thoroughly reviewed (Table 1).
Figure 1.
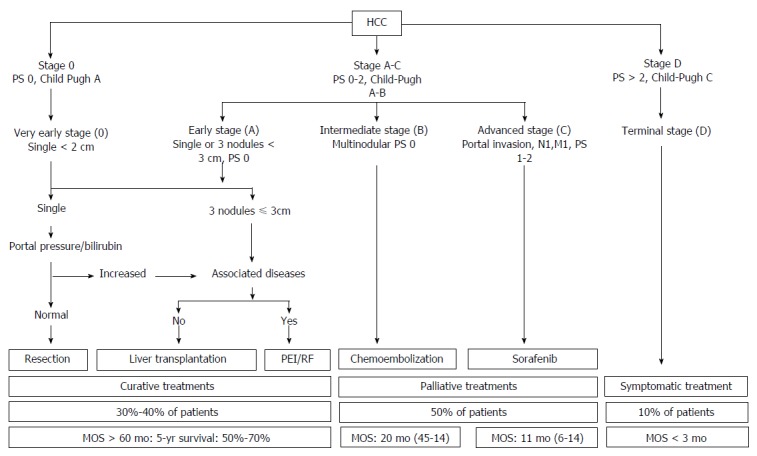
Updated barcelona clinic liver cancer staging system and treatment strategy. HCC: Hepatocellular carcinoma; RT: Radiation therapy.
Table 1.
Summary of combination treatments for hepatocellular carcinoma patients with portal vein tumor thrombosis
| Overall survival (mo) |
Extent of PVTT (mo) |
Ref. | ||
| Main PVTT | Branch PVTT | |||
| BSC | 2-4 | Llovet et al[3], Schöniger-Hekele et al[5] | ||
| Sorafenib | 6.5-8.1 | Llovet et al[9], Cheng et al[11] | ||
| TACE | 7-10 | 5.3 | 10 | Chung et al[21], Luo et al[22] |
| HAIC | 6.5-14 | Park et al[26], Ando et al[27], Eun et al[28] | ||
| RT | 9.6-10.9 | Toya et al[39], Nakazawa et al[40] | ||
| TARE | 6-16.9 | 7.7 | 16.9 | Salem et al[47], Kulik et al[49], Sangro et al[48], Memon et al[50] |
| TACE plus sorafenib | 11-13 | 3 | 13-15 | Pan et al[58], Zhu et al[59] |
| Sorafenib plus RT | 8.6-10.6 | Chen et al[53], Chow et al[61] | ||
| TACE plus RT | 10.6-12 | 12 | Yoon et al[64], Chung et al[72], Kim et al[73] | |
| HAIC plus RT | 12.1 | Fujino et al[76] | ||
BSC: Best supportive care; TACE: Transarterial chemoembolization; HAIC: Hepatic arterial infusion chemotherapy; RT: Radiation therapy; TARE: Transarterial radioembolization; PVTT: Portal vein thrombosis.
SYSTEMIC THERAPY
Sorafenib-an oral multi-kinase tyrosine inhibitor that demonstrates antiproliferative and antiangiogenic effects-is the only drug proven to improve overall survival in patients with advanced HCC, including those with PVTT[10]. The SHARP trial reported better median overall survival without significant drug toxicity in sorafenib-treated patients (10.7 mo in the sorafenib group vs 7.9 mo in placebo group; HR = 0.69; 95%CI: 0.55-0.87, P < 0.001)[9]. Subsequent subgroup analyses revealed that sorafenib consistently improved the median overall survival (OS) and median time to tumor progression (TTP) in comparison with the control group, irrespective of disease etiology, baseline tumor extent (e.g., presence of macroscopic vascular invasion), tumor stage (BCLC B-C), prior therapy, or performance status. In particular, patients with macrovascular invasion who were treated with sorafenib demonstrated a longer median OS (8.1 mo vs 4.9 mo) and TTP (4.1 mo vs 2.7 mo)[10]. Similar findings were also identified in an Asian-Pacific population (6.5 mo in the sorafenib group vs 4.2 mo in the placebo group; HR = 0.68; 95%CI: 0.50-0.93; P = 0.014)[9,11]. Regarding the safety profile, the most common adverse events included diarrhea, hand-foot skin reaction, fatigue, and skin rash. However, the incidence of serious or life-threatening complications was rare and not affected by the baseline patient characteristics. Therefore, it was suggested that sorafenib could be administered to a wide range of patients with HCC[10]. Since these 2 key studies were released, sorafenib has been considered the standard of care for HCC patients with PVTT by many treatment guidelines such as BCLC Staging System, European Association for the Study of Liver Disease (EASL), and American Association for the Study of Liver Diseases (AASLD)[8,12]. A retrospective study of 30 HCC patients with PVTT demonstrated that the median OS was 3.1 mo and the disease control rate was 33.3%, respectively[13]. Dose reduction was required in 13 patients due to fatigue, hand-foot syndrome, diarrhea, nausea, and skin rash, but no grade 4 adverse events occurred. Interestingly, 3 patients with a partial response achieved marked PVTT revascularization, and responsive patients demonstrated a significantly prolonged OS in comparison with nonresponders. It is assumed that sorafenib exerts antithrombotic effects on PVTT by inhibiting the vascular endothelial growth factor (VEGF) receptor pathway. Therefore, a sensitivity analysis is warranted in order to predict good responders to Sorafenib treatment.
Unfortunately, the observed survival benefits of sorafenib in HCC patients with PVTT are modest, and in recent years there have been efforts to combine systemic chemotherapy with other locoregional therapies in order to improve treatment outcomes. In addition, there are several other systemic agents under development, but none have demonstrated improved OS.
TRANSARTERIAL CHEMOEMBOLIZATION
Transarterial chemoembolization (TACE) refers to the percutaneous intraarterial introduction of an embolic agent that occludes tumor feeders in combination with an anticancer agent, with the aim of delivering sustained drug levels to the HCC. The anticancer agent is mixed with Lipiodol or loaded onto microspheres. Until now, TACE has been widely used to treat HCC in different stages and plays an established role in the treatment of unresectable HCC[14-18]. In the presence of PVTT, however, TACE is theoretically contraindicated because of the potential risk of hepatic insufficiency that results from ischemia following TACE. However, recent studies demonstrate that TACE can be safely performed in the presence of adequate collateral circulation around the occluded portal vein[19,20].
Chung et al[21] have investigated the efficacy and safety of TACE in patients with HCC and main PVTT and reported a median OS period of 3.7 mo. The median survival of the TACE group was significantly longer than the supportive care group (5.6 mo vs 2.2 mo). TACE and Child-Pugh A classification were independent predictive factors associated with better overall survival. Regarding complications, no procedure-related deaths were reported within 4 wk after TACE, and morbidity was 28.9%. A prospective comparative study investigated the efficacy and safety of administering TACE to HCC patients with PVTT in comparison with conservative management[22]. In that study, the TACE group demonstrated significantly better OS than the conservative treatment group (7.1 mo vs 4.1 mo), and TACE-related complications were adequately managed using conservative treatment. According to the subgroup analysis of segmental and major PVTT, the TACE group also demonstrated significantly better survival. Treatment type, PVTT extent, tumor size, and serum bilirubin were independent prognostic factors of survival on multivariate analysis. A recent meta-analysis, which included the aforementioned study, showed that patients who underwent a TACE procedure demonstrated a significantly better 1-year survival rate in comparison with patients who received conservative treatment (OR = 3.079; 95%CI: 1.094-8.662)[23].
As an alternative to conventional lipiodol-based TACE, nonresorbable microspheres can be loaded with an anticancer agent and intraarterially infused to increase the local drug concentration and reduce systemic toxicity[24]. These particles are known as drug-eluting beads (DEB). There are few studies on using DEB-TACE to treat HCC and PVTT. Kalva et al[25] evaluated the safety and efficacy of administering DEB-TACE to advanced HCC patients, including those with lobar PVTT. The median OS was 13.3 mo, and the presence of portal vein thrombosis demonstrated no statistically significant association with OS.
As demonstrated by various studies, TACE is considered safe and feasible for select patients with unresectable HCC, PVTT, and preserved liver function and collateral portal venous circulation. However, to date, the reported OS period for HCC patients with PVTT who receive TACE is slightly better than that of patients who receive sorafenib therapy, though this claim needs to be validated in a prospective study.
HEPATIC ARTERIAL INFUSION CHEMOTHERAPY
Hepatic arterial infusion chemotherapy (HAIC) using an implantable port system has been applied to treat advanced HCC with PVTT. HAIC is theoretically more effective against HCC than systemic chemotherapy because it provides the direct delivery of a high concentration of the anticancer agent to the tumor through the hepatic artery. HAIC also minimizes systemic toxicities due to first-pass effects[26]. HAIC is usually administered using 1 of the following 3 well-reported regimens: cisplatin alone, 5-FU plus cisplatin, or 5-FU plus interferon. Many studies have been conducted on advanced HCC with PVTT and demonstrate a median OS of 6.5-14 mo[26-29]. Quite recently, Song et al[30] conducted a multicenter study to compare the efficacy of sorafenib with HAIC in HCC patients with PVTT. In their study, the median OS (7.1 mo vs 5.5 mo, P = 0.011) and TTP were significantly longer in the HAIC group than in the sorafenib group (3.3 mo vs 2.2 mo, P = 0.034).
Regarding the safety profile of HAIC, hematologic complications (e.g., anemia, neutropenia, thrombocytopenia) and gastrointestinal toxicity (e.g., nausea, vomiting, abdominal pain) can occur. Most HAIC-related toxicities are transient, tolerable, and successfully controlled with conservative treatment, although some patients end up withdrawing from HAIC therapy. In addition, catheter-related complications (e.g., hematoma, catheter occlusion, infection) can also occur[31,32]. However, HAIC is not recommended as a standard treatment for HCC patients with PVTT, as these data are mostly from Japan and there is a lack of randomized controlled trials. To become an alternative to current Sorafenib treatment, the better outcomes observed in previous retrospective studies using HAIC need to be verified by future prospective studies and validated in a Western population.
EXTERNAL BEAM RADIATION
The role of external beam radiation therapy (RT) for HCC is limited due to the risks of radiation-induced liver disease and the low tolerance of the whole liver to RT[33]. However, rapid advances in radiotherapy techniques, including 3-dimensional conformal radiotherapy and image-guided radiotherapy, as well as knowledge on partial volume liver tolerance, have enabled the delivery of higher radiation doses to HCC than in the past, thereby allowing RT to be used as a potential standalone or adjunct treatment for HCC[34-36].
Notably, one of the primary indications for RT is the presence of PVTT, and previous studies report good treatment responses and promising outcomes using RT[37,38]. A retrospective study evaluated the treatment outcomes of RT in 38 patients with HCC with PVTT[39]. In that study, the treatment rate was 44.7% and OS was 9.6 mo. Nakazawa et al[40] recently compared standard sorafenib therapy to RT in patients with unresectable HCC with main or first-branch PVTT, and they reported a longer median OS in the RT group (10.9 mo vs 4.8 mo, P = 0.025) after performing propensity score analysis (28 pairs). In their study, whereas almost half the patients discontinued sorafenib due to adverse events, there was no grade 3 or higher gastrointestinal or hepatic toxicity and grade 3 leukocytopenia was only observed in 1 patient in the RT group. Because HAIC is not regarded as a standard therapeutic modality, future large-scale and prospective studies are warranted in order to test the clinical efficacy and safety of using RT to treat HCC with PVTT.
TRANSARTERIAL RADIOEMBOLIZATION
Transarterial radioembolization (TARE) and selective internal radiation therapy (SIRT) using beta-emitting yittrium-90 in resin microspheres or glass particles have been introduced as alternatives to TACE for HCC[41]. TARE differs from TACE in that it offers antitumor effects in the form of local beta radiation, not arterial obstruction. The embolic materials are loaded with yittrium-90 and administered via intraarterial injection, which allows lobar, segmental, and subsegmental therapy. The average penetration depth by this local radiation into the liver tissue is approximately 2.5 mm, thus sparing the normal adjacent liver from damage and obviating the need of postprocedure isolation. The half-life is 64 h, and almost the entire radiation dose is delivered within 14 d of the procedure[42-44]. There is some evidence that TARE results in encouraging outcomes, especially in patients with PVTT which is a contraindication to TACE[8,45,46]. Administering TARE to patients with advanced HCC has demonstrated a median OS of 6-10 mo, which is comparable to the 6.5-10.7 mo reported in landmark studies on sorafenib[9,11]. Because it produces much fewer embolic effects than TACE, PVTT is not a contraindication for TARE, but the presence and extent of PVTT does affect prognosis. Salem et al[47] studied the long-term outcomes of using TARE to treat HCC and reported a median OS of 16.6 mo in Child-Pugh A patients with branch PVTT vs 6.5 mo in Child-Pugh B patients. Among patients with main PVTT, the median OS decreased to 7.7 mo in Child-Pugh A patients and 4.5 mo in Child-Pugh B patients. Smaller studies using TARE report concordant results. When the distinction between main and branch PVTT was made, the median survival periods reported by Sangro et al[48] were 9.7 and 10.7 mo, and those reported by Kulik et al[49] were 4.4 and 9.9 mo. Memon et al[50] reported even higher median survival periods of 15.7 and 9 mo in Child-Pugh A patients, respectively. Mazzaferro et al[51] performed a single-center, prospective, phase II trial to study the efficacy of TACE on HCC patients with PVTT. They reported a median OS of 13 mo in 35 HCC patients with branch or main PVTT and better patient survival in Child-Pugh A patients (16 mo vs 6 mo). In a recent retrospective study, Gramenzi et al[52] compared the outcomes of sorafenib and TARE in patients with advanced HCC. The median OS values of the 2 groups were comparable: 13.2 mo in the TARE group (63 patients) and 14.4 mo in the sorafenib group (74 patients). Following propensity score analysis (38 pairs), the median OS did not differ between groups. Ongoing randomized controlled trials that compare standard sorafenib therapy to TARE as a first- or second-line treatment for HCC patients with PVTT are expected to define the populations that benefit from this therapeutic modality.
TARE is generally well tolerated, and the most common complication is postembolization syndrome which occurs in 20%-55% of patients. Postembolization syndrome consists of various symptoms (e.g., fatigue, fever, nausea, vomiting, abdominal pain), which are usually well-tolerated with conservative management. Other reported complications, such as radiation-induced liver disease, radiation pneumonitis, radiation cholecystitis, biloma, hepatic abscess, and biliary stricture, are uncommon[47,49]. TARE can lead to severe adverse events, such as gastrointestinal ulcerations, in < 5% of patients[53,54]. However, gastrointestinal toxicities may be prevented by carefully administering preemptive coil embolization.
EMERGENCE OF COMBINATION STRATEGIES FOR TREATING HCC WITH PVTT
As mentioned earlier, the current standard sorafenib treatment only provides modest survival benefits, and, thus, investigators have made concerted efforts to combine different modalities, which will be discussed in the following sections.
TACE IN COMBINATION WITH SORAFENIB
The efficacy of sorafenib in combination with TACE has been investigated, as these two therapeutic options are expected to work synergistically. TACE-induced hypoxia in surviving tumor cells results in the release of angiogenic growth factors, which contribute to tumor recurrence, metastasis, and worse outcomes[55,56]. Sorafenib suppresses tumor cell proliferation by exerting antiangiogenic effects through the blocking of VEGF receptor-2 and -3 and platelet-derived growth factor receptor tyrosine kinase[57]. A retrospective study on combining TACE and sorafenib to treat HCC patients with PVTT (branch and main PVTT) demonstrated a median OS of 13 mo and median TTP of 7 mo, respectively[58]. Procedure-related mortality and grade 4 adverse events did not occur. Child-Pugh class, extrahepatic metastasis, and gross morphologic type were prognostic factors. Zhu et al[59] compared the outcomes of sorafenib plus TACE to the outcomes of TACE alone in patients with HCC and PVTT. TACE plus sorafenib demonstrated significant survival benefits in comparison with TACE alone (11 mo vs 6 mo, P < 0.001). When considering first-, second-, and lower-order branch PVTT, subgroup analyses of OS in patients with different types of PVTT revealed that the median OS of patients treated with TACE plus sorafenib is significantly longer than that of patients treated with TACE alone (13 mo vs 6 mo for patients with first-order PVTT; 15 mo vs 10 mo for patients with second- or lower-order PVTT). In patients with main PVTT, no survival benefit was observed between groups (3 mo in both groups). The worsening of liver function after TACE-sorafenib treatment was only noted in patients with main PVTT, and sorafenib-related complications classified as grade 3 or higher occurred in 16 patients (35%).
A phase II prospective trial (START trial) is under way in which the efficacy of TACE plus sorafenib when administered to HCC patients (including those with branch PVTT) will be investigated. Interim analysis has indicated promising outcomes and acceptable adverse event rates, and, thus, it is expected that the role of combination therapy in HCC patients with PVTT will be determined within the foreseeable future.
SORAFENIB PLUS RADIOTHERAPY
The combination of sorafenib and radiotherapy is based on the finding that sorafenib enhances the radiosensitivity of human HCC cell lines by selectively inhibiting the radiation-induced activation of the VEGFR2 and extracellular signal-regulated kinase (ERK) pathways, thereby promoting radiation-induced apoptosis[60]. In this context, the concurrent administration of sorafenib and radiotherapy-in the form of either TARE or external beam radiation-is expected to work synergistically on advanced HCC. In a phase II study on combining sorafenib and external beam radiation therapy, the mean OS was 10.6 mo in Child-Pugh A patients with PVTT. Of the 40 patients analyzed, 4 (10%) and 6 patients (15%) experienced grade 3 or higher hepatic toxicities during or before RT, respectively. Therefore, special care needs to be taken when combination therapy is considered[53]. Combination of sorafenib and TARE therapy is reportedly well-tolerated, and the mean OS was reported to be 8.6 mo in advanced HCC patients[61]. However, that study only included patients with branch PVTT, not major PVTT. In a recent prospective study on the safety profile of combination TARE-sorafenib treatment, the incidences of total and > grade 3 adverse events did not statistically differ between the combination treatment and sorafenib-alone groups[62].
TACE PLUS RADIOTHERAPY
TACE in combination with radiotherapy is a newly introduced combination strategy that results in improved outcomes in HCC patients with PVTT[38,39,63-70]. The rationale behind this combined treatment is that reducing PVTT with radiotherapy may inhibit intravascular tumor growth and preserve adequate portal venous flow, thereby preventing the deterioration of liver function, limiting intrahepatic tumor spread, and facilitating subsequent treatments for the primary tumor[66,68]. In addition, radiotherapy may potentially increase the effects of subsequent chemoembolization by inducing the regression of the arterioportal shunt around the PVTT[71].
In a recent retrospective study, Chung et al[72] evaluated the safety and survival outcomes of TACE plus radiotherapy in patients with HCC invading the main portal vein. After chemoembolization, major complications occurred in 30 of 151 patients (19.9%) and were more frequently seen in Child-Pugh B patients. The 30-d mortality rate was 0.7%, and most adverse events were managed by conservative treatment. In addition, adjuvant RT for main PVTT after chemoembolization in 147 patients was uneventful without RT-associated adverse events. The median OS period was 12 mo (14 mo in Child-Pugh class A patients vs 8 mo in Child-Pugh class B patients). Yoon et al[64] also studied the efficacy of TACE in combination with RT for HCC with PVTT (main or bilateral/unilateral) and reported a 28.1% tumor response rate and OS of 10.6 mo. Kim et al[73] compared the efficacy of TACE with or without RT vs sorafenib for advanced HCC with PVTT. In that study, patients were divided into 3 different treatment pairs (TACE vs TACE + RT; TACE vs sorafenib; and TACE + RT vs sorafenib). According to the propensity score matched analysis, the group that received TACE in combination with radiotherapy demonstrated longer TTP and OS values than the groups that received TACE alone (102 pairs; 8.7 mo vs 3.6 mo, P < 0.01; 11.4 mo vs 7.4 mo, P = 0.023, respectively) or sorafenib-alone groups (30 pairs; 3.4 mo vs 1.8 mo, P < 0.01; 5.9 mo vs 4.4 mo, P = 0.03, respectively). Although these outcomes need to be verified by future randomized studies, TACE in combination with radiotherapy could serve as an alternative to current standard sorafenib therapy.
HEPATIC ARTERIAL INFUSION AND RADIOTHERAPY
Some investigators have combined HAIC with conformal radiotherapy to treat HCC patients with PVTT and reported the efficacy of arterial infusion chemotherapy plus radiotherapy[74,75]. In a recent retrospective study, Fujino et al[76] investigated the efficacy of combination therapy for major or first-order branch PVTT and reported that the combination group demonstrated a significantly longer median OS (12.1 mo vs 7.2 mo) and higher objective response rate than the HAIC-alone group when used to treat intrahepatic HCC patients who were nonresponsive to HAIC (objective response rate = 56.1% vs 33.3%; median OS = 8.6 mo vs 5 mo), but no significant differences were noted in intrahepatic responders. It is noteworthy that reducing PVTT volume with radiotherapy may help patients respond better to HAIC.
OTHER COMBINATION STRATEGIES
Various novel treatment modalities have been developed in an effort to reduce PVTT burden. According to the BCLC Staging System, radiofrequency ablation (RFA) is the standard care for early, stage A HCC. Recently, however, it was reported that RFA may improve patient survival and has come into use as a treatment modality for HCC patients with PVTT[77,78]. TACE in combination with RFA also confers survival benefits to PVTT patients[79]. Iodine-125 seeds have been used to treat solid tumors, and their use in HCC patients with PVTT is reportedly safe and feasible[80]. A recent prospective study compared the efficacy and safety of TACE in combination with the endovascular implantation of an iodine-125 seed strand for PVTT vs TACE alone. In that study, TACE in combination with iodine-125 seeds demonstrated better median OS than TACE alone[81].
GENERAL TREATMENT RECOMMENDATIONS FOR HCC WITH PVTT
Although the BCLC system, which is based on data from randomized controlled studies, has been widely validated, it does not reflect the diverse situations that present in clinical practice. In particular, because advanced HCC (i.e., BCLC C stage) affects heterogeneous patient populations, different treatment modalities or combination therapies have been advocated in order to obtain better treatment outcomes. In HCC patients with PVTT, the marginal survival benefits observed with sorafenib may be attributed to patient heterogeneity. Therefore, subclassification of BCLC stage C patients is thought to be the first step to providing more individualized treatments to patients with this stage of cancer. Under these circumstances, the recently introduced Hong Kong Liver Cancer Staging System subdivides macrovascular invasion into intrahepatic and extrahepatic vascular invasions and recommends administering more aggressive treatment to early- and intermediate-stage cancers[82]. Regarding the various therapeutic modalities reviewed above, it is not easy to reach a consensus regarding the best treatment options for individual patients and remains an area of active discussion because the data on each modality reflects regional differences in patient characteristics and clinical practice. In particular, the ongoing observational study (GIDEON) is expected to generate better understanding of the effectiveness and safety of the diverse treatment options for HCC patients with PVTT[83-85].
CONCLUSION
According to the BCLC Staging System, systemic therapy using sorafenib is considered the standard of care for patients with HCC and PVTT despite its modest survival benefits. Other treatment modalities for HCC with PVTT have continued to evolve in recent years (e.g., TACE, HAIC, TARE, radiotherapy, and various combination strategies), and the BCLC recommendations now seem very limiting. However, because there are few phase I or II studies on multimodal treatments, it is difficult to validate the findings of previous, retrospective, observational studies and thus to reach a consensus regarding the best options for advanced HCC with PVTT. Therefore, future prospective, randomized, controlled studies are warranted to compare the outcomes of diverse treatment modalities, and the observed findings may need to be incorporated into international guidelines.
Footnotes
Conflict-of-interest statement: The authors do not have any conflicts to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 27, 2015
First decision: September 11, 2015
Article in press: November 24, 2015
P- Reviewer: Kim YJ S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Cheung TK, Lai CL, Wong BC, Fung J, Yuen MF. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther. 2006;24:573–583. doi: 10.1111/j.1365-2036.2006.03029.x. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 4.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48:103–109. doi: 10.1136/gut.48.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, Burra P, Fagiuoli S, Farinati F, Rugge M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–159. doi: 10.1097/01.sla.0000109146.72827.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073–2080. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 12.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Jeong SW, Jang JY, Shim KY, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, et al. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein tumor thrombosis. Gut Liver. 2013;7:696–703. doi: 10.5009/gnl.2013.7.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 16.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 17.Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21:10327–10335. doi: 10.3748/wjg.v21.i36.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JW, Kim JH, Sung KB, Ko HK, Shin JH, Kim PN, Choi HK, Ko GY, Yoon HK, Chun SY, et al. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol. 2014;109:1234–1240. doi: 10.1038/ajg.2014.152. [DOI] [PubMed] [Google Scholar]
- 19.Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007;18:1517–1526; quiz 1527. doi: 10.1016/j.jvir.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Yoon HK, Kim SY, Kim KM, Ko GY, Gwon DI, Sung KB. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29:1291–1298. doi: 10.1111/j.1365-2036.2009.04016.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 23.Leng JJ, Xu YZ, Dong JH. Efficacy of transarterial chemoembolization for hepatocellular carcinoma with portal vein thrombosis: a meta-analysis. ANZ J Surg. 2014:Epub ahead of print. doi: 10.1111/ans.12803. [DOI] [PubMed] [Google Scholar]
- 24.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, Ganguli S, Wicky S, Blaszkowsky LS, Zhu AX. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014;37:381–387. doi: 10.1007/s00270-013-0654-7. [DOI] [PubMed] [Google Scholar]
- 26.Park JY, Ahn SH, Yoon YJ, Kim JK, Lee HW, Lee do Y, Chon CY, Moon YM, Han KH. Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:129–137. doi: 10.1002/cncr.22759. [DOI] [PubMed] [Google Scholar]
- 27.Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K, Sata M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 28.Eun JR, Lee HJ, Moon HJ, Kim TN, Kim JW, Chang JC. Hepatic arterial infusion chemotherapy using high-dose 5-fluorouracil and cisplatin with or without interferon-alpha for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis. Scand J Gastroenterol. 2009;44:1477–1486. doi: 10.3109/00365520903367262. [DOI] [PubMed] [Google Scholar]
- 29.Kim HY, Kim JD, Bae SH, Park JY, Han KH, Woo HY, Choi JY, Yoon SK, Jang BK, Hwang JS, et al. A comparative study of high-dose hepatic arterial infusion chemotherapy and transarterial chemoembolization using doxorubicin for intractable, advanced hepatocellular carcinoma. Korean J Hepatol. 2010;16:355–361. doi: 10.3350/kjhep.2010.16.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song do S, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, Lee SH, Park JY, Yim HJ, Cho SB, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015;50:445–454. doi: 10.1007/s00535-014-0978-3. [DOI] [PubMed] [Google Scholar]
- 31.Oh MJ, Lee HJ, Lee SH. Efficacy and safety of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma as first-line therapy. Clin Mol Hepatol. 2013;19:288–299. doi: 10.3350/cmh.2013.19.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song do S, Bae SH, Song MJ, Lee SW, Kim HY, Lee YJ, Oh JS, Chun HJ, Lee HG, Choi JY, et al. Hepatic arterial infusion chemotherapy in hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2013;19:4679–4688. doi: 10.3748/wjg.v19.i29.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 34.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan S, Dawson LA, Seong J, Akine Y, Beddar S, Briere TM, Crane CH, Mornex F. Radiotherapy for hepatocellular carcinoma: an overview. Ann Surg Oncol. 2008;15:1015–1024. doi: 10.1245/s10434-007-9729-5. [DOI] [PubMed] [Google Scholar]
- 36.Tse RV, Guha C, Dawson LA. Conformal radiotherapy for hepatocellular carcinoma. Crit Rev Oncol Hematol. 2008;67:113–123. doi: 10.1016/j.critrevonc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Huang YJ, Hsu HC, Wang CY, Wang CJ, Chen HC, Huang EY, Fang FM, Lu SN. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–1163. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 38.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 39.Toya R, Murakami R, Baba Y, Nishimura R, Morishita S, Ikeda O, Kawanaka K, Beppu T, Sugiyama S, Sakamoto T, et al. Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma. Radiother Oncol. 2007;84:266–271. doi: 10.1016/j.radonc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S, Koizumi W. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. doi: 10.1186/1471-230X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology. 2013;58:2188–2197. doi: 10.1002/hep.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A, Espat J, Bilbao JI, Sharma RA, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy AS, Salem R. Radioembolization (yttrium-90 microspheres) for primary and metastatic hepatic malignancies. Cancer J. 2010;16:163–175. doi: 10.1097/PPO.0b013e3181d7e8cf. [DOI] [PubMed] [Google Scholar]
- 45.Jelic S, Sotiropoulos GC. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v59–v64. doi: 10.1093/annonc/mdq166. [DOI] [PubMed] [Google Scholar]
- 46.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 49.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A, Nemcek AA, Gates VL, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 50.Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, Riaz A, Gupta R, Vouche M, Gates VL, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73–80. doi: 10.1016/j.jhep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 52.Gramenzi A, Golfieri R, Mosconi C, Cappelli A, Granito A, Cucchetti A, Marinelli S, Pettinato C, Erroi V, Fiumana S, et al. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int. 2015;35:1036–1047. doi: 10.1111/liv.12574. [DOI] [PubMed] [Google Scholar]
- 53.Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC, Chiou JF. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041–1047. doi: 10.1016/j.ijrobp.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Riaz A, Lewandowski RJ, Kulik LM, Mulcahy MF, Sato KT, Ryu RK, Omary RA, Salem R. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121–1130; quiz 1131. doi: 10.1016/j.jvir.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 55.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 56.Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan T, Li XS, Xie QK, Wang JP, Li W, Wu PH, Zhao M. Safety and efficacy of transarterial chemoembolization plus sorafenib for hepatocellular carcinoma with portal venous tumour thrombus. Clin Radiol. 2014;69:e553–e561. doi: 10.1016/j.crad.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, Cai M, Shan H. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology. 2014;272:284–293. doi: 10.1148/radiol.14131946. [DOI] [PubMed] [Google Scholar]
- 60.Yu W, Gu K, Yu Z, Yuan D, He M, Ma N, Lai S, Zhao J, Ren Z, Zhang X, et al. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2013;329:109–117. doi: 10.1016/j.canlet.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 61.Chow PK, Poon DY, Khin MW, Singh H, Han HS, Goh AS, Choo SP, Lai HK, Lo RH, Tay KH, et al. Multicenter phase II study of sequential radioembolization-sorafenib therapy for inoperable hepatocellular carcinoma. PLoS One. 2014;9:e90909. doi: 10.1371/journal.pone.0090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, Schott E, Schütte K, Verslype C, Walecki J, et al. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int. 2015;35:620–626. doi: 10.1111/liv.12622. [DOI] [PubMed] [Google Scholar]
- 63.Han K, Kim JH, Yoon HM, Kim EJ, Gwon DI, Ko GY, Yoon HK, Ko HK. Transcatheter arterial chemoembolization for infiltrative hepatocellular carcinoma: clinical safety and efficacy and factors influencing patient survival. Korean J Radiol. 2014;15:464–471. doi: 10.3348/kjr.2014.15.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 65.Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, Matsumoto S, Soejima T, Sugimura K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 66.Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, Sun HC, Wang BL, Zhang JY, Jiang GL, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 67.Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, Nihei K, Ito Y, Maru Y, Ikeda H. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189–193. doi: 10.1097/00000421-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 68.Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, Miyasaka Y, Nagayama K, Enomoto N, Sato C. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660–665. doi: 10.1046/j.1440-1746.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- 69.Nakazawa T, Adachi S, Kitano M, Isobe Y, Kokubu S, Hidaka H, Ono K, Okuwaki Y, Watanabe M, Shibuya A, et al. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology. 2007;73:90–97. doi: 10.1159/000120996. [DOI] [PubMed] [Google Scholar]
- 70.Yoon HM, Kim JH, Kim EJ, Gwon DI, Ko GY, Ko HK. Modified cisplatin-based transcatheter arterial chemoembolization for large hepatocellular carcinoma: multivariate analysis of predictive factors for tumor response and survival in a 163-patient cohort. J Vasc Interv Radiol. 2013;24:1639–1646. doi: 10.1016/j.jvir.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 71.Hsu HC, Chen TY, Chiu KW, Huang EY, Leung SW, Huang YJ, Wang CY. Three-dimensional conformal radiotherapy for the treatment of arteriovenous shunting in patients with hepatocellular carcinoma. Br J Radiol. 2007;80:38–42. doi: 10.1259/bjr/55395102. [DOI] [PubMed] [Google Scholar]
- 72.Chung SR, Kim JH, Yoon HK, Ko GY, Gwon DI, Shin JH, Song HY, Ko HK, Yoon SM. Combined Cisplatin-Based Chemoembolization and Radiation Therapy for Hepatocellular Carcinoma Invading the Main Portal Vein. J Vasc Interv Radiol. 2015;26:1130–1138. doi: 10.1016/j.jvir.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 73.Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, Ryoo BY, Kang YK, Lee D, Kim KM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320–329.e6. doi: 10.1016/j.jvir.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 74.Han KH, Seong J, Kim JK, Ahn SH, Lee do Y, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008;113:995–1003. doi: 10.1002/cncr.23684. [DOI] [PubMed] [Google Scholar]
- 75.Katamura Y, Aikata H, Takaki S, Azakami T, Kawaoka T, Waki K, Hiramatsu A, Kawakami Y, Takahashi S, Kenjo M, et al. Intra-arterial 5-fluorouracil/interferon combination therapy for advanced hepatocellular carcinoma with or without three-dimensional conformal radiotherapy for portal vein tumor thrombosis. J Gastroenterol. 2009;44:492–502. doi: 10.1007/s00535-009-0033-y. [DOI] [PubMed] [Google Scholar]
- 76.Fujino H, Kimura T, Aikata H, Miyaki D, Kawaoka T, Kan H, Fukuhara T, Kobayashi T, Naeshiro N, Honda Y, et al. Role of 3-D conformal radiotherapy for major portal vein tumor thrombosis combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2015;45:607–617. doi: 10.1111/hepr.12392. [DOI] [PubMed] [Google Scholar]
- 77.Hirooka M, Koizumi Y, Kisaka Y, Abe M, Murakami H, Matsuura B, Hiasa Y, Onji M. Mass reduction by radiofrequency ablation before hepatic arterial infusion chemotherapy improved prognosis for patients with huge hepatocellular carcinoma and portal vein thrombus. AJR Am J Roentgenol. 2010;194:W221–W226. doi: 10.2214/AJR.09.2852. [DOI] [PubMed] [Google Scholar]
- 78.Neeman Z, Libutti SK, Patti JW, Wood BJ. Percutaneous radiofrequency ablation of hepatocellular carcinoma in the presence of portal vein thrombosis. Clin Imaging. 2003;27:417–420. doi: 10.1016/S0899-7071(03)00015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng JS, Long J, Sun B, Lu NN, Fang D, Zhao LY, Du N. Transcatheter arterial chemoembolization combined with radiofrequency ablation can improve survival of patients with hepatocellular carcinoma with portal vein tumour thrombosis: extending the indication for ablation? Clin Radiol. 2014;69:e253–e263. doi: 10.1016/j.crad.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 80.Luo J, Yan Z, Liu Q, Qu X, Wang J. Endovascular placement of iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. J Vasc Interv Radiol. 2011;22:479–489. doi: 10.1016/j.jvir.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 81.Yang M, Fang Z, Yan Z, Luo J, Liu L, Zhang W, Wu L, Ma J, Yang Q, Liu Q. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: a two-arm, randomised clinical trial. J Cancer Res Clin Oncol. 2014;140:211–219. doi: 10.1007/s00432-013-1568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–1700.e3. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 83.Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609–617. doi: 10.1111/ijcp.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, Ladrón de Guevara L, Papandreou C, et al. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66:675–683. doi: 10.1111/j.1742-1241.2012.02940.x. [DOI] [PubMed] [Google Scholar]
- 85.Lencioni R, Marrero J, Venook A, Ye SL, Kudo M. Design and rationale for the non-interventional Global Investigation of Therapeutic DEcisions in Hepatocellular Carcinoma and Of its Treatment with Sorafenib (GIDEON) study. Int J Clin Pract. 2010;64:1034–1041. doi: 10.1111/j.1742-1241.2010.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


