Abstract
Endoscopic submucosal dissection (ESD) is a well-established treatment for superficial esophageal squamous cell neoplasms (SESCNs) with no risk of lymphatic metastasis. However, for large SESCNs, especially when exceeding two-thirds of the esophageal circumference, conventional ESD is time-consuming and has an increased risk of adverse events. Based on the submucosal tunnel conception, endoscopic submucosal tunnel dissection (ESTD) was first introduced by us to remove large SESCNs, with excellent results. Studies from different centers also reported favorable results. Compared with conventional ESD, ESTD has a more rapid dissection speed and R0 resection rate. Currently in China, ESTD for large SESCNs is an important part of the digestive endoscopic tunnel technique, as is peroral endoscopic myotomy for achalasia and submucosal tunnel endoscopic resection for submucosal tumors of the muscularis propria. However, not all patients with SESCNs are candidates for ESTD, and postoperative esophageal strictures should also be taken into consideration, especially for lesions with a circumference greater than three-quarters. In this article, we describe our experience, review the literature of ESTD, and provide detailed information on indications, standard procedures, outcomes, and complications of ESTD.
Keywords: Endoscopic submucosal tunnel dissection, Esophageal squamous cell neoplasms, Digestive endoscopic tunnel technique, Endoscopic submucosal dissection
Core tip: The digestive endoscopic tunnel technique (DETT) innovatively broke the traditional boundaries between medicine and surgery and has been a recent research hotspot. Based on the submucosal tunnel concept, endoscopic submucosal tunnel dissection (ESTD) was introduced by us to treat large superficial esophageal squamous cell neoplasms, with excellent results. Studies from different centers also achieved favorable results, and ESTD has become an important part of DETT in China. Therefore, we conducted a literature review and provided detailed information on indications, standard procedures, outcomes, and complications of ESTD.
INTRODUCTION
Endoscopic submucosal dissection (ESD) is acknowledged as the standard treatment for superficial esophageal squamous cell neoplasms (SESCNs)[1-3]. Compared with conventional endoscopic mucosal resection (EMR), ESD enables en bloc resection and precise pathological assessment, leading to a lower local recurrence rate[4]. However, with respect to large SESCNs, some frustrating problems arise, especially for tumors with a circumference that exceeds two-thirds of the esophageal lumen. During submucosal injection, rapid diffusion of submucosal liquid cushion after circumferential incision made the lifting-effect unsatisfactory. The submucosal endoscopic view was also not clear because the resected mucosa shrank and blocked the confined lumen[5,6]. Consequently, the ESD procedure is time consuming, has a high risk of adverse events, and requires highly skilled endoscopists. To overcome these problems, some endoscopic innovations were introduced, such as modified fishing-line traction system[6], peroral traction-assisted technique[7], clip-band technique[8], and medical ring system[9], but these were not suitable for extensive standardized application.
Early in 2009, Linghu et al[10,11] attempted to dissect a submucosal tunnel and successfully achieved en bloc removal of an 8 cm long circumferential SESCN. The results were presented as the “tunnel technique for circumferential esophageal lesions”, at the 2009 Beijing Annual Meeting of Digestive Endoscopy. The innovative technique was termed endoscopic submucosal tunnel dissection (ESTD)[5]. Although derived from ESD, ESTD with the submucosal tunnel concept changed the traditional procedures for ESD: marking-injection-circumferential incision-submucosal dissection became a new treatment strategy for superficial esophageal neoplasms.
The submucosal tunnel formed a bridge between medical treatment and surgery, which was a long-held ambition of endoscopists. Peroral endoscopic myotomy (POEM) for achalasia launched a new field in endoscopy of digestive endoscopic tunnel technique (DETT)[12]. Inspired by POEM, submucosal tunnel endoscopic resection (STER) was developed for the treatment of submucosal tumors of the muscularis propria (MP)[13,14]. Since we first reported our experience in ESTD[5], an increasing number of endoscopists have focused on the new treatment strategy for SESCNs[15-20]. Some believe that standardized ESTD has made esophageal ESD straightforward and less difficult, especially for Western endoscopists[15]. Currently in China, ESTD has become an important part of DETT, together with POEM and STER[10].
In this review, we describe the indications, procedures, outcomes, complications, advantages, and future perspectives of ESTD for SESCNs.
INDICATIONS
Generally, whether endoscopic resection is preferred for patients with SESCNs is determined by risk of lymph node metastasis and technical resectability[21]. Postoperative quality of life also should be taken into consideration. According to 2012 Japan Esophageal Society (JES) guidelines for treatment of esophageal carcinoma[22], lesions limited to the mucosal epithelium (m1) or the lamina propria mucosa (m2) have a low risk of lymph node and distant metastasis, and radical resection can be achieved endoscopically, with similar long-term survival to surgery. Therefore, these lesions are considered to be an absolute indication for endoscopic resection. As the risk of lymphatic metastasis increases to 10%-15%, endoscopic resection is relatively indicated for lesions invading the muscularis mucosae (m3) or submucosal layer < 200 μm (sm1), although Western endoscopists remain cautious and conservative (Figure 1)[1].
Figure 1.
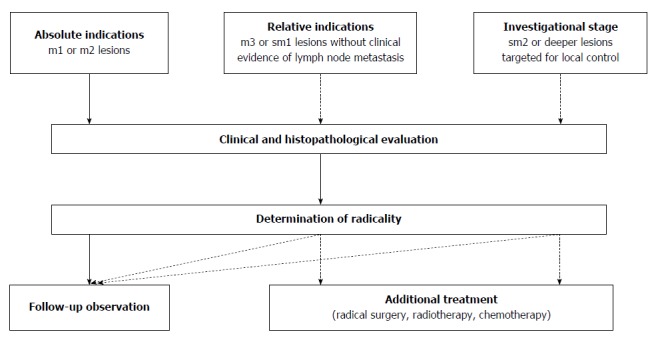
Indications for endoscopic resection by 2012 Japan Esophageal Society guidelines.
Technical resectability is often determined by circumferential extension of lesions, which is an important risk factor for postoperative stenosis[23,24]. As a result of advances in endoscopic techniques, the 2012 JES guidelines removed the restriction of lesion circumference in the 2007 edition, by which endoscopic resection was only indicated for m1 or m2 lesions not exceeding two-thirds of the esophageal circumference (absolute indication)[22,25]. We believe that it was the standardized ESTD that enabled lesion size no longer to be a barrier to endoscopic resection and significantly improved efficacy. Double-tunnel ESTD could further minimize operation duration for whole circumferential lesions[20]. Despite that, patients with lesions larger than three-quarters of the circumference should be informed of the risk of esophageal stenosis and prophylactic measures should be taken[23,24,26]. Lesions < 15 mm in diameter are not recommended for ESTD, although the technique is reported to be feasible[15]. The reasons are as follows: (1) EMR is easy to perform and achieves a favorable en bloc resection rate; and (2) excess normal mucosa is resected by the creation of a submucosal tunnel and is followed by more trauma and longer recovery. Based on calculation and our experience, the diameter of the submucosal tunnel should be ≥ 10 mm, and the resected area should be ≥ 20 mm in diameter, or one-third of the circumference[10,27].
Therefore, ESTD is indicated for: (1) lesions not invading deeper than sm1 and without clinical evidence of lymph node metastasis; and (2) lesions at least one-third of the esophageal circumference and ≥ 20 mm in diameter.
EQUIPMENT REQUIRED
A forward-viewing gastroscope (GIF 260J; Olympus, Tokyo, Japan) with a water-jet function is used, with a 9.8 mm outer diameter and a single 3.2 mm working channel. A transparent cap (D201-11804; Olympus) is fitted to the tip of the endoscope to provide a clear submucosal view. It also facilitates entering the tunnel and blunt dissection. Similar to ESD, an electrosurgical energy generator (ICC-200 or VIO 300D; Erbe, Tübingen, Germany) is connected to provide cutting or coagulation when needed. Various electrosurgical knives are available, including the dual knife, insulated-tip (IT) knife, the triangle-tip (TT) knife, and hook knife. The hybrid or flush knife, with a combined function of submucosal injection and electrosurgical knife, avoids frequent changing of tools, making the procedure simpler and more efficient. Selection of knives depends on the preference and specialty of the endoscopist. We prefer the dual knife for marking and mucosal incision and the IT knife for submucosal dissection and lateral resection.
A hemostatic forceps is used to handle active bleeding or large exposed vessels when a hand-held knife is insufficient. CO2 insufflation is strongly suggested because it decreases the risk of air-related adverse events, such as subcutaneous or mediastinal emphysema and even pneumothorax, and for its rapid absorption rate, around 150 times higher than air[28-30].
ESTD PROCEDURES
Patients are placed in the left lateral position. General anesthesia with mechanical ventilation is required in view of lesion location and long operation time. Endotracheal intubation prevents aspiration, and positive pressure ventilation reduces the risk of air-related adverse events[5,31]. ESTD procedures differ from ESD in the following ways (Figures 2 and 3)[5].
Figure 2.
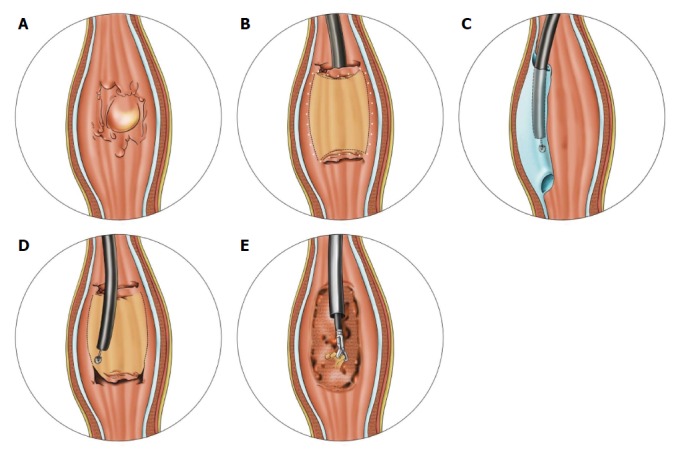
Schema of endoscopic submucosal tunnel dissection. A: Evaluating and delineating the neoplasm; B: After marking the lesion margin, mucosal incision was performed in the anal-oral sequence; C: A submucosal tunnel was created from the oral to anal side; D: Lateral resection with an insulated-tip knife for complete removal of the lesion; E: Preventive coagulation on artificial ulcer.
Figure 3.
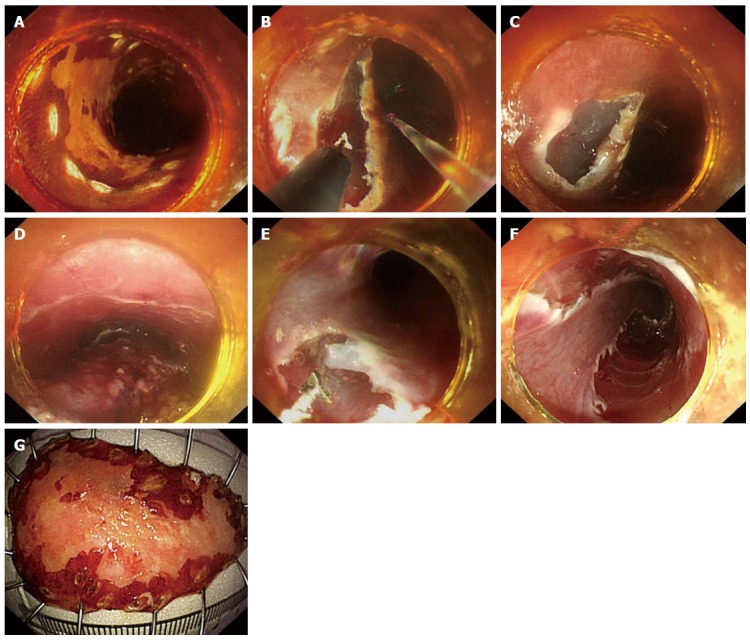
Endoscopic submucosal tunnel dissection with a single triangle-tip knife. A: Lugol staining to delineate the lesion, occupying almost half of the esophageal lumen. The margin was marked by argon plasma coagulation; B, C: Anal and oral incision was performed successively with a triangle-tip (TT) knife. Water jet helped entry into the submucosal tunnel; D: Submucosal tunnel was created from the oral to anal side; E: Lateral resection with a TT knife from the oral to anal side until reaching the anal incision; F: Complete en bloc resection was achieved in 52 min; G: The specimen, around 55 mm × 35 mm in size, was retrieved. Histopathological examination revealed a microinvasive squamous carcinoma limited in the lamina propria mucosa, free of lateral and vertical margin.
Marking lesion margin
Magnifying narrow-band imaging and lugol staining are carried out to delineate the lesion. Dots are made around 5 mm outside the margin with argon plasma coagulation (APC) or electrosurgical knives. For circumferential lesions, circular dots reveal the anal and oral margins.
Submucosal injection and mucosal incision
In the anal-oral sequence, the anal side of the mucosa is first cut open transversely with a dual knife after the submucosal fluid cushion reaches an acceptable level. In China, the frequently used injection fluid is a mixture of 100 mL normal saline solution or glycerol-fructose injection, 1 mg epinephrine, and indigo carmine or methylene blue, for its ease of application and low cost. Highly viscous hyaluronic acid can maintain a thick fluid cushion for a long time and is widely used by Japanese endoscopists. Subsequently, the same procedure is performed for incision of the oral side of the mucosa along the marked dots.
The anal side of the incision is a useful indicator of submucosal tunnel endpoint. More importantly, communication between the submucosal tunnel and esophageal lumen prevents gas accumulation and sharply rising tunnel pressure during dissection, avoids excessive normal mucosal separation, and reduces risk of air-related adverse events[5,32].
Creation of submucosal tunnel
Submucosal dissection was used to create a tunnel from the oral side to the anal side. Repeated submucosal injection is necessary to separate the mucosa from the MP to maintain an adequate space. IT knife (IT 2 or IT nano) with a small ceramic ball is a safe and efficient tool, offering both lateral and backward dissection. Submucosal dissection should be conducted close to the MP, where rich vascular networks are absent, which differs from the upper submucosal layer and muscularis mucosae. According to basic principles of DETT, at least one side of the submucosal tunnel should be intact as the only barrier to the mediastinum. Therefore, the blade should be parallel to the MP as much as possible and catch the submucosal fibers to the center of tunnel for electric cut to avoid MP injury. During lateral dissection of the tunnel, mucosal dots are a reminder of the tunnel boundary, and constant withdrawal of the endoscope from the tunnel also ensures that the tunnel is a consistent size, to avoid postoperative stenosis caused by excessive dissection.
Lateral resection
After completion of the tunnel, the endoscope is withdrawn, and the IT knife is used for lateral mucosal resection close to the markings from the anal to the oral side, until complete removal of the lesion. The resection is carried out simultaneously on the both sides of the tunnel. In this way, traction of the contralateral mucosa makes the procedure easier.
Management of artificial ulcer
After removal of the lesion, hemostatic forceps and APC are applied to coagulate the visible vessels on the surface of the artificial ulcer to prevent delayed bleeding. More attention should be given to vessels on the edge of the ulcer. Preventive clips should be placed when the MP layer is injured. Fibrin sealant or sucralfate can be used to protect the ulcer. For lesions more than three-quarters of the circumference, a fully covered esophageal metallic stent is conventionally placed to prevent postoperative stenosis in our endoscopy center.
As for double-tunnel ESTD, the difference is that circular incisions are successively performed at the anal and oral margin after marking. Then, the two opposite tunnels are dissected one after another from the oral to anal side. The procedures of double-tunnel ESTD are detailed in Figure 4.
Figure 4.
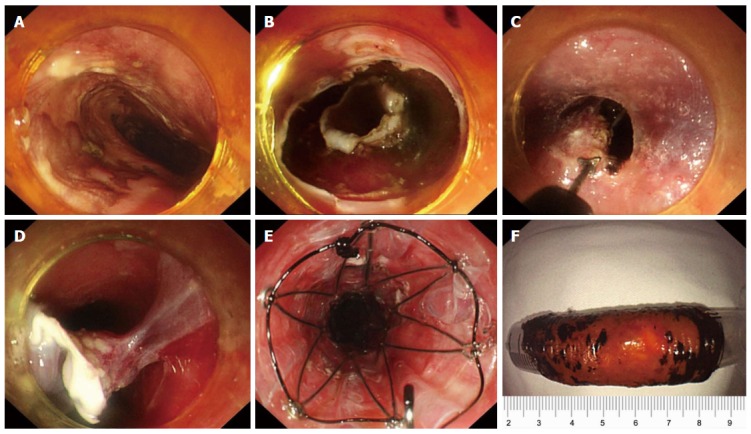
Double-tunnel endoscopic submucosal tunnel dissection for circumferential superficial esophageal neoplasms. A: An 8 cm circumferential superficial esophageal cancer was showed by iodine staining, at 28–36 cm from the incisors; B: Circular incisions were successively performed at the anal and oral margins after marking; C, D: Two submucosal tunnels were created opposite each other. Two tunnels nearly covered the whole esophageal lumen, and the borders were narrow enough to be resected easily; E: A 14 cm retrievable, fully-covered esophageal stent was placed to prevent postoperative stenosis. F: The lesion was resected circumferentially, about 60 mm in length. From Zhai et al[20].
OUTCOMES AND COMPARATIVE RESULTS
In our first report of ESTD, five SESCNs with a mean diameter of 57 mm (range: 40-80 mm) were en bloc and R0 resected without any complications[5]. Mean operation duration was 78.6 min (34-120 min) and no recurrence of SESCNs was observed with a mean follow-up of 7.4 mo (3-13 mo), preliminarily showing the feasibility and efficiency of ESTD. Several studies from different centers achieved similar favorable results. Xiong et al[19] reported that en bloc and R0 resection were achieved in all seven SESCNs treated with ESTD. In another retrospective study of 11 ESTD procedures by Pioche et al[17], en bloc and R0 resection rate were 100% and 81.8% (2/11), respectively. One patient (9.1%) experienced recurrence. Arantes et al[15] treated 25 esophageal neoplasms from 23 patients, ranging from 10 to 60 mm, with ESTD. En bloc resection was successfully performed in 23 lesions, and R0 resection was achieved in 22 lesions. Two of 23 patients experienced local recurrence, and one patient underwent reoperation, with supplementary EMR and radiofrequency ablation.
We performed a systematic literature review of ESTD from Chinese databases (CBM, Wanfang Data, CMJD and CNKI) and English databases (PubMed, EMBase and Cochrane Library). The review identified a total of 90 lesions (88 patients) from nine studies, with a mean size of 37.8 mm (range: 10-80 mm) (Table 1). The pooled en bloc, R0 resection and local recurrence rates were 97.8% (92%-100%), 85.6% (81.8%-100%), and 3.3% (0%-9.1%), respectively, which were at least comparable to those for ESD. In our retrospective comparative study[32], among 29 consecutive patients, 11 patients underwent ESTD and 18 ESD for lesions that were at least one-third the circumference and ≥ 20 mm in diameter. ESTD had a more rapid dissection speed, almost twice that of ESD (22.4 mm2/min vs 12.2 mm2/min). Curative resection was more likely to be achieved with ESTD (81.8% vs 66.7%). These advantages of ESTD may be attributed to the following factors[5,32]: (1) counter-traction of bilateral mucosae provides a clear submucosal vision for dissection; (2) the transparent cap and gas cushion created by CO2 insufflation can play a role in blunt dissection, accelerating the operation; (3) submucosal injection solution is retained longer because ESTD avoids conventional circumferential incision, and there is less need for repeated injection; and (4) because the dissection is deeper in the submucosa, close to the muscularis mucosae, and the mucosa together with most of the submucosa can be removed, making for more precise pathological assessment.
Table 1.
Systematic literature review of endoscopic submucosal tunnel dissection for superficial esophageal squamous cell neoplasms n (%)
| Ref. | Year | Lesions/cases | Mean size (mm) | Operation time (min) | En bloc resection | R0 resection | Complications | follow-up (mo) | Local recurrence |
| Linghu et al[5] | 2011 | 11/11 | 48.2 (30-80) | 78.6 (34-120) | 100% | 81.8% | Stenosis: 6 (54.5) | 13.5 (3-30) | 0% |
| Linghu et al[11] | 2013 | ||||||||
| Zhai et al[32] | 2014 | ||||||||
| Gao et al[18] | 2012 | 17/17 | 24 (20-50) | 128 (60-180) | 100% | 82.4% | Delayed bleeding: 1 (5.9) | NA | 0% |
| Xiong et al[19] | 2013 | 7/7 | 35.7 (20-40) | 61 (37-110) | 100% | 100% | Immediate bleeding: 2 (28.6) | 12 | 0% |
| Arantes et al[15] | 2013 | 25/23 | 25 (10-60) | 85 (60-210) | 92% | 84% | SE and ME: 2 (8) | 21.4 (3-36) | 2 (8) |
| Perforation: 1 (4) | |||||||||
| Pioche et al[17] | 2013 | 11/11 | 45 (27-80) | 76 (35-150) | 100% | 81.8% | SE: 9.1% | NA | 1 (9.1) |
| Stenosis: 4 (36.4) | |||||||||
| Tan et al[65] | 2014 | 1/1 | 50 | NA | 100% | 100% | None | 6 | 0% |
| Zhou et al[66] | 2014 | 18/18 | 58 (45-74) | 54.5 (32-85) | 100% | 100% | Immediate bleeding: 1 (5.6) | 6 | 0% |
| SE: 1 (5.6) | |||||||||
| Stenosis: 3 (16.7) | |||||||||
| Total | 90/88 | 37.8 (10-80) | 83.3 (32-180) | 88 (97.8) | 77 (85.6) | Immediate bleeding: 4 (4.4) | NA | 3 (3.3) | |
| Delay bleeding: 1 (1.1) | |||||||||
| Perforation: 1 (1.1) | |||||||||
| SE or ME: 3 (3.3) | |||||||||
| Stenosis: 9 (10) |
SE: Subcutaneous emphysema; ME: Mediastinal emphesema.
COMPLICATIONS
Bleeding and perforation are the common adverse events of ESTD, with a pooled incidence of 4.4% and 1.1%, respectively. These incidences do not differ significantly from those of ESD, which were reported as 0%-5.2% and 0%-6.9%, respectively[26,33-37]. Submucosal dissection is performed in the deeper submucosa, where transversed vessel trunk prevails. After exposure of the vessel trunk, prophylactic coagulation with hemostatic forceps should be undertaken in soft mode. For minor oozing bleeding, an electrosurgical knife in coagulation mode is usually sufficient, while for massive active bleeding, identification of bleeding spots with a water jet is more advisable than blind coagulation with hemostatic forceps. Clips are reserved for the last resort because they may hinder the next dissection procedure. Unstopped hemorrhage is rare and is mostly caused by delayed bleeding in the first 24 h postoperatively. In 17 patients with ESTD[18], one patient had delayed bleeding and was transferred to surgery after failed endoscopic hemostasis. Proton pump inhibitors are routinely administered, and emergency endoscopy should be performed once delayed bleeding is suspected during close surveillance.
Perforation during operation is usually < 10 mm, and endoscopic clips are the standard method for closing such perforations. Generally, multiple clips are required in a zipper fashion to ensure complete closure. Recently, a new over-the-scope clip has become clinically available and can successfully seal esophageal perforation up to 20 mm in size with high compression force[38-42]. For larger perforations, fully-covered self-expandable metal stents (FCSEMs) are effective, although they have a potential risk of stent migration[43]. For delayed perforation, early recognition determines treatment option and outcome. As a result of minor inflammation and content egression, delayed perforations within 12 h can be treated conservatively, including endoscopic closure with clips and percutaneous drainage. However, surgery is mandatory for patients with unstable hemodynamics, with perforations that are recognized after > 24 h, or who fail conservative treatment[44].
Although one tries to avoid touching the MP during dissection, air-related adverse events frequently occur; partly due to increased air pressure in the submucosal tunnel and thin MP without compact serosa. In 25 ESTDs with room-air insufflation by Arantes et al[15], two patients experienced subcutaneous and mediastinal emphysema, while 11 patients reported by us were uneventful with CO2 insufflation during ESTD[32]. Consensus has been reached that CO2 insufflation is strongly recommended in DETT[5,10,14,31,45]. The pooled incidence is 4.4% (0%-9.1%), which is lower than that of POEM or STER. All the emphysema was minor and could be absorbed spontaneously without additional treatment.
Postoperative esophageal stricture is of most concern for large mucosal defects after ESTD because it reduces quality of life. Circumference extent and histological depth are reliable predictors of postoperative stricture[23,24,46]. In a retrospective study of 84 esophageal ESDs by Ono et al[34], the incidence of lesions less than half, less than three-quarters, and more than three-quarters of the circumference was 2% (1/49), 20% (5/25), and 90% (9/10), respectively. Shi et al[24] also reported similar results in a review of 362 ESDs, in which lesions that were more than three-quarters of the circumference had a 93.3% (32/34) risk of stricture. Therefore, preventive intervention should be implemented for lesions that exceed three-quarters of the circumference or are less than three-quarters of the circumference but invade to a greater depth than m2[24,32,47].
Endoscopic pneumatic dilation (EPD) is taken as the standard treatment for postoperative esophageal strictures[48,49]. However, refractory strictures caused by large defects usually demand up to dozens of EPDs. This increases the risk of perforation as well as the treatment cost. In a study of 121 patients with 1337 EPD procedures for post-ESD stenosis[50], the incidence of perforation was 4.1% (5/121) per patient and 0.4% (5/1337) per procedure. Patients with perforations required more sessions of EPD than those without perforations [37 times (6-57) vs 7 times (1-70)]. Takahashi et al[51] also reported that seven patients developed perforation during 648 dilation procedures for post-EMR/ESD stricture, and multiple dilations and lower esophageal site were independent risk factors for perforation. Systemic administration or local endoscopic injection of steroid is effective, which not only lowers incidence of stricture, but also decreases extension of stricture and numbers of required endoscopic dilations[52-56]. In a recent randomized controlled trial by Takahashi et al[57], 32 patients with post-ESD defects more than three-quarters of the circumference were randomized to single triamcinolone acetonide injection (n = 16) and conventional EPD (n = 16). The steroid group required fewer dilatation sessions than the control group (6.1 sessions vs 11.5 sessions), without an increased risk of perforation. With respect to circumferential ESD, Sato et al[56] reported that oral steroid therapy with EPD was more effective than single EPD for the prevention of esophageal stricture. Given possible multiple local injections and mistaken deep injection into the MP, convenient oral steroid therapy with EBD on demand may be a preferred option for prevention and treatment of post-ESD stricture, in spite of a lack of studies comparing oral and local steroid injection. In our endoscopy center, FCSEMs are routinely placed for lesions more than three-quarters of the circumference with a period of 4-8 wk, which has been shown to decrease incidence of stricture and reduce the need for dilation[58]. Therefore, we suggest an algorithm for the prevention of postoperative stricture after endoscopic resection of SESCNs (Figure 5). Recently, other approaches, such as bioabsorbable poly-L-lactic acid stent placement[59], polyglycolic acid sheet with fibrin glue[60], and transplantation of tissue-engineered cell sheets[47,61], have shown promising results, but these approaches require further studies with large samples prior to extensive clinical use.
Figure 5.
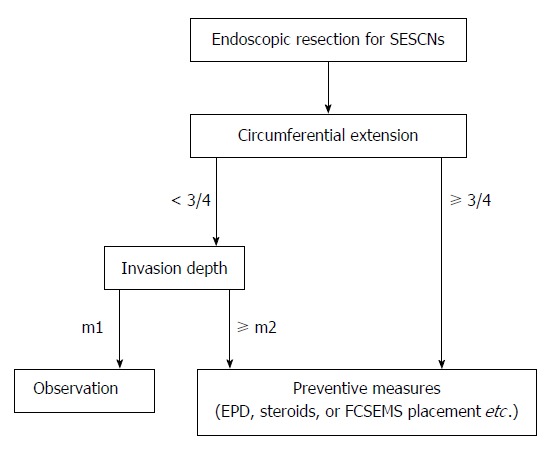
Proposed flow diagram of postoperative stricture prevention for superficial esophageal squamous cell neoplasms after endoscopic resection.
PERSPECTIVES AND OTHER APPLICATIONS
ESTD has established a new strategy for large SESCNs, which is different from that of traditional ESD, namely marking-circumferential incision-submucosal dissection. ESTD is derived from submucosal dissection techniques and advances in endoscopic equipment. As an important branch of DETT, ESTD is another good reflection of the submucosal tunnel concept, like popular POEM and STER. Compared with the latter procedures, ESTD does not have any operative difficulties and may be safer due to maintaining the MP layer untouched. Hence, we believe that endoscopists with experience of POEM or STER will find it easy to adopt ESTD, along with its parallel learning curve and training. Arantes et al[15] described their ESTD learning process, which involved several sessions of hands-on practice on animals; experience as first-assistant in human procedures; unsupervised ESD operations for gastric and rectal tumors (25 cases); and ESTD operations. With the excellent performance in 25 patients, Arantes et al[15] proposed ESTD as a standardized approach to facilitate ESD learning for Western endoscopists. Moreover, the promising results from both Asian and Western endoscopy centers and the advantages of ESTD over conventional ESD enable us to speculate that ESTD will replace ESD as a standardized method for large SESCNs. A multicenter prospective study of ESTD by us is ongoing, and other large-sample research from different centers is awaited. A prospective randomized study comparing ESTD with ESD is also eagerly anticipated.
ESTD can be also used for superficial lesions in other parts of the digestive tract. We have reported initial experience of ESTD for gastric lesions (three in the cardia and one in the lesser curvature), which were successfully removed in en bloc and R0 fashion without any complications[62]. The mean diameter was 43 mm (40-50 mm), and the mean operation time was 65 min (34-97 min). However, it is easy to miss lesions in the gastric tunnel by submucosal dissection, because the lesions are not always in a straight line, and superficial marks are invisible from the tunnel view. Frequent withdrawal of the endoscope from the tunnel is necessary. To overcome the limitations of submucosal fibrosis, Choi et al[63] utilized ESTD as a salvage technique to treat two patients with ulcerative early gastric cancer with fibrosis. The salvage technique made some modifications, by which the oral incision was 2-3 cm distal to lesions, and the anal was cut open in an arcuate style. To resect a large sessile duodenal adenoma in the bulb adjacent to a scar, Jin et al[16] created a submucosal tunnel through the pyloric ring for its removal. En bloc resection was achieved, and two minor perforations during hemostasis were completely clipped after submucosal dissection. The postprocedural course was uneventful, and the patient was discharged 8 d after ESTD. Hu et al[64] reported successful application of STER to resect eight rectal submucosal tumors. Despite absence of clinical use of ESTD in the colorectum, we still believe it is feasible to remove large, laterally spreading tumors from the rectum and distal colon. In our experience, given different anatomical features and potential risk, ESTD is currently indicated for lesions limited to the gastric cardia, lesser curvature of the gastric body, antrum, and rectum.
CONCLUSION
In conclusion, as a new treatment strategy, ESTD is an effective and safe method for large SESCNs. ESTD can thoroughly remove the obstacle of endoscopic resection due to lesion size and is recommended for lesions that do not invade deeper than sm1 and without clinical evidence of lymph node metastasis and at least one-third of the circumference of the esophagus and ≥ 20 mm in diameter. Postoperative esophageal stricture has received much attention, and preventive intervention should be implemented for lesions that exceed three quarters of the circumference, or lesions that are less than three-quarters of the circumference but invading deeper than m2. Although studies to date have been limited and larger studies with a high level of evidence are required, the promising results so far enable us to speculate that ESTD will replace traditional ESD as the standard treatment for large SESCNs in the near future.
Footnotes
Supported by National Natural Science Foundation of China, No. 81370584; and Military Major Projects of Clinical High-Tech Techniques, No. 431EG63G.
Conflict-of-interest statement: The authors declared that there is no conflict of interest relevant to this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 15, 2015
First decision: July 13, 2015
Article in press: September 15, 2015
P- Reviewer: Adachi Y S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S
References
- 1.Fernández-Esparrach G, Calderón A, de la Peña J, Díaz Tasende JB, Esteban JM, Gimeno-García AZ, Herreros de Tejada A, Martínez-Ares D, Nicolás-Pérez D, Nogales O, et al. Endoscopic submucosal dissection. Endoscopy. 2014;46:361–370. doi: 10.1055/s-0034-1364921. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and Techniques for Endoscopic Submucosal Dissection. Am J Gastroenterol. 2015;110:784–791. doi: 10.1038/ajg.2014.425. [DOI] [PubMed] [Google Scholar]
- 3.Maple JT, Abu Dayyeh BK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Konda V, Murad FM, Siddiqui UD, Banerjee S. Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:1311–1325. doi: 10.1016/j.gie.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066–1072. doi: 10.1016/j.gie.2008.03.1114. [DOI] [PubMed] [Google Scholar]
- 5.Linghu E, Feng X, Wang X, Meng J, Du H, Wang H. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy. 2013;45:60–62. doi: 10.1055/s-0032-1325965. [DOI] [PubMed] [Google Scholar]
- 6.Tsao SK, Toyonaga T, Morita Y, Fujita T, Hayakumo T, Azuma T. Modified fishing-line traction system in endoscopic submucosal dissection of large esophageal tumors. Endoscopy. 2011;43 Suppl 2 UCTN:E119. doi: 10.1055/s-0030-1256148. [DOI] [PubMed] [Google Scholar]
- 7.Jeon WJ, You IY, Chae HB, Park SM, Youn SJ. A new technique for gastric endoscopic submucosal dissection: peroral traction-assisted endoscopic submucosal dissection. Gastrointest Endosc. 2009;69:29–33. doi: 10.1016/j.gie.2008.03.1126. [DOI] [PubMed] [Google Scholar]
- 8.Parra-Blanco A, Nicolas D, Arnau MR, Gimeno-Garcia AZ, Rodrigo L, Quintero E. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video) Gastrointest Endosc. 2011;74:1137–1141. doi: 10.1016/j.gie.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto K, Nagahara A, Sakamoto N, Suyama M, Konuma H, Morimoto T, Sagawa E, Ueyama H, Takahashi T, Beppu K, et al. A new traction device for facilitating endoscopic submucosal dissection (ESD) for early gastric cancer: the “medical ring”. Endoscopy. 2011;43 Suppl 2 UCTN:E67–E68. doi: 10.1055/s-0030-1255923. [DOI] [PubMed] [Google Scholar]
- 10.Linghu E. Therapeutics of Digestive Endoscopic Tunnel Technique. Berlin: Springer Netherlands; 2014. [Google Scholar]
- 11.Linghu E, Li H, Huang Q, Wang X, Du H, Meng J, Kong J. Using tunnel technology dissecting long cirucumferencial lesions of esophagus. Zhongguo Jixu Yixue Jiaoyu. 2011;3:69–71. [Google Scholar]
- 12.Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 13.Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225–230. doi: 10.1055/s-0031-1291659. [DOI] [PubMed] [Google Scholar]
- 14.Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, et al. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos) Gastrointest Endosc. 2012;75:195–199. doi: 10.1016/j.gie.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Arantes V, Albuquerque W, Freitas Dias CA, Demas Alvares Cabral MM, Yamamoto H. Standardized endoscopic submucosal tunnel dissection for management of early esophageal tumors (with video) Gastrointest Endosc. 2013;78:946–952. doi: 10.1016/j.gie.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Jin P, Sheng J, Li A, Fu KI. Submucosal tunnel dissection through the pyloric ring for removal of a sessile duodenal adenoma adjacent to a scar. Endoscopy. 2013;45 Suppl 2 UCTN:E303–E304. doi: 10.1055/s-0033-1344557. [DOI] [PubMed] [Google Scholar]
- 17.Pioche M, Mais L, Guillaud O, Hervieu V, Saurin JC, Ponchon T, Lepilliez V. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy. 2013;45:1032–1034. doi: 10.1055/s-0033-1344855. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Shan H, Li Y, Luo G, Xu G. Application of submucosal tunneling endoscopic resection for early esophageal cancer and precan cerous lesions. Linchuang Waike Zazhi. 2012;20:491–492. [Google Scholar]
- 19.Xiong Y, Li Y, Yuan H, Geng Y, Zhang Z, Wang A. The application of endoscopic submucosal tunnel dissection (ESTD) in treating early esophageal cancer and precancerous lesions. Linchuang Xiaohuabing Zazhi. 2013;25:67–69. [Google Scholar]
- 20.Zhai Y, Linghu E, Li H. Double-tunnel endoscopic submucosal tunnel dissection for circumferential superficial esophageal neoplasms. Endoscopy. 2014;46 Suppl 1 UCTN:E204–E205. doi: 10.1055/s-0034-1365390. [DOI] [PubMed] [Google Scholar]
- 21.Ono S, Fujishiro M, Koike K. Endoscopic submucosal dissection for superficial esophageal neoplasms. World J Gastrointest Endosc. 2012;4:162–166. doi: 10.4253/wjge.v4.i5.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661–665. doi: 10.1055/s-0029-1214867. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T, Zhou JM, Chen TY, Zhong YS. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy. 2014;46:640–644. doi: 10.1055/s-0034-1365648. [DOI] [PubMed] [Google Scholar]
- 25.Kuwano H, Nishimura Y, Ohtsu A, Kato H, Kitagawa Y, Tamai S, Toh Y, Matsubara H. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus. April 2007 edition: Part I - Edited by the Japan Esophageal Society. Esophagus. 2008;5:61–73. [Google Scholar]
- 26.Isomoto H, Yamaguchi N, Minami H, Nakao K. Management of complications associated with endoscopic submucosal dissection/ endoscopic mucosal resection for esophageal cancer. Dig Endosc. 2013;25 Suppl 1:29–38. doi: 10.1111/j.1443-1661.2012.01388.x. [DOI] [PubMed] [Google Scholar]
- 27.Linghu E, Yang J, Zhang YC, Li W, Jin D, Sun Q. Endoscopic submucosal dissection through tunnel for esophageal lesions with diameter more than 2.5cm in pigs. Zhonghua Qiangjing Waike Zazhi. 2011;4:394–396. [Google Scholar]
- 28.Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638–1645. doi: 10.1007/s00464-009-0824-5. [DOI] [PubMed] [Google Scholar]
- 29.Maeda Y, Hirasawa D, Fujita N, Obana T, Sugawara T, Ohira T, Harada Y, Yamagata T, Suzuki K, Koike Y, et al. A pilot study to assess mediastinal emphysema after esophageal endoscopic submucosal dissection with carbon dioxide insufflation. Endoscopy. 2012;44:565–571. doi: 10.1055/s-0031-1291664. [DOI] [PubMed] [Google Scholar]
- 30.Bassan MS, Holt B, Moss A, Williams SJ, Sonson R, Bourke MJ. Carbon dioxide insufflation reduces number of postprocedure admissions after endoscopic resection of large colonic lesions: a prospective cohort study. Gastrointest Endosc. 2013;77:90–95. doi: 10.1016/j.gie.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Inoue H, Tianle KM, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Minami H, Kudo SE. Peroral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin. 2011;21:519–525. doi: 10.1016/j.thorsurg.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhai Y, Linghu E, Li H, Qin Z, Feng X, Wang X, Du H, Meng J, Wang H, Zhu J. Comparison of endoscopic submucosal tunnel dissection with endoscopic submucosal dissection for large esophageal superficial neoplasms. Nanfang Yike Daxue Xuebao. 2014;34:36–40. [PubMed] [Google Scholar]
- 33.Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 34.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–866. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 35.Sohara N, Hagiwara S, Arai R, Iizuka H, Onozato Y, Kakizaki S. Can endoscopic submucosal dissection be safely performed in a smaller specialized clinic? World J Gastroenterol. 2013;19:528–535. doi: 10.3748/wjg.v19.i4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda K, Akiho H. Endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. World J Gastrointest Pathophysiol. 2012;3:44–50. doi: 10.4291/wjgp.v3.i2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun F, Yuan P, Chen T, Hu J. Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis. J Cardiothorac Surg. 2014;9:78. doi: 10.1186/1749-8090-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901–2905. doi: 10.1007/s00464-011-1640-2. [DOI] [PubMed] [Google Scholar]
- 39.Matthes K, Jung Y, Kato M, Gromski MA, Chuttani R. Efficacy of full-thickness GI perforation closure with a novel over-the-scope clip application device: an animal study. Gastrointest Endosc. 2011;74:1369–1375. doi: 10.1016/j.gie.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 40.Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos) Gastrointest Endosc. 2012;76:202–208. doi: 10.1016/j.gie.2012.03.250. [DOI] [PubMed] [Google Scholar]
- 41.Stavropoulos SN, Modayil R, Friedel D. Closing perforations and postperforation management in endoscopy: esophagus and stomach. Gastrointest Endosc Clin N Am. 2015;25:29–45. doi: 10.1016/j.giec.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Verlaan T, Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P. Endoscopic closure of acute perforations of the GI tract: a systematic review of the literature. Gastrointest Endosc. 2015;82:618–628.e5. doi: 10.1016/j.gie.2015.03.1977. [DOI] [PubMed] [Google Scholar]
- 43.Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le Moine O, Devière J. Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc. 2011;73:890–899. doi: 10.1016/j.gie.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Baron TH, Wong Kee Song LM, Zielinski MD, Emura F, Fotoohi M, Kozarek RA. A comprehensive approach to the management of acute endoscopic perforations (with videos) Gastrointest Endosc. 2012;76:838–859. doi: 10.1016/j.gie.2012.04.476. [DOI] [PubMed] [Google Scholar]
- 45.Wang AY. Endoscopic submucosal tunnel dissection: the space between. Gastrointest Endosc. 2013;78:953–955. doi: 10.1016/j.gie.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 46.Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:626–631. doi: 10.1111/j.1442-2050.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 47.Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, Sasaki R, Namiki H, Okano T, Yamamoto M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588.e1-2. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 48.Wong VW, Teoh AY, Fujishiro M, Chiu PW, Ng EK. Preemptive dilatation gives good outcome to early esophageal stricture after circumferential endoscopic submucosal dissection. Surg Laparosc Endosc Percutan Tech. 2010;20:e25–e27. doi: 10.1097/SLE.0b013e3181c922a7. [DOI] [PubMed] [Google Scholar]
- 49.Ezoe Y, Muto M, Horimatsu T, Morita S, Miyamoto S, Mochizuki S, Minashi K, Yano T, Ohtsu A, Chiba T. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol. 2011;45:222–227. doi: 10.1097/MCG.0b013e3181f39f4e. [DOI] [PubMed] [Google Scholar]
- 50.Kishida Y, Kakushima N, Kawata N, Tanaka M, Takizawa K, Imai K, Hotta K, Matsubayashi H, Ono H. Complications of endoscopic dilation for esophageal stenosis after endoscopic submucosal dissection of superficial esophageal cancer. Surg Endosc. 2015;29:2953–2959. doi: 10.1007/s00464-014-4028-2. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi H, Arimura Y, Okahara S, Uchida S, Ishigaki S, Tsukagoshi H, Shinomura Y, Hosokawa M. Risk of perforation during dilation for esophageal strictures after endoscopic resection in patients with early squamous cell carcinoma. Endoscopy. 2011;43:184–189. doi: 10.1055/s-0030-1256109. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389–1393. doi: 10.1016/j.gie.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 53.Isomoto H, Yamaguchi N, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011;11:46. doi: 10.1186/1471-230X-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi N, Isomoto H, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73:1115–1121. doi: 10.1016/j.gie.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Hanaoka N, Ishihara R, Takeuchi Y, Uedo N, Higashino K, Ohta T, Kanzaki H, Hanafusa M, Nagai K, Matsui F, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy. 2012;44:1007–1011. doi: 10.1055/s-0032-1310107. [DOI] [PubMed] [Google Scholar]
- 56.Sato H, Inoue H, Kobayashi Y, Maselli R, Santi EG, Hayee B, Igarashi K, Yoshida A, Ikeda H, Onimaru M, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc. 2013;78:250–257. doi: 10.1016/j.gie.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi H, Arimura Y, Okahara S, Kodaira J, Hokari K, Tsukagoshi H, Shinomura Y, Hosokawa M. A randomized controlled trial of endoscopic steroid injection for prophylaxis of esophageal stenoses after extensive endoscopic submucosal dissection. BMC Gastroenterol. 2015;15:1. doi: 10.1186/s12876-014-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen J, Lu Z, Yang Y, Liu Q, Yang J, Wang S, Wang X, Du H, Meng J, Wang H, et al. Preventing stricture formation by covered esophageal stent placement after endoscopic submucosal dissection for early esophageal cancer. Dig Dis Sci. 2014;59:658–663. doi: 10.1007/s10620-013-2958-5. [DOI] [PubMed] [Google Scholar]
- 59.Saito Y, Tanaka T, Andoh A, Minematsu H, Hata K, Tsujikawa T, Nitta N, Murata K, Fujiyama Y. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci. 2008;53:330–333. doi: 10.1007/s10620-007-9873-6. [DOI] [PubMed] [Google Scholar]
- 60.Iizuka T, Kikuchi D, Yamada A, Hoteya S, Kajiyama Y, Kaise M. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Endoscopy. 2015;47:341–344. doi: 10.1055/s-0034-1390770. [DOI] [PubMed] [Google Scholar]
- 61.Maeda M, Kanai N, Kobayashi S, Hosoi T, Takagi R, Ohki T, Muragaki Y, Yamato M, Eguchi S, Fukai F, et al. Endoscopic cell sheet transplantation device developed by using a 3-dimensional printer and its feasibility evaluation in a porcine model. Gastrointest Endosc. 2015;82:147–152. doi: 10.1016/j.gie.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 62.Linghu E, Feng X, Wang X, Wang H, Meng J, Du H. Endoscopic submucosal tunnel dissection for gastric neoplastic lesions. Zhonghua Qiangjing Waike Zazhi. 2012;5:24–26. [Google Scholar]
- 63.Choi HS, Chun HJ, Seo MH, Kim ES, Keum B, Seo YS, Jeen YT, Lee HS, Um SH, Kim CD, et al. Endoscopic submucosal tunnel dissection salvage technique for ulcerative early gastric cancer. World J Gastroenterol. 2014;20:9210–9214. doi: 10.3748/wjg.v20.i27.9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu JW, Zhou PH, Yao LQ, Chen WF, Zhang YQ, Zhong YS, Xu MD. [Submucosal tunneling endoscopic resection in the treatment of rectal submucosal tumors originating from muscularis propria] Zhonghua Weichang Waike Zazhi. 2013;16:1155–1158. [PubMed] [Google Scholar]
- 65.Tan Y, Li C, Liu D, Huo J. Endoscopic submucosal tunnel dissection for one large high-grade intraepithelial neoplasia. Zhonghua Xiaohua Neijing Zazhi. 2014;31:415–416. [Google Scholar]
- 66.Zhou Z, Huang Z, Cheng H, Dai X, Li Y, Tang J. Endoscopic submucosal tunnel dissection for large early esophageal cancers and precancerous lesions. Zhonghua Xiaohua Neijing Zazhi. 2014;31:733–735. [Google Scholar]


