Abstract
Imipenem-resistant Pseudomonas aeruginosa (P. aeruginosa) has become an increasingly important problem in healthcare settings worldwide. The aim of the present study was to evaluate clonal spread among imipenem-resistant P. aeruginosa isolated from ICU-hospitalized patients. Totally, 150 wound specimens were analyzed. Antibiotic resistance profiles and clonal diversity were evaluated using Kirby-Bauer's disk diffusion method and Random Amplified Polymorphic DNA- (RAPD-) PCR, respectively. The isolates showed a high frequency of antibiotic resistance against meropenem, and imipenem (100%) followed by ciprofloxacin, and ceftazidime (90%); meanwhile resistance to polymyxin B was not observed. Eighteen (40%) of P. aeruginosa isolates were MBL-positive via ethylenediaminetetraacetic acid (EDTA) combined disk test. Our findings showed high genetic diversity, with 37 different RAPD types detected. RAPD typing results showed cross-acquisition of P. aeruginosa in investigated hospital, suggesting failure in infection control practices. Incidence of MBL-positive isolates is high and should be regarded as a threat to hospitalized patients.
1. Introduction
Pseudomonas aeruginosa (P. aeruginosa) is a well-known hospital-acquired pathogen that has been frequently isolated from different kinds of infections such as wound infections, urinary tract infections (UTIs), respiratory tract infections, and blood stream infections (BSI), especially in intensive care unit (ICU) patients [1–3]. Since the antibiotic resistance in P. aeruginosa against different classes of antibiotics is growing rapidly, its treatment is becoming more challenging with each passing year [4].
Carbapenem antibiotics such as imipenem and meropenem were very effective in the treatment of P. aeruginosa infections, when first introduced; however, after less than decade of use resistance to these antibiotics by metallo-β-lactamase- (MBL-) producing strains emerged in clinical settings. A variety of mechanisms of resistance to carbapenems have been observed, including MBL-enzymes, efflux pumps overexpression, OprD mutations, and class D β-lactamases [5, 6].
Treatment of infections caused by imipenem-resistant P. aeruginosa is difficult, owing to the fact that imipenem resistance genes are usually located in transferable genetic elements such as plasmids and integrons along with other antibiotic resistant genes [7, 8]. These observations are consistent with the high mortality and morbidity rates observed among patients infected with imipenem-resistant P. aeruginosa. Moreover production of MBL-enzymes confers resistance to virtually all β-lactam antibiotics, except for monobactam [9].
Molecular epidemiological methods such as Random Amplified Polymorphic DNA- (RAPD-) PCR are well suited to determination of transmission routes of P. aeruginosa in hospital wards [10, 11]. An evaluation of the incidence of imipenem-resistant P. aeruginosa and its transmission in hospitals is of particular importance for the effective management of hospital-acquired infections. The objective of this study was to evaluate the incidence of imipenem-resistant P. aeruginosa in ICU-hospitalized patients and to document the potential for clonal spread.
2. Methods
2.1. Specimen Collection and Bacterial Identification
In total, 150 wound swabs obtained from burned patients admitted to the ICU ward of a referral hospital in Isfahan, between January 2013 and July 2014, were evaluated. Wound infection was identified based on clinical signs, described previously [12]. Only one isolate per patient was included in the study. The study was approved by the ethics committee of the Isfahan University of Medical Sciences (number 392063).
Presumptive identification of P. aeruginosa was performed by using standard conventional biochemical test including Gram-staining, catalase, oxidase, pigment production, oxidative-fermentative (OF) tests, and growth at 42°C [13]. Subsequently, species-specific PCR was done using previously designed primer for ITS (16s–23s rRNA internal transcribed spacer) [14]. PCR mixture (25 μL) was made consisting of 200 μM dNTPs, 2.5 mM MgCl2, PCR buffer 1x, Taq DNA pol 1.5 unit (Cinna Gen, Iran), 10 pM of each primer (Metabion, Germany), and 40 ng DNA sample. The following cycling conditions were applied: initial denaturation at 95°C 5 min, 1 cycle, 35 cycles consisting of denaturation at 94°C 1 min, annealing at 58°C 45 s, extension at 72°C 45 s, and final extension at 72°C for 5 min.
2.2. DNA Extraction
Bacterial fresh colonies (two or three) were removed and suspended in 300 mL of lysis buffer containing Tris 100 mM, Nacl 50 mM, and EDTA 25 mM, pH = 7.5, completely. Then suspension was boiled at 95°C for 15 min. Equal volume of phenol/chloroform (25 : 24, pH = 7.5) was added, mixed thoroughly, and centrifuged at 9000 g for 5 min. Aqueous-viscous supernatant was transformed to a new micro tube and phenol/chloroform (25 : 24) was added again and centrifuged for 5 min at 9000 g. 600 μL cold and pure ethanol (Merck, Germany) was added and centrifuged at 13000 g, (4°C-30 min) to precipitate DNA. DNA washed twice in ethanol 70% and after quality check was stored at −20°C.
2.3. Antibiotic Susceptibility Test
Resistance to different antibiotics was tested by Kirby-Bauer's disk diffusion method based on CLSI (Clinical Laboratory Standard Institute) guidelines [15]. The following disks (MAST, UK) were applied: ceftazidime (CAZ, 30 μg), imipenem (IMP, 10 μg), meropenem (MEM, 10 μg), ciprofloxacin (CIP, 5 μg), aztreonam (ATM, 30 μg), polymyxin B (PB, 300 units), and amikacin (AMK, 30 μg). P. aeruginosa standard strain (ATCC 27853) was used as the quality control.
2.4. Detection of MBL
P. aeruginosa isolates that were resistant to imipenem and/or meropenem were subjected to phenotypical detection of MBL-producing isolates by imipenem-EDTA combined disk test, as described earlier [16]. Difference of ≥7 mm between the inhibition-zone diameter of the imipenem-EDTA disk and that of imipenem-only disk was considered as MBL-positive [16]. We used EDTA disk by itself and a strain of P. aeruginosa known to produce VIM-1 metallo-beta-lactamase as the negative and positive control, respectively.
2.5. Random Amplified Polymorphic DNA- (RAPD-) PCR
All P. aeruginosa isolates were submitted to RAPD-genotyping using primer 272-AGCGGGCCAA as previously described [17]. Briefly, optimized PCR mixture was made using 2.5 μL 10x PCR buffer, 2.5 mM MgCl2, 300 μM of dNTPs, 1.7 U Taq DNA polymerase (Cinna Gen, Iran), and 3 μL genomic DNA (40 ng) in 25 μL final volume. The following thermocycler program was used, (1) denaturation 5 min at 95°C, annealing 5 min at 36°C, and elongation 5 min at 72°C, for 4 cycles and (2) 31 cycles consisting of 94°C for 1 min, 45°C for 1 min, and 72°C for 2 min, followed by a final extension at 72°C for 10 min. To ensure reproducibility, each reaction was repeated three times. Similarity between isolates was evaluated based on Dice similarity coefficient and Unweighted Average Pair Group Method (UPGMA), using FreeTree and TreeView software [18, 19]. Only major reproducible bands regardless of intensity were considered for similarity matrix calculation [20] Cut-off value ≥80% was used for determination of potential clonal relatedness [21, 22].
3. Results
Out of 150 tested specimens, 45 (30%) patients had positive culture for P. aeruginosa. Only one isolate per patient was recruited for study. Of the 45 patients, 30 (66%) were females and 15 (34%) were males. The isolates showed a high frequency of antibiotic resistance against meropenem, and imipenem (100%) followed by ciprofloxacin, aztreonam, and ceftazidime (90%), whereas the lowest resistance rate 61.6% was seen against amikacin. Resistance to polymyxin B was not observed. Eighteen (40%) of imipenem-resistant isolates were MBL-positive, using IMP-EDTA combined disk test. This study revealed high diversity of RAPD types, with 37 different RAPD types (Figure 1). Twenty-nine strains of P. aeruginosa showed unique patterns, while the rest of the strains (16) formed 8 distinct clusters. Groups one and three are composed of 2 isolates; these isolates were also MBL-positive.
Figure 1.
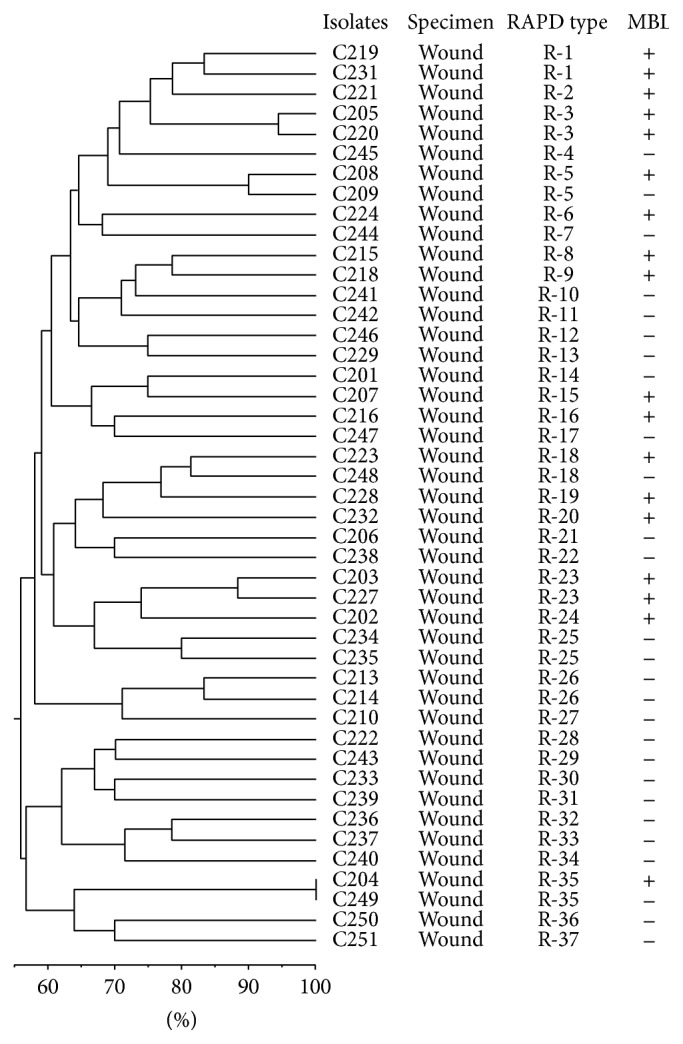
Dendrogram showing genetic diversity among 45 nonduplicate imipenem-resistant P. aeruginosa by RAPD-PCR.
4. Discussion
Hospitalized patients, particularly those who were admitted to the ICU, are mostly at risk for P. aeruginosa life-threatening infections [23]. Owing to the fact that imipenem resistance genes are usually located in the transposable genetic elements, along with other antibiotic resistance genes [24], the emergence of imipenem-resistant isolates of P. aeruginosa in healthcare settings is a serious problem.
In the present study, the highest percentages of resistant isolates were seen against aztreonam, meropenem, ceftazidime, and ciprofloxacin. Although this finding was supported by other studies carried out in Babol province (ceftazidime 92.6%) and Tehran province (imipenem and meropenem 94.7%, ceftazidime 89.4%, and ciprofloxacin 96.2%) of Iran, antibiotic resistance rates reported from Hamadan province (imipenem 7.5%, meropenem 13.2%, and ciprofloxacin 4.7%) were substantially lower than our resistance frequency [25–27]. In addition, our antibiotic resistance rates were significantly higher than those reported from some European countries [28–30]. This higher resistance rate probably resulted from indiscriminate use of antibiotics. According to independent study, presence of some risk factors, including use of catheter, ventilator, and previous consumption of antibiotics are associated with higher antibiotic resistance rates [31].
MBL was observed in 18 (40%) of the 45 imipenem-resistant isolates. This prevalence of MBL is much lower than that reported from other parts of Iran (Tehran province 94% and Zanjan province 87.8%) but is significantly higher than that reported from Sweden (1%) [32–34]. In MBL-negative isolates, probably other mechanisms such as OprD mutations, efflux pumps overexpression, or class D β-lactamase are responsible for carbapenems resistance [35]. In case of use of EDTA as MBL inhibitor due to inhibitory effect on bacterial growth, prevalence of MBL-positive isolates should be reported with precaution because it may lead to false-positive results.
Since determining bacterial genetic relatedness is essential for cross-infection evaluation, different genotyping methods have been established [36–39]. In spite of some of the limitations such as difficulty in interpretation of bands in some cases, RAPD-PCR have the advantage of being rapid, simple, and reproducible with high discriminatory power [36–39]. The RAPD-PCR fingerprinting technique yields reliable evaluations of clonal diversity [10, 40]. We detected the high genetic variability, with 37 distinct RAPD types among 45 isolates (82.2% of polymorphisms). Similarly, Silva reported 86 distinct RAPD types (89.6% of polymorphisms) among the 96 strains isolated from clinical specimens of different Brazilian hospitals [41]. In agreement with Pereira study in two referral hospitals of Portugal, our results did not reveal epidemic spread [11]. Our results demonstrate that most of the isolates probably originated from the patients themselves; however, cross-infection of P. aeruginosa between patients is possible to occur, suggesting nosocomial infection control problem.
5. Conclusion
According to our data incidence of imipenem-resistant isolates of P. aeruginosa is in alarming level. Our results did not demonstrate epidemic clone. Probable cross-acquisition has occurred and needs to be considered for future infection control procedure.
Acknowledgment
This study was supported by Isfahan University of Medical Sciences (Grant no. 392063).
Conflict of Interests
The authors declare no conflict of interests.
Authors' Contribution
Study design was carried out by Hamid Vaez, Sharareh Moghim, Bahram Nasr Esfahani, and Hajieh Ghasemian Safaei. Data interpretation and writing of paper were performed by Hamid Vaez and Hajieh Ghasemian Safaei. Laboratory procedure and support were done by Hamid Vaez, Sharareh Moghim, Bahram Nasr Esfahani, and Hajieh Ghasemian Safaei.
References
- 1.Kali A., Srirangaraj S., Kumar S., Divya H. A., Kalyani A., Umadevi S. Detection of metallo-β-lactamase producing Pseudomonas aeruginosa in intensive care units. Australasian Medical Journal. 2013;6(12):686–693. doi: 10.4066/amj.2013.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varaiya A., Kulkarni N., Kulkarni M., Bhalekar P., Dogra J. Incidence of metallo-beta-lactamase producing Pseudomonas aeruginosa in ICU patients. Indian Journal of Medical Research. 2008;127:398–402. [PubMed] [Google Scholar]
- 3.Rossolini G. M., Mantengoli E. Treatment and control of severe infections caused by multi-resistant Pseudomonas aeruginosa . Clinical Microbiology and Infection. 2005;11(4):17–32. doi: 10.1111/j.1469-0691.2005.01161.x. [DOI] [PubMed] [Google Scholar]
- 4.Ranjan S., Banashankari G. S., Sreenivasa Babu P. R. Comparison of epidemiological and antibiotic susceptibility pattern of metallo-beta-lactamase-positive and metallo-beta-lactamase-negative strains of Pseudomonas aeruginosa . Journal of Laboratory Physicians. 2014;6(2):109–113. doi: 10.4103/0974-2727.141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maltezou H. C. Metallo-β-lactamases in Gram-negative bacteria: introducing the era of pan-resistance? International Journal of Antimicrobial Agents. 2009;33(5):405.e1–405.e7. doi: 10.1016/j.ijantimicag.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Lister P. D., Wolter D. J., Hanson N. D. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clinical Microbiology Reviews. 2009;22(4):582–610. doi: 10.1128/cmr.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willmann M., Kuebart I., Marschal M., et al. Effect of metallo-β-lactamase production and multidrug resistance on clinical outcomes in patients with Pseudomonas aeruginosa bloodstream infection: a retrospective cohort study. BMC Infectious Diseases. 2013;13, article 515 doi: 10.1186/1471-2334-13-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babu K. V. Y., Visweswaraiah D. S., Kumar A. The influence of imipenem resistant metallo-beta-lactamase positive and negative Pseudomonas aeruginosa nosocomial infections on mortality and morbidity. Journal of Natural Science, Biology and Medicine. 2014;5(2):345–351. doi: 10.4103/0976-9668.136181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bebrone C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochemical Pharmacology. 2007;74(12):1686–1701. doi: 10.1016/j.bcp.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Nanvazadeh F., Khosravi A. D., Zolfaghari M. R., Parhizgari N. Genotyping of Pseudomonas aeruginosa strains isolated from burn patients by RAPD-PCR. Burns. 2013;39(7):1409–1413. doi: 10.1016/j.burns.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Pereira S. G., Reis T., Mendez I. P., Cardoso O. Prevalence and molecular epidemiology of imipenem-resistant Pseudomonas aeruginosa carrying metallo-beta-lactamases from two central hospitals in Portugal. Microbial Drug Resistance. 2013;19(5):392–396. doi: 10.1089/mdr.2013.0029. [DOI] [PubMed] [Google Scholar]
- 12.European Wound Management Association (EWMA) Position Document: Identifinig Criteria for Wound Infection. Aylesford, UK: MEP; 2005. [Google Scholar]
- 13.Mahon C., Lehman D., Manuselis G. Text Book of Diagnostic Microbiology. 4th. New York, NY, USA: Elsevier; 2011. [Google Scholar]
- 14.Tyler S. D., Strathdee C. A., Rozee K. R., Johnson W. M. Oligonucleotide primers designed to differentiate pathogenic Pseudomonads on the basis of the sequencing of genes coding for 16S–23S rRNA internal transcribed spacers. Clinical and Diagnostic Laboratory Immunology. 1995;2(4):448–453. doi: 10.1128/cdli.2.4.448-453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. CLSI Document. M100-S23. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2013. Performance standards for antimicrobial susceptibility testing; 23th informational supplement. [Google Scholar]
- 16.Yong D., Lee K., Yum J. H., Shin H. B., Rossolini G. M., Chong Y. Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. Journal of Clinical Microbiology. 2002;40(10):3798–3801. doi: 10.1128/jcm.40.10.3798-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahenthiralingam E., Campbell M. E., Foster J., Lam J. S., Speert D. P. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. Journal of Clinical Microbiology. 1996;34(5):1129–1135. doi: 10.1128/jcm.34.5.1129-1135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlíček A., Hrdá Š., Flegr J. FreeTree—freeware program for construction of phylogenetic trees on the basis of distance data and Bootstrap/Jackknife analysis of the tree robustness, application in the RAPD analysis of genus frenkelia. Folia Biologica. 1999;45(3):97–99. [PubMed] [Google Scholar]
- 19.Page R. D. TreeView: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12(4):357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 20.Ruimy R., Genauzeau E., Barnabe C., Beaulieu A., Tibayrenc M., Andremont A. Genetic diversity of Pseudomonas aeruginosa strains isolated from ventilated patients with nosocomial pneumonia, cancer patients with bacteremia, and environmental water. Infection and Immunity. 2001;69(1):584–588. doi: 10.1128/iai.69.1.584-588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giske C. G., Libisch B., Colinon C. Establishing clonal relationships between VIM-1-like metallo-β-lactamase-producing Pseudomonas aeruginosa strains from four European countries by multilocus sequence typing. Journal of Clinical Microbiology. 2006;44(12):4309–4315. doi: 10.1128/JCM.00817-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libisch B., Balogh B., Füzi M. Identification of two multidrug-resistant Pseudomonas aeruginosa clonal lineages with a countrywide distribution in Hungary. Current Microbiology. 2009;58(2):111–116. doi: 10.1007/s00284-008-9285-7. [DOI] [PubMed] [Google Scholar]
- 23.Souza Rodrigue A. C., Chang M. R., Nóbrega G. D., et al. Metallo-β-lactamase and genetic diversity of Pseudomonas aeruginosa in intensive care units in Campo Grande, MS, Brazil. Brazilian Journal of Infectious Diseases. 2011;15(3):195–199. doi: 10.1016/s1413-8670(11)70174-x. [DOI] [PubMed] [Google Scholar]
- 24.Odumosu B. T., Adeniyi B. A., Chandra R. Analysis of integrons and associated gene cassettes in clinical isolates of multidrug resistant Pseudomonas aeruginosa from Southwest Nigeria. Annals of Clinical Microbiology and Antimicrobials. 2013;12, article 29 doi: 10.1186/1476-0711-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouchaksaraei F. M., Shahandashti E. F., Molana Z., Kouchaksaraei M. M., Asgharpour F., Mojtahedi A. Molecular detection of Integron genes and pattern of antibiotic resistance in Pseudomonas aeruginosa strains isolated from intensive care unit, Shahid Beheshti Hospital, North of Iran. International Journal of Molecular and Cellular Medicine. 2012;1(4):209–217. [PMC free article] [PubMed] [Google Scholar]
- 26.Moazami-Goudarzi S., Eftekhar F. Assessment of carbapenem susceptibility and multidrug-resistance in Pseudomonas aeruginosa burn isolates in Tehran. Jundishapur Journal of Microbiology. 2013;6(2):162–165. doi: 10.5812/jjm.5036. [DOI] [Google Scholar]
- 27.Alikhani M. Y., Karimi Tabar Z., Mihani F., et al. Antimicrobial resistance patterns and prevalence of blaPER-1 and blaVEB-1 genes among ESBL-producing Pseudomonas aeruginosa isolates in West of Iran. Jundishapur Journal of Microbiology. 2014;7(1) doi: 10.5812/jjm.8888.e8888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanberger H., Burman L. G., Cars O., et al. Low antibiotic resistance rates in Staphylococcus aureus, Escherichia coli and Klebsiella spp but not in Enterobacter spp and Pseudomonas aeruginosa: a prospective observational study in 14 Swedish ICUs over a 5-year period. Acta Anaesthesiologica Scandinavica. 2007;51(7):937–941. doi: 10.1111/j.1399-6576.2007.01364.x. [DOI] [PubMed] [Google Scholar]
- 29.Glupczynski Y., Bogaerts P., Deplano A., et al. Detection and characterization of class a extended-spectrum-β-lactamase-producing Pseudomonas aeruginosa isolates in Belgian hospitals. Journal of Antimicrobial Chemotherapy. 2010;65(5):866–871. doi: 10.1093/jac/dkq048. [DOI] [PubMed] [Google Scholar]
- 30.Croughs P. D., Li B., Hoogkamp-Korstanje J. A. A., Stobberingh E. Thirteen years of antibiotic susceptibility surveillance of Pseudomonas aeruginosa from intensive care units and urology services in the Netherlands. European Journal of Clinical Microbiology & Infectious Diseases. 2013;32(2):283–288. doi: 10.1007/s10096-012-1741-4. [DOI] [PubMed] [Google Scholar]
- 31.Aloush V., Navon-Venezia S., Seigman-Igra Y., Cabili S., Carmeli Y. C. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrobial Agents and Chemotherapy. 2006;50(1):43–48. doi: 10.1128/aac.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saderi H., Lotfalipour H., Owlia P., Salimi H. Detection of metallo-β-lactamase producing Pseudomonas aeruginosa isolated from burn patients in Tehran, Iran. Laboratory Medicine. 2010;41(10):609–612. doi: 10.1309/lmqjf9j3t2oaacdj. [DOI] [Google Scholar]
- 33.Doosti M., Ramazani A., Garshasbi M. Identification and characterization of metallo-β-lactamases producing Pseudomonas aeruginosa clinical isolates in university hospital from Zanjan Province, Iran. Iranian Biomedical Journal. 2013;17(3):129–133. doi: 10.6091/ibj.1107.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erlandsson M., Gill H., Nordlinder D., et al. Antibiotic susceptibility patterns and clones of Pseudomonas aeruginosa in Swedish ICUs. Scandinavian Journal of Infectious Diseases. 2008;40(6-7):487–494. doi: 10.1080/00365540701864641. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Martínez J.-M., Poirel L., Nordmann P. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa . Antimicrobial Agents and Chemotherapy. 2009;53(11):4783–4788. doi: 10.1128/aac.00574-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onteniente L., Brisse S., Tassios P. T., Vergnaud G. Evaluation of the polymorphisms associated with tandem repeats for Pseudomonas aeruginosa strain typing. Journal of Clinical Microbiology. 2003;41(11):4991–4997. doi: 10.1128/jcm.41.11.4991-4997.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson S. L., Fry J. C., Dancer B. N. A comparative evaluation of five typing techniques for determining the diversity of fluorescent Pseudomonads . Journal of Microbiological Methods. 2002;50(1):9–22. doi: 10.1016/s0167-7012(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 38.Curran B., Jonas D., Grundmann H., Pitt T., Dowson C. G. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa . Journal of Clinical Microbiology. 2004;42(12):5644–5649. doi: 10.1128/jcm.42.12.5644-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Römling U., Tümmler B. Achieving 100% typeability of P. aeruginosa by pulsed-field gel electrophoresis. Journal of Clinical Microbiology. 2000;38(1):464–465. doi: 10.1128/jcm.38.1.464-465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitou K., Furuhata K., Fukuyama M. Genotyping of Pseudomonas aeruginosa isolated from cockroaches and human urine. Journal of Infection and Chemotherapy. 2010;16(5):317–321. doi: 10.1007/s10156-010-0055-7. [DOI] [PubMed] [Google Scholar]
- 41.Silva L. V., Galdino A. C. M., Nunes A. P. F., et al. Virulence attributes in brazilian clinical isolates of Pseudomonas aeruginosa . International Journal of Medical Microbiology. 2014;304(8):990–1000. doi: 10.1016/j.ijmm.2014.07.001. [DOI] [PubMed] [Google Scholar]


