Abstract Abstract
Macrolophus pygmaeus (Rambur, 1839) (Insecta, Heteroptera, Miridae) is a predator of key vegetable crop pests applied as a biocontrol agent in the Mediterranean region. Macrolophus pygmaeus and Macrolophus melanotoma (A. Costa, 1853) are cryptic species with great morphological similarity which results in their misidentification and negative consequences for the conservation of their populations on greenhouse and outdoor crops. In order to find out specific markers for their separation we studied the karyotype, male meiosis and heterochromatin composition of these species and additionally of a third species (as a reference one), Macrolophus costalis Fieber, 1858. We demonstrate here that all the three species share achiasmate male meiosis and sex chromosome pre-reduction. On the other hand, the species differ in karyotype, with 2n=28 (26+XY) in Macrolophus pygmaeus, 2n=27 (24+X1X2Y) in Macrolophus costalis, and 2n=34 (32+XY) in Macrolophus melanotoma, and heterochromatin distribution and composition. In addition, the species differ in sperm morphology: sperm cells of Macrolophus costalis are significantly longer with longer head and tail than those of Macrolophus melanotoma and Macrolophus pygmaeus, whereas sperm cells of Macrolophus melanotoma have a longer tail than those of Macrolophus pygmaeus. All these characters can be used as markers to identify the species, in particular the cryptic species Macrolophus melanotoma and Macrolophus pygmaeus.
Keywords: Macrolophus, Miridae, Heteroptera, karyotype, sex chromosomes, achiasmate meiosis, sex chromosome pre-reduction, sperm morphology
Introduction
The Miridae are the largest family of true bugs (Heteroptera, Cimicomorpha) with approximately 10000 species described (Schuh 1995). Cytogenetical data are presently available for about 200 species (Ueshima 1979, Nokkala and Nokkala 1986, Grozeva 2003, Grozeva et al. 2006, 2007, Grozeva and Simov 2008a, b, 2009, Kuznetsova et al. 2011). The mirid bugs share some cytogenetic characteristics with all the Heteroptera: they possess holokinetic (or holocentric) chromosomes and most of them are characterized by an inverted sequence of reductional and equational division of the sex chromosomes (post-reduction) in male meiosis (Ueshima 1979). On the other hand, they have some unique chromosomal characteristics. Chiasmata are absent in male meiosis, the achiasmate meiosis being of a collochore type (Nokkala and Nokkala 1986, Kuznetsova et al. 2011). In the three hitherto studied Macrolophus Fieber, 1858 species, Macrolophus costalis Fieber, 1858, Macrolophus pygmaeus (Rambur, 1839) and Macrolophus geranii Josifov, 1961, both autosomes and sex chromosomes divide pre-reductionally during the achiasmate male meiosis (Grozeva et al. 2006, 2007).
The species from the present study, Macrolophus costalis, Macrolophus melanotoma (A. Costa, 1853), and Macrolophus pygmaeus, occur on a variety of plant species in the Mediterranean region. Macrolophus pygmaeus is an efficient predator of several key vegetable crop pests in Europe produced commercially and used widely as a biocontrol agent (Alomar et al. 2002, 2006, van Lenteren 2003, Messelink et al. 2014). Macrolophus pygmaeus and Macrolophus melanotoma are cryptic species with great morphological similarity which results in their misidentification and negative consequences for the conservation of their populations on greenhouse and outdoor crops. In order to find specific markers for their separation we here studied the karyotype, male meiosis and heterochromatin composition of these species and additionally of a third species, Macrolophus costalis. The species have recently been separated based on differences of their genetic profiles, cuticular hydrocarbon composition and on the fact that interspecies crosses do not produce viable progeny (Martinez-Cascales et al. 2006, Gemeno et al. 2012, Castañé et al. 2013). Macrolophus costalis can be easily distinguished morphologically from Macrolophus pygmaeus or Macrolophus melanotoma by the black dot on the scutellum, but it was included in our study as a reference species. In earlier cytogenetic studies (Grozeva et al. 2006, 2007), karyotype of two of the three species here examined was reported. Such characters, as highly asymmetric karyotype (2n=24+X1X2X3Y) with two extra-large autosome pairs and interstitial distribution of C-heterochromatin in them (Grozeva et al. 2006), provide excellent cytogenetic markers to distinguish Macrolophus costalis from other Macrolophus species. The karyotype of Macrolophus pygmaeus (2n=26+XY) is asymmetric, as in Macrolophus costalis, but with different number of autosomes and a simple XY sex chromosome system (Grozeva et al. 2007). The species share sex chromosome pre-reduction, but can easily be differentiated by their karyotype and pattern of C-heterochromatin distribution.
Sperm morphology is significant in fertilization (Evans and Simmons 2008). Franco et al. (2011) have recently shown that the species in which sperm competition occurs also displayed the longest sperm length.
With the aim of distinguishing between the cryptic Macrolophus species, both karyotype and male meiosis were studied for the first time in Macrolophus melanotoma and reinvestigated in Macrolophus pygmaeus and (as a reference species) in Macrolophus costalis using standard chromosome staining and fluorochromes DAPI and CMA3. In addition, morphology of sperm cells was examined in each of the three species.
Material and methods
Insects
Males and females of Macrolophus costalis, Macrolophus pygmaeus and Macrolophus melanotoma were collected in Catalonia, NE of Spain, in the vicinity of Mataró (Barcelona) (41.556 North, 2.475 East) from Cistus albidus Linnaeus, 1753, commercial tomato fields and Dittrichia viscosa (Linnaeus) Greuter, respectively. Colonies from collected individuals were set-up under controlled conditions (25 ± 1°C, 70 ± 10% RH and L16:D8 photoperiod) on tobacco plants (Nicotiana tabacum Linnaeus, 1753) with Ephestia kuehniella Zeller, 1879 (Lepidoptera, Pyralidae) eggs as a prey (Agusti and Gabarra 2009a, b). All Macrolophus specimens were preliminarily identified following Josifov (1992). However, due to morphological similarity between Macrolophus melanotoma and Macrolophus pygmaeus, their identification was additionally tested by conventional PCR using methodology and specific primers described in Castañe et al. (2013).
Karyotype
The abdomen of 20 Macrolophus pygmaeus, 23 Macrolophus melanotoma and 13 Macrolophus costalis males were placed in 3:1 fixative (96% ethanol-glacial acetic mixture) and the thorax in 70% ethanol for later species identification by DNA analysis (Castañe et al. 2013). Dissected gonads were squashed in a small drop of 45% acetic acid. The cover slips were removed by the dry ice technique. Slides were dehydrated in fresh fixative (3:1) and air dried. Part of the preparations was stained using Schiff-Giemsa method of Grozeva and Nokkala (1996) to check the number of chromosomes and their behaviour in meiosis. For other slides, DNA- binding fluorochromes, GC-specific chromomycin A3 (CMA3) and AT-specific 4’-6’-diamino-2-phenylindole (DAPI) were applied following Schweizer (1976) and Donlon and Mafenis (1983), with minor modifications as described in Kuznetsova et al. (2001).
Chromosomes were analyzed using light/fluorescent microscopy (Axio Scope A1 – Carl Zeiss Microscope) at 100× magnification and documented with a ProgRes MFcool – Jenoptik AG digital camera. All cytogenetic preparations and remains of the specimens are stored at the Institute of Biodiversity and Ecosystem Research, BAS in Sofia.
Sperm morphology
In every species, sperm cells from 10 males (other than those used for the karyotype analysis) were measured following Franco et al. (2011). On a slide, one drop of Beadle saline solution (128.3 mM NaCl, 4.7 mM KCl, 23 mM CaCl2) was added to the male abdomen. The seminal vesicle was extracted and opened in 20 μl of saline solution to allow the sperm out. The sperm were diluted carefully with the aid of a fine needle, and then one drop was transferred and smeared across a microscope slide, allowed to dry and rinsed. Sperm cells were analyzed using a Leica DM 4000 light microscope. Twelve sperm cells per individual were measured (head and tail length) at 400x under dark field using the QWin 6.0 (Leica Microsystems, Germany) software package. In order to reduce measurement variation, each component was measured five times for each sperm cell. Data were analyzed by a one-way ANOVA and means separation by Tukey multiple range test.
Results
Karyotype
Macrolophus costalis, 2n=27 (24+X1X2Y)
Published data: 2n=28 (24+X1X2X3Y) (Grozeva et al. 2006)
Spermatogonial metaphases consisted of 5 large (incl. Y) and 22 similar in size chromosomes (incl. two X) (Fig. 1). In meiosis, condensation stage was most abundant and showed 12 autosomal bivalents plus a positively heteropycnotic sex chromosome body (Fig. 2). Size differences between the bivalents were observed. The complement included two extremely large bivalents, four to five times the size of the other 10 similar size bivalents. Bivalents consisted of parallel-aligned homologous chromosomes without chiasmata, i.e. the male meiosis was achiasmate. After the Schiff-Giemsa staining, it was easy to see that conspicuous interstitial heterochromatic bands in both large bivalents divide them into the three almost equal parts. At metaphase I (MI), the sex chromosomes were seen either as a trivalent (Fig. 3a, b) or as a bivalent (Fig. 3c). They clearly segregated at anaphase I (Fig. 4a) resulting in two types of metaphase II, with 12 autosomes plus two X chromosomes (Fig. 4b) and with 12 autosomes plus the Y (Fig. 4c). Male meiosis was hence pre-reductional both for autosomes and sex chromosomes. After second division, every cell produced daughter cells possessing either 2X or Y chromosome respectively (Fig. 5). Thus, the chromosome formula of male Macrolophus costalis was determined as 2n=27 (24+X1X2Y) in contrast to 2n=28 (24+X1X2X3Y) earlier reported in Grozeva et al. (2006).
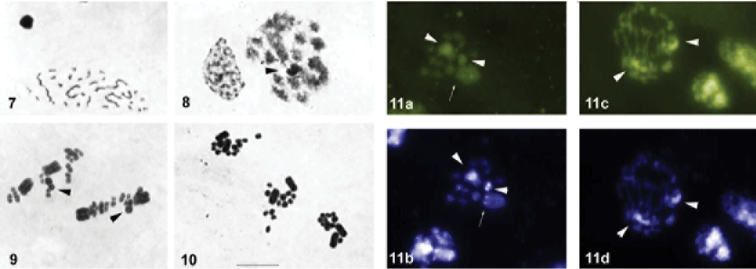
Figures 1–6.
Male meiosis in Macrolophus costalis. (1–5 conventional staining 6 fluorochrome staining: 6a, c CMA3 6b, d DAPI) 1 spermatogonial metaphase 2 condensation stage 3a–c metaphase I 4a–c metaphase II 5 telophase 6a, b condensation stage 6c, d metaphase I. Sex-chromosomes are indicated by arrowheads. Heterochromatin blocks are indicated by arrows. Bar = 10 µm.
After staining with fluorochromes, bright DAPI- and CMA3- positive bands were observed in the same locations on the larger autosomal bivalents and sex chromosomes (Fig. 6a–d).
Macrolophus pygmaeus, 2n=28 (26+XY)
Published data: 2n=28 (26+XY) (Grozeva et al. 2007)
Spermatogonial metaphases consisted of 5 large (incl. X) and 23 similar size chromosomes (incl. Y) (Fig. 7). In meiosis, condensation stage showed 13 autosomal bivalents and a positively heteropycnotic sex chromosome body (Fig. 8). The complement included two extremely large bivalents (~five times the size of the others), and eleven bivalents similar in size. The bivalents consisted of parallel-aligned homologous chromosomes; chiasmata were absent and the male meiosis was achiasmate. MI was nonradial (i.e., the autosomes did not form a ring), and the sex chromosomes formed a pseudobivalent (Fig. 9). The sex chromosomes segregated at AI resulting in two MII cells each with 14 chromosomes (13 + X or Y) (Fig. 10). Male meiosis was hence pre-reductional both for autosomes and sex chromosomes. Thus, the chromosome formula of Macrolophus pygmaeus was confirmed as 2n=28 (26+XY) in line with that earlier reported in Grozeva et al. (2007).
Figures 7–11.
Male meiosis in Macrolophus pygmaeus. (7–10 conventional staining 11 fluorochrome staining: 11a, c CMA3 11b, d DAPI) 7 spermatogonial metaphase 8 condensation stage 9 metaphase I 10 metaphase II 11a, b metaphase I 11c, d anaphase. Sex-chromosomes are indicated by arrowheads. CMA3/DAPI signals are indicated by arrows. Bar = 10 µm.
After staining with fluorochromes, bright DAPI- and CMA3 -positive bands were observed on both sex chromosomes (Fig. 11a–d). In addition, a weak DAPI- positive / CMA3 -negative signal was registered in a telomere of one of the larger bivalents (Fig. 11a, b).
Macrolophus melanotoma 2n=34 (32+XY)
Published data: absent
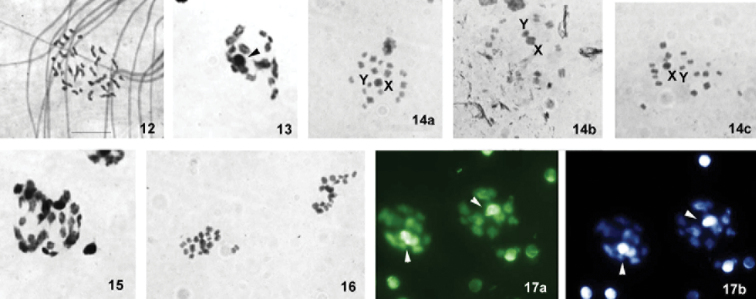
At spermatogonial metaphase, there were 34 chromosomes (Fig. 12) gradually decreasing in size and the sex chromosomes were difficult to distinguish. At meiotic condensation stage, autosomal bivalents consisted of parallel lying homologs, and the sex chromosomes appeared as a heteropycnotic body (Fig. 13). At MI, there were 16 autosomal bivalents and X and Y chromosomes (Fig. 14a–c). Note that both sex chromosomes were occasionally placed in the center of a ring formed by autosomal bivalents (Fig. 14b). The autosomal bivalents constituted a decreasing size series and the X was more than twice the size of the Y. As a result of pre-reductional division of sex chromosomes at AI (Fig. 15), two types of MII (Fig. 16) raised, both with 17 chromosomes while with X or Y chromosome respectively.
Figures 12–17.
Male meiosis in Macrolophus melanotoma. (12–16 conventional staining 17 fluorochrome staining: 17a CMA3 17b DAPI) 12 spermatogonial metaphase 13 condensation stage 14a–c metaphase I 15 anaphase I 16 metaphase II 17 condensation stage. Sex-chromosomes are indicated by arrowheads. Bar = 10 µm.
After staining with fluorochromes, bright DAPI- and CMA3 bands were observed on the sex chromosomes (Fig. 17).
Sperm morphology
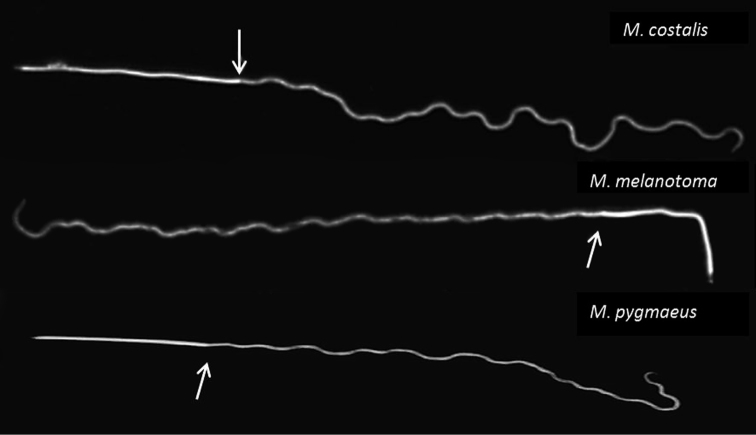
Sperm cells of the species studied were of similar shape, with a long and filiform head (Fig. 18). However, species are different in the total length (mean ± SE) of the sperm cells (F2,27=15.53; P<0.0001), in the length of head (F2,27=38.89; P<0.0001) and tail (F2,27=25.43; P<0.01) (Table 1).
Figure 18.
Sperm morphology of Macrolophus species. The arrow shows the end of sperm head.
Table 1.
Sperm cells lenght (means ± standard error) of Macrolophus costalis, Macrolophus melanotoma and Macrolophus pygmaeus. Within each row, means followed by the same letter do not differ significantly (P≤0.005).
| Sperm length (µm) | Macrolophus costalis | Macrolophus melanotoma | Macrolophus pygmaeus |
|---|---|---|---|
| Total | 236±0.9 a | 220.4±0.9 b | 215±1.4 c |
| Head | 61.3±0.4 a | 51.3±0.4 b | 50.6±0.4 b |
| Tail | 174.7±0.8 a | 169.1±1 b | 164.6±1.2 c |
Discussion
The genus Macrolophus belongs to the tribe Dicyphini of the subfamily Bryocorinae (Heteroptera, Miridae). In Bryocorinae, besides the modal for the Miridae chromosome number 2n=34 (32+XY), some higher (2n=36+XY and 2n=46+XY/X1X2Y) and lower (2n=16-26+XY) chromosome numbers have also been described (Ueshima 1979, Grozeva 2003, Grozeva et al. 2006, 2007, 2008a). The chromosome formula of the reference species Macrolophus costalis was reported earlier as 2n=28 (24+X1X2X3Y) for a Bulgarian population collected from the tobacco plants, which was the first report of three X chromosomes in the family (Grozeva et al. 2006). In contrast, the chromosome formula of the NE Spanish population here studied appeared to be different, with two instead of three X chromosomes. As the specimens come from different geographic regions we could speculate that they represent chromosomal races within a species. At taxonomic level, they may probably be considered as subspecies, but to clarify this hypothesis a chromosomal analysis of individuals (males and females) from natural populations of this species over the whole distribution range will be necessary. Macrolophus pygmaeus showed 2n=28 (26+XY) both in Bulgaria (Grozeva et al. 2007) and Spain (present study). The third species, Macrolophus melanotoma, studied here for the first time, appeared to differ from Macrolophus costalis and Macrolophus pygmaeus in karyotype. Besides difference in the chromosome number, Macrolophus melanotoma lacks two large autosome pairs characteristic of the two other species allowing for the cryptic Macrolophus melanotoma and Macrolophus pygmaeus to be reliably differentiated.
Differences in molecular organisation of chromatin revealed after fluorochrome staining, suggest an additional chromosome marker to differentiate Macrolophus species. In Macrolophus costalis, bright DAPI/CMA3 bands were observed in the same locations on the large autosomal bivalents and sex chromosomes whereas in two remaining species bright fluorescent bands were observed only on the sex chromosomes. In turn, Macrolophus pygmaeus differed from Macrolophus melanotoma in that it showed some additional weak DAPI-positive signals in a telomeric region of a larger bivalent.
Franco et al. (2011) have recently reported data on sperm morphology in Macrolophus pygmaeus and another dicyphine species Nesidiocoris tenuis Reuter, 1895. Macrolophus pygmaeus males were shown to have significantly smaller sperm cells (213.18 µm), with longer (50.94 µm) and wider heads and shorter tails (162.94 µm) than Nesidiocoris tenuis. In our study, the data on Macrolophus pygmaeus sperm cell size were confirmed. On the other hand, we found that Macrolophus costalis males have significantly longer sperm cells, with a longer head and tail compared to Macrolophus melanotoma and Macrolophus pygmaeus. In turn, Macrolophus melanotoma males have significantly longer sperm tails compared to Macrolophus pygmaeus (Table 1).
Conclusion
As mentioned in Introduction, the cryptic species Macrolophus pygmaeus and Macrolophus melanotoma can be differentiated from each other based on the cuticular hydrocarbon profiles and specific molecular primers (Gemeno et al. 2012, Castañé et al. 2013). In our study, we provide some alternative characters, such as karyotype (number and size of chromosomes, sex chromosome system, and amount and distribution of heterochromatin) and sperm cells’ morphology, allowing for reliable identification of Macrolophus pygmaeus, Macrolophus melanotoma and Macrolophus costalis.
Acknowledgments
This study has been funded by the Spanish Ministry of Economy and Competitiveness (MINECO) (Project AGL2011-24349), and by a travel grant from the University of Lleida - Fundació “La Caixa” to Dr Grozeva. The chromosome analysis was performed using microscope Axio Scope A1 – Carl Zeiss Microscopy upgraded by the project WETLANET (FP7 CSA – SUPPORT ACTION, GA 229802). We thank cordially Prof. Dr V. Kuznetsova for the valuable advices to improve the manuscript.
Citation
Jauset AM, Edo-Tena E, Castañé C, Agustí N, Alomar O, Grozeva S (2015) Comparative cytogenetic study of three Macrolophus species (Heteroptera, Miridae). Comparative Cytogenetics 9(4): 613–623. doi: 10.3897/CompCytogen.v9i4.5530
References
- Agustí N, Gabarra R. (2009a) Puesta a punto de una cría masiva del depredador polífago Dicyphus tamaninii Wagner (Heteroptera: Miridae). Boletín de Sanidad Vegetal (PLAGAS) 35: 205–218. [Google Scholar]
- Agustí N, Gabarra R. (2009b) Effect of adult age and insect density of Dicyphus tamaninii Wagner (Heteroptera: Miridae) on progeny. Journal of Pest Science 82(3): 241–246. doi: 10.1007/s10340-009-0245-1 [Google Scholar]
- Alomar O, Goula M, Albajes R. (2002) Colonisation of tomato fields by predatory mirid bugs (Hemiptera: Heteroptera) in northern Spain. Agriculture, Ecosystems and Environment 89: 105–115. doi: 10.1016/S0167-8809(01)00322-X [Google Scholar]
- Alomar O, Riudavets J, Castañe C. (2006) Macrolophus caliginosus in the biological control of Bemisia tabaci on greenhouse melons. Biological Control 36(2): 154–162. doi: 10.1016/j.biocontrol.2005.08.010 [Google Scholar]
- Castañé C, Agustí N, Arnó J, Gabarra R, Riudavets J, Comas J, Alomar O. (2013) Taxonomic identification of Macrolophus pygmaeus and Macrolophus melanotoma based on morphometry and molecular markers. Bulletin of Entomological Research 103(2): 204–215. doi: 10.1017/S0007485312000545 [DOI] [PubMed] [Google Scholar]
- Donlon TA, Mafenis RE. (1983) Methyl green is a substitute for distamycin A in the formation of distamycin A/DAPI C-bands. Human Genetics 65: 144–146. doi: 10.1007/BF00286651 [DOI] [PubMed] [Google Scholar]
- Evans JP, Simmons LW. (2008) The genetic basis of traits regulating sperm competition and polyandry: can selection favour the evolution of good- and sexy-sperm? Genetica 134: 5–19. doi: 10.1007/s10709-007-9162-5 [DOI] [PubMed] [Google Scholar]
- Franco K, Jauset A, Castañé C. (2011) Monogamy and polygamy in two species of mirid bugs: a functional-based approach. Journal of Insect Physiology 57: 307–315. doi: 10.1016/j.jinsphys.2010.11.020 [DOI] [PubMed] [Google Scholar]
- Gemeno C, Laserna N, Riba M, Valls J, Castañé C, Alomar O. (2012) Cuticular hydrocarbons discriminate cryptic Macrolophus species (Hemiptera: Miridae). Bulletin of Entomological Research 102: 624–631. doi: 10.1017/S0007485312000193 [DOI] [PubMed] [Google Scholar]
- Grozeva S. (2003) Karyotype of three endemic Mediterranean Miridae species (Heteroptera) from Bulgaria. Acta Zoologica Bulgarica 10(3): 19–27. [Google Scholar]
- Grozeva S, Nokkala S. (1996) Chromosomes and their meiotic behaviour in two families of the primitive infraorder Dipsocoromorpha (Heteroptera). Hereditas 125: 31–36. doi: 10.1111/j.1601-5223.1996.t01-1-00031.x [Google Scholar]
- Grozeva S, Nokkala S, Simov N. (2006) First evidence of sex chromosomes pre-reduction in male meiosis in the Miridae bugs (Heteroptera). Folia Biologica (Kraków) 54(1-2): 9–12. doi: 10.3409/173491606777919166 [DOI] [PubMed] [Google Scholar]
- Grozeva S, Simov N, Josifov M. (2007) Karyotaxonomy of some European Macrolophus species (Heteroptera, Miridae). Mainzer Naturwissenschaftliches Archiv/Beiheft 31: 81–87. [Google Scholar]
- Grozeva S, Simov N. (2008a) Cytogenetic Studies of Bryocorinae Baerensprung, 1860 true bugs (Heteroptera, Miridae). Acta Zoologica Bulgarica, Suppl 2: 61–70.
- Grozeva S, Simov N. (2008b) Cytotaxonomy of two Cremnocephalus species (Heteroptera, Miridae). In: Grozeva S, Simov N. (Eds) Advances in Heteroptera Research. Festschrift in Honour of 80th Anniversary of Michail Josifov, 171–179.
- Grozeva S, Simov N. (2009) Cytogenetic study of two species of Dryophilocoris: D. luteus and D. flavoquadrimaculatus (Insecta, Heteroptera, Miridae). Genetics and Breeding 38(1): 41–45. [Google Scholar]
- Josifov M. (1992) Zur Taxonomie der paläarktischen Macrolophus-Arten (Insecta, Heteroptera: Miridae). Reichenbachia 29(1): 1–4. [Google Scholar]
- Kuznetsova VG, Westendorff M, Nokkala S. (2001) Patterns of chromosome banding in the sawfly family Tenthredinidae (Hymenoptera, Symphyta). Folia biologica (Kraków) 54(3): 227–233. doi: 10.1080/00087114.2001.10589230 [Google Scholar]
- Kuznetsova VG, Grozeva S, Nokkala S, Nokkala C. (2011) Cytogenetics of the true bug infraorder Cimicomorpha (Hemiptera, Heteroptera): a review. ZooKeys 154: 31–70. doi: 10.3897/zookeys.154.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cascales JI, Cenis JL, Cassis G, Sánchez JA. (2006) Species identity of Macrolophus melanotoma (Costa 1853) and Macrolophus pygmaeus (Rambur 1839) (Insecta: Heteroptera: Miridae) based on morphological and molecular data and bionomic implications. Insect Systematics and Evolution 37: 385–404. doi: 10.1163/187631206788831470 [Google Scholar]
- Messelink GJ, Bloemhard CMJ, Hoogerbrugge H, Van Schelt J, Ingegno BL, Tavella L. (2014) Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. Journal of Applied Entomology 139(5): 333–341. doi: 10.1111/jen.12170 [Google Scholar]
- Nokkala S, Nokkala C. (1986) Achiasmatic male meiosis of collochore type in the heteropteran family Miridae. Hereditas 105: 193–197. doi: 10.1111/j.1601-5223.1986.tb00661.x [Google Scholar]
- Schuh RT. (1995) Plant bugs of the world (Insecta: Heteroptera: Miridae). Systematic Catalog Distributions, Host list, and Bibliography. The New York Entomological Society, xii+1329 pp. [Google Scholar]
- Schweizer D. (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58: 307–324. doi: 10.1007/BF00292840 [DOI] [PubMed] [Google Scholar]
- Ueshima N. (1979) Insecta 6. Hemiptera II. Heteroptera. In: John B. Animal Cytogenetics. Gebruder Borntraeger, Berlin, Stuttgart, 1–118. [Google Scholar]
- van Lenteren JC. (2003) Commercial availability of biological control agents. In: van Lenteren JC. (Ed.) Quality Control and Production of Biological Control Agents, Theory and Testing Procedures. Oxon, UK, CABI Publishing, 167–179. doi: 10.1079/9780851996882.0167 [Google Scholar]