Abstract
In canine visceral leishmaniasis a diffuse chronic inflammatory exudate and an intense parasite load throughout the gastrointestinal tract (GIT) has been previously reported. However, these studies did not allow a properly description of canine cellular morphology details. The aim of our study was to better characterize these cells in carrying out a qualitative and quantitative histological study in the gastrointestinal tract of dogs naturally infected with Leishmania infantum by examining gut tissues embedded in glycol methacrylate. Twelve infected adult dogs were classified in asymptomatic and symptomatic. Five uninfected dogs were used as controls. After necropsy, three samples of each gut segment, including oesophagus, stomach, duodenum, jejunum, ileum, cecum, colon, and rectum were collected and fixed in Carnoy’s solution for glycol methacrylate protocols. Sections were stained with hematoxylin-eosin, toluidine blue borate, and periodic acid-Schiff stain. Leishmania amastigotes were detected by immunohistochemistry employed in both glycol methacrylate and paraffin embedded tissues. The quantitative histological analysis showed higher numbers of plasma cells, lymphocytes and macrophages in lamina propria of all segments of GIT of infected dogs compared with controls. The parasite load was more intense and cecum and colon, independently of the clinical status of these dogs. Importantly, glycol methacrylate embedded tissue stained with toluidine blue borate clearly revealed mast cell morphology, even after mast cell degranulation. Infected dogs showed lower numbers of mast cells in all gut segments than controls. Despite the glycol methacrylate (GMA) protocol requires more attention and care than the conventional paraffin processing, this embedding procedure proved to be especially suitable for the present histological study, where it allowed to preserve and observe cell morphology in fine detail.
Key words: Glycol methacrylate, embedding, light microscopy, canine visceral leishmaniasis, dogs
Introduction
Distribution of cells in the canine gastrointestinal tract (GIT) was first reported in 1969. Leucocyte subgroups and mast cells are variably distributed in distinct regions such as villus and crypts of each GIT segment, stomach through colon.1,2 These authors discussed the importance of both qualitative and quantitative information with respect to the cell population of canine intestinal lamina propria and epithelium, and suggest that, in chronic intestinal infection and disease, these cells populations may be altered in number, morphology, anatomical distribution, and immunological activation. In canine visceral leishmaniasis (CVL) this has not been extensively investigated.3 Visceral leishmaniasis disorders occur in humans (HVL)4-9 and in dogs.10-18 Some works described a chronic inflammatory exudate composed of macrophages, plasma cells, and lymphocytes throughout the lamina propria of the large intestine in a dog naturally infected with L. infantum in Europe.13 However, reports of this GIT involvement in CVL consist of rare individual case studies targeting a specific region of the GIT, and this finding in CVL is little reported15 as a clinical manifestation.16 Recent studies have demonstrated the presence of Leishmania amastigotes in the lamina propria of dog GIT associated with inflammation, demonstrating that CVL affects more than 70% of the GIT of both symptomatic and asymptomatic infected dogs.3,16,19
In common with other reports,11,13,15 we have shown3,16,19 an increased cellularity, predominately mononuclear cells, associated with the intensity of parasitism throughout all segments of GIT mucosa and submucosa. However, these semi-quantitative studies do not describe morphological details of the mononuclear exudate because they employed routine histological techniques using paraffin-embedding process. Some works embedded rat tissues in glycol methacrylate (GMA) resin that has advantages in studies of cell morphology, morphometric analysis, and immunohistochemical studies of semi-thin sections. The reported advantages compared to paraffin include: i) rapid processing; ii) ease of handling; iii) no requirement for high temperatures for infiltration and soluble polymerization assays; iv) ability to obtain semi-thin sections of 0.5 µm; v) less distortion and artifact; and vi) better resolution with light microscopy.20
Thus, considering that GMA improves histological evaluation mainly due to the greater definition of cytological details.
The aim of our study was to carry out a qualitative and quantitative histological study in the gastrointestinal tract of dogs naturally infected with Leishmania infantum by examining gut tissues embedded in glycol methacrylate.
Materials and Methods
Naturally infected animals
Twelve adult mixed-breed dogs of unknown age naturally infected with Leishmania were identified during an epidemiological survey of CVL carried out by the municipality of Ribeirão das Neves, Belo Horizonte metropolitan area, Minas Gerais (MG) State, Brazil. All dogs were IgG positive using IFAT (titers >1:80) and ELISA (Optical Density >100 >1:400 dilutions). Parasitism was assessed through cytology of aspirate of bone marrow and cervical lymph nodes and immunohistochemistry on ear skin. Clinical examinations were carried out on all the infected dogs, which were subsequently divided into two groups: Group I comprised six dogs that exhibited classical signs of CVL, including lymphadenopathy, cutaneous changes (alopecia, exfoliative dermatitis, or ulcers), onychogryphosis, keratoconjunctivitis, cachexia and clinical anemia. Group II comprised six dogs with no clinical symptoms.
Control dogs
Five dogs of unknown age were obtained from the Zoonosis Control Center of the municipality of Carandaí, a non-endemic area for CVL, Minas Gerais State, Brazil. Serological (IFAT, ELISA) and parasitological (cytology of bone marrow and immunohistochemistry of ear skin) examinations were negative for Leishmania infection. All uninfected dogs were euthanized in accordance with the protocols of the Brazilian College of Animal Experimentation (COBEA) and were approved by the Ethics Committee on Animal Experimentation / Federal University of Minas Gerais CETEA / UFMG, protocol 218/2009. This procedure was performed in the Experimental Kennel Department of Parasitology (UFMG).
Ethical considerations
Treatment of animals with CVL is prohibited by the Brazilian Health Ministry and discouraged by Conselho Federal de Medicina Veterinária (CRMV). The Ministry of Health policy requires rapid evaluation based on immunochromatographic tests and association between ELISA for epidemiology. Infected and uninfected dogs were obtained from the Control Zoonosis Center of the Municipality of Ribeirão das Neves, Belo Horizonte Metropolitan area and Carandaí. The study was submitted to and approved by the CETEA/UFMG (Comite de Etica em Experimentação Animal/Universidade Federal de Minas Gerais), protocol 218/2009. All procedures involving animals were conducted according to the guidelines of the Colégio Brasileiro de Experimentação Animal (COBEA). Euthanization was conducted under Ethics Committee approval.
Animals procedures
Dogs were maintained in a period of three months at the Experimental Kennel Department of Parasitology (UFMG). Vaccination (anti-rabies Defensor® and anti-infections, Vanguard®, Pfizery, Brazil), and oral anthelmintic broad spectrum (pyrantel pamoate, praziquantel and febantel, Helfine Cãesy, Agener Union, Brazil) were used in all dogs. Topical products ant parasitic effect (Top Line®, Merial Animal Health) and sandflies repellent (Scalibor®, Intervet, The Netherlands) were used in all dogs. They all received industrialized food where it was distributed in collective kennels, respecting the capacity of five dogs/kennel (dimension 3x5 meters). Water was offered ad libitum. These kennels were cleaned daily with sodium hypochlorite at 1% and sprayed every months with pyrethroids.
Two veterinarians (first and second authors) were responsible for all dog procedures throughout the experiment where it was carried out always inside the kennel.
Anaesthetic and euthanasia protocol
Dogs were anesthetized for the cytological exams, skin biopsies and euthanasia with a combination of 1.0 mg/kg xylazine (2%) (Anesedan®, Vetbrands, Brazil) and 10mg / kg of ketamine hydrochloride (10%) (Ketamine Agener®, Agener Union, Brazil), i.m. Euthanasia was done in according to Conselho Federal de Medicina Veterinária (CFMV), Brazil, following the Resolution No. 1000/2012 of 17 May 2012. After this anaesthetic protocol overdose of fenobarbituric Tiopental (Tiopenthax®,) 2.5% Sodium (1mL/kg) was administrated i.v.
Necropsy, parasitological diagnosis, and fixation
After necropsy, samples of liver, spleen, nose skin, ear skin, bone marrow, and lymph nodes were collected. Other samples of bone marrow and lymph nodes were stained with Giemsa for parasitological diagnosis. Amastigotes of Leishmania were detected in all animals using oil immersion light microscopy (1000 × magnification). In addition, three samples of each GIT segment, including oesophagus, stomach, duodenum, jejunum, ileum, cecum, colon, and rectum were collected and fixed in i) Carnoy’s solution (absolute ethanol, absolute chloroform and acetic acid, 6:3:1) at 4ºC for 1 to 4 h; ii) 10% buffered formalin at 4ºC for 48 h; and iii) absolute acetone at -20ºC for 7 days.
Glycol methacrylate resin embedding
Before embedding in GMA, tissue fragments were cut to appropriate size for use in the KIT Historesin Leyca KIT (Leika Historesin, Embedding Kit, Heidelberg, Germany) as previously described.20 Subsequently, tissues were dehydrated in absolute ethanol in three steps of 30 min each and two baths of xylene at 15 min each. After dehydration, the tissue samples were GMA-embedded using the Historesin Solution Kit. A solution, designated as the infiltration solution (1 packet activator diluted in 50 ml basic resin/liquid) was created to promote the penetration of the resin into the tissue before it solidifies. Samples were kept in the solution for 16 h under constant agitation at 4°C. After 16 h, the infiltration solution was renewed and maintained for 24 h at 4°C under constant agitation. Samples were placed in plastic forms of approximately 2.5 cm. An inclusion solution comprising 15 ml infiltration solution was added to the form along with 1ml hardener. This procedure is carried out on ice, as the reaction is exothermic and rapidly polymerizes at room temperature. The forms were immediately removed from the ice and left at room temperature for 24 h until the solution solidified to create the GMA block. The form contains an upper chamber to which an acrylic dental resin (Jet Classic, Brazil) is added to create a larger unit, which is attached to a stub to hold the GMA block for cutting. Sections of embedded tissue were cut on a Reichert Jung 1140 semi-automatic microtome. To provide 1-2 m sections, a glass knife was used according to previous procedure.20
Histological staining of tissues embedded in GMA
Sections were stained with hematoxylin-eosin, toluidine blue borate, and periodic acid-Schiff stain (PAS). Hematoxylin-eosin staining was conducted as follows: several drops of mordent solution (iron alum 2%) were placed on the section on a slide; after 10 min, the slide was rinsed in running water for 5 min; then, several drops of hematoxylin solution were added and after 15 min, the slide was rinsed in running water for 5 min.; thereafter, several drops of eosin solution were added, after 30 s the slide was rinsed under running water to removed excess stain and excess water was removed by gently pressing the slide with tissue, facing down, over filter paper. Finally, the slide was left drying completely at room temperature and mounted with a coverslip using ERV-Mount (EasyPath, Erviegas, São Paulo, Brazil).
Toluidine staining blue borate was as follows: several drops of toluidine blue-borate were placed on the sections on a slide; after 1 min the slide was rinsed under running water to remove excess stain, then excess water was removed by gently pressing the slide with tissue, facing down, over filter paper. Finally, the slide was left drying completely at room temperature and mounted with a coverslip using ERV-Mount (EasyPath, Erviegas, São Paulo, Brazil).
PAS staining was conducted with the following procedure: several drops of periodic acid solution were placed over sections; after 20 min, the slide was rinsed with distilled water for 5 min; then, several drops of Schiff reagent were placed on the slide and after 60 min the slide was rinsed in three baths of differentiator solution. Thereafter, the slide was rinsed in running water for 30 min, then several drops of hematoxylin were placed on the slide. After 10 min, the slide was rinsed under running water for 30 min and excess water was removed by gently pressing the slide with tissue, facing down, over filter paper. Finally, the slide was left drying completely at room temperature and mounted with a coverslip using ERV-Mount (EasyPath, Erviegas, São Paulo, Brazil).
Immunohistochemical identification of Leishmania amastigotes in tissue samples embedded in GMA and paraffin
The streptavidin peroxidase reaction for immunohistochemical reaction in paraffin embedded tissues was conducted in accordance with our group.21 For identification in GMA embedded tissues, we replaced the second antibody with polyclonal goat anti-dog biotin-conjugated IgG (dilution 1:50), heavy and light chains (Nordic Immunological Laboratories, Netherlands; batch, 5071; code, GAD/IgG(H+ L)/Bio).
Morphometric analysis of Leishmania amastigotes in paraffin embedded tissues
Immunohistochemical reactions in 20 random images (size 1048 x 746) of stomach mucosa, duodenum, jejunum, ileum, cecum, colon, and rectum for identification of Leishmania amastigotes were evaluated through at 40x objective magnification. Analysis of the esophageal submucosa was made using a sequence of algorithm programs, KS400 (Carl Zeiss, Germany). The area of immunohistochemical staining (µm2) of each image was calculated by selecting those pixels with shades of brown, and a binary image was created.2
Morphometric analysis of cells in the lamina propria of the GIT in stained tissue sections
Histological sections of stomach, duodenum, jejunum, ileum, cecum, colon and rectum (oesophagus was excluded since no histological alteration or parasitism was observed), embedded in GMA and stained with H&E and toluidine blue borate, were evaluated by microscopy at 40x objective magnification. Twenty random images (1024 x 768 pixels) of mucosa (lamina propria) of each GIT segment (submucosa of esophagus) were scanned. The image was viewed actual size with KS 400 (Carl Zeiss, Jena, Germany) software. This morphometric analysis was carried out for: i) Epithelial layer area (m2) of all GIT segments (excluding esophagus); ii) plasma cells, lymphocytes, macrophages, granulocytes, mast cells counted semi-automatically viewed on a computer video screen and lamina propria area (μm2) of all GIT segments (excluding oesophagus).
Statistical analysis
According to the nature of the data, the comparison of the three groups was performed using the Kruskal Wallis test for multiple comparisons. The Tukey test was used to analyse the difference between intestinal segments in the same group. For nonparametric data (parasitism in both groups) used the Mann-Whitney test. The analyses were performed using the statistical computer program Prism 5.0, GraphPad Instat, and Minitab 1.4, adopting a significance level of P<0.05.
Results
Macroscopic evaluation
Macroscopic evaluation of the GIT did not reveal significant abnormalities in any dog. In three symptomatic and two asymptomatic dogs, Dipylidium caninum were observed in the small and large intestine but without mucosal alterations such as hyperaemia, oedema, or petechial haemorrhage.
Histopathology
Infected animals showed diffuse chronic inflammatory reaction in the lamina propria and submucosa throughout the GIT, except oesophagus, in comparison to controls. No infections, or associated histological abnormalities, were observed in oesophagus, including by immunohistochemistry. There was a predominance of mononuclear cells and, rarely, polymorphic nuclear cells such as neutrophils and eosinophils. Macrophages exhibiting atypical morphology were observed: large nuclei with loose chromatin and visible cytoplasm parasites sometimes in vacuoles or not. The presence of epithelioid cells and/or giant cells typically associated with a discrete mononuclear infiltrate was observed in both asymptomatic and symptomatic groups. Microscopic examination revealed parasitism in at least one segment of GIT of all infected animals. Despite the inflammatory reaction no large lesions such as erosions or ulcers in the intestinal mucosa were observed (Figure 1). Naturally infected animals showed the presence of Leishmania amastigotes, particularly within macrophages in the lamina propria of the GIT segments, regardless of clinical manifestations.
Figure 1.
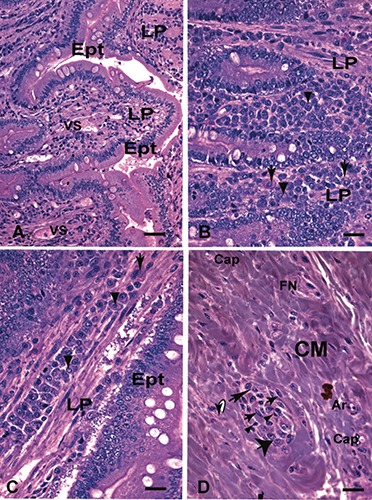
Small intestine (duodenum) embedding in glycol methacrylate (GMA) of dog naturally infected with Leishmania (L. infantum) symptomatic. A) Panoramic intact glandular epithelium; scale bar: 32 µm. B-D) Details of panel A showing the presence of mononuclear cells distributed diffusely in the lamina propria; in panel B note the presence of plasma cells with peculiar morphology: some with acidophilic cytoplasm characterized in hyaline degeneration cells (Russel’s corpuscle) (arrows) and the other with the formation of intracytoplasmic vacuoles that histologically suggest the expansion of the Golgi complex (arrowheads); in panel C (centre) observe plasma cells with basophilic cytoplasm arranged in a row in the lamina propria; there are also plasma cells in hyaline degeneration (Russel corpuscle) (arrow) and the other with the formation of intracytoplasmic vacuoles (arrowheads) in (D) formation of a granuloma in the muscular layer composed of epithelioid cells (thick arrows), macrophages (thin arrow), plasma cells (large arrow) and small lymphocytes (arrowheads); Hematoxylin -eosin (H&E); scale bars: 16 µm. Ept, epithelium; LP, lamina propria; MM, muscular mucosal; CM, muscular layer; Cap, capillary; Air, arteriole; FN, bundle nervous; VS, blood vessel.
Morphometric analysis of parasitism in paraffin-embedded tissue
In the symptomatic group, the segment exhibiting the highest parasite load was the cecum, followed by the duodenum, colon, stomach, rectum, and ileum. In the asymptomatic group, a higher parasite load was found in duodenum and cecum of a single dog (defined as CL46), which showed conspicuous infection in the duodenum mucosa and submucosa layers (Figure 2). Colon, rectum, stomach, and ileum also showed positive immunolabelling in both infected groups (amastigotes of Leishmania were not observed in the oesophagus or jejunum).
Figure 2.
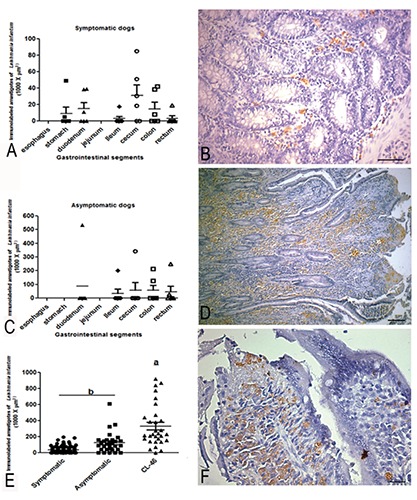
Parasite load of gastrointestinal tract (GIT) of dogs naturally infected with Leishmania (L.) infantum. A) Graph showing parasite load throughout oesophagus to rectum; in the symptomatic group, the segment of highest parasite load was the cecum, followed by the duodenum, colon, stomach, rectum, and ileum (statistical difference among groups (P≤0.001). B) Panoramic view of TGI mucosae (lower magnification) showing a presence of Leishmania amastigotes nearby muscular layer of mucosae; scale bar: 32 µm. C) Graph showing higher parasite load in duodenum and cecum in asymptomatic groups (P≤0-001). D) Higher magnification of mucosae (lamina propria) showing numerous amastigotes within macrophages brown colour highlighted by blue background colour. E) Graph showing a comparison between symptomatic, asymptomatic and CL46 dog case (P≤0.001). F) Besides a high presence of parasites, note the integrity of the epithelium lining with integrity of the intestinal glands, without ulceration or necrosis; scale bar: 16 µm. Immunohistochemistry staining by streptavidin peroxidase method.
Immunohistochemistry of tissue embedded in GMA compared to paraffin-embedded tissue
Detection of amastigotes in tissue embedded in GMA was unsatisfactory.
Immunolabeled amastigotes showed low colour intensity compared to the paraffin-embedded tissues. In GMA-embedded tissue it was common to observe both labelled and unlabelled Leishmania amastigotes in the same field under high magnification (Figure 3).
Figure 3.
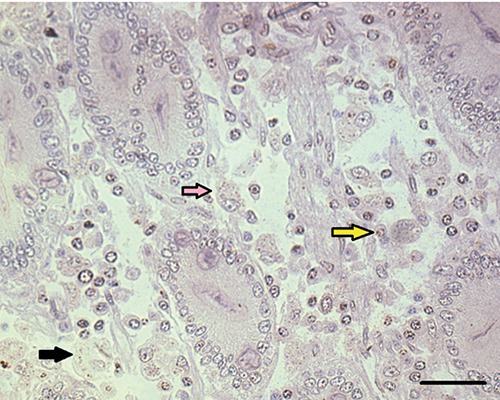
Small intestine of dog naturally infected with Leishmania infantum. A) Observe positive immunolabeled amastigotes (black arrows) in the lamina propria of the duodenum of dog naturally infected with Leishmania infantum; scale bar: 16 μm Note positive immunolabeled amastigotes forms of Leishmania (black arrows) and nnon-specific labelling of a nucleus (yellow arrow) and unlabelled amastigotes (pink arrow). Immunohistochemistry Streptoavidin-biotin reaction method.
Tissue embedded in GMA compared to tissue embedded in paraffin using haematoxylin-eosin (H&E), toluidine blue borate, and periodic acid Schiff (PAS) stain. GMA-embedded tissue showed greater morphological detail with all employed stains compared to the same tissue embedded in paraffin (Figure 4). Epithelial cells, stromal cells, and inflammatory cells such as plasma cells, lymphocytes and lymphoblasts, macrophages, granulocytes (neutrophils or eosinophils), and mast cells were observed. Morphological aspects of cytoplasm limits of cells and amastigotes of Leishmania were preserved.
Figure 4.
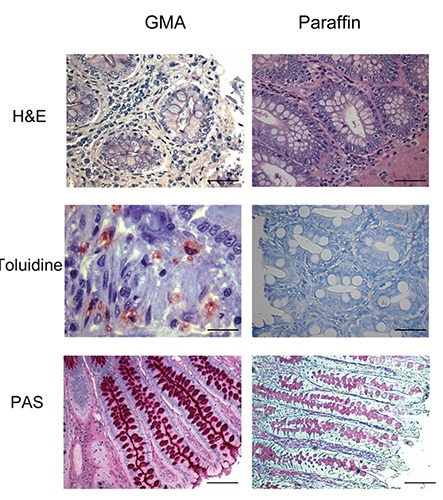
Large intestine sections of dogs naturally infected with Leishmania infantum. Compare distinct staining’s as Hematoxylin-Eosin (H&E), Toluidine Blue and Periodic Acid Schifft (PAS) in tissue samples embedding in glycol methacrylate (GMA) and paraffin. Scale bars: H&E) 16 µm; Toluidine Blue) 8 and 16 µm; PAS) 16 µm.
Best results were obtained using Carnoy’s fixative, but it requires that tissue fragments be held for a maximum of 4 h at 4°C. The GMA protocol is labour-intensive.
Morphometric analysis of inflammatory cells in GIT embedded in GMA
A higher cellularity in the lamina propria was observable in infected dogs in both paraffin- and GMA-embedded GIT segments. However, GMA provided better visualization of cell types and distribution. The lamina propria comprised more than 90% mononuclear cells consisting of plasma cells, lymphocytes, and macrophages.
As predicted by our light microscopy observation, the morphometric analysis confirmed the predominance of plasma cells in lamina propria of all segments of GIT in all dogs. However, in infected dogs, regardless of clinical manifestations, the number of plasma cells was always higher than in controls (plasmocytosis) and was significantly higher in stomach, duodenum, ileum, cecum, and rectum (Figure 5). Plasma cells in infected dogs showed distinct characteristics: displaced nuclei, acidophilic cytoplasm, and sometimes vacuolated or with protein corpuscles (Russell body). Lymphocyte occurrence was also higher in infected than in uninfected animals and observed in large numbers, mainly in the large intestine associated with parasitism (Figure 6). Macrophages were observed in the lamina propria and were higher in infected than in uninfected dogs. It was possible to identify parasitized macrophages containing cytoplasmic vacuoles of Leishmania amastigotes in segments of small and large intestine (Figure 7). There was no difference in the number of polymorphonuclear cells among groups. A lower number of polymorphonuclear cells were seen compared to other inflammatory cells.
Figure 5.
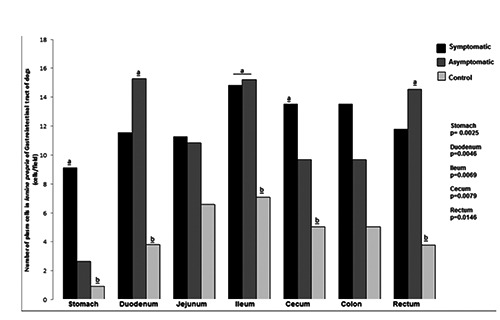
Number of plasma cells in the lamina propria of gastrointestinal tract of dogs naturally infected with Leishmania infantum and controls. Higher number of plasma cells in the lamina propria of GIT of infected dogs than controls in stomach, duodenum, ileum, cecum and rectum segments.
Figure 6.
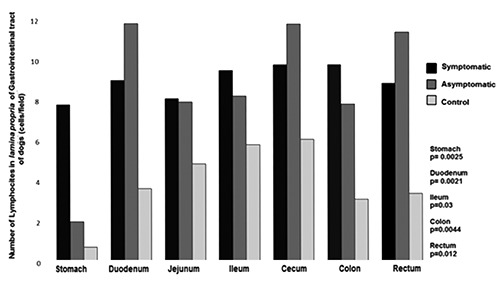
Number of lymphocytes in the lamina propria of gastrointestinal tract of dogs naturally infected with Leishmania infantum and controls. Higher number of lymphocyte in infected dogs than controls in stomach, duodenum, ileum, colon and rectum.
Figure 7.
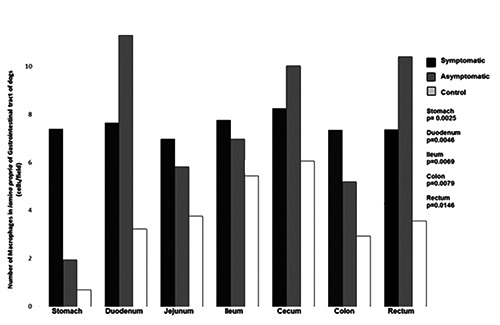
Number of macrophages in the lamina propria of gastrointestinal tract of dogs naturally infected with Leishmania infantum and controls. Higher number of macrophages in infected dogs than controls in stomach, duodenum, ileum, colon and rectum.
The number of mast cells in non-infected dogs was higher than in infected dogs. Diffuse mast cells filled with granules were distributed throughout the lamina propria of uninfected animals, permeating the tissue beneath the epithelium (sub-epithelial mast cells). The number of mast cells was lower in infected animals, and sometimes showed reduced granulation (Figures 8 and 9).
Figure 8.
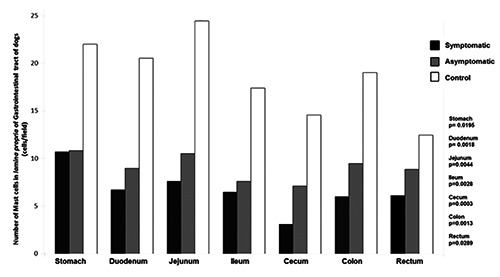
Number of mast cells in the lamina propria of gastrointestinal tract of dogs naturally infected with Leishmania infantum and controls. Lower number of mast cells in infected dogs than controls in all gastrointestinal tract segments.
Figure 9.
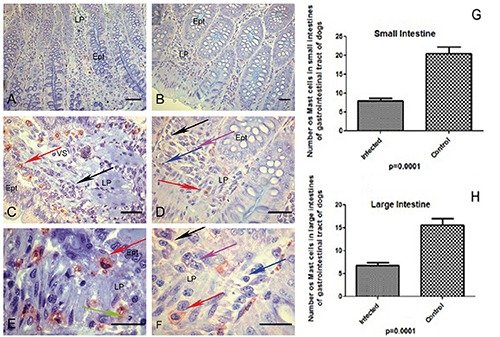
A-H) Canine tissues included in glycol methacrylate (GMA) resin of dogs naturally infected with Leishmania infantum and controls. A,B) Photomicrograph of infected dog with Leishmania (L.) infantum of ileum segment; in the lamina propria observe metachromatic mast cells (red) and epithelial cells preserved in blue background (blue toluidine); scale bars: 32 µm. C) Higher magnification showing stained metachromatic mast cells (red arrow) and plasma cells in the lamina propria (black arrow); scale bars: 16 µm. D,E,F) Observe distinct mononuclear inflammatory cells: plasma cells (black arrow), macrophages (pink arrow), lymphocytes (blue arrow) and mast cells (red arrow); rare neutrophils could be seen in lamina propria (green arrows); scale bars: D) 16 µm; E,F) 8 µm. G) Graph showing a lower number of mast cells in small intestine (duodenum, jejunum and ileum) of all infected dogs (P≤0.0001). H) Graph showing a lower number of mast cells in large intestine (caecum, colon and rectum) of all infected dogs groups (P≤0.0001).
A homogenous pattern of the epithelia throughout stomach to rectum in control dogs was observed. Atrophy of the epithelial layers was observed in symptomatic infected animals. We have depicted these analyses of the area of epithelia (m2) considering all the segments of GIT together (Figure 9).
Discussion
Macroscopic observations of CVL in the GIT have been reported by many authors to include hyperaemic areas of erosion of the mucosa, thickening of the wall of segments including colon and rectum, oedema and haemorrhage.3,12,13,15,23 Also, gastric haemorrhage was described in a jackal Canis aureus with visceral leishmaniasis.14 In the present study, no gross anatomical lesions were found in any dog. However, qualitative and quantitative microscopic analysis revealed increasing cellularity of the lamina propria and submucosa of GIT segments, except oesophagus, of all infected dogs. The lamina propria, the intermediate layer of the mucosa of the GIT, has both structural and immunological functions. It rests upon the muscularis mucosa, surrounds the crypts, and extends upward as the core of the intestinal villus. The most conspicuous feature of the lamina propria is its abundant immune and migratory cell component.24 In humans24,25 and dogs1,3,26,27 five types of immune or inflammatory cells normally occur in the lamina propria: plasma cells, lymphocytes, eosinophils, macrophages, and mast cells. Neutrophils are not usually present in the lamina propria or anywhere in the small intestine wall, with the exception of those confined to vascular lumina.15
In our study, increased cellularity, especially plasma cells (plasmocytosis), was observed in the GIT of infected dogs in both paraffin and GMA embedded tissue. Plasma cells appeared with abundant pink cytoplasm and a characteristic hypercromatic peripheral nucleus. Many plasma cells showed small intracytoplasmic vacuoles, suggestive of hypertrophy of the Golgi complex, or homogeneous eosinophilic inclusions called Russell bodies. Plasmocytosis is a classical pathology described in visceral leishmaniasis involving various organs as well as spleen, lymph node, and bone marrow in dogs both naturally and experimentally infected with L. infantum in Europe10,11,15 and in Brazil.28,29 Under normal conditions, lymphocytes of the lamina propria are small, showing dense nuclei with pale cytoplasm, and often forming dense infiltrates adjacent to the muscularis mucosae. In infected dogs, lymphocytes were observed in higher numbers and larger size, with a higher nucleus to cytoplasm ratio (lymphoblast). Macrophages of atypical morphology with intracytoplasmic vacuoles containing Leishmania amastigotes were frequently found, independent of animal clinical status. Epithelioid cells (macrophages increased in size, pale cytoplasm, and vesicular nucleus with loose chromatin) and/or foreign body giant cells (fusion of macrophages and young monocytes) usually associated with mononuclear infiltrate were also observed as previously reported.3,11
In the present study, the presence of polymorphonuclear cells such as neutrophils and/or eosinophils was rare. These cells can be considered accessory cells, although with sporadic granulomas, but were not prominent in the lamina propria of any GIT segment. This result is in agreement with other works that observed eosinophils associated with a predominant infiltrate of mononuclear cells in the intestinal mucosa.15 In Greece, authors described pyogranulomatous formation as the most common histological feature (90%) in the colonic mucosa observed by biopsy in CVL.23 However, in the present study, granulomas composed of plasma cells, macrophages (epithelioid cells), and small lymphocytes, but not pyogranulomas, were found in 16% of cases. Our results are in agreement with those of other studies.3,15,27,30
In six beagles experimentally infected with L. infantum, ulcerative colitis with atrophic epithelial cells and intense hydropic degenerative lesions in GIT were observed.12 Herein, we found atrophic epithelial cells in colon of symptomatic dogs. We also observed decreasing epithelial mucosal area throughout GIT of symptomatic dogs. These results associated with the chronic inflammatory GIT response might suggest that it could be related the weight loss, a classical clinical sign of CVL.18 GMA-embedded tissue stained with toluidine blue borate clearly revealed mast cell morphology. We easily identified mast cells diffusely distributed in the lamina propria of mucosa and submucosa of GIT of all dogs. Infected dogs showed lower numbers of mast cells in all GIT segments than did controls. The reduced numbers may have been due to mast cell degranulation, as detected under microscopy with the GMA protocol. GMA provided preservation of cytoplasmic boundaries even after mast cell degranulation. There are few works related to mast cells in GIT of dogs with visceral leishmaniasis.31,32,33 However, some works with naturally infected dogs in Brazil, observed lower numbers of mast cells in the ear skin of infected dogs than in controls.34,35 The role of these cells in visceral leishmaniasis is not clear.36
Our observations of GIT parasite load in CVL were similar to results of other research. Studies in Europe and in Brazil have shown parasitism in large intestine in asymptomatic or symptomatic dogs.3,13,15,30,34 We observed that large intestine was predominantly affected, with the exception of a single dog with an intense parasite load in duodenum, previously reported as unusual pathology.16 Some works use immunohistochemistry on paraffin sections of TGI in healthy dogs, identified a panel of leucocytes (Lymphocytes T CD4+; CD8+, CD3, CD5, B cells) and macrophages (antigen L1).1,2 Authors also used conventional histology to investigate neutrophils, eosinophils, and mast cells. Despite the GMA protocol requires more attention and care when compared to conventional histological processing, especially considering the stage of the inclusion process at the ideal temperature of the plastic resin, we can say that GMA is surely more advantageous, as it allows the identification of cells only by histological morphology.1,2 In CVL intestinal disorders, the chronic infiltrate consists of plasma cells, lymphocytes, and macrophages, independent of clinical manifestation. The GMA protocol proved to be more suitable in histological studies that require observation of cells in fine detail.
Figure 10.
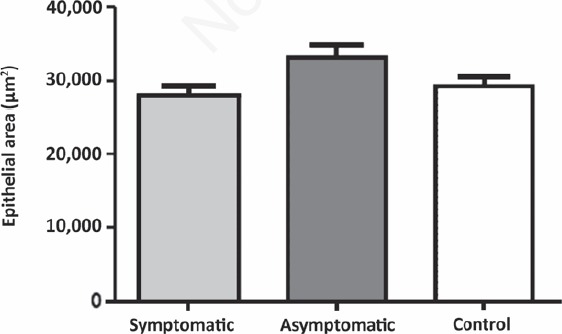
Epithelial area of gastrointestinal tract of dogs naturally infected with Leishmania infantum and controls. The epithelial area is lower in symptomatic group than asymptomatic and control groups (P=0.0238).
Funding Statement
Funding: this research was supported by grants Conselho Nacional de Desenvolvimento da Pesquisa Tecnológica e Científica (CNPq 473601/2009-5; 474665/2012-7; 303022/2013-2); Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG/APQ-01378-12); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Edital CAPES/PNPD 2011 (Processo 2258/2011) and Pró-reitoria de Pesquisa da Universidade Federal de Minas Gerais, Brazil. The authors would like to thank the Control Zoonosis Center of the Municipality of Ribeirão das Neves, Belo Horizonte Metropolitan area, Minas Gerais, Brazil.
References
- 1.German AJ, Hall EJ, Moore PF, Ringler DJ, Newman W, et al. The distribution of lymphocytes expressing alphabeta and gammadelta T-cell receptors, and the expression of mucosal addressin cell adhesion molecule-1 in the canine intestine. J Comp Pathol 1999;121:249-63. [DOI] [PubMed] [Google Scholar]
- 2.Kleinschmidt S, Meneses F, Nolte I, Hewicker-Trautwein M. Distribution of mast cell subtypes and immune cell populations in canine intestines: evidence for age-related decline in T cells and macrophages and increase of IgA-positive plasma cells. Res Vet Sci 2008;84:41-8. [DOI] [PubMed] [Google Scholar]
- 3.Pinto AJ, Figueiredo MM, Silva FL, Martins T, Michalick MS, et al. Histopathological and parasitological study of the gastrointestinal tract of dogs naturally infected with Leishmania infantum. Acta Vet Scand 2001;53:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal PJ, Chaisson RE, Hadley WK, Leech JH. Rectal leishmaniasis in a patient with acquired immunodeficiency syndrome. Am J Med 1988;84:307-9. [DOI] [PubMed] [Google Scholar]
- 5.Laguna F, Garcia-Samaniego J, Soriano V, Valencia E, Redondo C, et al. Gastrointestinal leishmaniasis in human immunodeficiency virus-infected patients: report of five cases and review. Clin Infect Dis 1994;19:48-53. [DOI] [PubMed] [Google Scholar]
- 6.Laguna F, Soriano V, Valencia E, Gonzalez-Lahoz JM. [Gastrointestinal leishmaniasis in patients infected with the human immunodeficiency virus]. [Article in Spansih]. Rev Clin Esp 1994;194:510-1. [PubMed] [Google Scholar]
- 7.Villanueva JL, Torre-Cisneros J, Jurado R, Villar A, Montero M, et al. Leishmania esophagitis in an AIDS patient: an unusual form of visceral leishmaniasis. Am J Gastroenterol 1994;89:273-5. [PubMed] [Google Scholar]
- 8.Alonso MJ, Munoz E, Picazo A, Abad MM, Gomez F, et al. Duodenal leishmaniasis diagnosed by biopsy in two HIV-positive patients. Pathology, research and practice 1997;193:43-7; discussion 49-50. [DOI] [PubMed] [Google Scholar]
- 9.Hamour AA, Skelly R, Jowitt SN, Wilson GE, Curry A, et al. Visceral leishmaniasis (Kala-azar) in two patients with HIV-1 infection: atypical features and response to therapy. J Infect 1998;36:217-20. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DC, Buckner RG, Glenn BL, MacVean DW. Endemic canine leishmaniasis. Vet Pathol 1980;7:94-6. [DOI] [PubMed] [Google Scholar]
- 11.Keenan CM, Hendricks LD, Lightner L, Johnson AJ. Visceral leishmaniasis in the German shepherd dog. II. Pathology. Vet Pathol 1984;21:80-6. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez JL, Fermin ML, Garcia P, Rollan E, Castano M. Erosive colitis in experimental canine Leishmaniasis. Zentralbl Veterinarmed B 1990;37:377-82. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer L, Juanola B, Ramos JA, Ramis A. Chronic colitis due to Leishmania infection in two dogs. Vet Pathol 1991:28:342-3. [DOI] [PubMed] [Google Scholar]
- 14.Hervás J, Mendez A, Carrasco L, Gomez-Villamandos JC. Pathological study of visceral leishmaniasis in a jackal (Canis aureus). Vet Rec 1996;139:293-5. [DOI] [PubMed] [Google Scholar]
- 15.Toplu N, Aydogan A. An immunohistochemical study in cases with usual and unusual clinicopathological findings of canine visceral leishmaniosis. Parasitol Res 2011;109:1051-7. [DOI] [PubMed] [Google Scholar]
- 16.Pinto AJW, Figueiredo MM, Ferreira RA, Caliari MV, Tafuri WL. Unsual Small Intestine inflamatory Lesions in dogs with visceral leishmaniasis. Braz J Vet Pathol 2013;6:19-25. [Google Scholar]
- 17.Viegas C, Requicha J, Albuquerque C, Sargo T, Machado J, Dias I, et al. Tongue nodules in canine leishmaniasis - a case report. Parasite Vectors 2012;15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi G, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 2011;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueiredo MM, Amorim IF, Pinto AJ, Barbosa VS, Pinheiro Lde J, et al. Expression of Toll-like receptors 2 and 9 in cells of dog jejunum and colon naturally infected with Leishmania infantum. BMC Immunol 2013;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiarini-Garcia H, Parreira GG, Almeida FR. Glycol methacrylate embedding for improved morphological, morphometrical, and immunohistochemical investigations under light microscopy: testes as a model. Methods Mol Biol 2011;689:3-18. [DOI] [PubMed] [Google Scholar]
- 21.Tafuri WL, Santos RL, Arantes RM, Goncalves R, de Melo MN, et al. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J Immunol Methods 2004;292:17-23. [DOI] [PubMed] [Google Scholar]
- 22.Lima WG, Oliveira PS, Caliari MV, Goncalves R, Michalick MS, et al. Histopathological and immunohistochemical study of type 3 complement receptors (CD11b/CD18) in livers and spleens of asymptomatic and symptomatic dogs naturally infected with Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol 2007;117:129-36. [DOI] [PubMed] [Google Scholar]
- 23.Adamama-Moraitou KK, Rallis TS, Koytinas AF, Tontis D, Plevraki K, et al. Asymptomatic colitis in naturally infected dogs with Leishmania infantum: a prospective study. Am J Trop Med Hyg 2007;76:53-7. [PubMed] [Google Scholar]
- 24.Platt AM, Mowat AM. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol Lett 2008;119:22-31. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology 2011;140:1768-75. [DOI] [PubMed] [Google Scholar]
- 26.Stonehewer J, Simpson JW, Else RW, Macintyre N. Evaluation of B and T lymphocytes and plasma cells in colonic mucosa from healthy dogs and from dogs with inflammatory bowel disease. Res Vet Sci 1998;65:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueiredo MM, Barbosa VS, Amorim IFG, Deotti B, Michalick MS, Faria AMC, et al. Obtaining cells from colon of dog with leishmaniasis for flow cytometric analysis. Nature Protocols Exchange 2011;273:2275. [Google Scholar]
- 28.Tafuri WL, Tafuri WL, Barbosa AJ, Michalick MS, Genaro O, et al. Histopathology and immunocytochemical study of type 3 and type 4 complement receptors in the liver and spleen of dogs naturally and experimentally infected with Leishmania (Leishmania) chagasi. Rev Inst Med Trop Sao Paulo 1996;38:81-9. [DOI] [PubMed] [Google Scholar]
- 29.Tafuri WL, de Oliveira MR, Melo MN, Tafuri WL. Canine visceral leishmaniosis: a remarkable histopathological picture of one case reported from Brazil. Vet Parasitol 2001;96:203-12. [DOI] [PubMed] [Google Scholar]
- 30.Silva FL, Tafuri WL, Oliveira MR, Tafuri WL. Histopathological and immunohistochemical study of the gastrointestinal tract from a dog naturally infected with Leishmania (Leishmania) chagasi: a case report. Arq Bras Med Vet Zootec 2002;54:340-4. [Google Scholar]
- 31.Kube P, Audige L, Kuther K, Welle M. Distribution, density and heterogeneity of canine mast cells and influence of fixation techniques. Histochem Cell Biol 1998;110:129-35. [DOI] [PubMed] [Google Scholar]
- 32.Locher C, Tipold A, Welle M, Busato A, Zurbriggen A, et al. Quantitative assessment of mast cells and expression of IgE protein and mRNA for IgE and interleukin 4 in the gastrointestinal tract of healthy dogs and dogs with inflammatory bowel disease. Am J Vet Res 2001;62:211-6. [DOI] [PubMed] [Google Scholar]
- 33.Noviana D, Mamba K, Makimura S, Horii Y. Distribution, histochemical and enzyme histochemical characterization of mast cells in dogs. J Mol Histol 2004;35:123-32. [DOI] [PubMed] [Google Scholar]
- 34.Figueiredo MM, Moura EP, Costa MM, Ribeiro VM, Michalick MS, et al. Histopathological and parasitological investigations of ear healthy skin of dogs naturally and experimentally infected with Leishmania (Leishmania) chagasi. Histol Histopathol 2010;25:877-87. [DOI] [PubMed] [Google Scholar]
- 35.Menezes-Souza D, Guerra-Sa R, Carneiro CM, Vitoriano-Souza J, Giunchetti RC, et al. Higher expression of CCL2, CCL4, CCL5, CCL21, and CXCL8 chemokines in the skin associated with parasite density in canine visceral leishmaniasis. PLoS Negl Trop Dis 2012;6:e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calabrese KS, Cortada VM, Dorval ME, Souza Lima MA, Oshiro ET, et al. Leishmania (Leishmania) infantum/chagasi: Histopathological aspects of the skin in naturally infected dogs in two endemic areas. Exp Parasitol 2010;124:253-7. [DOI] [PubMed] [Google Scholar]


