Abstract
Toll-like receptors (TLR) are essential for the innate immune response against invading pathogens and have been described in immunocompetent cells of areas affected by periapical disease. Besides initiating the inflammatory response, they also directly regulate epithelial cell proliferation and survival in a variety of settings. This study evaluates the in situ expression of TLR4 in periapical granulomas (PG) and radicular cysts, focusing on the epithelial compartment.
Twenty-one periapical cysts (PC) and 10 PG were analyzed; 7 dentigerous non-inflamed follicular cyst (DC) served as control. TLR4 expression was assessed by immunohistochemistry. TLR4 immunoreaction products were detected in the epithelium of all specimens, with a higher percentage of immunostained cells in PG. Although TLR4 overexpression was detected in both PG and PC, there were differences that seemed to be related to the nature of the lesion, since in PG all epithelial cells of strands, islands and trabeculae were strongly immunoreactive for TLR4, whereas in PC only some areas of the basal and suprabasal epithelial layers were immunostained. This staining pattern is consistent with the action of TLR4: in PG it could promote formation of epithelial cell rests of Malassez and in epithelial strands and islands the enhancement of cell survival, proliferation and migration, whereas in PC TLR4 could protect the lining epithelium from extensive apoptosis. These findings go some way towards answering the intriguing question of why many epithelial strands or islands in PG and the lining epithelium of apical cysts regress after non-surgical endodontic therapy, and suggest that TLR4 plays a key role in the pathobiology of the inflammatory process related to periapical disease.
Key words: TLR4, periapical inflammatory granulomas, radicular cysts
Introduction
Periapical granuloma (PG) and periapical cyst (PC) are the most common inflammatory jaw lesions. They are usually initiated by the mixed microflora of infected root canals, which includes Gram-positive and Gram-negative bacteria.1 The invasion of bacteria or bacterial toxins into the periapical region involves initially non-specific inflammatory reactions and is followed by specific inflammatory reactions.2
In a non-specific inflammatory reaction, the innate immune system recognizes highly conserved pathogen-associated molecular patterns (PAMP) from bacteria through a variety of pattern recognition receptors (PRR); recognition triggers the inflammatory reaction.3 Therefore, PRR such as toll-like receptors (TLR) are essential for the innate immune response. TLR are type I transmembrane proteins that play a critical role in the early innate immune response by sensing microorganism;2 13 distinct TLR have been identified, 10 of which are characterized in humans. Representative bacterial PAMP are lipopolysaccharides (LPS) from Gram-negative bacteria, lipotheichoic acid from Gram-positive bacteria and peptidoglycan (PGN) from either Gram-positive or Gram-negative bacteria.4 In humans, PAMP recognition by TLR activates the inflammatory reaction, including up-regulation in immunocompetent cells of the genes encoding inflammatory cytokines.5-7
Previous investigations have addressed TLR expression in periodontal8 and periapical disease.5, 9-11 In the latter, TLR2 and TLR4 involvement has been demonstrated at the onset of the experimentally induced furcation lesions of endodontic origin.11 Furthermore, TLR2 and TLR4 up-regulation has been described in PG and PC from patients,5,9 demonstrating that their expression is associated with reactive periapical inflammation. Yet, where periapical disease is concerned, such investigations have largely focused on TLR expression in immunocompetent cells, whereas their association with microbial pathogens and their expression in the epithelium of inflammatory periapical lesions (PL) have been neglected.TLR are also expressed in epithelial cells of other tissues including tumor cells.12-14 Thus, even though inflammatory periapical disease is likely to be sustained by the immune system via reaction to bacterial antigens through TLR, TLR expression in the epithelium has not yet been reported. Further investigation is thus required to elucidate some aspects concerning the proliferation of epithelial cell rests and the formation of apical cysts. Accordingly, TLR4 expression was examined by immunohistochemistry in PG with proliferating epithelium and in radicular cysts with emphasis on the epithelial compartment.
Materials and Methods
Study design and tissue samples
This cross-sectional study was performed using tissue blocks from surgical samples of inflammatory radicular cysts and PG archived in 2001-2012.15-18 Tissue blocks and data regarding patients and samples were obtained from the Department of Dentistry, Federal University of Bahia, Brazil. PL were diagnosed based on clinical and histopathological findings, as previously described.9 Inclusion criteria were sufficient material for immunohistochemistry and presence of epithelial cell rests of Malassez (ERM) in PG. Exclusion criteria were evidence of previous endodontic treatment, refractory endodontic disease and specimens with sinus tract.9 To choose specimens meeting the inclusion and exclusion criteria, a serial section per block was stained with hematoxylin-eosin and microscopically evaluated. The study sample therefore consisted of 21 PC cysts and 10 PG from 31 patients (18 males and 13 females). The mean age of patients was 49.6±8.4 (range 35-63). Dentigerous non-inflamed follicular cyst (DC, n=7) served as control. The study was approved under Ethical Approval number 646.081 by the School of Dentistry, Federal University of Bahia, Brazil.
Histological staining and immunohistochemical reactions
Four-micron serial sections from each case were cut from formalin-fixed, paraffin-embedded blocks of representative PG and PC areas. One section per tissue block was stained with hematoxylin-eosin to select specimens. The other sections were mounted on poly-L-lysinecoated glass slides to be further processed for immunohistochemical reaction. TLR4 antibody (H-80; sc-10741) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); the dilution was established based on negative and positive controls (1/40 for TLR4). Immunohistochemistry was performed with a Benchmark® XT autostainer (Ventana Medical Systems, Tucson, AZ, USA). Deparaffinization was done in xylene and rehydration with graded alcohol series. The standard linked streptavidin-biotin horseradish peroxidase technique (LSAB-HRP) with the iView DAB Detection Kit (Ventana Medical Systems Tucson, AZ, USA) was used to detect staining. Sections were counterstained with type-II-Gill’s hematoxylin, dehydrated with ethanol and permanently coverslipped. Positive controls for immunohistochemistry consisted of tissue sections of oral squamous cell carcinoma. A negative control was obtained in all cases by substituting the primary antibody with normal mouse serum.
Evaluation of immunohistochemistry
Slides were examined under a light microscope (Olympus Cx31; Tokyo, Japan) at 400x magnification. Immunoreactivity was assessed by two expert pathologists, who were blinded to the clinical-pathological data, and scored by a semi-quantitative scale (extent score, ES). Cases were assigned to one of 4 categories: 0 (0 to <5% immunostained cells), 1 (6% to <40% immunostained cells, 2 (41% to <70% immunostained cells) or 3 (>71% immunostained cells). The percentage of positive cells was determined from the analysis of 100 cells in 10 random areas at 40x magnification. For each patient the highest ES was selected for further statistical analysis.
Statistical analysis
Means and standard deviations were calculated for ES in each sample (Table 1). Data were analyzed using the Kruskal-Wallis test, to compare ES in the epithelial linings of PG, PC and DC. Statistical analysis was conducted using SPSS (SPSS® release 16.0, Chicago, IL, USA).
Table 1.
Clinical-pathological data of the specimens, with mean and standard deviations of the extent score. Differences among groups were significant; extent score was significantly greater in periapical cysts and granulomas than in follicular cysts (0.001;<P<0.01); extent score of periapical granulomas was significantly greater (0.01;<P<0.05) than that of periapical cysts.
| Periapical lesions | ES |
|---|---|
| Periapical granulomas | 2.7±0.4 |
| Periapical granulomas | 1.8±0.6 |
| Periapical granulomas | 0.28±0.4 |
ES, Extent score; PC, periapical cysts; DC, follicular cysts.
Results
The study sample consisted of 10 PG and 21 PC. PG were characterized by a cell-rich granulation tissue and ERM, which formed strands or islands of epithelium. PC exhibited fully developed cavities lined by stratified squamous epithelium with variable thickness and fibrous connective tissue walls. In all specimens inflammatory cell infiltrate density ranged from moderate to intense.
TLR4 immunoreaction products were detected in the epithelium of all specimens, though with different percentages of immunostained cells. TLR4 immunolabeling in PG was seen in nearly all epithelial cells forming strands and islands, whereas in PC it was mostly in basal and parabasal layers of the cystic epithelial lining, but not all cells were immunolabeled; indeed, areas showing immunostained cells interspersed with groups of unstained cells were frequently detected. The ES was highest in PG (2.7±0.4); in PC it was 1.8±0.6 (Figure 1). Very few cells in control samples showed immunostaining (ES, 0.28±0.4 for DC). Differences among groups were significant; the ES of PC and PG were significantly greater than the one of DC (0.001<P<0.01). The ES of PG was significantly greater than that of PC (0.01<P<0.05). TLR4 was always localized in the cytoplasm and cell membrane.
Figure 1.
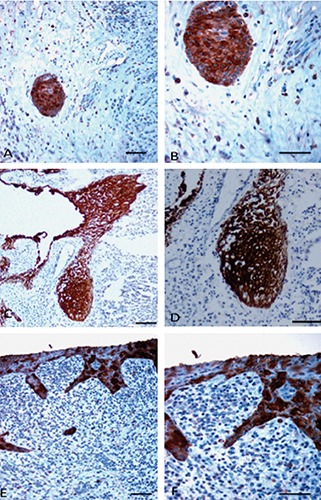
Toll-like receptor 4 (TLR4) immunoreactivity (brown reaction product) in periapical lesions. A,B) Islands of epithelial cell rests of Malassez. C,D) Strands of epithelial cell rests of Malassez. E,F) Epithelial lining of a radicular cyst; note the strong TLR4 expression in epithelial cell rests of Malassez (A-D). TLR4 expression is sparse but strong in radicular cyst epithelium (E,F). Scale bars: 50 µm.
Discussion
TLR are transmembrane proteins expressed on sentinel cells of the immune system such as macrophages and dendritic cells.19 They are also found in non-immune cells such as keratinocytes of skin and oral mucosa and gastrointestinal and female reproductive tract lining.20-23 On these lining epithelia TLR act as pathogen sensors. Recently, it has become increasingly apparent that TLR also recognize damage/danger-associated molecular patterns (DAMP), endogenous molecules released from damaged and dying cells.12 These events can trigger sterile inflammation without detectable infection.24 Novel TLR functions in cell proliferation, cell survival and tissue repair have also recently been described.25-26
The growth rate or activity of inflammatory PL depends on the balance between cell proliferation and death; therefore proliferation markers, besides apoptotic reactions, have been extensively investigated.18,27,28 These studies have suggested that regression of epithelial strands in PG and of the lining epithelium in apical cysts are most likely caused by programmed cell death. After non-surgical endodontic therapy the restricted-potential basal stem cells in the epithelial strands or lining epithelium of cysts stop proliferating due to a decline of inflammatory mediators, proinflammatory cytokines, and growth factors. Furthermore, the terminally differentiated squamous cells in the epithelial strands and lining epithelium of a cyst will die of programmed cell death, similar to that of surface epithelial cells of the oral epithelium.18,27,28 TLR, which are also involved in epithelial cell proliferation, cell survival and tissue repair, could therefore be of interest to understand the pathobiology of periapical inflammatory disease.
Among TLR, TLR2 and TLR4 have been studied in the inflammatory infiltrate of PC and PG. The presence of TLR2-expressing inflammatory cells described in PG and PC indicated that the disease is likely to be sustained by the immune system via reactions to bacterial antigens.5,9,10 The same conclusions have been drawn in the only TLR4 study,9 where TLR4 has been found to be constitutive and non-constitutively expressed in human epithelial cells;13,20,21,23,29,30 no data are available about its expression in ERM and the epithelium cyst lining of PC. The present study examined its expression in PL by immunohistochemistry focusing on the epithelial compartment.
Our findings demonstrated TLR4 overexpression both in PG and PC, although with differences related to the nature of the lesion. In fact, in PG all epithelial cells of strands, islands and trabeculae were strongly immunoreactive for TLR4, whereas in PC only some areas of the basal and suprabasal layers of epithelium showed TLR4 immunostaining. This staining pattern is consistent with the known functions of TLR4, since TLR4 could promote cell survival, proliferation and migration of epithelial strands and islands in ERM, whereas in PC it could protect the lining epithelium from extensive apoptosis. This agrees with its overexpression in PG and its moderate expression in PC, where it may participate in maintaining the thickness of radicular cyst lining epithelium, which depends on the balance between cell proliferation and apoptosis. These findings go some way toward answering the intriguing question of why many epithelial strands or islands in PG and the lining epithelium of apical cysts regress after non-surgical endodontic therapy. TLR4 ligation leads to activation and translocation of the nuclear transcription factor- B (NF- B) from cytoplasm to the nucleus, where it induces the expression of a set of target genes.12,31 Therefore, activated NF- B plays an important role in cell differentiation and proliferation, in response to a variety of physiological and pathological stimuli.12 Activated NF- B has been described in some odontogenic lesions and among these in PC;32 the TLR4 upregulation described in this study in PL epithelium could thus be a pathway of NF- B activation.
Based on our findings and on previous evidence linking PL to chronic inflammation via TLR - which would play a significant role in the recognition of endodontic pathogens and trigger adaptive immune responses against endodontic pathogens2 - it is reasonable to assume a key role for TLR4 in the pathobiology of the inflammatory processes related to periapical disease. In this very complex interplay of many bioactive molecules, the up- or downregulation of these bioactive molecules might be a key mechanism in the regulation of inflammatory.9 Further work harnessing additional investigation methods is required to elucidate the possible contribution of TLR4 to inflammatory PL.
References
- 1.Marton IJ, Kiss C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol 2000;15:139-50. [DOI] [PubMed] [Google Scholar]
- 2.Desai SV, Love RM, Rich AM, Seymour GJ. Antigen recognition and presentation in periapical tissues: a role for TLR expressing cells? Int Endod J 2011;44:87-99. [DOI] [PubMed] [Google Scholar]
- 3.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782-7. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999;11:443-51. [DOI] [PubMed] [Google Scholar]
- 5.Desai SV, Love RM, Rich AM, Seymour GJ. Toll-like receptor 2 expression in refractory periapical lesions. Int Endod J 2011;44:907-16. [DOI] [PubMed] [Google Scholar]
- 6.Vitale M, Gobbi G, Mirandola P, Ponti C, Sponzilli I, Rinaldi L, et al. TNF-related apoptosis-inducing ligand (TRAIL) and erythropoiesis: a role for PKC epsilon. Eur J Histochem 2006;50:15-8. [PubMed] [Google Scholar]
- 7.Loreto C, Almeida LE, Migliore MR, Caltabiano M, Leonardi R. TRAIL, DR5 and caspase 3-dependent apoptosis in vessels of diseased human temporomandibular joint disc. An immunohistochemical study. Eur J Histochem 2010;54:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krauss JL, Potempa J, Lambris JD, Hajishengallis G. Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol 2000 2010;52:141-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira Rde C, Beghini M, Borges CR, Alves PM, de Araujo MS, Pereira SA, et al. Higher expression of galectin-3 and galectin-9 in periapical granulomas than in radicular cysts and an increased tolllike receptor-2 and toll-like receptor-4 expression are associated with reactivation of periapical inflammation. J Endod 2014;40:199-203. [DOI] [PubMed] [Google Scholar]
- 10.da Silva RA, Ferreira PD, De Rossi A, Nelson-Filho P, Silva LA. Toll-like receptor 2 knockout mice showed increased periapical lesion size and osteoclast number. J Endod 2012;38:803-13. [DOI] [PubMed] [Google Scholar]
- 11.Chokechanachaisakul U, Kaneko T, Okiji T, Kaneko R, Kaneko M, Kawamura J, et al. Increased gene expression of Toll-like receptors and antigen-presenting cell-related molecules in the onset of experimentally induced furcation lesions of endodontic origin in rat molars. J Endod 2010;36:251-5. [DOI] [PubMed] [Google Scholar]
- 12.Rich AM, Hussaini HM, Parachuru VP, Seymour GJ. Toll-like receptors and cancer, particularly oral squamous cell carcinoma. Front Immunol 2014;5:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Guo S, Ranzer MJ, DiPietro LA. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol 2013;133:258-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mempel M, Voelcker V, Kollisch G, Plank C, Rad R, Gerhard M, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol 2003;121:1389-96. [DOI] [PubMed] [Google Scholar]
- 15.Leonardi R, Caltabiano R, Loreto C. Collagenase-3 (MMP-13) is expressed in periapical lesions: an immunohistochemical study. Int Endod J 2005;38:297-301. [DOI] [PubMed] [Google Scholar]
- 16.Leonardi R, Caltabiano M, Pagano M, Pezzuto V, Loreto C, Palestro G. Detection of vascular endothelial growth factor/ vascular permeability factor in periapical lesions. J Endod 2003;29:180-3. [DOI] [PubMed] [Google Scholar]
- 17.Leonardi R, Villari L, Caltabiano M, Travali S. Heat shock protein 27 expression in the epithelium of periapical lesions. J Endod 2001;27:89-92. [DOI] [PubMed] [Google Scholar]
- 18.Loreto C, Galanti C, Leonardi R, Musumeci G, Pannone G, Palazzo G, et al. Possible role of apoptosis in the pathogenesis and clinical evolution of radicular cyst: an immunohistochemical study. Int Endod J 2013;46:642-8. [DOI] [PubMed] [Google Scholar]
- 19.Montero Vega MT, de Andres Martin A. The significance of toll-like receptors in human diseases. Allergol Immunopathol (Madr) 2009;37:252-63. [DOI] [PubMed] [Google Scholar]
- 20.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013;153:812-27. [DOI] [PubMed] [Google Scholar]
- 21.Begon E, Michel L, Flageul B, Beaudoin I, Jean-Louis F, Bachelez H, et al. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol 2007;17:497-506. [DOI] [PubMed] [Google Scholar]
- 22.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol 2003;148: 670-9. [DOI] [PubMed] [Google Scholar]
- 23.Li JP, Chen Y, Ng CH, Fung ML, Xu A, Cheng B, et al. Differential expression of Toll-like receptor 4 in healthy and diseased human gingiva. J Periodontal Res 2014;49:845-54. [DOI] [PubMed] [Google Scholar]
- 24.Wagner H. Endogenous TLR ligands and autoimmunity. Adv Immunol 2006;91:159-73. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine 2010;49:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonardi R, Perrotta RE, Crimi S, Matthews JB, Barbato E, dos Santos JN, et al. Differential expression of TLR3 and TLR4 in keratocystic odontogenic tumor (KCOT): A comparative immunohistochemical study in primary, recurrent, and nevoid basal cell carcinoma syndrome (NBCCS)--associated lesions. J Craniomaxillofac Surg 2015;43:733-7. [DOI] [PubMed] [Google Scholar]
- 27.Lin LM, Huang GT, Rosenberg PA. Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J Endod 2007;33:908-16. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Kumamoto H, Kunimori K, Ooya K. Immunohistochemical analysis of apoptosis-related factors in lining epithelium of radicular cysts. J Oral Pathol Med 2005;34:46-52. [DOI] [PubMed] [Google Scholar]
- 29.Panzer R, Blobel C, Folster-Holst R, Proksch E. TLR2 and TLR4 expression in atopic dermatitis, contact dermatitis and psoriasis. Exp Dermatol 2014;23:364-6. [DOI] [PubMed] [Google Scholar]
- 30.Beklen A, Hukkanen M, Richardson R, Konttinen YT. Immunohistochemical localization of Toll-like receptors 1-10 in periodontitis. Oral Microbiol Immunol 2008;23:425-31. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Luo J, Ni J, Tang L, Zhang HP, Zhang L, et al. MMP-7 is upregulated by COX-2 and promotes proliferation and invasion of lung adenocarcinoma cells. Eur J Histochem 2014;58:2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Andrade Santos PP, de Aquino AR, Oliveira Barreto A, de Almeida Freitas R, Galvao HC, de Souza LB. Immunohistochemical expression of nuclear factor kappaB, matrix metalloproteinase 9, and endoglin (CD105) in odontogenic keratocysts, dentigerous cysts, and radicular cysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:476-83. [DOI] [PubMed] [Google Scholar]


