Abstract
Background
Previous studies have not shown a consistent link between body mass index (BMI) and outcomes such as mortality and kidney disease progression in non-dialysis-dependent chronic kidney disease (CKD) patients. Therefore, we aimed to complete a systematic review and meta-analysis study on this subject.
Methods
We searched MEDLINE, EMBASE, Web of Science, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL), and screened 7,123 retrieved studies for inclusion. Two investigators independently selected the studies using predefined criteria and assessed each study's quality using the Newcastle-Ottawa quality assessment scale. We meta-analyzed the results based on the BMI classification system by the WHO.
Results
We included 10 studies (with a total sample size of 484,906) in the systematic review and 4 studies in the meta-analyses. The study results were generally heterogeneous. However, following reanalysis of the largest reported study and our meta-analyses, we observed that in stage 3-5 CKD, being underweight was associated with a higher risk of death while being overweight or obese class I was associated with a lower risk of death; however, obesity classes II and III were not associated with risk of death. In addition, reanalysis of the largest available study showed that a higher BMI was associated with an incrementally higher risk of kidney disease progression; however, this association was attenuated in our pooled results. For earlier stages of CKD, we could not complete meta-analyses as the studies were sparse and had heterogeneous BMI classifications and/or referent BMI groups.
Conclusion
Among the group of patients with stage 3-5 CKD, we found a differential association between obesity classes I-III and mortality compared to the general population, indicating an obesity paradox in the CKD population.
Key Words: Chronic kidney disease, Body mass index, Mortality, End-stage renal disease, Obesity paradox
Introduction
Obesity is a major risk factor for cardiovascular disease and death in the general population [1,2]. It is also associated with a higher risk of de novo chronic kidney disease (CKD) and end-stage renal disease (ESRD) [3,4,5]. However, once ESRD is developed, obesity is paradoxically associated with greater survival [6]. This phenomenon is been described as the ‘obesity paradox’, and it has been observed in certain populations such as in incident hemodialysis patients, hospitalized patients, elderly nursing home residents, and those with stroke and chronic heart failure [6,7,8,9,10,11,12,13,14]. This reversal of the obesity-mortality association has been particularly robust in hemodialysis patients [6,15,16], and it is also observed in peritoneal dialysis patients over the short term [17,18,19]. Nevertheless, current evidence has not provided a consistent link between obesity and clinical outcomes in non-dialysis-dependent (NDD) CKD patients. Hence, we aimed to complete a systematic review and meta-analysis of the association of the body mass index (BMI), an indicator of obesity, with mortality and progression to ESRD in this population.
Methods
Search Strategies
We searched for studies investigating the link between BMI and mortality in all CKD patients including those with NDD CKD. We searched MEDLINE (PubMed), Web of Science, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane Central Register of Controlled Trials (CENTRAL) with no limitation in the study type, language, and geographical area. Detailed search strategies are explained in a previous article (online suppl. table 1; see www.karger.com/doi/10.1159/000437277 for all online suppl. material) [20]. The corresponding authors of all included studies as well as two field experts (C.P.K. and K.K.Z.) were consulted to identify any unidentified relevant study.
Study Selection
After removal of the duplicated records using EndNote software (Thomson Reuters, New York, N.Y., USA), two investigators (S.F.A. and G.Z.), blinded to the study authors and journals, independently screened the studies for inclusion. The included studies were required to describe data from case-control, cohort, or clinical trial studies, and to study the association of either baseline BMI or change in BMI with all-cause mortality in NDD CKD patients. We excluded the studies with mixed NDD CKD and dialysis patients as well as the studies with a sample size <1,000, as small studies are more likely to be influenced by publication (reporting) bias. Any discrepancies between the two reviewers on study eligibility were resolved by discussion and consensus.
Data Abstraction and Quality Assessment
We extracted and tabulated the main characteristics and findings of the included studies (table 1; online suppl. tables 2 and 3). In addition to all-cause mortality, we also extracted the summary estimates of progression to ESRD (i.e. the start of the dialysis therapy) when available. The corresponding authors of the studies with incomplete data were contacted in order to request further data. To assess the quality of the included studies, two investigators (S.F.A. and E.A.) independently applied the Newcastle-Ottawa quality assessment scale [21] assigning a quality score of 0-9 to each study (online suppl. table 4). The quality score was based on three major components: selection of study participants (0-4 points), quality of the adjustment for confounding (0-2 points), and ascertainment of the exposure or outcome of interest in case-control studies or cohorts, respectively (0-3 points). The maximum score was 9 points, representing the highest methodological quality. Disagreements in the scores were resolved by discussion and consensus.
Table 1.
Characteristics of the included studies
| Authors [Ref.], year | Study design and settings | Participants | Demographics | Major comorbidities | Main analyses | Quality score | Summary of results |
|---|---|---|---|---|---|---|---|
| Studies included in meta-analyses | |||||||
| Lu et al. [22], 2014 | Cohort study: data from Veterans Affairs national data sets and US Renal Data System (USRDS), USA; recruitment: 2004–2006; follow-up: until 2012 | 453,946 patients with CKD stages 3–5 (eGFR <60), no history of RRT, and available BMI measurements | Mean age: 73.9±9.3 yo; female: 2.8%; ethnicities: AA: 9.1%, W: 87.0% | CVD: 43.50%, DM: 40.93%, lung disease: 24.53%, Cancer: 18.39%, CHF: 15.39% | Multivariate Cox regression to calculate HRs of all-cause mortality and progression to ESRD from baseline BMI categories | 7 | In patients with CKD stages 3–5, BMI showed a U-shaped relationship with all-cause mortality in which compared to BMIs of 30–<35, both higher and lower BMIs were associated with higher mortality; however, in a subgroup of patients with CKD stages 4 and 5 (eGFR <30), higher BMIs were not associated with any significantly different mortality; also, lower BMIs were associated with lower progression to ESRD but higher BMIs were not associated with any significantly different progression to ESRD |
| Muntner et al. [24], 2013 | Cohort study: data from Reasons for Geographic and Racial Differences in Stroke (REGARDS) study and US Renal Data System (USRDS), USA; recruitment: 2003–2007; follow-up: until 2009 | 3,093 patients ≥45 yo with CKD stages 3–5 (eGRF <60) and no history of RRT | Mean age: 72.2±8.7 yo; female: 54.9%; ethnicities: AA: 42.3% | HTN: 80.8%, DM: 35.1%, CHD: 31.7%, stroke: 13.0% | Multivariate Cox regression to calculate HRs of all-cause mortality and progression to ESRD from baseline BMI categories | 7 | In patients with CKD stages 3–5, compared to BMIs of ≥30, lower BMIs were not associated with any significantly different mortality or progression to ESRD |
| Ricardo et al. [25], 2013 | Cohort study: data from Third National Health and Nutrition Examination Survey (NHANES III) and National Death Index, USA; recruitment: 1988–1994; follow-up: until 2006 | 2,288 male or nonpregnant female patients ≥18 yo with CKD stages 3 and 4 (eGFR <60) or microalbuminuria (UACR >30); Cox regression models included a subset of 2,145 participants | Mean age: 59 yo (SD: N/A); female: 60%; ethnicities: AA: 12% MA: 4% W: 77% | HTN: 59.1%, DM: 23.3%, CVD: 16.3% | Multivariate Cox regression to calculate HRs of all-cause and cardiovascular mortality from baseline BMI categories | 7 | In patients with CKD stages 3 or 4 or microalbuminuria and BMI ≥18.5, compared to BMIs of 22–<25, BMIs of 18.5–22 were associated with higher all-cause and cardiovascular mortality, and higher BMIs (i.e. ≥25) were not associated with any significantly different mortality |
| De Nicola et al. [26], 2012 | Cohort study: data from Targer Blood Pressure Levels (TABLE) study, Italy; recruitment: 2003; follow-up: until 2011 | 1,248 patients with CKD stages 3–5 (eGFR <60) or proteinuria ≥3 months, ≥1 year duration between 1st nephrology clinic visit and enrollment, no history of acute kidney injury during 6 months before enrollment | Mean age: 67±14 yo; female: 52.5%; ethnicities: N/A | Prior CV event: 31.8%, DM: 27.7% | Multivariate Cox regression to calculate HRs of all-cause mortality and progression to ESRD from baseline BMI values | 7 | In patients with CKD stages 3–5, BMI had no linear relationship with mortality but higher BMIs were associated with slightly lower rates of progression to ESRD |
| Babayev et al. [23], 2013 | Cohort study: data from National Kidney Foundation Kidney Early Evaluation Program (NKF KEEP] and US Renal Data System (USRDS], USA; recruitment and follow-up: 2000–2009 | 12,534 AA and Whites with CKD stages 3 or 4 (eGFR 15–59), no history of RRT, and available BMI measurements | Mean age: 66.8±11.7 yo (AA), 70.9±11.2 yo (W); female: 73.7% (AA), 66.6% (W); ethnicities: AA: 28%, W: 72% | HTN: 92.94%, DM: 42.12% | Multivariate Cox regression to calculate HRs of all-cause mortality and progression to ESRD from baseline BMI categories | 8 | In patients with CKD stages 3 and 4, compared to BMIs of <30, BMIs of 30–34.9 were associated with lower mortality and BMIs of ≥35 were not associated with any significantly different mortality; also, higher BMIs were not associated with any significantly different progression to ESRD |
| Dalrymple et al. [27], 2011 | Cohort study: data from Cardiovascular Health Study (CHS] and US Renal Data System (USRDS], USA; recruitment: 1989–1990 and 1992–1993; follow-up: until 2003 | 1,268 patients ≥65 yo with CKD stages 3–5 (eGFR <60], available baseline creatinine measurements and cause of death, and no history of RRT, chemoradiation therapy for cancer, hospice care, and wheelchair use within home | Mean age: 75 yo (SD: N/A); female: 54%; ethnicities: N/A | HTN: 69%, CVD: 33%, DM: 17% | Multivariate Cox regression to calculate HRs of all-cause mortality and progression to ESRD from baseline BMI categories | 7 | In patients with CKD stages 3–5, compared to BMIs of 18.5–24.9, lower BMIs were associated with higher mortality but higher BMIs were not associated with any significantly different mortality; also, higher BMIs were associated with higher progression to ESRD but the study was inconclusive regarding BMIs <18.5 |
| Kramer et al. [28], 2011 | Cohort study: data from Reasons for Geographic and Racial Differences in Stroke (REGARDS) study and National Death Index, USA; recruitment and follow-up: 2003–2007 | 3,093 patients ≥45 yo with CKD stages 1–4 (eGRF <60] or microalbuminuria (UACR ≥30], BMI ≥18.5, no history of RRT, and available measurements for serum creatinine, albumin, BMI, and waist circumference | N/A | N/A | Multivariate Cox regression to calculate HRs of all-cause mortality from baseline BMI categories and values | 8 | In patients with CKD stages 1–4 and BMIs ≥18.5, compared to BMIs of 25–29.9, the highest BMI category (i.e. BMIs of ≥40) was associated with higher mortality, but other categories were not associated with any significantly different mortality; also, each 1-unit increase in BMI was associated with decreased mortality |
| Obermayr et al. [29], 2009 | Cohort study: data from Vienna Health Screening Initiative study and Austrian Death Registry, Austria; recruitment and follow-up: 1990–2006 | 2,879 subjects with CKD stages 1–3 (GFR >30 and proteinuria) | Mean age: 40±12 yo (CKD 1–2], 62±15 yo (CKD 3); female: N/A; ethnicities: N/A | N/A | Multivariate restricted cubic spline analysis to predict HR of cardiovascular mortality from baseline BMI values | 8 | In patients with CKD stages 1–3, BMIs close to 25 were associated with the least cardiovascular mortality and lower/higher BMIs were associated with higher cardiovascular mortality |
| Weiner et al. [31], 2008 | Cohort study: data from Atherosclerosis Risk in Communities (ARIC) study and Cardiovascular Health Study (CHS), USA; recruitment: 1987–1990 and 1992–1993; follow-up: N/A | 1,678 patients >45 yo with CKD stages 3 or 4 (eGFR 15–59.9) | Mean age: 51±13 yo; female: 40%; ethnicities: W: 80% | HTN: 74%, CVD: 34%, DM: 15% | Multivariate Cox regression to calculate HRs of all-cause mortality for each 5-unit increase in BMI | 8 | In patients with CKD stages 3 or 4, increase in BMI is associated with lower all-cause mortality |
| Madero et al. [30], 2007 | Cohort study: data from Modification of Diet in Renal Disease (MDRD) study and National Death Index, USA; recruitment: 1989–1993; follow-up: until 2000 | 1,759 CKD patients 18–70 yo with serum creatinine of 1.2–7 mg/dl, available BMI measurements, and without: type 1 DM, GN due to autoimmune disease, obstructive uropathy, renal artery stenosis, proteinuria >10 g/dl, MAP >126, and history of renal transplantation | Mean age: 51±13 years; female: 40%; ethnicities: W: 80% | N/A | Multivariate Cox regression to calculate HRs of all-cause mortality from baseline BMI categories | 8 | In patients with earlier stages of CKD, compared to BMIs of 18.5–24.9, higher BMI categories were not associated with any significantly different mortality; also, compared to the 1st BMI quartile, higher BMI quartiles were not associated with any significantly different mortality |
eGFR = Estimated GFR; RRT = renal replacement therapy; AA = African-American; W = White; CVD = cardiovascular disease; DM = diabetes mellitus; CHF = chronic heart failure; yo = years old; HTN = hypertension; CHD = coronary heart disease; UACR = urine albumin-to-creatinine ratio; MA= Mexican-American; GN = glomerulonephritis; MAP = mean arterial pressure; N/A = not assessed.
Data Analysis and Synthesis
We quantified the interrater agreement for study selection and quality assessment. For meta-analyses, we pooled the summary estimates representing the association of BMI (a priori outcome) or progression to ESRD (post hoc outcome) with mortality. We assessed statistical heterogeneity using the I2 statistic. Summary estimates with a corresponding I2 ≤25% were pooled using fixed-effects meta-analysis while those with a corresponding I2 >25% were pooled using the random-effects model. The risk of publication (reporting) bias was assessed using Begg's test and funnel plots. Statistical significance was defined as a 95% confidence interval with no overlap with the null effect value [hazard ratio (HR) = 1]. For statistical procedures, we used Stata 12 (StataCorp, College Station, Tex., USA).
Results
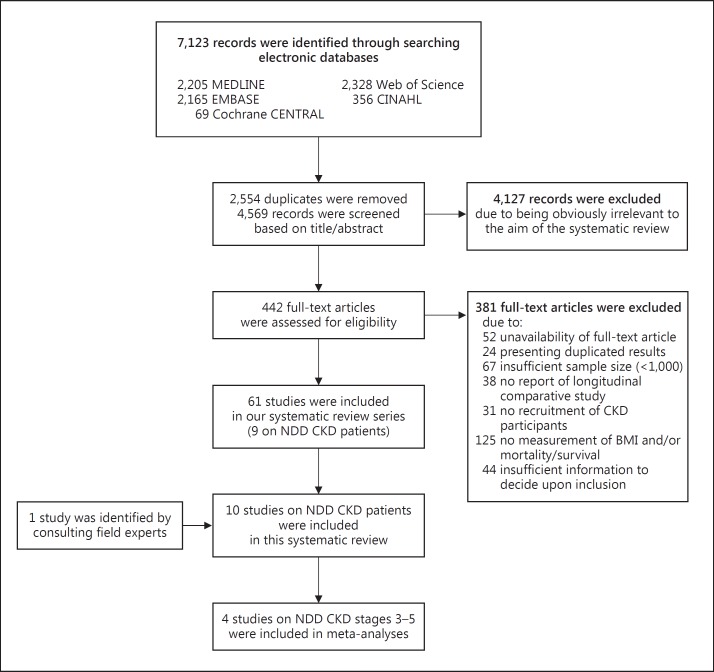
We retrieved 7,123 citations in total, removed the duplicated records, and screened the remaining records for inclusion based on their titles and/or abstracts. Afterwards, we evaluated a subset of 442 citations based on their full-text article (fig. 1). Finally, we included 10 studies examining the association of BMI with mortality in NDD CKD patients (table 1) [22,23,24,25,26,27,28,29,30,31]. The included studies comprised cohorts from preexisting registry data that reported HRs of mortality from Cox regression models [22,23,24,25,26,27,28,30,31] or restricted cubic spline analysis [29]. Also, Newcastle-Ottawa Scale scores of 7 or 8 out of 9 indicated a low risk of bias across the included studies (table 1). Interrater agreement was 94% (kappa: 0.77) for study selection and 90% (kappa: 0.82) for quality assessment.
Fig. 1.
Study flow diagram.
Association of BMI with Mortality
The included studies were diverse in terms of their BMI classification and referent BMI class. Here, we use the WHO BMI classification system [32] for ease of reading. Four studies examined stage 3-5 CKD [22,24,26,27]. The largest study by Lu et al. [22] used obesity class I as the referent group and observed a U-shaped relationship between BMI and risk of death. Dalrymple et al. [27] observed that being underweight was associated with a higher mortality rate, but did not detect a significant association between being overweight or obese and mortality. The two remaining studies reported no significant association [24,26].
Another three studies examined stage 3-4 CKD. Babayev et al. [23] used combined underweight, normal BMI, and overweight status as its referent and observed a higher risk of death in obesity class I but a similar risk in combined obesity classes II/III. Ricardo et al. [25] divided the normal BMI class into two subclasses and observed that the low-normal BMI is associated with higher all-cause and cardiovascular mortality. Weiner et al. [31] examined BMI change in lieu of baseline BMI, and found that increased BMI was associated with a lower risk of death.
Kramer et al. [28] examined stage 1-4 CKD and linked only obesity class III with higher mortality. However, they excluded underweight patients from analyses and set the overweight class as their referent group. They also linked high waist circumference to higher mortality. Obermayr et al. [29] observed that among patients with stage 1-3 CKD, the association of BMI with cardiovascular mortality is U-shaped in those with higher glomerular filtration rates (GFRs) or no proteinuria; however, there was an inverse association between BMI and mortality risk in those with lower GFRs and proteinuria. Finally, Madero et al. [30] found no BMI-mortality association in nonunderweight CKD patients.
Association of BMI with Progression to ESRD
Not all included studies evaluated the risk of progression to ESRD as it was not an a priori outcome. Among the studies of stage 3-5 CKD, Lu et al. [22] reported that being underweight, normal, or overweight (reference: obesity class I) was associated with a lower risk of ESRD. However, obesity class II or higher (i.e. BMI ≥35) was not associated with a significantly different risk. Dalrymple et al. [27] linked being overweight or obese to a higher risk of ESRD. Muntner et al. [24] and De Nicola et al. [26] reported no significant association. Also, in stage 3 and 4 CKD, Babayev et al. [23] did not observe a significant association. The other included studies did not evaluate the risk of progression to ESRD.
Reanalysis of the Data by Lu et al.
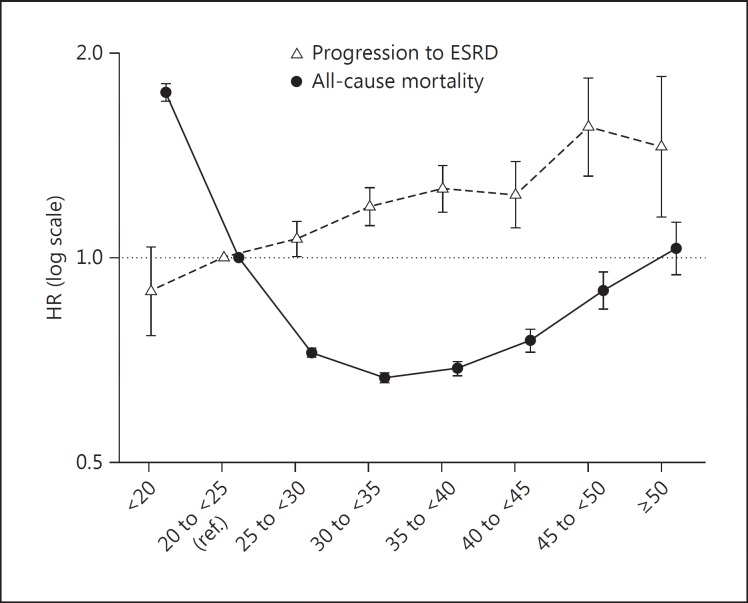
As the study by Lu et al. [22] was large (n = 453,946), with ‘obesity class I’ as its referent group, we reanalyzed their data using the same analytical approach, however, this time with ‘normal BMI’ as the referent group. Compared to having a normal BMI, being underweight was associated with a higher risk of death; in addition, all overweight and obese groups were associated with lower risks of death except for extremely obese patients (i.e. BMI ≥50) in whom risk of death was not significantly different. For progression to ESRD, the BMI-outcome association was grossly linear, and overweight/obese groups were associated with a higher risk. For the underweight group, the association did not reach statistical significance (fig. 2; online suppl. table 5).
Fig. 2.
Reanalysis of the data of the largest included study by Lu et al. [22] showed a survival advantage of obesity in patients with CKD stages 3-5, whereas higher BMI classes were associated with incrementally higher risks of progression to ESRD.
Meta-Analysis of Study Results
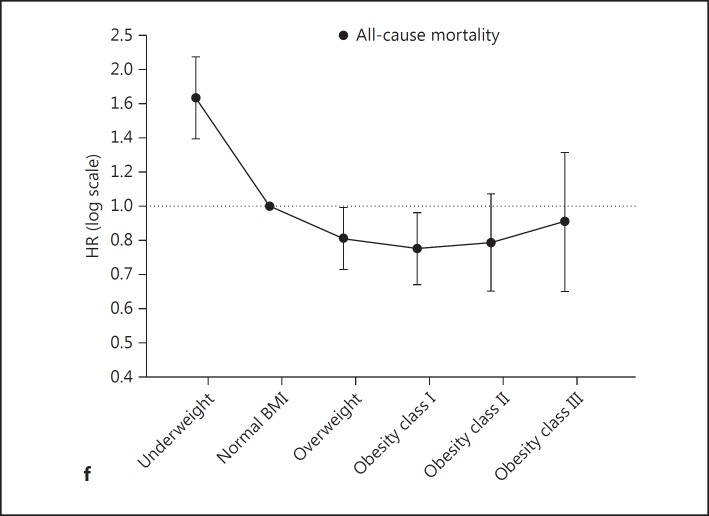
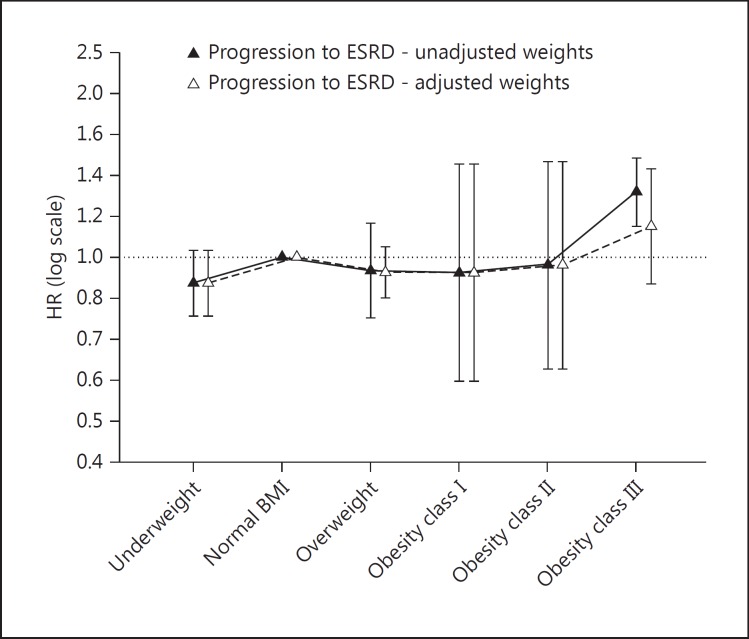
Given that the studies used heterogeneous BMI classifications, we requested further results based on the WHO classification system [32]. We obtained and pooled additional results from 4 studies [22,24,25,26]. Our meta-analyses showed that, compared to normal BMI, being underweight was associated with higher mortality, and being overweight or obese class I was associated with lower mortality; also, obesity classes II and III were not associated with a significantly different mortality risk (fig. 3). For progression to ESRD, not all HRs were significant except for obesity class III; however, when the weight in the study of Lu et al. [22] was limited to 50% and other study weights were adjusted accordingly, this association became nonsignificant (fig. 4; online suppl. fig. 6a–e). Finally, Begg's tests did not find evidence of publication bias (online suppl. fig. 7a–e).
Fig. 3.
Meta-analysis of HRs of all-cause mortality. a-e The meta-analysis of the HRs of all-cause mortality in underweight, overweight, and obesity classes I-III, respectively, compared to normal BMI. The BMI cutoff points between the above-mentioned BMI classes were 18.5 [24,25,26] or 20 [22], 25, 30, 35, and 40, respectively. f The results of a-e put together, showing the pooled HRs of all-cause mortality.
Fig. 4.
Pooled HRs of progression to ESRD in underweight, overweight, and obesity classes I-III, respectively, compared to normal BMI. The BMI cutoff points between the above-mentioned BMI classes were 18.5 [24,25,26] or 20 [22], 25, 30, 35, and 40, respectively. Pooled HRs with adjusted weights derived from meta-analyses in which study weights were limited to ≤50%.
Discussion
We generally observed heterogeneous findings across the included studies. However, reanalysis of data from Lu et al. [22] and our meta-analyses showed that in stage 3-5 CKD, being underweight was associated with a higher risk of death while being overweight or obese class I was associated with lower risk; however, obesity classes II and III were not associated with a significantly different risk of death. In addition, reanalysis of the largest included study showed that a higher BMI was associated with an incrementally higher risk of disease progression; however, this association was attenuated in our pooled results. For earlier stages of CKD, we could not complete meta-analyses as the studies were sparse and had heterogeneous BMI classifications and/or referent BMI groups. Nevertheless, a high-quality study of stage 1-3 CKD by Obermayr et al. [29] showed that the BMI-mortality association was U-shaped in those with higher GFRs or no proteinuria whereas BMI had an inverse linear association with mortality in those with lower GFRs and proteinuria. Also, another included study [31] suggested that an increase in BMI was associated with a lower risk of death in stage 3 and 4 CKD.
The association of being underweight with higher mortality is well documented in the general population as well as in hemodialysis patients, peritoneal dialysis patients, and kidney transplant recipients [2,16,19,20], and we observed a similar association of being underweight with a higher risk of death in NDD CKD patients. On the other hand, we observed that in stage 3-5 CKD, overweight and obesity class I were associated with a lower risk of death, whereas obesity classes II or III were not associated with risk of death. It should be noted that a meta-analysis in the general population has shown that being overweight is associated with a lower (rather than higher) risk of death and that obesity class I does not bear a higher risk of mortality [2]. Nevertheless, patients with stage 3-5 CKD have a differential association between obesity classes I-III and mortality as compared to the general population, indicating an obesity paradox in the CKD population.
Obesity can lead to various adverse sequelae given the direct metabolic effects of increased adiposity, as well as its predisposition to diabetes mellitus and hypertension [33,34]. Hence, our results seem paradoxical, as BMI levels in the overweight/mildly obese range were associated with the best outcomes, whereas morbid obesity was not associated with adverse outcomes. Several explanations have been posited for this so-called obesity paradox. For example, advanced CKD patients have a shorter life span that may provide little time for obesity to cause its adverse metabolic effects, and, at the same time, obesity may improve survival by providing better nutritional reserves for such patients [35]. Other potential underlying causes of the obesity paradox are explained elsewhere [6].
In this study, we searched multiple databases using sensitive search strategies and included large studies (n ≥ 1,000) providing a higher accuracy and lower likelihood of publication bias. Also, our study selection and quality assessment were carried out in duplicate. However, our study possesses the inherent limitations of systematic reviews of observational studies. Despite being large and robust, the included studies' results were adjusted for only recognized and measured confounders. In addition, BMI was considered a surrogate of obesity even though it does not provide accurate information about adiposity or body composition. In fact, BMI reflects both fat mass and lean body mass; higher fat mass is associated with cardiovascular sequelae and unclear nutritional effects, whereas higher lean body mass provides higher nutritional reserves and protects against malnutrition-inflammation complex syndrome in patients with CKD as well as against a number of other chronic conditions [6,35,36]. Also, higher lean body mass generally represents more muscle mass and indicates better ‘fitness’, which is reportedly protective against the adverse effects of obesity [37,38]. Nevertheless, BMI is commonly used in clinical practice to drive weight management and hence our results have clinical relevance.
In conclusion, in stage 3-5 CKD, being underweight was associated with a higher risk of death, and being overweight or obese class I was associated with a lower risk of death; also, being obese class II or III was not associated with a different risk of death. As studies examining earlier stages of CKD were few, we did not draw net conclusions regarding the BMI-mortality association in this population. Our results indicated that in patients with NDD CKD, traditional weight management strategies may not be appropriate. The ideal BMI and the best ways to achieve it should be identified from randomized controlled trials. Before such information becomes available, a cautious approach to BMI-based weight management seems necessary in this population.
Supplementary Material
Supplementary data
References
- 1.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 4.Foster MC, Hwang S-J, Larson MG, Lichtman JH, Parikh NI, Vasan RS, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52:39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PWF, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 6.Park J, Ahmadi S-F, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56:415–425. doi: 10.1016/j.pcad.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Rhee CM, Amin AN. To legitimize the contentious obesity paradox. Mayo Clin Proc. 2014;89:1033–1035. doi: 10.1016/j.mayocp.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, Jin DC, Molnar MZ, Dukkipati R, Kim Y-L, Jing J, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc. 2013;88:479–486. doi: 10.1016/j.mayocp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke. 2015;10:99–104. doi: 10.1111/ijs.12016. [DOI] [PubMed] [Google Scholar]
- 10.Herselman M, Esau N, Kruger JM, Labadarios D, Moosa MR. Relationship between body mass index and mortality in adults on maintenance hemodialysis: a systematic review. J Ren Nutr. 2010;20:281–292. doi: 10.1053/j.jrn.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49:968–979. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 14.Landi F, Onder G, Gambassi G, Pedone C, Carbonin P, Bernabei R. Body mass index and mortality among hospitalized patients. Arch Intern Med. 2000;160:2641–2644. doi: 10.1001/archinte.160.17.2641. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46:489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Li T, Liu J, An S, Dai Y, Yu Q. Body mass index and mortality in patients on maintenance hemodialysis: a meta-analysis. Int Urol Nephrol. 2014;46:623–631. doi: 10.1007/s11255-014-0653-x. [DOI] [PubMed] [Google Scholar]
- 17.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64:1838–1844. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 18.Mehrotra R, Chiu Y-W, Kalantar-Zadeh K, Vonesh E. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int. 2009;76:97–107. doi: 10.1038/ki.2009.94. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadi S-F, Zahmatkesh G, Streja E, Mehrotra R, Rhee CM, Kovesdy CP, et al. : Association of body mass index with mortality in peritoneal dialysis patients: a systematic review and meta-analysis. Perit Dial Int, Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 20.Ahmadi S-F, Zahmatkesh G, Streja E, Molnar MZ, Rhee CM, Kovesdy CP, et al. Body mass index and mortality in kidney transplant recipients: a systematic review and meta-analysis. Am J Nephrol. 2014;40:315–324. doi: 10.1159/000367812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spergel LM, Holland JE, Fadem SZ, McAllister CJ, Peacock EJ. Static intra-access pressure ratio does not correlate with access blood flow. Kidney Int. 2004;66:1512–1516. doi: 10.1111/j.1523-1755.2004.00946.x. [DOI] [PubMed] [Google Scholar]
- 22.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25:2088–2096. doi: 10.1681/ASN.2013070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babayev R, Whaley-Connell A, Kshirsagar A, Klemmer P, Navaneethan S, Chen S-C, et al. Association of race and body mass index with ESRD and mortality in CKD stages 3-4: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2013;61:404–412. doi: 10.1053/j.ajkd.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 24.Muntner P, Judd SE, Gao L, Gutiérrez OM, Rizk DV, McClellan W, et al. Cardiovascular risk factors in CKD associate with both ESRD and mortality. J Am Soc Nephrol. 2013;24:1159–1165. doi: 10.1681/ASN.2012070642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricardo AC, Madero M, Yang W, Anderson C, Menezes M, Fischer MJ, et al. Adherence to a healthy lifestyle and all-cause mortality in CKD. Clin J Am Soc Nephrol. 2013;8:602–609. doi: 10.2215/CJN.00600112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Nicola L, Minutolo R, Chiodini P, Borrelli S, Zoccali C, Postorino M, et al. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int. 2012;82:482–488. doi: 10.1038/ki.2012.174. [DOI] [PubMed] [Google Scholar]
- 27.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26:379–385. doi: 10.1007/s11606-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer H, Shoham D, McClure LA, Durazo-Arvizu R, Howard G, Judd S, et al. Association of waist circumference and body mass index with all-cause mortality in CKD: the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2011;58:177–185. doi: 10.1053/j.ajkd.2011.02.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermayr RP, Temml C, Gutjahr G, Kainz A, Klauser-Braun R, Függer R, et al. Body mass index modifies the risk of cardiovascular death in proteinuric chronic kidney disease. Nephrol Dial Transplant. 2009;24:2421–2428. doi: 10.1093/ndt/gfp075. [DOI] [PubMed] [Google Scholar]
- 30.Madero M, Sarnak MJ, Wang X, Sceppa CC, Greene T, Beck GJ, et al. Body mass index and mortality in CKD. Am J Kidney Dis. 2007;50:404–411. doi: 10.1053/j.ajkd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51:212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Body Mass Index Classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed May 9, 2013).
- 33.Haslam DW, James WPT. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 34.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–442. doi: 10.1097/MCO.0b013e3281a30594. [DOI] [PubMed] [Google Scholar]
- 36.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 37.Lavie CJ, De Schutter A, Patel DA, Milani RV. Does fitness completely explain the obesity paradox? Am Heart J. 2013;166:1–3. doi: 10.1016/j.ahj.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 38.McAuley PA, Beavers KM. Contribution of cardiorespiratory fitness to the obesity paradox. Prog Cardiovasc Dis. 2014;56:434–440. doi: 10.1016/j.pcad.2013.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data