Abstract
Two new species isolated from plant leaves belonging to Talaromyces section Talaromyces are reported, namely T. neofusisporus (ex-type AS3.15415 T = CBS 139516 T) and T. qii (ex-type AS3.15414 T = CBS 139515 T). Morphologically, T. neofusisporus is featured by forming synnemata on CYA and YES, bearing appressed biverticillate penicilli and smooth-walled fusiform conidia about 3.5–4.5 × 2–2.5 μm; and T. qii is characterized by velutinous colony texture, yellowish green conidia, yellow mycelium and ovoid to subglobose echinulate conidia measuring 3–3.5 μm. Phylogenetically, T. neofusisporus is such a unique species that no close relatives are found according to CaM, BenA and ITS1-5.8S-ITS2 as well as the combined three-gene sequences; and T. qii is related to T. thailandensis according to CaM, BenA and the combined sequence matrices, whereas ITS1-5.8S-ITS2 sequences do not support the close relationship between T. qii and T. thailandensis.
The species bearing symmetrical biverticillate penicilli, acerose phialides, with mycelium showing yellow, orange, pink or red tints, and the ascocarps, when present, being gymnothecial had been included in Penicillium section Biverticillata-Symmetrica by Raper and Thom1. Due to the rules of dual nomenclature, Pitt2 discriminated the anamorphic state from the teleomorphic state and placed the species only presenting anamorphic state in Penicillium subgenus Biverticillium and those with the teleomorphic state in the genus Talaromyces. However, the intrinsic difference between the species showing the above characters and other penicillia had long been well evidenced either by traditional characters [e. g.,3] or molecular phylogenetics [e. g.,4,5,6,7]. Recently, the Melbourne nomenclatural code abolished dual naming system and decided using a single name for a single species8, thusTalaromyces became the valid genus name for these species. In 2011, Samson et al. accepted 71 species in Talaromyces7. Later in 2012, Visagie and Jacobs established 3 new species9. In 2013, Manoch et al. reported 2 new members isolated from Thailand10. Then Peterson and Jurjević added another new member to the genus11. Afterwards, Sang et al. described 2 new taxa from Korea12 and Frisvad et al. reported a distinct species producing red pigment13. This year, Yilmaz et al. discovered 4 new members of the genus14. In a monographic study, Yilmaz et al. listed 88 species and divided Talaromyces into 7 sections, i. e. sections Talaromyces, Helici, Purpurei, Trachyspermi, Bacillispori, Subinflati and Islandici, among which, section Talaromyces included 36 species15. Just recently, Visagie et al. added 5 new members to this section16.
In the survey of phylloplane moulds in China, we discovered certain isolates showing the characters of the genus Talaromyces. Here, we report 2 additional new taxa of section Talaromyces, namely T. neofusisporus sp. nov. and T. qii sp. nov.
Results and Discussion
PCR amplification produced amplicons of the partial calmodulin gene (CaM) ca. 660 bp, partial β-tubulin gene (BenA) about 650 bp using primers I2 and Bt2b, and ca. 410 bp using primers Bt2a and Bt2b, the ITS1-5.8S-ITS2 region of the rDNA (ITS1-5.8S-ITS2) about 540 bp. The trimmed alignments of CaM, BenA, ITS1-5.8S-ITS2 and the combined three-gene sequences contained 553, 416, 459 and 1432 characters with gaps, respectively.
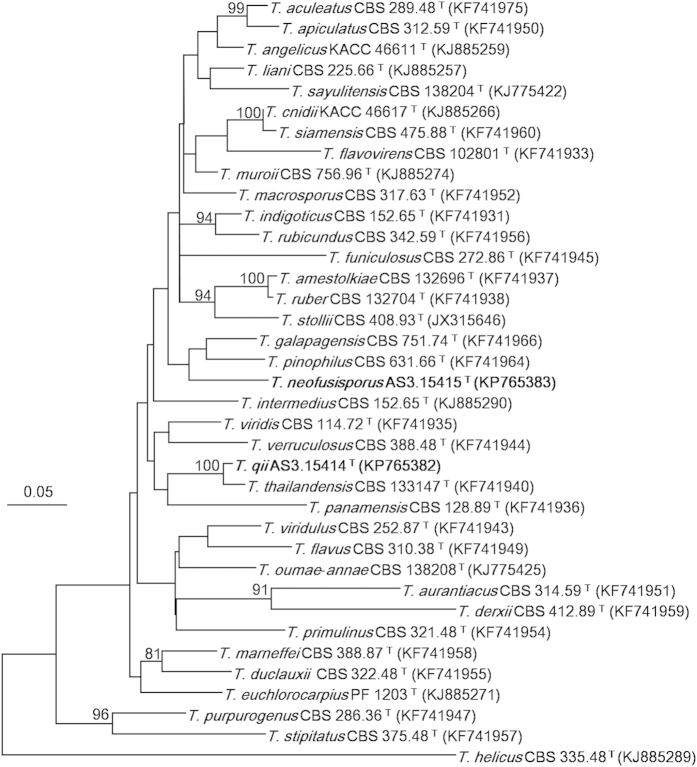
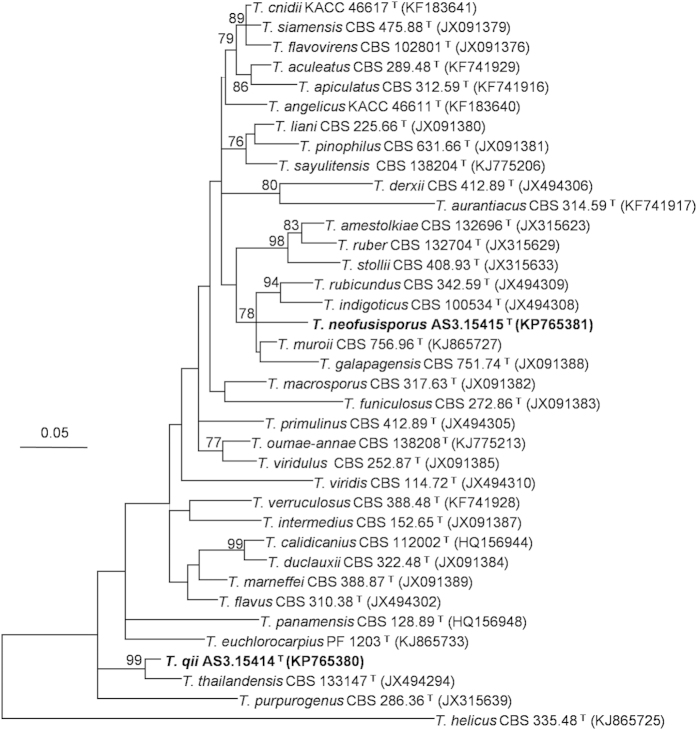
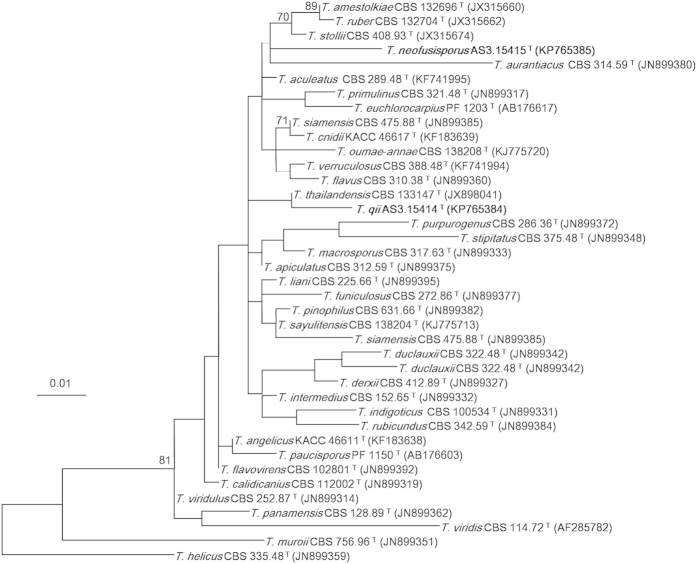
The Maximum Likelihood (ML) phylograms resulting from CaM, BenA, ITS1-5.8S-ITS2 and the three-gene matrices all showed that T. neofusisporus was a unique species without close relatives; and T. qii was closely related to T. thailandensis with 100%, 99% and 99% bootstrap support according to CaM, BenA and the three-gene sequences, respectively. However in the phylogram based on ITS1-5.8S-ITS2 region, T. qii had no close relatives. On the whole, either the individual or the combined analyses of the three genes supported T. neofusisporus and T. qii as valid new species (Figs 1, 2, 3, Supplementary Figure S1).
Figure 1. ML phylogram inferred from partial CaM sequences.
Bootstrap percentages over 70% derived from 1000 replicates are indicated at the nodes. Bar = 0.05 substitutions per nucleotide position.
Figure 2. ML phylogram inferred from partial BenA sequences.
Bootstrap percentages over 70% derived from 1000 replicates are indicated at the nodes. Bar = 0.05 substitutions per nucleotide position.
Figure 3. ML phylogram inferred from partial ITS1-5.8S-ITS2 sequences.
Bootstrap percentages over 70% derived from 1000 replicates are indicated at the nodes. Bar = 0.01 substitutions per nucleotide position.
Description of Talaromyces neofusisporus L. Wang, sp. nov
MycoBank: MB 811447
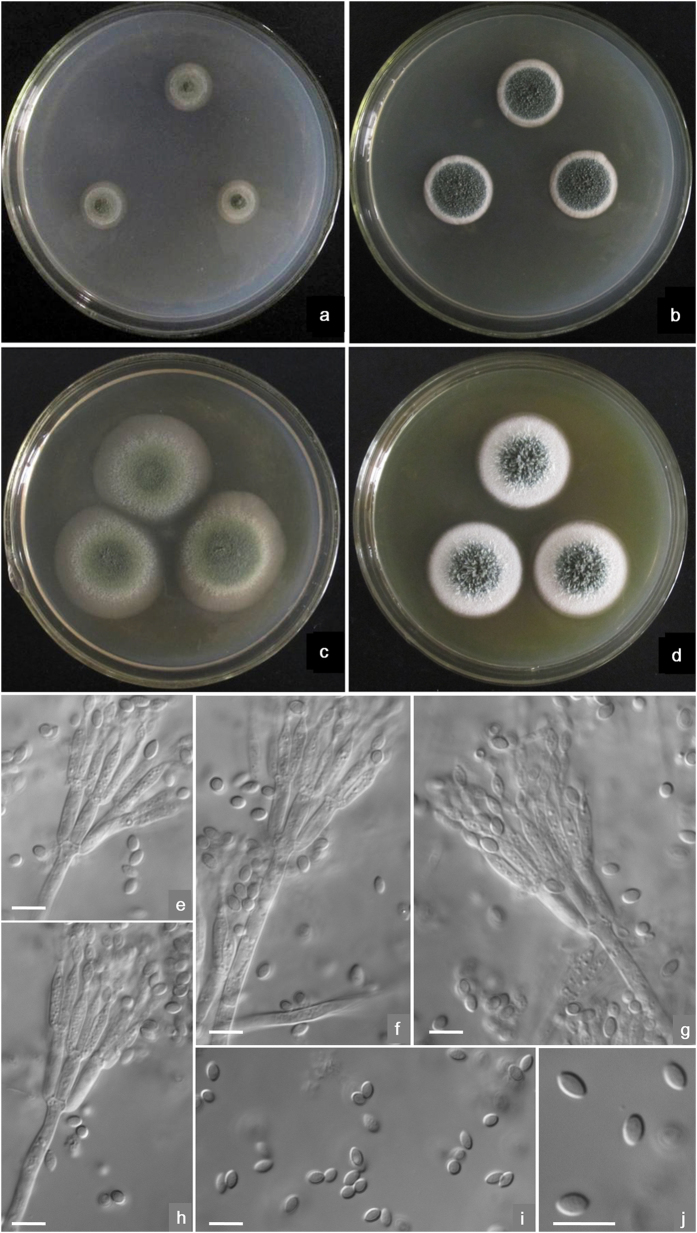
Etymology: The specific epithet is derived from the fusiform-shaped conidia of this species.(Fig. 4)
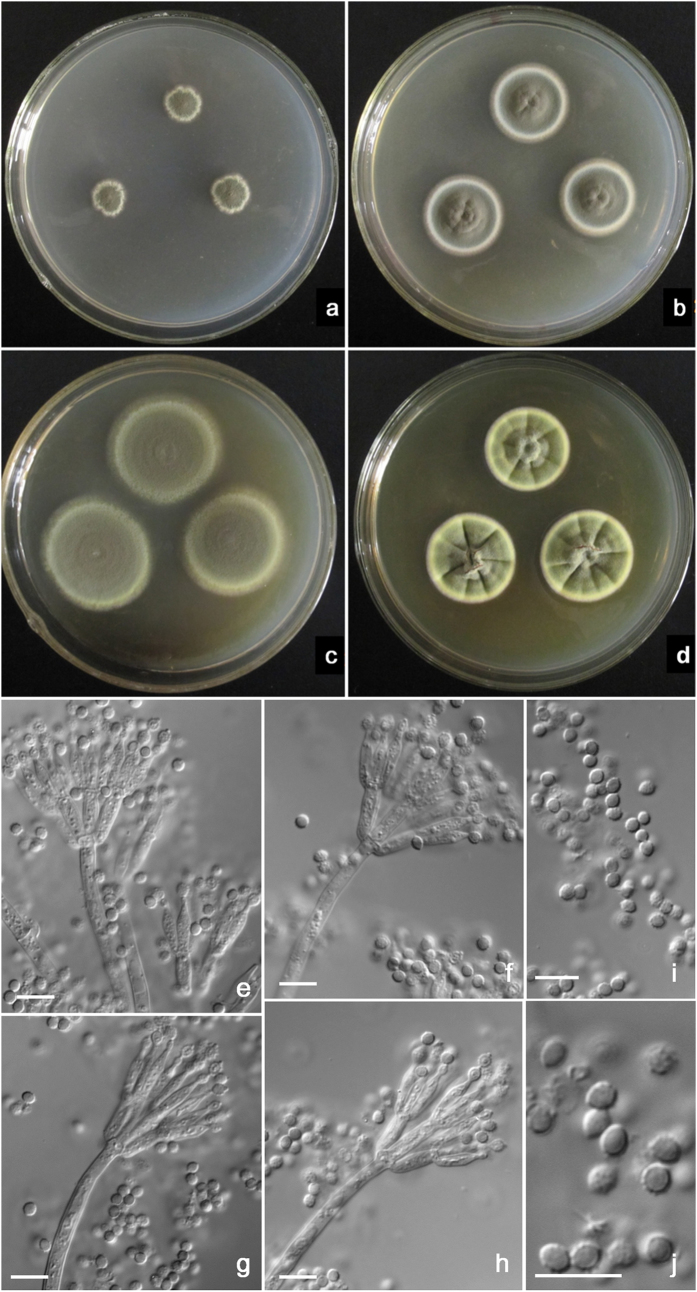
Figure 4. Morphological characters of T. neofusisporus AS3.15415 T.
(a) On Cz at 25 °C after 7 d; (b) On CYA at 25 °C after 7 d; (c) On MEA at 25 °C after 7 d; (d) On YES at 25 °C after 7 d; (e–h) Conidiophores; (i–j) Conidia. Bar = 10 μm.
Holotype: HMAS246033
On Cz at 25 °C after 7 d: Colonies 13–14 mm diam, plane, low, sparse, margins submerged; velutinous; conidiogenesis moderate at central areas, coloured near Grayish Olive (R. Pl. XLVI); mycelium white; no exudate and soluble pigment; reverse coloured Pale to Light Grayish Olive (R. Pl. XLVII). On CYA at 25 °C after 7 d: Colonies 19–20 mm diam, plane, low; surface appearing velutinous and granular due to synnemata about 1–2 mm long; conidiogenesis abundant, near Russian Green (R. Pl. XLII); mycelium white; no exudate and soluble pigment; reverse coloured near Cream Color (R. Pl. XVI). On MEA at 25 °C after 7 d: Colonies 33–36 mm diam, low, plane, margins submerged; velutinous; conidiogenesis abundant, near Deep Dull Yellow-Green (1) (R. Pl. XXXII); mycelium white; no exudate and soluble pigment; reverse coloured near Naphthalene Yellow (R. Pl. XLI). On YES at 25 °C after 7 d: Colonies 26–28 mm diam, loose and deep; mycelium white, aggregated into synnemata about 2–3 mm long in central areas; conidiogenesis moderate at central areas, near Russian Green (R. Pl. XXXII); mycelium white; no exudate and soluble pigment; reverse coloured Ochraceous-Buff to Light Ochraceous-Buff (R. Pl. XV). No growth at 5 °C on CYA. On CYA at 37 °C after 7 d, colonies 2–3 mm diam with white mycelium only.
Conidiophores arising from agar surface and synnemata; stipes 120–180 (−200) × 3–3.5 μm when from surface, but 85–120 μm when from synnemata, smooth-walled; penicilli biverticillate; appressed metulae 4–6 (−8) per stipe, 9 –11 × 2.5–3 μm; phialides 2–4 per metula, acerose with distinguishable collula, 9–11 × 2.5–3 μm; conidia fusiform, (3.5−) 4–4.5 (−5) × 2–2.5 μm, smooth-walled, born in irregularly tangled chains about 120 μm forming loose brushes. Teleomorphic state unknown.
Strains examined. CHINA. Tibet: Motuo County, 29°41′37″N 94°43′36″E, 3700 m; ex-type culture AS3.15415 T = CBS 139516 T from an unidentified leaf sample no. 150C6, 19 Sep 2014, Q-M. Wang. (HOLOTYPE: HMAS 246033, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China; dried culture of ex-type AS3.15415 T on Cz).
Notes. T. neofusisporus is characterized by synnemata, fusiform conidia, and the growth at 37 °C.
Description of Talaromyces qii L. Wang, sp. nov
MycoBank: MB 811448
Etymology: The specific epithet is in honour of Prof. Zu-Tong Qi, who made great contribution to the Aspergillus and Penicillium taxonomy in China. (Fig. 5)
Figure 5. Morphological characters of T. qii AS3.15414 T.
(a) On Cz at 25 °C after 7 d; (b) On CYA at 25 °C after 7 d; (c) On MEA at 25 °C after 7 d; (d) On YES at 25 °C after 7 d; (e–h) Conidiophores; (i–j) Conidia. Bar = 10 μm.
Holotype: HMAS246032
On Cz at 25 °C after 7 d: Colonies 10–13 mm diam, thin, plane, margins irregular, submerged; velutinous; conidiogenesis abundant, coloured near Dark Dull Yellow-Green (R. Pl. XXXII) or Light Hellebore Green (R. Pl. XVII); mycelium coloured near Pale Dull Green-Yellow (R. Pl. XVII); no exudate and soluble pigment; reverse coloured Orange in central areas and Maize Yellow at marginal areas (R. Pl. XV). On CYA at 25 °C after 7 d: Colonies 23–24 mm diam, thin, plane but protuberant centrally with slightly radial and annular plicates; margins regular and submerged; velutinous; conidiogenesis abundant, near Dark Dull Yellow-Green (R. Pl. XXXII); mycelium coloured near Pale Dull Green-Yellow (R. Pl. XVII); no exudate and soluble pigment; reverse coloured near Hay’s Russet (R. Pl. XIV), with a Light Ochraceous-Salmon tint at marginal areas (R. Pl. XV). On MEA at 25 °C after 7 d: Colonies 33–35 mm diam, low, plane, margins regular and submerged; velutinous; conidiogenesis abundant, near Pois Green (R. Pl. XLI); mycelium with a Chalcedony Yellow tint (R. Pl. XVII); no exudate and soluble pigment; reverse coloured near Deep Colonial Buff (R. Pl. XXX). On YES at 25 °C after 7 d: Colonies 25–27 mm diam, low, slightly with radial plicates, protuberant in centers, margins regular; velutinous, conidiogenesis abundant, Pois Green (R. Pl. XLI); mycelium with a Clear Yellow-Greeen (R. Pl. VI) tint; no exudate and soluble pigment; reverse coloured near Hay’s Russet (R. Pl. XIV) with Ochraceous-Buff to Light Ochraceous-Buff colour at margins. No growth at 5 °C and 37 °C on CYA.
Conidiophores arising from substrate; stipes (150–) 200–300 (−360) × 3.5–4 μm, smooth-walled; with biverticillate penicilli; metulae 4–6 (−8) per stipe, 7–11 (−13) × 2.5–3 μm; phialides 2– 4 per metula, acerose to ampulliform with short collula, 7–9 × 2–2.5 (−3) μm; conidia ovoid to suglobose, 3–3.5 μm, walls echinulate, born in irregularly tangled chains forming loose masses about 120 μm. Teleomorphic state unknown.
Strains examined. CHINA. Tibet: Motuo County, 29°16′30″N 95°15′04″E, 1211 m; ex-type culture AS3.15414 T = CBS 139515 T from an unidentified leaf sample no. 125E25, 19 Sep 2014, Q-M. Wang. (HOLOTYPE: HMAS 246032, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China; dried culture of ex-type AS3.15414 T on Cz).
Notes. T. qii is characterized by the velvety colony texture, yellow-coloured mycelium and ovoid to subglobose echinulate conidia.
T. neofusisporus and T. qii are located in Talaromyces section Talaromyces, which including 41 species according to the most recent phylogenetic studies by Yilmaz et al. and Visagie et al.15,16.
Yilmaz et al. reported 12 species that can produce synnemata in the genus Talaromyces, and that only 4 members belong to section Talaromyces, namely T. calidicanius, T. duclauxii, T. flavovirens, and T. panamensis15. In this paper, another synnema-producing member is added, i. e. T. neofusisporus. In addition to the phylogenetic evidence of Figs 1, 2, 3 and Supplementary Figure S1 indicating that T. neofusisporus is not related to the above 4 species, this new taxon can also be readily distinguished from them morphologically.
T. neofusisporus can be distinguished from T. calidicanius in that it grows slowly on standard media at 25 °C (Cz 13–14 mm, CYA 19–20 mm, MEA 33–36 mm, YES 26–28 mm) showing the synnematous colony texture only on CYA and YES, bears exclusive appressed biverticillate penicilli with smooth-walled fusiform conidia. However, T. calidicanius grows faster (CYA 27–30 mm, MEA 47–48 mm, YES 40–41 mm), presenting funiculose and floccose texture with long synnemata about 6 mm on all standard media; additionally, it produces a minor portion of biverticillate penicilli bearing subterminal branches, and rough-walled to striate-walled conidia15,17.
T. duclauxii is also a species that produces long synnemata up to 5 mm within 7 days on all standard media, giving a deep fluffy colony appearance; it also bears a portion of biverticillate penicilli with subterminal branches on sinuous stipes, and ellipsoidal to subglobose, smooth to finely rough-walled conidia. However, T. neofusisporus only produces discernible synnematous colony texture on CYA and YES, and shows velvety texture on Cz and MEA; besides, it produces strictly biverticillate penicilli and straight conidiophore stipes, with smooth-walled fusiform conidia1,2,15.
Although the characters of low growth rate, velvety colony appearance on Cz and MEA, and abundant sporulation fairly resemble those of T. flavovirens, T. neofusisporus bears relatively long synnemata about 2–3 mm on YES that are obvious to the naked eye within 7 days, with no gymnothecia observed neither in nature nor on artificial media, yet T. flavovirens bears shorter synnemata only 750 μm in length after prolonged culturing and produces ascomata on Quercus suber leaf litter according to Visagie et al. Moreover, the mycelium of T. neofusisporus is in white colour, while that of T. flavovirens is with a yellow tint. Furthermore, the penicilli are strictly biverticillate and conidia are fusiform-shaped in T. neofusisporus, but in T. flavovirens a minor portion of biverticillate penicilli with subterminal branches and ellipsoidal conidia are observed15,18.
The low growth rate, fusiform conida and grayish-coloured conidia en masse of T. neofusisporus are similar to those of T. panamensis. However, T. neofusiporus gives a moderate to abundant sporulation on culturing media, does not form synnemata on MEA, but instead bears obvious synnemata on YES without visible yellow stalks. On the contrary, T. panamensis sporulates sparsely on all media, does not produce synnemata on YES, while produces yellow-stalked synnemata on MEA. Furthermore, T. neofusisporus bears only biverticillate penicilli on relatively longer stipes commonly about 85–120 μm even from synnemata, whereas T. panamensis produces a minor portion of biverticillate penicilli with subterminal branches on shorter stipes ca. 40–85 μm15,19.
In the phylograms inferred from CaM, BenA and the combined three-gene sequences, T. qii is closely related to T. thailandensis with 100%, 99% and 99% bootstrap support, respectively. However the phylogram based on ITS1-5.8S-ITS2 does not support a close relationship between them, because though they fall in the same clade, there is no significant bootstrap support for this clade (Fig. 3). In addition, T. qii and T. thailandensis can be readily distinguished from each other morphologically in that T. thailandensis produces yellow gymnothecia, ellipsoidal spiny ascospores, but no sexual state is found in T. qii. Additionally, T. qii grows more slowly (CYA 23–24 mm, MEA 33–35 mm, YES 25–27 mm) than T. thailandensis (CYA 45–47 mm, MEA 40–42 mm, YES 47–50 mm), and produces abundant echinulate conidia on all the standard media, while the sporulation of T. thailandensis is almost absent or sparse and its conidia are smooth-walled10,15.
The moderate growth rate at 25 °C and no growth at 37 °C, as well as the ovoid to subglobose rough-walled conidia of T. qii indicate certain similarities to T. kendrickii. But T. qii shows a velvety colony texture with heavy sporulation and without exudate, while in T. kendrickii, the colony texture is floccose, the sporulation is sparse and abundant reddish to pinkish exudate is obviously present. Moreover in micromorphology, T. qii bears only biverticillate penicilli, but some monoverticillate penicilli are present in T. kendrickii. Further, the stipe lengths of T. qii are shorter (150–360 μm) than those of T. kendrickii (150–500 μm), and T. qii produces larger conidia (3–3.5 μm) than those of T. kendrickii (2.5–3 μm).
The characters of the plane dense colonies, heavy sporulation, conidia en masse coloured dull green with a yellowish green tint of T. qii remind of the resemblance to T. diversus and T. cnidii. However, T. qii grows much faster on CYA (23–24 mm) and YES (25–27 mm) than T. diversus on CYA (7–10 mm) and YES (8–10 mm). Besides, T. qii does not grow at 37 °C, while T. diversus can form colonies about 2–8 mm in diam. Still, the penicilli are strictly biverticillate in T. qii, but T. diversus bears a portion of biverticillate penicilli with subterminal branches. In addition to these morphological differences, the phylogenetic work of Yilmaz et al. indicates that T. diversus is a member of section Trachyspermi, which is phylogenetically fairly distant to section Talaromyces where T. qii is located1,2,15. Comparing with T. cnidii, the growth rate of T. qii is slower (CYA 23–24 mm, MEA 33–35 mm, YES 25–27 mm) than that of T. cnidii (CYA 30–35 mm, MEA 38–43 mm, YES 40–45 mm), and T. qii does not excrete soluble pigment on CYA, while T. cnidii produces strong red pigment. Additionally, the conidia of T. qii are ovoid to subglobose with echinulate walls, whereas those of T. cnidii are ellipsoidal with smooth to finely rough walls12,15. Besides the morphological disparity, our phylograms resulted from CaM, BenA, ITS1-5.8S-ITS2 and the combined sequences all demonstrate that T. qii and T. cnidii are located in different clades in section Talaromyces (Figs 1, 2, 3, Supplementary Figure S1).
The characters of similar growth rate, velvety colony texture, yellowish dull green conidia en masse and yellowish mycelium also present certain resemblance between T. qii and T. ruber. However, the colony reverse of T. qii on Cz and CYA shows a tint of orange colour but that of T. ruber coloured cherry red or brownish red. Besides, the conidia of T. qii are ovoid to subglobose with echinulate walls, but those of T. ruber are ellipsoidal and smooth-walled1,15. In addition to the morphological evidence, our molecular phylograms inferred separately from the three loci and the combined sequences all indicate that T. qii and T. ruber lie in well-separated clades (Figs 1, 2, 3, Supplementary Figure S1).
Plant phylloplane is one major natural habitat for bacteria, yeasts and filamentous fungi20,21. The phylloplane microbial species are recruited from the airborne taxa in the environment and then a distinct phylloplane microbial community is constructed. During this process, plant genotypes play an important role as the selection force by providing specific nutrients and habitats22,23,24. Thus, more new or distinctive microbial taxa might be discovered from phyllosphere where the plant diversity is abundant. Correspondingly, habitant microbes produce growth hormones for host plants and assist host plants in the antagonism against pathogens in many respects25,26. In this sense, the discovery of new phylloplane taxa may provide potential biocontrol agents.
Materials and Methods
Isolation of strains
Leaf samples from living trees were collected and kept in sterilized plastic bags and reserved in a mobile refrigerator. The isolation of phylloplane fungi followed the method of Nakase and Takashima27. No specific permissions were required for these locations/activities. The field studies did not involve endangered or protected species and the GPS coordinates of the specific locations in our study are 29°41′37″N 94°43′36″E and 29°16′30″N 95°15′04″E. The isolates were deposited in China General Microbiological Culture Collection (CGMCC), Institute of Microbiology, Chinese Academy of Sciences, Beijing, China. The ex-type cultures of T. neofusisporus AS3.15415 T and T. qii AS3.15414 T were also deposited in the culture collection of the CBS-KNAW Fungal Biodiversity Centre in The Netherlands as CBS 139516 T and CBS 139515 T, respectively. The cultures are also reserved at the corresponding author’s laboratory and will be supplied upon request for educational or scientific purpose.
Morphological studies
Colony characters were examined using Czapek agar (Cz, Raper and Thom1), Czapek yeast autolysate agar (CYA, Pitt2), 2% malt extract agar (MEA, malt extract (Difco), Pitt2), YES (yeast extract sucrose agar, yeast extract (Oxoid), Frisvad and Samson28). Colour names were referred to Ridgway29. Wet mounts were made using material from colonies growing on MEA at 25 °C after 7 d and mounted in 85% lactic acid without dye. The microscopic characters were examined and photographed using Axioplan2 imaging and Axiophot2 universal Microscope (Carl Zeiss Shanghai Co. Ltd.).
Molecular studies
Genomic DNA extraction method was referred to Scott et al.30. BenA sequences were amplified with the sense primers btI231 or Bt2a, and the antisense primer Bt2b32; ITS1-5.8S-ITS2 sequences were obtained using the primers ITS5 and ITS433; the CaM PCR amplification was achieved with the primers AD1 and Q134. PCR reaction was employed in 20 μL reaction mixture containing 0.5 μL of each primer (10 pM/μL), 1.0 μL of genomic DNA (10 ng/μL), 8 μL of 2 × PCR MasterMix buffer (0.05 u/μL Taq polymerase, 4 mM MgCl2, 0.4 mM dNTPs), and 10 μL of double-distilled water (Tsingke Biotechnologies Co., Ltd., Beijing, China). Amplifications were performed in a PTC-150 thermocycler (MJ Research, Watertown, Massachusetts, USA), which was programmed for 3-min. denaturation at 94 °C followed by 34 cycles of 94 °C for 30 s, 50 °C for 30 s and extension for 45 s at 72 °C, with a final 5 min. elongation step at 72 °C. After amplification, the PCR fragments were electrophoresed in 2.0% agarose gels with a 100 bp DNA ladder (MBI Fermentas) at 80 V for 20 min. Gels were stained in 0.5 μg/mL ethidium bromide water solution for 15 min and checked under 254 nm UV with a portable UV light. Samples showing one clear, single band of the anticipated length on the gel were then purified and sequenced in double direction with an ABI 3700 DNA analyzer (Applied Biosystems, Inc., Foster City, California, USA). Raw sequences were proof-read and edited manually with BioEdit 7.0.935. Edited sequences were aligned using muscle implemented in MEGA version 536. The new species together with 34 ex-type strains without T. calidicanius and T. paucisporus were included in CaM analysis; with 34 ex-type isolates of section Talaromyces without T. paucisporus and T. stipitatus were in BenA analysis; and with 36 ex-type cultures were subjected to ITS1-5.8S-ITS2 analysis; while with 33 ex-type isolates lacking T. calidicanius, T. paucisporus and T. stipitatus were in the combined three-gene analysis. T. helicus of section Helici15 was as the outgroup in all these analyses (Figs 1, 2, 3, Supplementary Figure S1). First, these sequence data were determined for the most optimal substitution model and the rates among sites using the function “Find the Best DNA/Protein Models (ML)” of MEGA version 5, and Kimura-2 parameter substitution model and Gamma distributed with Invariant sites (G + I) for rates among sites were found to be the best, gaps were treated as partial deletion according to Hall37. All the sequence matrices were analyzed using the ML method and subjected to 1000 bootstrap replications.
Additional Information
How to cite this article: Wang, Q.-M. et al. Talaromyces neofusisporus and T. qii, two new species of section Talaromyces isolated from plant leaves in Tibet, China. Sci. Rep. 6, 18622; doi: 10.1038/srep18622 (2016).
Supplementary Material
Acknowledgments
Yun Yu did some molecular work.
Footnotes
Author Contributions Conceived and designed the experiments: L.W. Performed the experiments: Q.M.W., Y.H.Z. and B.W. Analyzed the data: Q.M.W., Y.H.Z. and B.W. Contributed reagents/materials/analysis tools: Q.M.W. and L.W. Wrote the paper: L.W.
References
- Raper K. B. & Thom C. A manual of the penicillia (Williams and Wilkins, 1949). [Google Scholar]
- Pitt J. I. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces (Academic Press, 1979). [Google Scholar]
- Malloch D. The trichocomaceae: relationships with other Ascomycetes. In Advances In Penicillium and Aspergillus systematics. eds Samson R. A., Pitt J. I. pp. 365–382, Plenum Press, New York (1985). [Google Scholar]
- LoBuglio K. F., Pitt J. I & Taylor J. W. Phylogenetic analysis of two ribosomal DNA regions indicates multiple independent losses of a sexual Talaromyces state among asexual Penicillium species in Subgenus Biverticillium. Mycologia 85, 592–604 (1993). [Google Scholar]
- Wang L. & Zhuang W.-Y. Phylogenetic analyses of penicillia based on partial calmodulin gene sequences. BioSystems 88, 113–126 (2007). [DOI] [PubMed] [Google Scholar]
- Houbraken J. & Samson R. A. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud. Mycol. 70, 1–55 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R. A. et al. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 70, 159–183 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill J. et al. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum Vegetabile 154 (Koeltz Scientific Books, 2012). [Google Scholar]
- Visagie C. M. & Jacobs K. Three new additions to the genus Talaromyces isolated from Atlantis sandveld fynbos soils. Persoonia 28, 14–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoch L., Dethoup T., Yilmaz N., Houbraken J. & Samson R. A. Two new Talaromyces species from soil in Thailand. Mycoscience 54, 335–342 (2013). [Google Scholar]
- Peterson S. W. & Jurjević Ž. Talaromyces columbinus sp. nov., and genealogical concordance analysis in Talaromyces Clade 2a. Plos ONE 8(10), e78084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang H. et al. Two novel Talaromyces species isolated from medicinal crops in Korea. J. Microbiol. 51, 704–708 (2013). [DOI] [PubMed] [Google Scholar]
- Frisvad J. C. et al. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 8(12), e84102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz N. et al. Taxonomic re-evaluation of species in Talaromyces section Islandici, using a polyphasic approach. Persoonia 36, 637–656 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz. N., Visagie C. M., Houbraken J., Frisvad J. C & Samson R. A. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 78, 175–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie C. M. et al. Five new Talaromyces species with ampulliform-like phialides and globose rough conidia resembling T. verruculosus. Mycoscience 56, 486–502 (2015). [Google Scholar]
- Chen L., Yen J. H., Lin W. S. & Ku W. L. A new synnematous species of Penicillium from soil in Taiwan. Mycologia 94, 866–872 (2002). [PubMed] [Google Scholar]
- Visagie C. M., Llimona X., Vila J., Louis-Seize G. & Seifert K. A. Phylogenetic relationships and newly discovered sexual state of Talaromyces flavovirens, comb. nov. Mycotaxon 122, 399–411 (2012). [Google Scholar]
- Samson R. A., Stolk A. C. & Frisvad J. C. Two new synnematous species of Penicillium. Stud. Mycol. 31, 133–143 (1989). [Google Scholar]
- Vorholt J. A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840 (2012). [DOI] [PubMed] [Google Scholar]
- Bringel F. & Couée I. Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Front. Microbiol. 6, 486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maignien L., DeForce E. A, Chafee M. E, Eren A. M. & Simmons S. L. Ecological succession and stochastic variation in the assembly of Arabidopsis thaliana phyllosphere communities. mBio 5(1), e00682–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenhausen N. Bortfeld-Miller M., Ackermann M. & Vorholt J. A. A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet. 10(4), e1004283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota R., Knorr K., Jørgensen L. N., O’Hanlon K. A. & Nicolaisen M. Host genotype is an important determinant of the cereal phyllosphere mycobiome. New Phytol. 207, 1134–1144 (2015). [DOI] [PubMed] [Google Scholar]
- Berg G. Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 84, 11–18 (2009). [DOI] [PubMed] [Google Scholar]
- Pieterse C. M., Van der Does D., Zamioudis C., Leon-Reyes A.& Van Wees S. C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521 (2012). [DOI] [PubMed] [Google Scholar]
- Nakase T. & Takashima M. A simple procedure for the high frequency isolation of new taxa of ballistosporous yeasts living on the surfaces of plants. RIKEN Review 3, 33–34 (1993). [Google Scholar]
- Frisvad J. C. & Samson R. A. Polyphasic taxonomy of Penicillium subgen. Penicillium. A guide to identification of food and air-borne terverticillate penicillia and their mycotoxins. Stud. Mycol. 49, 1–173 (2004). [Google Scholar]
- Ridgway R. Color standards and color nomenclature (Published by the author, 1912). [Google Scholar]
- Scott J., Malloch D., Wong B., Sawa T. & Straus N. DNA heteroduplex fingerprinting in Penicillium. in Integration of modern taxonomic methods for Penicillium and Aspergillus classification. eds Samson R. A., Pitt J. I. pp. 225–236, Harwood Academic Publishers, Amsterdam (2000). [Google Scholar]
- Wang B. & Wang L. Penicillium kongii, a new terverticillate species isolated from plant leaves in China. Mycologia 105, 1547–1554 (2013). [DOI] [PubMed] [Google Scholar]
- Glass N. L. & Donaldson G. C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. App. Environ. Microbiol. 61, 1323–1330 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. J., Bruns T., Lee S. & Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: a guide to methods and applications. eds Innis M. S., Gelfand D. H. pp. 315–322, Academic Press, New York (1990).
- Wang L. Four new records of Aspergillus section Usti from Shandong Province, China. Mycotaxon 120, 373–384 (2012). [Google Scholar]
- Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 41, 95–98 (1999). [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 30, 1229–1235 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.