Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is an effective therapy for patients with reversible cardiac and/or respiratory failure. Acute kidney injury (AKI) often occurs in patients supported with ECMO; it frequently evolves into chronic kidney damage or end-stage renal disease and is associated with a reported 4-fold increase in mortality rate. Although AKI is generally due to the hemodynamic alterations associated with the baseline disease, ECMO itself may contribute to maintaining kidney dysfunction through several mechanisms.
Summary
AKI may be related to conditions derived from or associated with extracorporeal therapy, leading to a reduction in renal oxygen delivery and/or to inflammatory damage. In particular, during pathological conditions requiring ECMO, the biological defense mechanisms maintaining central perfusion by a reduction of perfusion to peripheral organs (such as the kidney) have been identified as pretreatment and patient-related risk factors for AKI. Hormonal pathways are also impaired in patients supported with ECMO, leading to failures in mechanisms of renal homeostasis and worsening fluid overload. Finally, inflammatory damage, due to the primary disease, heart and lung crosstalk with the kidney or associated with extracorporeal therapy itself, may further increase the susceptibility to AKI. Renal replacement therapy can be integrated into the main extracorporeal circuit during ECMO to provide for optimal fluid management and removal of inflammatory mediators.
Key Messages
AKI is frequently observed in patients supported with ECMO. The pathophysiology of the associated AKI is chiefly related to a reduction in renal oxygen delivery and/or to inflammatory damage. Risk factors for AKI are associated with a patient's underlying disease and ECMO-related conditions.
Key Words: Acute kidney injury, Renal replacement therapy, Extracorporeal membrane oxygenation, Carbon dioxide removal, Systemic inflammation
Introduction
Extracorporeal membrane oxygenation (ECMO) is an artificial extracorporeal system capable of providing temporary respiratory and circulatory support [1,2]. Acute kidney injury (AKI) is frequently observed during ECMO therapy [3]; several studies report a high incidence of AKI in patients supported with ECMO [4,5], with a reported 4-fold increase in mortality rate [4].
Askenazi et al. [6] reported a mortality rate of 27.4 and 41.6%, respectively, for neonatal and pediatric patients supported with ECMO for noncardiac indications. In these groups, an odds ratio for death of 3.2 and 1.7, respectively, was observed if AKI was present. The importance of AKI in adult ECMO patients as a factor for mortality appears to be similar. In a retrospective analysis, Kielstein et al. [7] showed that in adult patients receiving ECMO, AKI requiring renal replacement therapy (RRT) was associated with an overall 98-day survival of only 17%.
Moreover, the need for RRT during ECMO is considered an independent risk factor associated with failure to wean from ECMO [8] and an independent predictor of mortality [9]. Considering that an additional organ failure has a detrimental effect on patient outcome, the extraordinary increase in mortality due to the association between ECMO and RRT is not surprising [7].
Apart from evidence of an increased mortality in ECMO patients suffering from AKI, the pathophysiological mechanisms of kidney injury during ECMO remain poorly understood. Certainly, AKI in patients treated with ECMO is mainly due to the hemodynamic alterations associated with the baseline disease. However, despite the fact that ECMO effectively supports organ function in these patients, it may contribute to maintaining the kidney's dysfunction through several mechanisms. The possible causative effects of ECMO on the kidney require an articulated discussion and need to be elucidated. Furthermore, the concomitant risk derived from a preexisting renal dysfunction should be evaluated in light of a possible increased susceptibility of the kidney to considerable exposure to a stressor such as the ECMO circuit.
The aim of this report is to review various mechanisms that may be responsible for AKI in ECMO patients and to identify their specific pathophysiological pathways.
Physiological and Pathological Changes
Alterations in kidney function in patients supported with ECMO may be related to several conditions (table 1) derived from or associated with the extracorporeal therapy (fig. 1) which potentially lead to a reduction in renal DO2 (fig. 2) and/or to inflammatory damage (fig. 3). In particular, the incidence of AKI in these patients may be due to an imbalance between factors able to protect renal function (e.g. the increase in kidney perfusion obtained with high-support venoarterial ECMO in patients with cardiogenic shock) and factors able to induce a kidney injury or impairment (e.g. ischemia-reperfusion damage or systemic inflammation).
Table 1.
Potential pathophysiological pathways that correlate ECMO and AKI
| Patient-related (ECMO-independent) variables | |
| Pretreatment factors | Hypoperfusion, loss of autoregulation Hypoxia |
| Nephrotoxic drugs Systemic inflammation | |
| ECMO-related variables | |
| Hemodynamic factors | Blood flow alterations |
| Hormonal factors | Renin-angiotensin-aldosterone dysregulation ANP downregulation |
| ECMO-related | Blood shear stress |
| systemic inflammation | Exposure to a non-self membrane Blood/air interface |
| Organ crosstalk | Cardiorenal syndromes Lung/kidney interactions |
| Circuit-related factors | Hypermyoglobinemia |
| Embolism | |
| Hemolysis | |
Fig. 1.
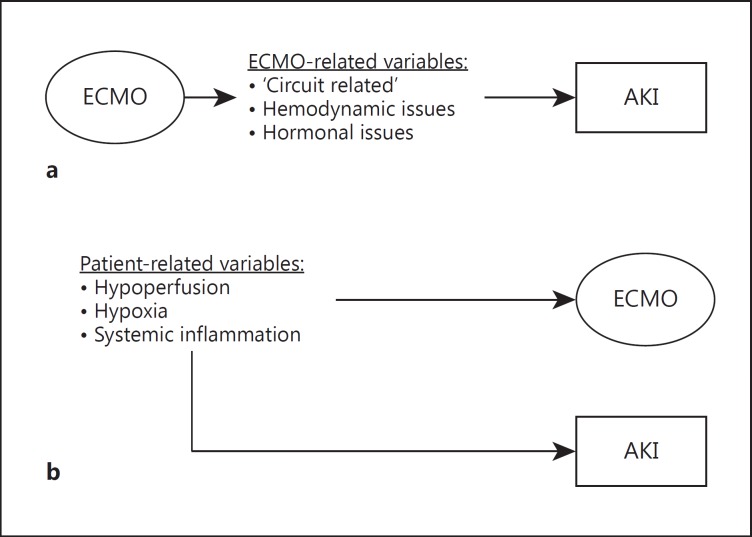
Relationship between ECMO and AKI. a Variables directly derived from ECMO therapy may cause AKI; in this hypothesis, ECMO contributes to maintaining kidney dysfunction and causally participates in the development of AKI. b Pretreatment variables or variables which lead to ECMO initiation may also cause AKI development; in this hypothesis, AKI is an epiphenomenon of ECMO and derives from ECMO-independent variables. Theoretically, a third hypothesis may be presented, in which patients with AKI may further require ECMO initiation (e.g. for a cardiorenal syndrome type 3), but this hypothesis is beyond the intention of this review.
Fig. 2.
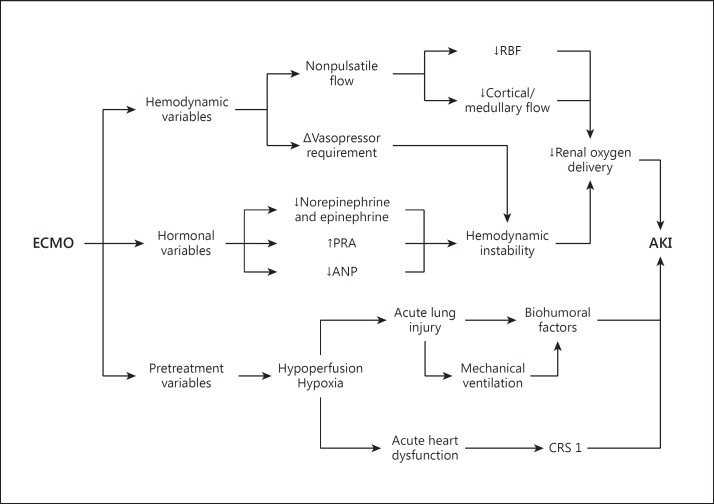
Major hemodynamic factors related to the development of AKI in patients treated with ECMO. CRS 1 = Cardiorenal syndrome type 1; RBF = renal blood flow.
Fig. 3.
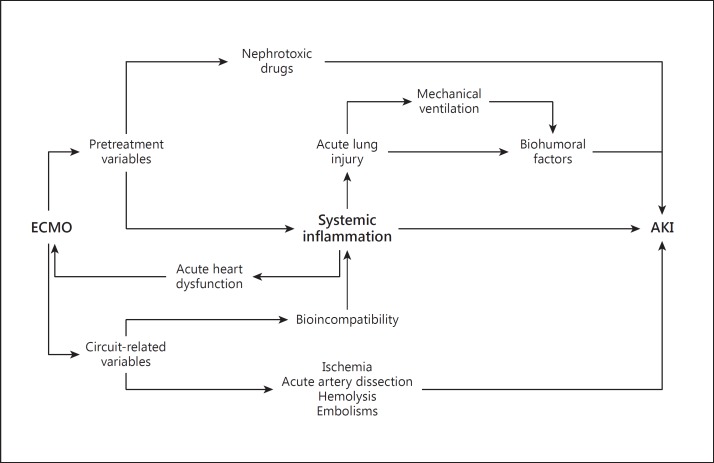
Major inflammatory factors related to the development of AKI in patients treated with ECMO.
Patient-Related Variables
Several conditions in patients to be treated with ECMO may induce AKI before ECMO is initiated; in particular, AKI in patients treated with ECMO is generally due to the hemodynamic alterations associated with the baseline disease. In patients with low cardiac output, biological defense mechanisms maintain perfusion to central organs such as the heart and brain by reducing perfusion to peripheral organs such as the kidney. In such situations, autoregulation leads to a decrease in renal cortical blood flow [10] and may be responsible for clinical AKI (fig. 2).
Intensive life-sustaining therapy has usually been performed on critically ill patients with refractory cardiopulmonary failure before ECMO is initiated, and vasopressor therapy, as well as the use of nephrotoxic medications, certainly increases the risk for development of AKI [3]. Moreover, organ ischemia, respiratory failure, cardiac failure and systemic inflammation leading to multiorgan dysfunction could have an additional role (fig. 3).
In a retrospective study, Lin et al. [4] observed that AKI is commonly present before ECMO in patients with postcardiotomy cardiogenic shock. Furthermore, a higher RIFLE stage at the onset of ECMO was associated with a significant increase in mortality rate for all patients (20% for non-AKI, 57.1% for RIFLE-R, 72.2% for RIFLE-I and 100% for RIFLE-F).
ECMO-Related Variables
Hemodynamic Variables
During ECMO, there are important changes in hemodynamics and in the perfusion of specific organs [3]. Although venoarterial ECMO is instituted to improve overall perfusion in patients with cardiac failure and hemodynamic shock, the continuous flow generated by the ECMO system may not be sufficient to maintain adequate tissue perfusion and DO2 in peripheral organs such as the kidney (fig. 2) [11]. Pulsatile blood flow is important in preserving renal cortical blood flow [12]. The importance of pulsatile perfusion in maintaining renal function has been extensively demonstrated [13,14]. Using an animal model of cardiogenic shock, Nemoto [12] studied renal perfusion obtained with different kinds of extracorporeal ventricular support. In particular, in this study inadequate renal artery flow was evident in pigs with cardiogenic shock supported with a continuous-flow extracorporeal support. Although a higher level of renal perfusion was obtained by increasing the percentage of extracorporeal support, only with an assist rate greater than 80% the renal perfusion was not statistically different from that obtained with a more physiological pulsatile flow. Moreover, the renal cortical/medullar blood flow ratio, which was reduced during clinical shock, was further decreased during the first 3 h of extracorporeal support with low-level assistance and only moderately increased with high-level assistance [12]. On the other hand, in hemodynamically stable patients requiring venovenous ECMO, the native pulsatile cardiac output is maintained with minimal repercussions on renal perfusion [3]. However, these results have not been uniformly confirmed in the literature [15].
During the early phase of ECMO support, close attention to hemodynamic monitoring is important, as the reduction in vasoactive drugs may increase a patient's susceptibility to hemodynamic fluctuations with considerable impairment of renal blood flow, leading to ischemia-reperfusion-associated AKI [16].
Hormonal Variables
Several studies in the literature have analyzed hormonal changes during extracorporeal ventricular support. A downregulation of hormonal mechanisms – which are capable of inducing cardiac and renal dysfunction if chronically activated – has been recognized during long-term ventricular support with left ventricular assist devices [10]. However, different effects on hormonal pathways have been identified for acute ventricular support with ECMO (fig. 2).
In particular, renin-angiotensin-aldosterone downregulation has been clearly demonstrated to influence volume load and cardiac and renal functions early and positively in chronic heart failure patients supported with a left ventricular assist device. The same is not uniformly recognized during ECMO. Contrasting results have been reported in the literature regarding renin levels in ECMO patients. An upregulation of plasma renin activity (PRA) is identified as a possible cause of the acute hypertension observed in children with respiratory failure supported with venoarterial ECMO [17]. Based on an analysis of renin levels in healthy sheep treated with venoarterial ECMO, Saito et al. [18] hypothesized that upregulation of PRA may be an adaptive response to lack of pulsatile pressure. Different results were observed by Semmekrot et al. [19]. These authors observed a reduction of PRA and angiotensin II levels in patients treated with venoarterial ECMO. In the same study, PRA and angiotensin II levels were negatively correlated with mean arterial pressure, suggesting mean arterial pressure could directly and indirectly affect global and intrarenal perfusion in patients treated with venoarterial ECMO.
In addition to the effects on the renin-angiotensin-aldosterone pathway, several studies have indicated impairments to the atrial natriuretic peptide (ANP) pathway during ECMO (fig. 2). Analyzing neonatal patients with acute respiratory failure supported with venoarterial ECMO, Semmekrot et al. [19] observed that ANP levels were reduced during the treatment. Considering the physiological ANP effects, an impairment of intrarenal blood flow regulation is likely in this situation. Moreover, in that ANP is thought to attenuate kidney-lung crosstalk [20], a particular potential corresponding risk of this ANP reduction with ECMO is a reduction in the renoprotective and anti-inflammatory effects of ANP in acute respiratory failure patients requiring ECMO [20]. Since atrial distention is the main stimulus to ANP release, the ANP reduction could be related to drainage of blood by the venous cannula in the right atrium. Moreover, a significant correlation with central venous pressure (CVP) values was observed in this study and explained by the direct venous catheter drainage. As in other critical care settings, the use of CVP as an indicator of circulating volume during ECMO is flawed, and its value should be interpreted carefully. The relationships between circulating volume, CVP and ANP may be further complicated in venovenous ECMO, where the draining and reinfusing cannulae may both affect the local pressure.
Systemic Inflammation
The systemic inflammatory response syndrome universally occurs during ECMO therapy and is associated with systemic organ dysfunctions (e.g. AKI) and considerable mortality [21]. As in other extracorporeal treatments, the blood shear stress [21], the exposure to non-self surfaces and the air/blood interface may cause a hypercoagulable state [16] as well as systemic inflammation (fig. 3) [22]. The activation of neutrophils is a pivotal event in ECMO-related systemic inflammatory response syndrome [21]. With adherence to the capillary/venular endothelium, the activated neutrophils release cytokines, arachidonic acid metabolites and reactive oxygen species, causing widespread microvascular injury [23], capillary leaks [24] and multiorgan dysfunction [25].
In an experimental animal model, McIlwain et al. [21] observed a significant upregulation of CD18, CD35, CD62L and CD11b expression (i.e. indirect markers of neutrophil activation) in pigs treated with ECMO. Moreover, increases in leukocyte infiltration and focal hemorrhages were evident in the kidney just 2 h after the initiation of ECMO [21]. Consistent with these findings, thrombocytes and complement pathways are all activated and accompanied by histopathological inflammatory changes and increases in plasma concentrations of proinflammatory cytokines [26].
Specific coatings including poly(2-methoxyethylacrylate), albumin, heparin or phosphorylcholine have been proposed for disposables in the extracorporeal circuit to reduce all these effects. In a historical case-control study, Deptula et al. [27] compared a group of pediatric patients undergoing extracorporeal circulation with a poly(2-methoxyethylacrylate) surface coating the circuits with a group of patients treated with standard circuits. Their results suggested possible favorable effects of surface-treated circuits, mainly in terms of transfusion requirement and ICU stay.
Organ Crosstalk
Considering the potential pathophysiological side effects of ECMO, important changes in kidney function should be anticipated. The acute changes in myocardial physiology that are seen with both venovenous and venoarterial ECMO may produce variable grades of kidney impairment by organ crosstalk mechanisms. In particular, acute ventricle distention, which may occur during ECMO, can involve both the right and the left ventricles. Schwarz et al. [28] observed acute left ventricle distention in 10.9% of patients treated with venoarterial ECMO. In these patients, increased left ventricular afterload, a progressive increase in end-diastolic volume, ventricular distention and pulmonary edema were all evident. In these conditions, the left ventricular preload is increased and accompanied by a rise in right ventricle afterload, which is further increased by preexisting severe lung injury with hypoxemia. Hemodynamic changes, neurohormonal activation, hypothalamic-pituitary stress reaction, inflammation and immune cell signaling derived from ventricular dysfunction may all produce AKI as a manifestation of cardiorenal syndrome type 1 (fig. 2) [29]. Furthermore, several additional ongoing conditions may lead to myocardial dysfunction requiring ECMO and accompanying AKI. Among these conditions, sepsis is certainly the most common one causing cardiorenal syndrome type 5 (fig. 3) [30].
Patients with the acute respiratory distress syndrome typically require mechanical ventilation and, in particularly severe cases, may require ECMO. Such patients on ECMO are at particular risk of developing AKI. The effects of mechanical ventilation on kidney function are mediated through hemodynamic (fig. 2) and biohumoral changes, blood gas variations and biotrauma (fig. 3). In these conditions, important components of lung-kidney crosstalk are related to disturbances in the innate immune/inflammatory response, oxidative stress and cellular necrosis/apoptosis.
However, an important benefit of the carbon dioxide removal and oxygenation obtained through venovenous or venoarterial ECMO is allowing patient treatment with ‘lung protective ventilation’ [31]. As demonstrated in several trials, lung protective ventilation is a valuable therapeutic approach to mechanical ventilation which increases patient survival by reducing baro- and biotrauma of the lung and attenuating the pathophysiological components of organ crosstalk.
Circuit-Related Variables
In patients supported with venoarterial ECMO, ischemic complications related to the arterial vascular access may develop (fig. 3). In a recent analysis of complications observed in patients who underwent ECMO support, Cheng et al. [32] reviewed 20 studies encompassing 1,866 patients. Thirteen of the studies (including 677 patients) reported extremity ischemia as a complication with a pooled estimate rate of 16.9%. Moreover, a pooled estimate rate of 10.3% was observed for lower extremity fasciotomy or compartment syndrome. In these clinical situations, several biochemical insults, such as hypermyoglobinemia or ischemia-reperfusion injury, may precipitate AKI.
Other unusual but potentially severe complications related to ECMO support can cause kidney damage. In particular, Cheng et al. [32] reported that in about 2% of patients treated with venoarterial ECMO, an aortic retrograde dissection develops which may involve the renal arteries. In addition, systemic thrombotic or air microembolism can have a causative role in the development of AKI in patients treated with venoarterial ECMO [33]. Finally, it is known that during prolonged use of ECMO, the negative pressure generated by the roller or centrifugal pumps can produce hemolysis with hemoglobinuria, which may itself cause kidney damage (fig. 3) [34].
Changes in Renal Function after ECMO
Several studies in the literature have outlined the prevalence of chronic kidney disease (CKD) in patients who have undergone ECMO [35]. The rate of RRT dependence among patients treated with ECMO ranges between 2 and 65% [36,37]. However, experiences in the pediatric population suggest that primary renal disease at presentation is the main risk factor for CKD in these patients [35]. Limited information is available on the need for RRT in adult patients who have undergone ECMO. In a review of complications after ECMO in adult patients, Cheng et al. [32] observed a pooled estimate rate of 46% for RRT dependence after ECMO. Certainly, AKI as observed in this condition may produce an irreversible loss of nephron units with deleterious effects on renal reserve and/or overall long-term renal function [38], leading to CKD.
RRT in ECMO
RRT can be integrated into the main extracorporeal circuit during ECMO [39]. Although several RRT modalities can be used to support ECMO patients with AKI (table 2), comparative studies are not available, and clinical practice is based on expert opinion and local experience [3,40].
Table 2.
Treatments for AKI in ECMO
| Treatment | Pros | Cons |
|---|---|---|
| IHD | Potentially joinable with the main ECMO circuit | Rapid fluid removal and hemodynamic instability |
| Reduced downtime | Disequilibrium syndrome and potential risk of cerebral edema | |
| Lower costs than CRRT | Technically more complex and demanding | |
| Prolonged IHD | Potentially joinable with the main ECMO circuit | Potentially associated with hypotension in high-risk patients |
| Slower volume and solute removal than IHD | Technically more complex and demanding | |
| Reduced downtime and costs compared with CRRT | ||
| CRRT | Potentially joinable with the main ECMO circuit | Patient immobilization Increased risk of hypothermia High costs |
| Continuous removal of toxins Hemodynamic stability Tight and easy control of fluid balance Gentle solute removal avoiding disequilibrium syndrome Potentially allows blood purification therapies for systemic inflammation | ||
| Potentially allows blood purification therapies for systemic inflammation | ||
| PD | Hemodynamic stability | Mainly restricted to the pediatric population and to patients already treated with chronic PD |
| Technically simple | Requires specific intraperitoneal catheters Risk of peritonitis | |
| Lower cost | Impairs diaphragmatic movements, potentially prolonging the weaning from ECMO | |
IHD = Intermittent hemodialysis; PD = peritoneal dialysis.
Although the indications for beginning RRT in ECMO patients are relative similar to those for starting RRT in non-ECMO patients, the timing of RRT and the targets for fluid removal need to be adjusted to the overall clinical situation, which is usually complex. The supporting role of RRT in these conditions could reduce the renal metabolic requirement and inflammatory mediators, improve the neurohormonal pathway related to fluid overload and, consequently, enhance renal recovery from AKI. As observed in non-ECMO patients, staff availability, local expertise and experience with RRT in complex situations influence the timing of treatment [3]. Despite important variations between participating centers in analyzing Extracorporeal Life Support Organization (ELSO) data, Fleming et al. [41] reported that treatment of fluid overload has a major role in the decision to initiate RRT in ECMO patients. In particular, fluid overload was the main RRT indication reported for 43% of the patients, while AKI and electrolyte disorders were the indications for 35 and 4% of the patients, respectively. Several studies have suggested that cumulative fluid overload in critically ill patients is an independent factor associated with mortality, worse oxygenation, longer length of stay and mechanical ventilation [42]. Moreover, fluid overload prolongs ECMO treatment [43], while its reduction is associated with an improvement in lung function and with more rapid weaning from ECMO [44].
Reports in the literature indicate that in pediatric patients, concomitant RRT-ECMO treatment is associated with less fluid overload compared with ECMO alone [45]. In addition, RRT when combined with ECMO may improve outcomes as a result of more optimal administration of nutrition, medications and blood products without further increasing the fluid overload. Finally, as suggested by data from two large ECMO center studies which considered ECMO patients treated with RRT [15], the likelihood of chronic long-term RRT for ECMO survivors is low in the absence of primary renal disease.
Continuous RRT is certainly the most common modality utilized in these hemodynamically unstable patients. It can be provided through the use of an in-line hemofilter or, in most cases, through a continuous RRT (CRRT) machine connected to the ECMO extracorporeal circuit [3,39]. As recently reported in an international survey, 50.8% of the 65 ECMO centers participating in the survey use CRRT, while 21.5% use an in-line hemofilter. In this review, 23% of the centers did not use RRT during ECMO treatment [41]. When an in-line hemofilter is used in the ECMO circuit, it is typically placed between the pump (which provides the forward blood flow) and the oxygenator (which traps clots and air, preventing possible complications). The purified blood is usually returned to the prepump limb of the circuit, creating a shunt and producing an overestimation of blood flows delivered to the patient [3]. Alternatively, a CRRT machine can be connected to the venous limb of the ECMO circuit [39]. The blood treated by CRRT is collected and reinfused into the venous limb of the ECMO circuit before the pump. However, if a centrifugal pump is employed in the ECMO circuit, it is suggested that the CRRT machine be placed after the pump to reduce the risk of air embolism.
Conclusions
ECMO is an effective support therapy for patients with reversible cardiac and/or respiratory failure. AKI is frequently observed in patients undergoing ECMO and may be related to several conditions derived from or associated with the extracorporeal therapy; they are mainly hemodynamic, hormonal and inflammatory in nature. The severe conditions of patients treated with ECMO and the different variables of this technically complex extracorporeal treatment usually do not allow us to exactly identify the pathophysiological mechanism mainly responsible for AKI development during ECMO. Furthermore, different pathophysiological pathways might explain the development of AKI during the treatment and at the same time produce the pathological conditions requiring ECMO. Despite the fact that all of these are critical gaps in our understanding of the relationship between ECMO and kidney function, the actual need to provide renal support therapy during ECMO nowadays appears clearer. In particular, RRT is usually required and provides for the management of fluid overload as well as solute control and the removal of inflammatory mediators. Multidimensional evaluations and multimodal therapies should be integrated in a flexible and patient-tailored multiorgan support therapy.
Disclosure Statement
No grants and no relationships with the industry are declared.
References
- 1.Gattinoni L, Carlesso E, Langer T. Clinical review: extracorporeal membrane oxygenation. Crit Care. 2011;15:243. doi: 10.1186/cc10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richard C, Argaud L, Blet A, Boulain T, Contentin L, Dechartres A, et al. Extracorporeal life support for patients with acute respiratory distress syndrome: report of a Consensus Conference. Ann Intensive Care. 2014;4:15. doi: 10.1186/2110-5820-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askenazi DJ, Selewski DT, Paden ML, Cooper DS, Bridges BC, Zappitelli M, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 2012;7:1328–1336. doi: 10.2215/CJN.12731211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C, Chen Y, Tsai F, Tian Y, Jenq C, Fang J, et al. RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol Dial Transplant. 2006;21:2867–2873. doi: 10.1093/ndt/gfl326. [DOI] [PubMed] [Google Scholar]
- 5.Yan X, Jia S, Meng X, Dong P, Jia M, Wan J, et al. Acute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: evaluation of the RIFLE classification and the Acute Kidney Injury Network criteria. Eur J Cardiothorac Surg. 2010;37:334–338. doi: 10.1016/j.ejcts.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Askenazi D, Ambalavanan N, Hamilton K, Cutter G, Laney D, Kaslow R, et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:e1–e6. doi: 10.1097/PCC.0b013e3181d8e348. [DOI] [PubMed] [Google Scholar]
- 7.Kielstein JT, Heiden AM, Beutel G, Gottlieb J, Wiesner O, Hafer C, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 2013;28:86–90. doi: 10.1093/ndt/gfs398. [DOI] [PubMed] [Google Scholar]
- 8.Wu M, Lin P, Tsai F, Haung Y, Liu K, Tsai F. Impact of preexisting organ dysfunction on extracorporeal life support for non-postcardiotomy cardiopulmonary failure. Resuscitation. 2008;79:54–60. doi: 10.1016/j.resuscitation.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Lan C, Tsai P, Chen Y, Ko W. Prognostic factors for adult patients receiving extracorporeal membrane oxygenation as mechanical circulatory support – a 14-year experience at a medical center. Artif Organs. 2010;34:E59–E64. doi: 10.1111/j.1525-1594.2009.00909.x. [DOI] [PubMed] [Google Scholar]
- 10.Mao H, Katz N, Kim C, Day S, Ronco C. Implantable left ventricular assist devices and the kidney. Blood Purif. 2014;37:57–66. doi: 10.1159/000357970. [DOI] [PubMed] [Google Scholar]
- 11.Sezai A, Shiono M, Orime Y, Nakata K, Hata M, Yamada H, et al. Renal circulation and cellular metabolism during left ventricular assisted circulation: comparison study of pulsatile and nonpulsatile assists. Artif Organs. 1997;21:830–835. doi: 10.1111/j.1525-1594.1997.tb03752.x. [DOI] [PubMed] [Google Scholar]
- 12.Nemoto M. Experimental evaluation of the influence of complete artificial circulation on renal circulation and tissue metabolism – comparative study of pulsatile vs nonpulsatile circulation. Ann Thorac Cardiovasc Surg. 2003;9:355–364. [PubMed] [Google Scholar]
- 13.Song Z, Wang C, Stammers A. Clinical comparison of pulsatile and nonpulsatile perfusion during cardiopulmonary bypass. J Extra Corpor Technol. 1997;29:170–175. [PubMed] [Google Scholar]
- 14.Undar A, Masai T, Yang S, Goddard-Finegold J, Frazier OH, Fraser CD. Effects of perfusion mode on regional and global organ blood flow in a neonatal piglet model. Ann Thorac Surg. 1999;68:1336–1342. doi: 10.1016/s0003-4975(99)00913-3. [DOI] [PubMed] [Google Scholar]
- 15.Ingyinn M, Rais-Bahrami K, Evangelista R, Hogan I, Rivera O, Mikesell G, et al. Comparison of the effect of venovenous versus venoarterial extracorporeal membrane oxygenation on renal blood flow in newborn lambs. Perfusion. 2004;19:163–170. doi: 10.1191/0267659104pf736oa. [DOI] [PubMed] [Google Scholar]
- 16.Keckler S, Laituri C, Ostlie D, St Peter S. A review of venovenous and venoarterial extracorporeal membrane oxygenation in neonates and children. Eur J Pediatr Surg. 2010;20:1–4. doi: 10.1055/s-0029-1231053. [DOI] [PubMed] [Google Scholar]
- 17.Heggen BJA, Fortenberry JD, Tanner AJ, Reid CA, Mizzell DW, Pettignano R. Membrane oxygenation for pediatric respiratory failure. J Pediatr Surg. 2004;39:1626–1631. doi: 10.1016/j.jpedsurg.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Saito S, Westaby S, Piggot D, Dudnikov S, Robson D, Catarino PA, et al. End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg. 2002;74:1080–1085. doi: 10.1016/s0003-4975(02)03846-8. [DOI] [PubMed] [Google Scholar]
- 19.Semmekrot B, Pesman G, Span P, Sweep C, van Heijst A, Monnens L, et al. Serial plasma concentrations of atrial natriuretic peptide, plasma renin activity, aldosterone, and antidiuretic hormone in neonates on extracorporeal membrane. ASAIO J. 2002;48:26–33. doi: 10.1097/00002480-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Tulafu M, Mitaka C, Hnin Si MK, Abe S, Kitagawa M, et al. Atrial natriuretic peptide attenuates kidney-lung crosstalk in kidney injury. J Surg Res. 2014;186:217–225. doi: 10.1016/j.jss.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 21.McIlwain R, Timpa J, Kurundkar A, Holt D, Kelly D, Hartman Y, et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest. 2010;90:128–139. doi: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurundkar AR, Killingsworth CR, McIlwain RB, Timpa JG, Hartman YE, He D, et al. Extracorporeal membrane oxygenation causes loss of intestinal epithelial barrier in the newborn piglet. Pediatr Res. 2010;68:128–133. doi: 10.1203/PDR.0b013e3181e4c9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graulich J, Walzog B, Marcinkowski M, Bauer K, Kössel H, Fuhrmann G, et al. Leukocyte and endothelial activation in a laboratory model of extracorporeal membrane oxygenation (ECMO) Pediatr Res. 2000;48:679–684. doi: 10.1203/00006450-200011000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Kim T, Lim C, Park I, Kim DJ, Jung Y, Park K. Prognosis in the patients with prolonged extracorporeal membrane oxygenation. Korean J Thorac Cardiovasc Surg. 2012;45:236–241. doi: 10.5090/kjtcs.2012.45.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mildner R, Taub N, Vyas J, Killer H, Firmin R, Field D, et al. Cytokine imbalance in infants receiving extracorporeal membrane oxygenation for respiratory failure. Biol Neonate. 2005;88:321–327. doi: 10.1159/000087630. [DOI] [PubMed] [Google Scholar]
- 26.Fortenberry JD, Bhardwaj V, Niemer P, Cornish JD, Wright JA, Bland L. Neutrophil and cytokine activation with neonatal extracorporeal membrane oxygenation. J Pediatr. 1996;128:670–678. doi: 10.1016/s0022-3476(96)80133-8. [DOI] [PubMed] [Google Scholar]
- 27.Deptula J, Glogowski K, Merrigan K, Hanson K, Felix D, Hammel J, et al. Evaluation of biocompatible cardiopulmonary bypass circuit use during pediatric open heart surgery. J Extra Corpor Technol. 2006;38:22–26. [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz B, Mair P, Margreiter J, Pomaroli A, Hoermann C, Bonatti J, et al. Experience with percutaneous venoarterial cardiopulmonary bypass for emergency circulatory support. Crit Care Med. 2003;31:758–764. doi: 10.1097/01.CCM.0000053522.55711.E3. [DOI] [PubMed] [Google Scholar]
- 29.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 30.Chelazzi C, Villa G, De Gaudio AR. Cardiorenal syndromes and sepsis. Int J Nephrol. 2011;2011:652967. doi: 10.4061/2011/652967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care. 2014;18:203. doi: 10.1186/cc13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock adult patients. Ann Thorac Surg. 2014;97:610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 33.MacLaren G, Combes A, Bartlett R. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012;38:210–220. doi: 10.1007/s00134-011-2439-2. [DOI] [PubMed] [Google Scholar]
- 34.Toomasian J, Bartlett R. Hemolysis and ECMO pumps in the 21st century. Perfusion. 2011;26:5–6. doi: 10.1177/0267659110396015. [DOI] [PubMed] [Google Scholar]
- 35.Paden M, Warshaw B, Heard M, Fortenberry J. Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:153–158. doi: 10.1097/PCC.0b013e3181e2a596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diez C, Haneya A, Brünger F, Philipp A, Hirt S, Ruppecht L, et al. Minimized extracorporeal circulation cannot prevent acute kidney injury but attenuates early renal dysfunction after coronary bypass grafting. ASAIO J. 2009;55:602–607. doi: 10.1097/MAT.0b013e3181bbcd3e. [DOI] [PubMed] [Google Scholar]
- 37.Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010;139:302–311. doi: 10.1016/j.jtcvs.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 38.Ronco C, Rosner MH. Acute kidney injury and residual renal function. Crit Care. 2012;16:144. doi: 10.1186/cc11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seczyńska B, Królikowski W, Nowak I, Jankowski M, Szułdrzyński K, Szczeklik W. Continuous renal replacement therapy during extracorporeal membrane oxygenation in patients treated in medical intensive care unit: technical considerations. Ther Apher Dial. 2014;18:523–534. doi: 10.1111/1744-9987.12188. [DOI] [PubMed] [Google Scholar]
- 40.Sasser W, Robert S, Askenazi D, O'Meara L, Borasino S, Alten J. Peritoneal dialysis: an alternative modality of fluid removal in neonates requiring extracorporeal membrane oxygenation after cardiac surgery. J Extra Corpor Technol. 2014;46:157–161. [PMC free article] [PubMed] [Google Scholar]
- 41.Fleming G, Askenazi D, Bridges B, Cooper D, Paden M, Selewski D, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention during Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J. 2012;58:407–414. doi: 10.1097/MAT.0b013e3182579218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann H, Wheeler A, Bernard G, Thompson B, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 43.Blijdorp K, Cransberg K, Wildschut ED, Gischler SJ, Houmes RJ, Wolff ED, et al. Haemofiltration in newborns treated with extracorporeal membrane oxygenation: a case-comparison study. Crit Care. 2009;13:R48. doi: 10.1186/cc7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson H, 3rd, Coran A, Drongowski R, Ha H, Bartlett R. Extracellular fluid and total body water changes in neonates undergoing extracorporeal membrane oxygenation. J Pediatr Surg. 1992;27:1003–1007. doi: 10.1016/0022-3468(92)90547-k. [DOI] [PubMed] [Google Scholar]
- 45.Hoover N, Heard M, Reid C, Wagoner S, Rogers K, Foland J, et al. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 2008;34:2241–2247. doi: 10.1007/s00134-008-1200-y. [DOI] [PubMed] [Google Scholar]