Abstract
Hypothesis/Introduction
Angiotensin II (Ang II) has been shown to control erythropoietin (EPO) synthesis as Ang II type 1 receptor (AT1R) blockers block Ang-II-induced EPO oversecretion. To further explore the involvement of AT1R in processes controlling EPO levels, plasma EPO and mononuclear cell NADPH oxidase 4 (NOX4) – a NOX family member involved in oxygen sensing, which is a process central to controlling EPO levels – were assessed in Bartter's/Gitelman's syndrome (BS/GS) patients, a human model of endogenous AT1R antagonism and healthy subjects. Heme oxygenase (HO)-1, antioxidant and anti-inflammatory factor related to NOX4 activation, and the relationship of EPO and NOX4 to HO-1 were also assessed.
Materials and Methods
EPO was measured by chemiluminescent immunoassay, HO-1 by sandwich immunoassay and NOX4 protein expression by Western blot.
Results
EPO was increased in BS/GS patients compared to healthy subjects (7.64 ± 2.47 vs. 5.23 ± 1.07 U/l; p = 0.025), whereas NOX4 did not differ between BS/GS and healthy subjects (1.76 ± 0.61 vs. 1.65 ± 0.54 densitometric units; p = n.s.), and HO-1 was increased in BS/GS patients compared to healthy subjects (9.58 ± 3.07 vs. 5.49 ± 1.04 ng/ml; p = 0.003). NOX4 positively correlated with HO-1 only in BS/GS patients; no correlation was found between EPO and either NOX4 or HO-1 in those two groups.
Conclusions
The effect of the renin-angiotensin system on EPO cannot be solely mediated by Ang II via AT1R signaling, but rather, EPO levels are also determined by a complex interrelated set of signals that involve AT2R, nitric oxide levels, NOX4 and HO-1 activity.
Key Words: Angiotensin II, Angiotensin II receptors, Erythropoietin, NOX4, Nitric oxide, Heme oxygenase-1
Introduction
Angiotensin II (Ang II), the principal effector of the renin-angiotensin system (RAS), signals via two main receptor subtypes, the widely expressed type 1 receptors (AT1R) that mediate vasoconstriction, sympathetic nervous system activation and cardiovascular remodeling, and via type 2 receptors (AT2R) that counteract AT1R signaling by being involved in vasodilation, apoptosis and antiproliferative effects [1]. RAS has become increasingly recognized for playing an important role in hematopoiesis-related processes, as erythropoietin (EPO) secretion has been linked to Ang II via AT1R. The Ang-II-AT1R-EPO pathway was proposed by Vlahakos et al. [2] based in large measure on the inhibitory effects of losartan, an AT1R blocker, on EPO levels [3,4,5,6,7].
In an extensive series of studies on Bartter's/Gitelman's syndrome (BS/GS) patients, we have documented that these patients are a human model of endogenous AT1R antagonism [8,9,10]. BS/GS patients, notwithstanding their increased Ang II level, have, in fact, a blunted short- and long-term Ang II signaling via AT1R [8,10], alongside an activation of Ang II signaling via AT2R [10,11], a lack of cardiovascular remodeling [12,13], an upregulation of the nitric oxide (NO) system [8,10] and an increased NO-mediated vasodilation [8,10]. Given the proposed AT1R-EPO connection, we thought it would be of interest to examine the level of EPO in these unique patients, thus providing a test for the role of AT1R in the EPO system. In addition, BS/GS patients have reduced oxidative stress [10,13] and an increased expression of heme oxygenase (HO)-1 [10,14], which protects against oxidative stress and has anti-inflammatory effects [15], and the role of reactive oxidant species (ROS) in physiological pathways such as RAS has become increasingly important [1]. A significant source of cellular ROS is NADPH oxidase 4 (NOX4), a member of the NOX family that is highly expressed in cardiovascular tissues and produces a unique spectrum of ROS products [16,17]. Given that NOX4 is involved in oxygen sensing, a process central to controlling EPO levels, along with cellular proliferation, fibrosis and angiogenesis [16,17], and that HO-1 is an effector of NOX4 activity [18], we thought it would also be of interest to examine the level of NOX4 in these unique patients.
The current study reports plasma levels of EPO as well as mononuclear cell levels of NOX4 in BS/GS patients, comparing them to the levels in healthy subjects. It also reports HO-1 levels in the two groups considered and examines the relationship between EPO and NOX4 and other related effectors such as HO-1.
Patients and Methods
Patients
We tested 11 patients from our cohort of BS/GS patients (1 BS and 10 GS), including 6 men and 5 women, with an age range of 27-58 years; the same patients have been enrolled in a recently published study [14] strictly related to the present study. A full biochemical characterization of all patients was obtained (table 1), with 10 GS patients having undergone full genetic analysis and 1 BS patient awaiting the results of the genetic screenings (table 2). The patients' blood pressure ranged from 100/65 to 120/70 mm Hg.
Table 1.
Clinical and laboratory data on BS/GS patients and healthy subjects included in the study
| BS/GS patients | Sex | Age, years | Plasma electrolytes, mmol/l |
Urinary electrolytes, mmol/day |
PRA, ng Ang I/ml/h | Aldosterone, nmol/l | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Cl– | Mg++ | Na+ | K+ | Cl– | Ca++ | |||||
| BS patient | No. | |||||||||||
| 1 | F | 27 | 138 | 2.3 | 96 | 0.93 | 160 | 38.6 | 190 | 3.8 | 12 | 0.94 |
| GS patient | No. | |||||||||||
| 1 | F | 30 | 137 | 2.9 | 99 | 0.63 | 255 | 38.0 | 200 | 1.9 | 9 | 0.70 |
| 2 | M | 44 | 140 | 2.8 | 98 | 0.60 | 198 | 30.8 | 220 | 2.0 | 7 | 0.88 |
| 3 | F | 31 | 138 | 2.7 | 98 | 0.60 | 297 | 42.8 | 289 | 2.1 | 10 | 0.78 |
| 4 | M | 32 | 138 | 3.0 | 99 | 0.56 | 200 | 81.5 | 239 | 2.0 | 6 | 0.67 |
| 5 | F | 58 | 140 | 3.0 | 100 | 0.55 | 190 | 42.5 | 220 | 2.0 | 6 | 0.70 |
| 6 | M | 29 | 139 | 3.0 | 100 | 0.57 | 190 | 42.5 | 218 | 2.1 | 6 | 0.75 |
| 7 | M | 31 | 139 | 3.1 | 100 | 0.58 | 192 | 42.8 | 210 | 2.2 | 6 | 0.75 |
| 8 | M | 43 | 139 | 3.0 | 98 | 0.58 | 200 | 45.4 | 197 | 2.0 | 5.8 | 0.80 |
| 9 | M | 39 | 139 | 2.8 | 97 | 0.59 | 195 | 47.4 | 198 | 2.0 | 5.8 | 0.81 |
| 10 | F | 30 | 139 | 2.9 | 100 | 0.60 | 196 | 48.8 | 199 | 2.0 | 6.1 | 0.77 |
| Controls | 6 M/ | 46.2± | 140± | 4.1± | 99± | 0.99± | 180± | 52.8± | 179.8± | 4.5± | 0.73± | 0.18± |
| (n = 10) | 4 F | 10.5 | 1.0 | 0.2 | 0.97 | 0.2 | 16.5 | 4.8 | 19.6 | 0.5 | 0.13 | 0.02 |
The table reports single data on patients and controls. PRA = Plasma renin activity. Normal values for plasma renin activity and plasma aldosterone in our laboratory are 0.2–2.8 ng Ang I/ml/h and 0.08–0.29 nmol/l, respectively. Normal values for plasma Na+, K+, Cl– and Mg++ are 136–145, 3.5–5, 96–108 and 0.65–1.05 mmol/l, respectively. Normal values for urinary Na+, K+, Cl– and Ca++ excretion are 40–220, 25–125, 110–250 and 2.5–7.5 mmol/day, respectively.
Table 2.
SLC12A3 mutations identified in the patients with GS
| Patient No. | Exon | Mutation at nucleotide | Homo-/heterozygous | Predicted effect on protein |
|---|---|---|---|---|
| 1 | 23 | 2736G→A | homozygous | Arg904Gln |
| 2 | 22 | 2579C→T | heterozygous | Arg852Cys |
| 23 | 2736G→A | heterozygous | Arg904Gln | |
| 3 | 15 | 1950G→A | heterozygous | Arg642His or splice donor site truncated SLC12A3 protein |
| 18 | 2246G→A | heterozygous | Gly741Arg | |
| 4 | 22 | 2579C→T | heterozygous | Arg852Cys |
| 23 | 2736G→A | homozygous | Arg904Gln | |
| 5 | 22 | 2579C→T | heterozygous | Arg852Cys |
| 23 | 2736G→A | heterozygous | Arg904Gln | |
| 6 | 21 | 2542G→T | heterozygous | Asp848Tyr |
| 10 | c.1196_1202dup, 7 bp | heterozygous | Ser402X | |
| 7 | 21 | 2542G→T | heterozygous | Asp848Tyr |
| 10 | c.1196_1202dup, 7 bp | heterozygous | Ser402X | |
| 8 | 17 | c.2089_2095del, 7 bp | heterozygous | pThr697fs |
| 26 | 2985G→A | heterozygous | Ser402X | |
| 9 | 17 | c.2089_2095del, 7 bp | heterozygous | pThr697fs |
| 26 | 2985G→A | heterozygous | Ser402X | |
| 10 | 10 | 1195C→T | heterozygous | pArg399Cys |
| 15 | 1220C→T | heterozygous | pArg642His | |
A total of 10 normotensive healthy individuals (6 males and 4 females, aged 46.2 ± 10.5 years) from the staff of the Department of Medicine, University of Padova, Padova, Italy, were used as the control group. Their blood pressure ranged from 122/82 to 134/85 mm Hg.
BS/GS patients were only taking potassium and magnesium supplements, and all patients abstained from food, alcohol and caffeine-containing drinks for at least 12 h before the study. Informed consent was obtained from all the study participants, and the study protocol was approved by our institutional authorities.
Methods
Plasma EPO Determination
The plasma level of EPO was measured by Access Immunoassay Systems, with Unicel DXI 800 (Beckman Coulter), a chemiluminescent immunoassay with paramagnetic particles for the quantitative determination of the level of EPO in human serum and plasma. The range of sensitivity was 2.6-18.5 U/l. The intra-assay coefficient of variation was up to 4.6%, and the interassay coefficient of variation was 6%.
Mononuclear Cell NOX4 Protein Expression
Peripheral blood mononuclear cells were isolated by Ficoll-Paque PLUS gradient (GE Healthcare, Uppsala, Sweden) from 35 ml of EDTA anticoagulated blood. Mononuclear cell NOX4 protein expression was performed using Western blot analysis. Total protein extracts were obtained by cell lysis with an ice-cold buffer (Tris-HCl 20 mM, NaCl 150 mM, EDTA 5.0 mM, Niaproof 1.5%, Na3VO4 1.0 mM, SDS 0.1% and PMSF 0.5 mM), with protease inhibitors added (Complete Protease Inhibitor Cocktail, Roche Diagnostics, Mannheim, Germany). Proteins were separated by SDS-PAGE, transferred onto nitrocellulose membranes (Hybond ECL, Amersham, Uppsala, Sweden) and blocked overnight with nonfat milk (5% in Tween-PBS). Membranes were probed with primary polyclonal antibody anti-NOX4 (Santa Cruz Biotechnology, Santa Cruz, Calif., USA). After incubation with proper secondary antibodies, horseradish peroxidase-conjugated (Amersham Biosciences, Uppsala, Sweden) immunoreactive proteins were visualized with chemiluminescence using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, Ill., USA). GAPDH was used as loading control (Millipore, Billerica, Mass., USA). NOX-4 protein expression was quantified with a densitometric semiquantitative analysis using NIH image software and normalized to GAPDH used as the loading control.
HO-1 Protein Quantification
Total protein extracts from peripheral blood mononuclear cells were obtained by cell lysis with an ice-cold buffer (Tris-HCl 20 mM, NaCl 150 mM, EDTA 5.0 mM, Niaproof 1.5%, Na3VO4 1.0 mM and SDS 0.1%), with protease inhibitors added (Complete Protease Inhibitor Cocktail, Roche Diagnostics). The protein concentration was evaluated by bicinchoninic acid assay (BCA Protein Assay, Pierce).
An equal amount of total protein was used for the determination of HO-1, using a sandwich immunoassay for the detection and quantitation of human HO-1 protein in cell lysates, according to the manufacturer's specifications (Stressgen Bioreagents, Ann Arbor, Mich., USA). After the test, absorbance was measured at 450 nm. The resulting readings were plotted against a standard curve to determine the concentration of HO-1 in each sample (ng/ml). The intra- and interassay coefficients of variation were both <10%.
Statistical Analysis
Data are expressed as means ± SDs and analyzed using the JMP (version 9.0; SAS, Cary, N.C., USA) statistical package running on a Mac Pro (Apple, Cupertino, Calif., USA). Student's t test for unpaired data and linear regression analysis were used. Values at a <5% level (p < 0.05) were considered significant.
Results
EPO and NOX4 Levels in BS/GS and Healthy Subjects
Based on the inhibitory effects of the blocking of AT1R on EPO levels [3,4,5,6,7], EPO secretion has been linked to Ang II signaling via AT1R; we examined the level of EPO in the unique cohort of BS/GS patients, a human model of endogenous AT1R antagonism [8,9,10], providing a test for the role of AT1R in the EPO system.
In addition, NOX4 – a member of the NOX family which is involved in oxygen sensing [16,17], a process central to controlling EPO levels – produces H2O2, which does not only react with and thereby destroy NO but also activates eNOS [16]. Thus, we have evaluated the level of NOX4 in BS/GS patients, who show reduced oxidative stress and activation of the NO system [10,13]. The levels of both EPO and NOX4 in the two groups of patients studied are presented in figure 1.
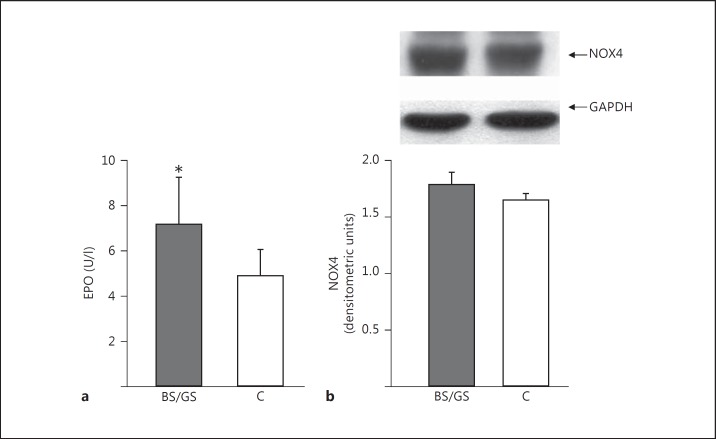
Fig. 1.
a Plasma levels of EPO in BS/GS patients and healthy subjects (C). * p = 0.011 versus healthy subjects. b Mononuclear cell NOX4 protein expression in BS/GS patients and healthy subjects (C). The top part shows the representative Western blot product of 1 BS/GS patient and 1 healthy subject.
The EPO plasma level was increased in BS/GS patients compared to healthy subjects (7.64 ± 2.47 vs. 5.23 ± 1.07 U/l; p = 0.011), whereas the NOX4 protein level did not differ between BS/GS and healthy subjects (1.76 ± 0.61 vs. 1.65 ± 0.54 densitometric units; p = n.s.).
HO-1 in BS/GS Patients and Healthy Subjects, and Analysis of the Relationship of EPO and NOX4 to HO-1
Given that BS/GS patients have been reported with increased expression of HO-1 [10,14], which is an effector of NOX4 activity [18] and protects against oxidative stress [15], we have quantified the HO-1 level and examined the relationship of EPO and NOX4 levels to HO-1.
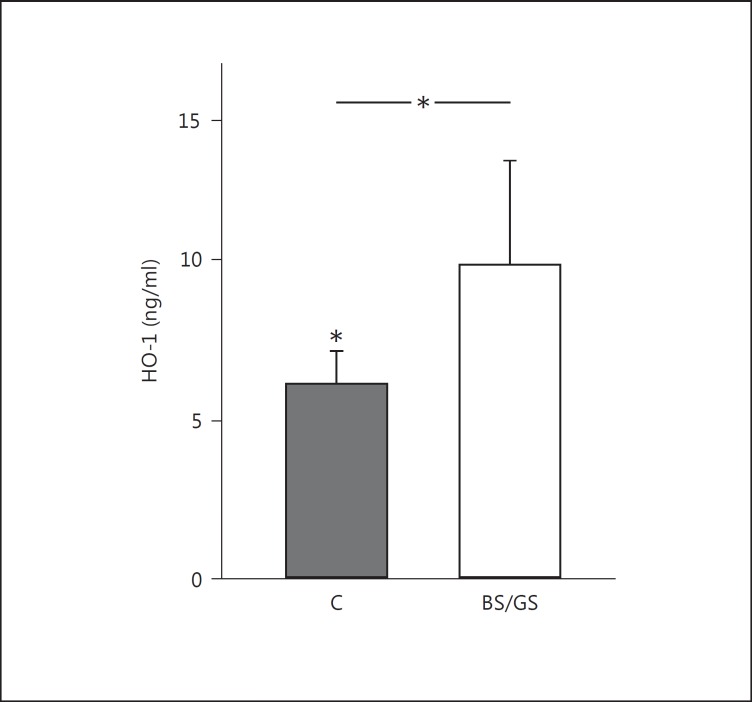
HO-1 levels were increased in BS/GS patients as compared to those in healthy subjects (9.58 ± 3.07 vs. 5.49 ± 1.04 ng/ml; p = 0.003; fig. 2). The levels of EPO and NOX4 were also analyzed with respect to the levels of HO-1 in the same subjects.
Fig. 2.
Mononuclear cell HO-1 protein levels in BS/GS patients and normotensive healthy subjects (C). * p = 0.003.
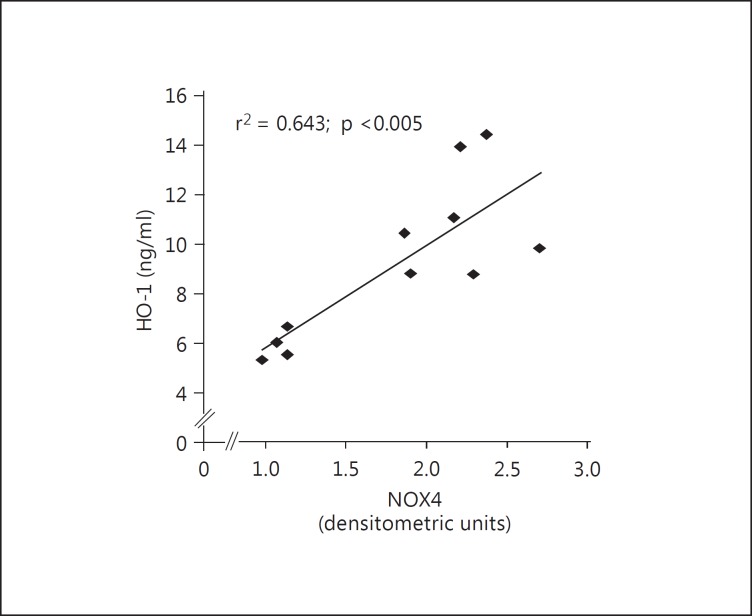
As presented in figure 3, in BS/GS patients, correlation analysis found that NOX4 levels were positively correlated with HO-1 levels (r2 = 0.643; p < 0.005). In contrast, in BS/GS patients, EPO was not correlated with either NOX4 (r2 = 0.22; p > 0.1) or HO-1 (r2 = 0.001; p > 0.9; data not shown). In healthy subjects, no correlation was observed between NOX4 and HO-1.
Fig. 3.
Correlation analysis showing the relationship between NOX4 and HO-1 in BS/GS patients.
The absence of a relationship between EPO and NOX4 is consistent with the lack of any difference in NOX4 activity between healthy subjects and BS/GS patients, while EPO levels were different between healthy subjects and BS/GS patients.
Discussion
Evidence for Ang II as a physiologically important regulatory peptide in erythropoiesis has recently been provided [2]. Ang II acts not only directly as an EPO secretagogue but also as a growth factor for erythroid progenitors [2]. A major basis for these conclusions were the reports showing that the effect of Ang II on EPO production was abolished by AT1R blockers [3,4,5,6,7]. In addition, evidence also comes from studies using transgenic mice harboring human renin and angiotensinogen genes, which found that these animals exhibited both erythrocytosis as well as hypertension [6]. Plasma EPO levels and renal EPO mRNA expression in the double-transgenic mice were increased compared with those of the wild-type control, while the elevated plasma EPO levels were attenuated in the compound mice. Thus, based on the data provided by Kim et al. [7], RAS was held to enhance erythropoiesis mainly through the AT1R-mediated signaling in EPO-producing renal cells. Based on these reports, one would predict that EPO should be reduced in BS/GS, a model of endogenous ATR1 antagonism [8,9,10], if EPO were only regulated via AT1R signaling. Plasma EPO levels in BS/GS patients are instead higher compared to those in healthy subjects. The elevation of EPO in BS/GS patients, in spite of their antagonism of AT1R signaling, provides evidence that AT1R-mediated Ang II signaling is unlikely to be the only mechanism to play a major role in EPO physiology.
The findings in BS/GS patients suggest that alternative mechanisms must be involved in addition to those reported on erythropoiesis upon pharmacological AT1R blockade. One potential mechanism is that elevated EPO in BS/GS patients may reflect the effects of unopposed AT2R signaling when AT1R signaling is blocked [11]. It is well recognized that the gene transcription of EPO is stimulated by hypoxia-inducible factor (HIF)-1α. Ang II stimulates HIF-1α expression by a posttranscriptional mechanism either via direct Ang II crosstalk or via ACE2 products of Ang II on AT2R [19]. ATR2 signaling increases HIF-1 abundance by interfering with its degradation [19].
Another potential mechanism linked to HIF-1 is the result of the ability of the AT1R blockers to indirectly increase NO levels via effects of unopposed AT2R signaling when AT1R signaling is blocked [11,20]. This may happen via stimulatory effects on NO production and/or by reducing oxidant levels and, therefore, avoiding the loss of NO via reactions with oxidants, which then ultimately results in increased NO bioavailability [19,21]. The increased level of NO slows HIF degradation, allowing HIF-1 to accumulate and translocate to the nucleus, where it induces EPO gene transcriptional activation. Thus, the increase in EPO found in our BS/GS cohort may also be linked to their increased NO production [8,10]. In addition, Craige et al. [22] have recently found that NO produced by eNOS links NOX4 and angiogenesis. This is of interest, as NOX family enzymes are involved in Ang-II-induced hypertension and blood pressure regulation [23]. Interestingly, NOX4 specifically produces H2O2, which does not only react with and destroy NO but rather serves, via activation of Akt, to activate eNOS [16]. These effects on NO may be significant, as both EPO and HO-1, effectors of NOX4 activity [18], share Akt as a common pathway [24].
The results of this study show that the NOX4 level in BS/GS patients was the same as that in healthy controls. The fact that the NOX4 levels in BS/GS patients were indistinguishable from those in healthy subjects despite the presence of elevated Ang II provides further confirmation of BS/GS patients' endogenous AT1R signaling blockade and suggests that, in humans, elevated Ang II may contribute to the reduction in NOX4 levels. The increased level of HO-1 in BS/GS patients compared with the levels found in healthy subjects might also be related to the increased NOX4 expression found in these patients, as the maintenance/increase in HO-1 expression has been shown to be a downstream effect of NOX4 activity [18]. In this regard, the existence of a relationship between HO-1 and NOX4 is further bolstered by the significant linear correlation found between HO-1 and NOX4 in BS/GS patients.
Finally, the combined presence in BS/GS patients of reduced oxidative stress, blunted AT1R signaling, increased levels of HO-1, upregulation of the NO system, activated AT2R signaling [8,9,10,11] and, as shown in this study, increased NOX4 levels suggests that multiple pathways contribute to the increased EPO level. Ang II acts as proinflammatory mediator [25] via induction of nuclear factor-κB [26], and inflammation and increased inflammatory cytokines are known to exert a suppressive effect on erythropoiesis [27]. However, despite their high Ang II levels, the inflammatory cytokine levels of BS/GS patients are not different from those in healthy subjects [10], and, more importantly, levels of IκB, the inhibitory subunit of nuclear factor-κB, are increased [10].
In conclusion, the results of this study using BS/GS patients, a human model of the endogenous blockade of Ang II signaling via AT1R, which is an opposite image of hypertension, provide additional elements that link vascular tone, RAS and EPO. These results expand our understanding of the role(s) of RAS and Ang II in EPO biology as they indicate that the effect(s) of RAS on EPO levels likely represent(s) a summation of a complex interrelated set of signals involving not only Ang II but also AT2R, NO levels, NOX4 and HO-1 activity, although the final pathways remain to be clarified. Their understanding is likely to have important implications on clinical grounds as well.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
The authors are grateful to Mrs. Chiara Berton, the nurse in charge of our outpatient ambulatory clinic, who performed the blood sampling from the BS/GS patients and the healthy subjects participating in this study.
References
- 1.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 2.Vlahakos DV, Marathias KP, Madias NE. The role of the renin-angiotensin system in the regulation of erythropoiesis. Am J Kidney Dis. 2010;56:558–565. doi: 10.1053/j.ajkd.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 3.Freudenthaler SM, Schreeb K, Körner T, Gleiter CH. Angiotensin II increases erythropoietin production in healthy human volunteers. Eur J Clin Invest. 1999;29:816–823. doi: 10.1046/j.1365-2362.1999.00530.x. [DOI] [PubMed] [Google Scholar]
- 4.Freudenthaler SM, Lucht I, Schenk T, Brink M, Gleiter CH. Dose-dependent effect of angiotensin II on human erythropoietin production. Pflugers Arch. 2000;439:838–844. doi: 10.1007/s004249900238. [DOI] [PubMed] [Google Scholar]
- 5.Gossmann J, Burkhardt R, Harder S, Lenz T, Sedlmeyer A, Klinkhardt U, Geiger H, Scheuermann EH. Angiotensin II infusion increases plasma erythropoietin levels via an angiotensin II type 1 receptor-dependent pathway. Kidney Int. 2001;60:83–86. doi: 10.1046/j.1523-1755.2001.00773.x. [DOI] [PubMed] [Google Scholar]
- 6.Kato H, Ishida J, Imagawa S, Saito T, Suzuki N, Matsuoka T, Sugaya T, Tanimoto K, Yokoo T, Ohneda O, Sugiyama F, Yagami K, Fujita T, Yamamoto M, Nangaku M, Fukamizu A. Enhanced erythropoiesis mediated by activation of the renin-angiotensin system via angiotensin II type 1a receptor. FASEB J. 2005;19:2023–2025. doi: 10.1096/fj.05-3820fje. [DOI] [PubMed] [Google Scholar]
- 7.Kim YC, Mungunsukh O, McCart EA, Roehrich PJ, Yee DK, Day RM. Mechanism of erythropoietin regulation by angiotensin II. Mol Pharmacol. 2014;85:898–908. doi: 10.1124/mol.113.091157. [DOI] [PubMed] [Google Scholar]
- 8.Calò LA. Vascular tone control in humans: the utility of studies in Bartter's/Gitelman's syndromes. Kidney Int. 2006;69:963–966. doi: 10.1038/sj.ki.5000253. [DOI] [PubMed] [Google Scholar]
- 9.Calò LA, Pessina AC. RhoA/Rho-kinase pathway: much more than just a modulation of vascular tone. Evidence from studies in humans. J Hypertens. 2007;25:259–264. doi: 10.1097/HJH.0b013e328010d4d2. [DOI] [PubMed] [Google Scholar]
- 10.Calò LA, Davis PA, Rossi GP. Understanding the mechanisms of angiotensin II signaling involved in hypertension and its long-term sequelae: insights from Bartter's and Gitelman's syndromes, human models of endogenous angiotensin II signaling antagonism. J Hypertens. 2014;32:2109–2119. doi: 10.1097/HJH.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 11.Calò LA, Schiavo S, Davis PA, Pagnin E, Mormino P, D'Angelo A, Pessina AC. Angiotensin II signaling via type 2 receptors in a human model of vascular hyporeactivity: implications for hypertension. J Hypertens. 2010;28:111–118. doi: 10.1097/HJH.0b013e328332b738. [DOI] [PubMed] [Google Scholar]
- 12.Calò LA, Maiolino G. Mechanistic approach to the pathophysiology of target organ damage in hypertension from studies in a human model with characteristics opposite to hypertension: Bartter's and Gitelman's syndromes. J Endocrinol Invest. 2015;38:711–716. doi: 10.1007/s40618-015-0249-z. [DOI] [PubMed] [Google Scholar]
- 13.Maiolino G, Azzolini M, Paolo Rossi G, Davis PA, Calò LA: Bartter/Gitelman syndromes as a model to study systemic oxidative stress in humans. Free Radic Biol Med 2015, Epub ahead of print. [DOI] [PubMed]
- 14.Calo LA, Davis PA, Pagnin E, Dal Maso L, Caielli P and Rossi GP. Calcitonin gene-related peptide, heme oxygenase-1, endothelial progenitor cells and nitric oxide-dependent vasodilation relationships in a human model of angiotensin II type-1 receptor antagonism. J Hypertens. 2012;30:1406–1413. doi: 10.1097/HJH.0b013e32835414f7. [DOI] [PubMed] [Google Scholar]
- 15.Kim YM, Pae HO, Park JE, Lee YC, Woo JM, Kim NH, Choi YK, Lee BS, Kim SR, Chung HT. Heme oxygenase in the regulation of vascular biology: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;14:137–167. doi: 10.1089/ars.2010.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F, Haigh S, Barman S, Fulton DJ. From form to function: the role of Nox4 in the cardiovascular system. Front Physiol. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandes RP, Takac I, Schröder K. No superoxide – no stress? Nox4, the good NADPH oxidase! Arterioscler Thromb Vasc Biol. 2011;31:1255–1257. doi: 10.1161/ATVBAHA.111.226894. [DOI] [PubMed] [Google Scholar]
- 18.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Wolf G, Schroeder R, Stahl RA. Angiotensin II induces hypoxia-inducible factor-1 alpha in PC 12 cells through a posttranscriptional mechanism: role of AT2 receptors. Am J Nephrol. 2004;24:415–421. doi: 10.1159/000080086. [DOI] [PubMed] [Google Scholar]
- 20.Huisamen B, Pêrel SJ, Friedrich SO, Salie R, Strijdom H, Lochner A. ANG II type I receptor antagonism improved nitric oxide production and enhanced eNOS and PKB/Akt expression in hearts from a rat model of insulin resistance. Mol Cell Biochem. 2011;349:21–31. doi: 10.1007/s11010-010-0656-6. [DOI] [PubMed] [Google Scholar]
- 21.Ho JJ, Man HS, Marsden PA. Nitric oxide signaling in hypoxia. J Mol Med (Berl) 2012;90:217–231. doi: 10.1007/s00109-012-0880-5. [DOI] [PubMed] [Google Scholar]
- 22.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calò LA, Davis PA, Piccoli A, Pessina AC. A role for heme oxygenase-1 in the antioxidant and antiapoptotic effects of erythropoietin: the start of a good news/bad news story? Nephron Physiol. 2006;103:107–111. doi: 10.1159/000092213. [DOI] [PubMed] [Google Scholar]
- 25.Das UN. Is angiotensin II an endogenous pro-inflammatory molecule? Med Sci Monit. 2005;11:RA155–162. [PubMed] [Google Scholar]
- 26.Schmeisser A, Soehnlein O, Illmer T, Lorenz HM, Eskafi S, Roerick O, Gabler C, Strasser R, Daniel WG, Garlichs CD. ACE inhibition lowers angiotensin II-induced chemokine expression by reduction of NF-kB activity and AT1 receptor expression. Biochem Biophys Res Commun. 2004;325:532–540. doi: 10.1016/j.bbrc.2004.10.059. [DOI] [PubMed] [Google Scholar]
- 27.Smrzova J, Balla J, Barany P. Inflammation and resistance to erythropoiesis-stimulating agents – what do we know and what needs to be clarified? Nephrol Dial Transplant. 2005;20:2–7. doi: 10.1093/ndt/gfh1109. [DOI] [PubMed] [Google Scholar]