Abstract
Background
The osteocyte-derived hormone, fibroblast growth factor 23 (FGF23), regulates the phosphorus metabolism and suppresses 1,25-dihydroxyvitamin D production, thereby mitigating hyperphosphatemia in patients with renal disorders. An elevated FGF23 level is suggested to be an early biomarker of altered phosphorus metabolism in the initial stages of chronic kidney disease (CKD) and acts as a strong predictor of mortality in dialysis patients. In the Saudi population, there is no report on the FGF23 level in CKD patients to date. This study aims to estimate the plasma FGF23 levels in the Saudi population and to correlate it with its clinical manifestations in order to ascertain its role in the pathogenesis of CKD patients.
Methods
The FGF23 level in the plasma samples was determined using ELISA in a diverse cohort of 89 cases with stage 3-5 CKD and 100 healthy subjects. The plasma FGF23 level was correlated with other biochemical parameters.
Results
The results revealed that the FGF23 level was markedly elevated among CKD patients compared to the control group, and a significant inverse correlation was observed between the FGF23 level and glomerular filtration rate. FGF23 elevation was approximately 40-fold among stage 5 patients compared to the control, while the elevation of phosphate, parathyroid hormone (PTH) and alkaline phosphatase was 2-, 3- and 8-fold in this stage, respectively.
Conclusion
Elevated FGF23 levels may have a strong correlation with the disease pathogenesis. In addition, FGF23 might be a future therapeutic target to intervene against the progression of CKD as well as to increase patient survivability.
Key Words: FGF23, Chronic kidney disease, Phosphate, Parathyroid hormone, Calcitriol, Mortality
Introduction
Chronic kidney disease (CKD) predisposes people to a higher risk for cardiovascular diseases, left ventricular hypertrophy and end-stage renal disease (ESRD), leading to premature mortality; it is thus considered a public health epidemic worldwide. Numerous epidemiological studies have underlined the importance of a recently identified hormone, fibroblast growth factor 23 (FGF23), which is associated with declining renal function.
The main functions of FGF23 are the regulation of systemic phosphate homeostasis and vitamin D metabolism. FGF23, predominately expressed in bone osteocytes and osteoblasts, is a key molecule in the counterregulation of vitamin D and parathyroid hormone (PTH) biosynthesis, thereby regulating the phosphate metabolism parallel to the 1,25-dihydroxyvitamin D3 (calcitriol) and PTH-regulating phosphate and calcium. Klotho, which is an important cofactor of FGF23, is expressed in the kidney and parathyroid gland and suppresses calcitriol and PTH synthesis, respectively, thereby increasing the FGF23 expression in the bones [1]. FGF23 inhibits the synthesis of calcitriol by inhibiting the activity of the renal 25-hydroxyvitamin D-1α-hydroxylase enzyme. The stimulation of FGF23 secretion is triggered or repressed by sustained increase or decrease in dietary phosphorus intake, respectively. The aberrant function of FGF23 has been shown to result in several clinical manifestations [2].
Several cross-sectional studies have demonstrated that there is an inverse association between renal function and FGF23 levels. High FGF23 levels in patients with CKD and ESRD have been reported to be linked with various adverse events including mortality [3]. A Swedish population study identified FGF23 as an independent predictor of cardiovascular mortality, supporting its potential role in cardiovascular disease [4]. Elevated FGF23 has also been associated with proteinuria and albuminuria levels in patients with CKD and IgA nephropathy in Dutch and Swedish populations [5,6]. The Mild to Moderate Kidney Disease study including patients from Germany, Austria and Italy showed FGF23 as a novel independent predictor of progression of renal disease in patients with nondiabetic CKD [7]. Altogether, FGF23 is emerging as a novel and exciting biomarker that may help to identify which CKD patients might benefit most from the aggressive management of the disordered phosphorus metabolism [8]. Few studies have examined or reviewed the potential role of FGF23 as a prognostic marker in CKD progression [5,6,7,8,9]. A single study from a Saudi population with respect to FGF23 levels on chronic obstructive pulmonary disease (COPD) reported a significant increase in the FGF23 level among the COPD patients [10]. Thus, our study is the first of its kind to correlate the FGF23 level among CKD patients in this indigenous population of Saudi Arabia. Limited data are available to determine whether FGF23 measurements are clinically relevant in CKD patients. Therefore, in the present study, we have evaluated the role of FGF23 as a biomarker of CKD progression and its severity in a Saudi Arabian patient population, which may be used to find strategies for a better management of CKD patients. The current study is an approach to validate the relationship between FGF23 elevations and CKD progression.
Methods
Study Population
Eighty-nine CKD patients were recruited from the Department of Nephrology at King Fahd Hospital of the University, Al-Khobar, Saudi Arabia. We categorized the patients into three groups according to the modified National Kidney Foundation staging system, which is based on estimated glomerular filtration rate (eGFR) values (stage 3: eGFR 30-60 ml/min/1.73 m2; stage 4: eGFR 15-29 ml/min/1.73 m2, and stage 5: eGFR <15 ml/min/1.73 m2). It is noteworthy that due to the lack of symptoms, stage 1 and 2 patients were not reported to register and were thus excluded. The patient group had a mean age of 55 ± 15 years and included stage 3 (n = 20), stage 4 (n = 18) and stage 5 (n = 51) CKD patients. The control group was composed of 100 healthy individuals with no history of CKD.
Patients with a known history of any phosphate wasting disorder (e.g., tumor-induced osteomalacia, X-linked hypophosphatemia, autosomal dominant hypophosphatemic rickets or untreated primary hyperthyroidism) and those currently treated with renal replacement therapy were excluded from the study.
Sample Collection
Blood samples were collected in EDTA vacutainers, and plasma was extracted and stored at −80°C until the day of FGF23 estimation. Anthropometric details and biochemical parameters, including levels of eGFR, creatinine, phosphate, PTH, alkaline phosphatase (ALP), albumin and calcium were documented.
Measurement of FGF23 and 1,25-Hydroxyvitamin D3 Levels in Plasma
Levels of native human FGF23 in plasma samples were determined by human FGF23 ELISA assay kit (catalogue No. EZHFGF23-32K; Millipore, USA). The assay was conducted according to the manufacturer's instructions, with the intensity of color produced read on a plate reader absorbance set at 450 and 590 nm (ELx800; Biotek). Levels of vitamin D3 were measured by HPLC according to the manufacturer's instructions (Recipe HPLC kit for Vitamin D Analysis, Germany).
Statistical Analysis
Continuous variables are reported as the mean ± SD, and categorical variables are presented as numbers and percentages. Linear regression analysis was performed using FGF23 as a dependent variable. Since plasma FGF23 levels showed an asymmetrical distribution, their logarithmic conversion was taken in a customizable manner to a linear model. Survival curves were plotted by the Kaplan-Meier method and assessed by the log-rank test. Differences with p values <0.05 were considered statistically significant. The averaged biochemical levels of the deceased patients were compared using the two-sided t test. Statistical analyses were carried out using GraphPad Prism 5 software (GraphPad Software, USA).
Results
Clinical Parameters
Clinical patient data were obtained from the medical records of the Department of Nephrology at King Fahd Hospital of the University, Al Khobar, Saudi Arabia. Clinical and demographic characteristics of the study subjects are presented in table 1, including 63 males and 26 females of Arabian ethnicity. The mean body mass index was 27.7 ± 6.7, which is considered overweight. The cause of renal failure for the majority of the patients was diabetes (48.3%), followed by hypertension (36.0%). The majority have anemia (34.8%), secondary hyperparathyroidism (28.1%) and coronary artery disease (20.2%) as coexisting conditions.
Table 1.
Characteristics of the study population
| Total (n = 89) | Men (n = 63) | Women (n = 26) | |
|---|---|---|---|
| Demographic data | |||
| Age, years | 54.3±15.5 | 54.1±16.1 | 55.0±13.6 |
| Age at diagnosis, years | 42.6±15.6 | 40.7±14.9 | 45.1±14.9 |
| Body mass index | 27.7±6.7 | 27.4±5.6 | 29.0±8.7 |
| Primary cause of CKD, n (%) | |||
| Diabetes | 43 (48.3) | 32 (50.8) | 11 (42.3) |
| Hypertension | 32 (36.0) | 25 (39.7) | 7 (26.9) |
| Glomerulonephritis | 9 (10.1) | 4 (6.3) | 5 (17.2) |
| Other | 5 (5.6) | 2 (3.17) | 3 (11.5) |
| Coexisting conditions, n (%) | |||
| Coronary artery disease | 18 (20.2) | 16 (25.4) | 2 (7.7) |
| Congestive heart failure | 7 (7.9) | 6 (9.5) | 1 (3.8) |
| Stroke | 2 (2.2) | 1 (16) | 1 (3.8) |
| Secondary hyperparathyroidism | 25 (28.1) | 17 (27.0) | 8 (30.8) |
| Anemia | 31 (34.8) | 22 (34.9) | 9 (34.6) |
| Systolic blood pressure, mm Hg | 146.3±22.4 | 146.5±23.6 | 146.1±20.5 |
| Diastolic blood pressure, mm Hg | 78.3±13.5 | 77.0±12.7 | 82.0±15.1 |
FGF23 Levels and Mineral Metabolite in Relation to Stages
FGF23 levels and other biochemical parameters including creatinine, PTH, phosphate, calcium, ALP, vitamin D3 and albumin of patients classified into the three stages and the control group are shown in table 2. Data indicate that the circulating FGF23 level gradually increased with declining renal function. The mean FGF23 level in the control group was 39.66 ± 17.38 pg/ml, and an exponential increase was observed between stage 3 (mean 61.20 ± 14.10 pg/ml), stage 4 (mean 118.50 ± 63.20 pg/ml) and stage 5 (mean 1,526.40 ± 1,456.00 pg/ml), with a concurrent decline in eGFR levels and an increase in creatinine level. Phosphate, PTH and ALP increased concurrently with an increase in the FGF23 level. However, the increase in PTH and ALP levels was considerably higher than that in the phosphate levels. On the other hand, the levels of calcium, albumin and vitamin D3 remained unaltered compared to controls.
Table 2.
Biochemical parameters according to the levels of renal function
| CKD groups | Control | Stage 3 | Stage 4 | Stage 5 |
|---|---|---|---|---|
| Subjects, n (%) | 100 | 20 (22.5) | 18 (20.2) | 51 (57.3) |
| Age, years | 44.90±20.30 | 55.88±15.64 | 55.00±14.36 | 53.57±16.03 |
| Males/females | 68/32 | 16/4 | 12/6 | 35/16 |
| eGFR, ml/min/1.73 m2 | <60 | 43.70±8.30 | 21.00±4.40 | 8.63±1.50 |
| Biochemical data | ||||
| Creatinine, mg/dl | 0.69±0.19 | 1.60±0.30 | 3.00±0.60 | 10.10±3.60 |
| PTH, pmol/l | 5.85±2.94 | 10.30±5.60 | 19.90±12.00 | 44.38±38.00 |
| Phosphate, mg/dl | 3.84±0.62 | 3.60±0.80 | 4.30±0.60 | 4.81±1.20 |
| Calcium, mg/dl | 9.07±0.46 | 9.00±0.40 | 8.70±0.80 | 8.80±1.20 |
| ALP, U/l | 88.21±45.57 | 89.80±37.80 | 154.30±150.40 | 173.55±123.30 |
| Vitamin D3, ng/ml | 18.78±6.00 | 20.90±14.10 | 17.40±5.40 | 22.90±12.90 |
| Albumin, g/dl | 3.50±0.40 | 3.70±0.50 | 3.50±0.60 | 3.30±0.50 |
| Intact FGF23, pg/ml | 39.66±17.38 | 61.20±14.10 | 118.50±63.20 | 1,526.40±1,456.00 |
To further assess the strength of correlation between an elevated FGF23 level with other biochemical parameters, we performed a linear regression analysis with FGF23 as the dependent variable and the levels of eGFR, creatinine, PTH, phosphate, calcium, ALP, vitamin D3 and albumin as independent variables irrespective of stage. Regression analysis showed that there is a significant negative correlation of FGF23 level with eGFR (R2 = 0.2144, p < 0.0001), and a significant positive correlation with creatinine (R2 = 0.4622, p < 0.0001) and phosphate (R2 = 0.12610, p < 0.0011). In addition, the analysis did not show any significant correlation with the levels of other analytes, such as PTH, calcium, ALP, albumin or vitamin D3 (table 3).
Table 3.
Linear regression analysis with levels of eGFR, creatinine, PTH, phosphate, calcium, ALP, vitamin D3 and albumin as independent variables versus the FGF23 level as dependent variable
| Analyte | β value | R2 value | p value |
|---|---|---|---|
| FGF23 | Ref. | Ref. | Ref. |
| eGFR | −0.0239 | 0.2144 | <0.0001 |
| Creatinine | 0.0052 | 0.4622 | <0.0001 |
| PTH | 1.0370 | 0.0028 | 0.6285 |
| Phosphate | 0.0023 | 0.1261 | 0.0011 |
| Calcium | −0.1606 | 0.0014 | 0.7318 |
| ALP | −2.7760 | 0.0102 | 0.3637 |
| Vitamin D3 | 0.0611 | 0.0228 | 0.1920 |
| Albumin | −0.0038 | 0.0102 | 0.3637 |
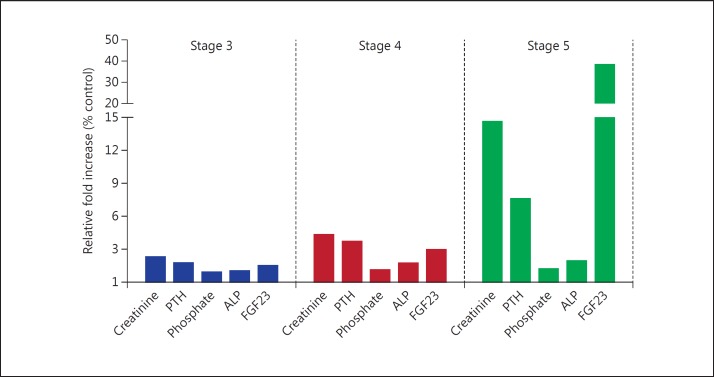
Several studies have demonstrated that as the renal function declines, the level of creatinine, PTH, phosphate, ALP and FGF23 increases in the blood. To illustrate the rate of increase in the level of these parameters in each stage, we plotted a graph representing the fold increase of each parameter based on their control on the y-axis and the three stages on the x-axis (fig. 1). Like other parameters, a gradual increase in the FGF23 level can be observed in stages 3 and 4, but the fold increase suddenly shot up more than 10 times at stage 5.
Fig. 1.
Relative fold increase of the biochemical parameters, namely creatinine, PTH, phosphate, ALP and FGF23, in three different stages of CKD.
Relationship between Elevated FGF23 and Mortality
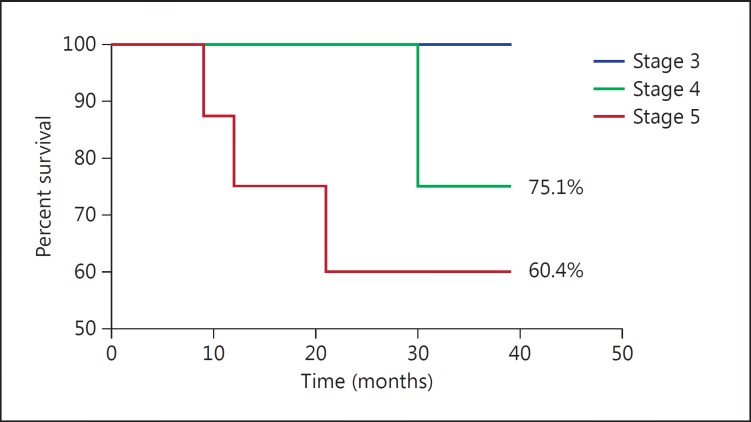
To verify that there is any association between this uncontrolled elevation of FGF23 with mortality, we followed up patients over a period of 36 months and collected the survival data. From these data, we found that 14 out of 89 patients had died. A Kaplan-Meier analysis was performed using their time of death (p = 0.0373), and the results of the survival function are presented in figure 2. We found that percent survival was in the following order: stage 3 (100%) > stage 4 (75.1%) > stage 5 (60.4%). The log-rank trend was significant.
Fig. 2.
Kaplan-Meier survivability curve in stage 3, 4 and 5 CKD patients.
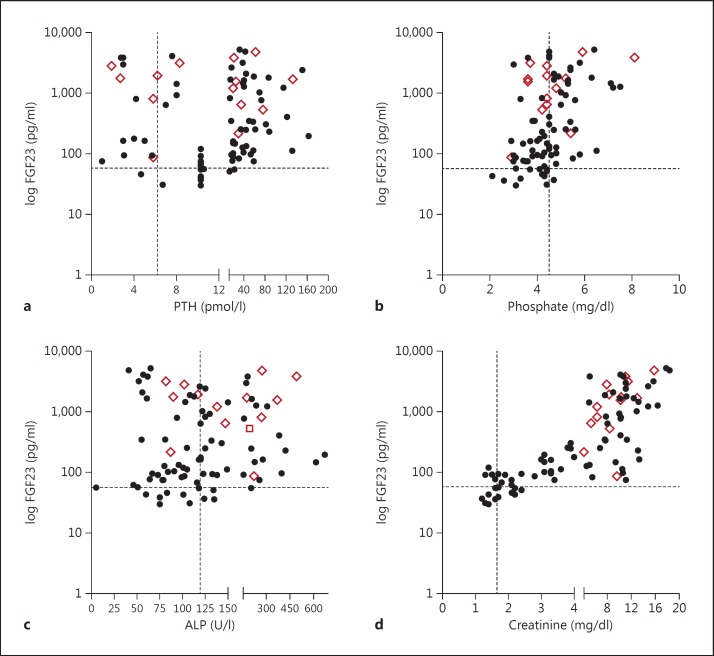
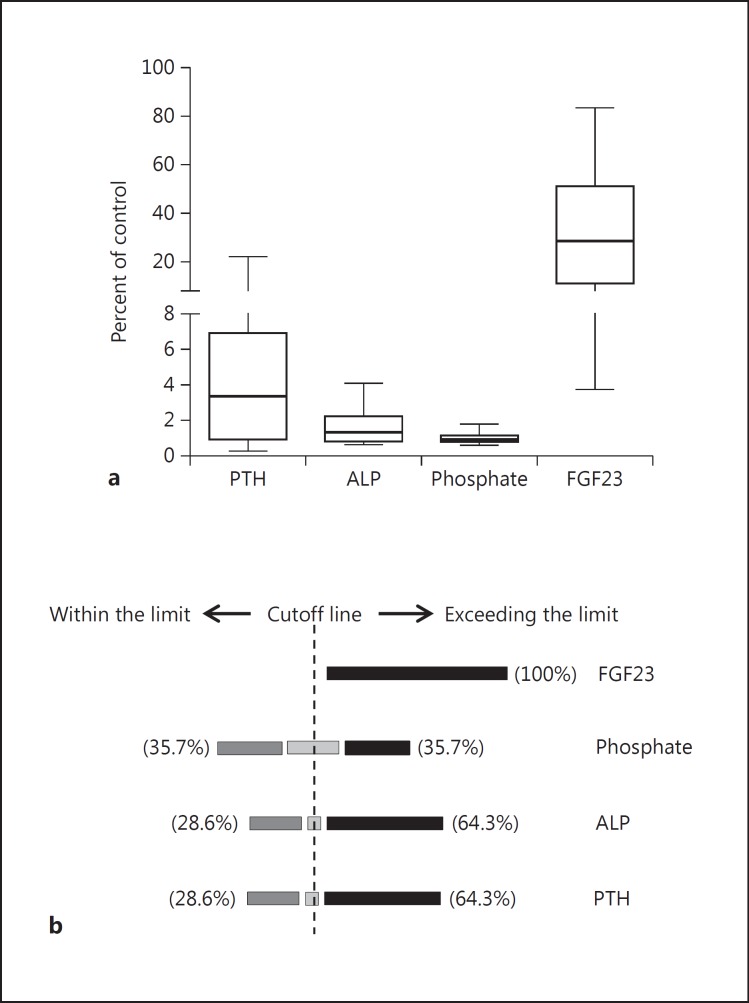
We noticed that the FGF23 level in the deceased group was considerably high, and the majority (65%) had >1,000 pg/ml of FGF23. To evaluate the status of other parameters compared to FGF23 in this group, the FGF23 level of the patient cohort was plotted against different parameters, such as creatinine, PTH, ALP and phosphate (fig. 3). The upper limit of normal range of the control group for each biochemical parameter was set as the cutoff mark for analysis (i.e. FGF23 = 60 pg/ml, PTH = 9 pmol/l, phosphate = 4.5 mg/dl, ALP = 120 U/l, and creatinine = 1.6 mg/dl). The level of FGF23 of all the deceased patients was above the cutoff mark compared to all other parameters, showing a distribution on both lobes, except for creatinine which is a certain indicator of renal function failure. To reinforce this phenomenon, we drew a box plot using percent of control values for all biochemical parameters of the deceased group, as shown in figure 4a. The mean value and the standard deviation of FGF23, PTH, ALP and phosphate in the deceased group was 32.03 ± 23.89, 5.141 ± 6.154, 1.641 ± 0.999 and 1.032 ± 0.283%, respectively, and the p value of FGF23 was 0.0002, and that of PTH, ALP and phosphate was 0.0080, <0.0001 and <0.0001, respectively. Additionally, a schematic has been made to show how this deceased group was distributed around the cutoff line based on their biochemical parameters (fig. 4b). Except for FGF23, in which 100% are exceeding the cutoff line, the percentages for the other parameters are distributed on both sides of the cutoff line.
Fig. 3.
Distribution of biochemical parameters such as PTH (a), phosphate (b), ALP (c) and creatinine (d) related to their FGF23 levels in patients regardless of their CKD stages. The dotted line represents the cutoff value for each parameter, and the diamonds indicate the coordinates of those who died during the 36-month period.
Fig. 4.
a Levels of the biochemical parameters in the deceased groups based on their percent of control. Boxes with lines at the lower quartile, median and the upper quartile. b Distribution of the dead patients around the cutoff line of each parameter. The cutoff line represents the highest end point of the examined biochemical parameters in the control group. The rest of the percentages with other parameters falls on the cutoff line.
Discussion
These results are of great interest for the identification of new biomarkers, which may have a better diagnostic sensitivity for the detection of CKD. The importance of measuring the FGF23 level is reflected by several studies that have shown that FGF23 has an inevitable role in the progressive derangement of mineral homeostasis, such as phosphate and calcium, in addition to phosphotropic hormones [11]. Recent studies have shown that FGF23 elevation is significantly associated with declining renal function [7,11].
The secretion of PTH, vitamin D3 and FGF23 is regulated in a classic negative endocrine pathway. FGF23 secretion is stimulated by PTH, both directly and indirectly, via a PTH-mediated increase in vitamin D3[12]. The vitamin D3 response element in the FGF23 promoter region controls the regulation of FGF23 transcription. Similarly, an increased FGF23 level lowers the level of vitamin D3[13] and inhibits PTH secretion by an FGFR-Klotho-dependent pathway [14]. It is well established that higher levels of FGF23 are consistently associated with higher serum phosphate, PTH and creatinine levels as well as with lower levels of vitamin D3 and eGFR [13,15]. Similarly, in the present study, the FGF23 level increases with the advancement of CKD, with the most pronounced increase shown at stage 5 CKD. This increase occurs concurrently with an increase in the levels of creatinine, phosphate, PTH and ALP during CKD development.
We observed that the FGF23 levels were not reversed even in the patients who were undergoing hemodialysis (stage 5). Plasma FGF23 concentrations have been found to predict mortality not only among dialysis patients but among predialysis CKD patients as well, and has been identified as an independent risk factor for ESRD [16]. FGF23 elevation was primarily described in the context of impaired renal function, but it has been observed that the elevation is being associated with worse clinical outcomes in patients with cardiac disease and/or CKD. A very recent study has hypothesized that a single FGF23 measurement itself is enough of a prognostic tool to predict complications, mortality and the clinical course in patients undergoing elective cardiac surgery [17]. Similarly, another study reported that the FGF23 level in stage 5 was much higher in patients with severe secondary hyperparathyroidism and independently associated with decreased heart rate variability [18]. In our study cohort, the FGF23 level of 50% of patients in stage 5 was >1,000 pg/ml, and 30% of them have coronary artery disease and 50% secondary hyperparathyroidism as coexisting conditions. Within 36 months, 26% of stage 5 patients had died, of whom 65% had an FGF23 level >1,000 pg/ml. This result further strengthens the previous findings that FGF23 indeed is a prognostic marker of CKD complications and mortality. The biochemical mechanism behind this elevated FGF23 in stage 5 CKD, and its association with mortality, is still unclear. However, the above-mentioned studies and the present results reinforce the importance of quantifying FGF23 levels over the other parameters in each CKD stage to provide better strategies, to prevent the patient's health from worsening and to avert the mortal consequences.
Gutiérrez et al. [19] reported that an increased C-terminal FGF23 level was associated with a monotonically increasing risk of death, with highest-quartile FGF23 levels nearly 600% increased compared to subjects with lowest-quartile FGF23 levels. FGF23 levels lacked any significant interaction with ethnic group and mortality; however, a significant interaction with race (p = 0.048) was seen, with the median FGF23 level being significantly lower among Blacks and Hispanics than among Whites [19]. Three more studies reported an association between higher serum FGF23 levels and mortality risk among chronic hemodialysis patients. Higher serum FGF23 levels, independent of the serum phosphate level, were associated with increased all-cause mortality, but also related to vascular calcification in the French population [20]. On the other hand, in the Japanese population, Sugimoto et al. [21] found no association between higher serum FGF23 levels and increased mortality risk until affected by previous CVD and also showed a gender impact on the association between serum FGF23 levels and mortality. Similarly, Olauson et al. [22] reported a lack of association between serum FGF23 levels and increased mortality risk in a Swedish population, but the level of FGF23 was a risk factor for mortality in crude models among men with previous CVD [22]. In contrast, a recent study determining if peptide biomarkers can be useful to identify the risk of mortality among hemodialysis patients in a German population revealed that among the modern peptide biomarkers, only mid-regional pro-adrenomedullin and FGF23 showed an association with mortality [23]. These studies and our observed data indicate that though the determination of the FGF23 level in hemodialysis patients is controversial, it still holds a significant place in diagnosis.
While FGF23 appears to have significant potential as a diagnostic marker of CKD, it is uncertain whether it is causally underpinning CKD or simply associated with the process. The analysis methodology of Mendelian randomization can strengthen inferences drawn from observational studies and has the ability to deconvolute the relationship between causality and association [24], since Mendelian randomization relies on the random assortment of alleles at gametogenesis and assesses the distributions of genetic variants that are generally independent of behavioral and environmental factors which typically confound epidemiological associations between postulated risk factors and diseases of interest. This method has been used to show that well-established factors that are strongly associated with a condition's outcome are not causally related to the condition, whilst a new factor is shown to be causally related [25], and this may be a means to assess the potential of FGF23 as a potential drug target. Good physiological and molecular studies in this area are still on demand to answer the actual mechanism behind this FGF23 elevation at stage 5 and its impact on patient survival.
Conclusions
An elevated FGF23 level is significantly associated with declining renal function. The rate of FGF23 elevation at different stages of CKD and its correlation with other biochemical parameters shows that FGF23 is associated with PTH, creatinine and phosphate levels during CKD development and precedes all these parameters at stage 5. The level of FGF23 was associated with an increased mortality risk in this cohort of ESRD patients. FGF23 can be used as a potential biomarker to guide strategies for a better management of patients with CKD. In addition, FGF23 might be a future therapeutic target to intervene against the progression of CKD as well as to increase patient survivability.
Statement of Ethics
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of the University of Dammam and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Disclosure Statement
The authors report no conflicts of interest.
Acknowledgement
This work was supported by a grant received from the Deanship of Scientific Research, University of Dammam, Dammam, Saudi Arabia.
References
- 1.Kuro-o M. Klotho and βklotho. Adv Exp Med Biol. 2012;728:25–40. doi: 10.1007/978-1-4614-0887-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 3.Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, et al. Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int. 2005;67:1120–1125. doi: 10.1111/j.1523-1755.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 4.Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, Larsson TE. Higher fibroblast growth factor-23 increases the risk of all cause and cardiovascular mortality in the community. Kidney Int. 2013;83:160–166. doi: 10.1038/ki.2012.327. [DOI] [PubMed] [Google Scholar]
- 5.Vervloet MG, van Zuilen AD, Heijboer AC, ter Wee PM, Bots ML, Blankestijn PJ, Wetzels JF, MASTERPLAN group study fibroblast growth factor 23 is associated with proteinuria and smoking in chronic kidney disease: an analysis of the MASTERPLAN cohort. BMC Nephrol. 2012;13:20. doi: 10.1186/1471-2369-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundberg S, Qureshi AR, Olivecrona S, Gunnarsson I, Jacobson SH, Larsson TE. FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol. 2012;7:727–734. doi: 10.2215/CJN.10331011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 8.Wolf M. Fibroblast growth factor 23 and the future of phosphorus management. Curr Opin Nephrol Hypertens. 2009;18:463–468. doi: 10.1097/MNH.0b013e328331a8c8. [DOI] [PubMed] [Google Scholar]
- 9.Smith ER. The use of fibroblast growth factor 23 testing in patients with kidney disease. Clin J Am Soc Nephrol. 2014;9:1283–1303. doi: 10.2215/CJN.10941013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsammak MY, Attia A, Suleman M. Fibroblast growth factor-23 and hypophosphatemia in chronic obstructive pulmonary disease patients. J Med Biochem. 2012;31:12–18. [Google Scholar]
- 11.Nitta K, Nagano N, Tsuchiya K. Fibroblast growth factor 23/klotho axis in chronic kidney disease. Nephron Clin Pract. 2014;128:1–10. doi: 10.1159/000365787. [DOI] [PubMed] [Google Scholar]
- 12.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Tang W, Zhou J, Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 14.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427–1435. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Chronic Renal Insufficiency Cohort (CRIC) Study Group Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speer T, Groesdonk HV, Zapf B, Buescher V, Beyse M, Duerr L, Gewert S, Krauss P, Poppleton A, Wagenpfeil S, Fliser D, Schaefers HJ, Klingele M. A single preoperative FGF23 measurement is a strong predictor of outcome in patients undergoing elective cardiac surgery: a prospective observational study. Crit Care. 2015;19:190. doi: 10.1186/s13054-015-0925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang LN, Yang G, Cheng C, Shen C, Cui YY, Zhang J, et al. Plasma FGF23 levels and heart rate variability in patients with stage 5 CKD. Osteoporos Int. 2015;26:395–405. doi: 10.1007/s00198-014-2862-7. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–2796. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto H, Ogawa T, Iwabuchi Y, Otsuka K, Nitta K. Relationship between serum fibroblast growth factor-23 level and mortality in chronic hemodialysis patients. Int Urol Nephrol. 2014;46:99–106. doi: 10.1007/s11255-013-0386-2. [DOI] [PubMed] [Google Scholar]
- 22.Olauson H, Qureshi AR, Miyamoto T, Barany P, Heimburger O, Lindholm B, Stenvinkel P, Larsson TE. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: are gender and cardiovascular disease confounding the relationship? Nephrol Dial Transplant. 2010;25:3033–3038. doi: 10.1093/ndt/gfq191. [DOI] [PubMed] [Google Scholar]
- 23.Artunc F, Nowak A, Müller C, Peter A, Heyne N, Häring HU, Friedrich B. Mortality prediction using modern peptide biomarkers in hemodialysis patients – a comparative analysis. Kidney Blood Press Res. 2014;39:563–572. doi: 10.1159/000368468. [DOI] [PubMed] [Google Scholar]
- 24.Smith GD, Ebrahim S. Mendelian randomization prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 25.Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–550. doi: 10.1093/eurheartj/eht571. [DOI] [PMC free article] [PubMed] [Google Scholar]