Abstract
The antioxidant potential of mistletoe (Viscum album L. var. coloratum Ohwi; VAL) extract in uncooked pork patties was evaluated. Three concentrations of VAL extract (0.1 [T1], 0.5% [T2] and 1.0% [T3]) along with 0.02% ascorbic acid as a positive control (V) were added to ground pork and pork patties were prepared. Incorporation of VAL extract decreased (p<0.05) the pH of the pork patties throughout the storage time and reduced (p<0.01) the thiobarbituric acid reactive substance values after day 14 of storage. Total plate counts of the VAL extract-treated samples and V-treated samples were also significantly lower (p<0.01) than that of the control (C) throughout the storage period. In addition, odor scores of the VAL extract-treated patties were lower than those of the C- or V-treated samples on 3rd day of the storage period. These results demonstrated that the VAL extract acts as a natural antioxidant in uncooked pork products.
Keywords: Mistletoe Extract, Antioxidant, Lipid Oxidation, Microbial Counts, Uncooked Pork Patties
INTRODUCTION
Synthetic and natural antioxidants have been successfully utilized to prevent or delay auto-oxidation in meat (McCarthy et al., 2001a). Addition of synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tertiary butylhydroquinone has been reported to be very effective for preventing oxidative changes in meat products (Khalil and Mansour, 1998). However, use of these synthetic antioxidants has been restricted due to possible health risks and toxicity (Buxiang and Fukuhara, 1997). Furthermore, consumers are increasingly demanding additive-free or natural meat products (Ahn et al., 2002). A large number of naturally occurring compounds such as flavonoids, catechins, lignans, and phenolic acids contained in herbs and spices have antioxidant properties. Natural antioxidants have recently become a major area of research. The term ‘mistletoe’ is generally applied to plants with a similar hemiparasitic lifestyle and a certain degree of taxonomical relationship in three families: Loranthaceae, Viscaceae, and Eremolepidaceae (Bar-Sela, 2011). Mistletoe is capable of photosynthesis but relies on xylem sap of host plants to obtain water and mineral nutrients (Ehleringer and Marshall, 1995). It was reported that mistletoe contains lectins (Franz et al., 1981), alkaloids (Khwaja et al., 1980), viscotoxins (Romagnoli et al., 2000), and polysaccharides (Mueller and Anderer, 1990). Clinical and experimental studies showed that extracts from Viscum album L. (VAL), a type of mistletoe that lives on the Quercus acutissima tree, exert cytotoxic effects (Jung et al., 1990) and possess immunostimulatory properties (Mannel et al., 1991). Despite the fact that heat-treated mistletoe extracts are widely consumed as tea or medical reagents, the ability of mistletoe extracts to help preserve meat products is unclear. In the present study, the antioxidant activity of VAL extract in meat was investigated. In addition, the color, total plate count (TPC), coliform, and 2-thiobarbituric acid reactive substances (TBARS) of uncooked pork patties treated with VAL extract were evaluated during a 14-d chilled storage period.
MATERIALS AND METHODS
Materials
Catechin, quercetin, Folin–Ciocalteu reagent, deoxyribose, deoxyribose, xanthine oxidase, hypoxantine, aluminium chloride (AlCl3), sodium hydroxide (NaOH), ethylenediaminetetraacetic acid (EDTA), iron chloride (FeCl3), L-ascorbic acid, BHA, thiobarbituric acid (TBA), trichloroacetic acid (TCA) and nitroblue tetrazolium (NBT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Sodium chloride (NaCl) and sodium nitrite (NaNO2) were purchased from Nacalai Tesque Inc. (Kyoto, Japan). Methanol and ethanol used was of HPLC grade and all other solvents used in this study were of analytical grade.
VAL extract preparation
The stems of V. album that lives on the Quercus acutissima tree were purchased from a local market (Sanchung market, Jinju, Republic of Korea). The plant materials were air-dried for 7 d at room temperature in the dark, and then powdered with a mill (Shinil SFM-555SP, Hwasung, Korea). The dried and powdered plant material (100 g) was subjected to extraction with 70% ethanol (900 mL) at room temperature for 7 d with occasional stirring. The sample was filtered (Whatman Ltd, Maidstone, Kent, England) and the ethanol was removed (RW-0524G; Heidolph, Germany) at 40°C. The dried extract was re-dissolved in water (~2 g of extract was first dissolved in a minimum amount of ethanol and then 200 mL of water were added), dried in a freeze-drier (PVTFD10R, ILSIN Bio Co., Dongduchun, Korea), and stored at −20°C until further use.
Total phenolic contents of VAL extract
Total phenolic contents of the VAL extracts were determined spectrophotometrically according to the Folin-Ciocalteu colorimetric method (Singleton and Rossi, 1965). Because catechin a polyphenolic compounds, total phenolic contents of the VAL extract were expressed as μg catechin equivalents (CE)/g extract. Different concentrations of (+)-catechin were prepared in 80% methanol, and absorbance was recorded at 765 nm. For analysis, 100 μL of sample (1 mg/mL) was dissolved in 500 μL (1:10 dilution) of Folin–Ciocalteu reagent and 1,000 μL of double distilled water (DDW), mixed, and incubated at room temperature for 1 min. Next, 1,500 μL of 20% sodium carbonate (Na2CO3) solution was added. The mixture was shaken and then incubated for 2 h in the dark at room temperature. Absorbance of all samples was measured using a spectrophotometer (Ultrospec 2100 pro; Amersham Pharmacia Biotech Co., Piscataway, NJ, USA).
Total flavonoid contents of VAL extract
Total flavonoid contents of the VAL extract were determined using the method described by Meda et al. (2005) with minor modifications. Briefly, 0.25 mL of sample (1 mg/mL) was added to a tube containing 1 mL of DDW. Next, 0.075 mL of 5% NaNO2, 0.075 mL of 10% AlCl3, and 0.5 mL of 1 M NaOH were sequentially added at 0, 5, and 6 min. Finally, the volume of the solution was adjusted to 2.5 mL with DDW. Absorbance of the solution was measured at a wavelength of 410 nm with a spectrophotometer (Ultrospec 2100 pro, Amersham Pharmacia Biotech Co., USA). Quercetin, a ubiquitous flavonoid present in many plant extracts, was used as a standard to quantify the total flavonoid contents of the VAL extract. The results are expressed as μg quercetin equivalents (QE)/g of extract. The total flavonoid content was measured five times and the experiment was repeated three times.
Free radical scavenging activity of VAL extract
Free radical scavenging activity (FRSA) of the VAL extract (1 mg/mL) was measured using the method described by Brand-Williams et al. (1995). Briefly, a 100 mL solution of VAL extract (1 mg/mL) in ethanol was mixed with 100 μL of 0.1 mM 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) in ethanol. The mixtures were shaken vigorously and allowed to stand at room temperature in the dark for 25 min. Thereafter, the absorbance of the assay mixture was measured at 518 nm against each blank in a spectrophotometer (Ultrospec 2100 pro; Amersham Pharmacia Biotech Co., USA). L-ascorbic acid was used as a positive control. The percent inhibition was calculated using the following equation:
Hydroxyl radical scavenging activity of VAL extract
Hydroxyl radical scavenging activity (HRSA) of the VAL extract was measured using the deoxyribose method (Halliwell, 1996) with a slight modification. The assay was performed with 10 mM phosphate buffer (pH 7.4) containing 2.5 mM deoxyribose, 1.5 mM H2O2, 100 μM FeCl3, 104 μM EDTA, and the test sample (0.5 mg/mL). The reaction was initiated by adding ascorbic acid at a final concentration of 100 μM. The reaction mixture was then incubated for 1 h at 37°C in a water bath. After incubation, color was developed by the addition of 0.5% TBA followed by ice-cold 2.8% TCA in 25 mM NaOH and heating for 30 min at 80°C. A sample of the extract without any additions was used as the control (A1). Sample A2 was cooled on ice and the absorbance was measured at 532 nm. The HRSA was calculated as follows:
Superoxide anion radical scavenging activity of VAL extract
Superoxide anion radicals were generated using a modified method by Liu et al. (1997). VAL extract samples (0.5 mg/mL in dimethyl sulfoxide were added to a reaction solution containing 100 μL of 30 mM EDTA (pH 7.4), 10 μL of 30 mM hypoxantine in 50 mM NaOH, and 200 μL of 1.42 mM NBT; mixed, and preincubated at room temperature for 3 min. Next, 100 μL of 0.5 U/mL xanthine oxidase was added to the mixture and the volume was brought up to 3 mL with 50 mM phosphate buffer (pH 7.4). The solution was then incubated at room temperature for 20 min and absorbance was measured at 560 nm. A sample of reaction mixture without xanthine oxidase was used as a blank (A1). VAL extract (A2) was added to the reaction mixture, and superoxide anion radical scavenging was measured by assessing the inhibition of NBT reduction. Absorbance was measured and decreases in superoxide anions were calculated as follows:
Preparation of fresh minced pork
Pork (longissimus dori) and back-fat were obtained from a meat processing company (Asian C&A Co. Ltd., Jinju, Korea) and chilled overnight at 4°C. Meat and back-fat were separately minced twice (with a 10-mm steel plate followed by 5-mm steel plate) in a mincer (model MGB-32; Fugee, Uijeongbu, Korea). The minced meat (85%) was thoroughly mixed with back-fat (15%) and refined wheat flour (3%) in a silent cutter (AS-30; Ramon Co., Oiartzum, Spain). The minced meat was then divided into five aliquots: control sample (no functional ingredients were added, C), minced meat mixed with 0.02% ascorbic acid (V); and minced meat mixed with 0.1% (T1), 0.5% (T2), or 1.0% (T3) of dry VAL extract powder (dry base, w/w). The concentrations of VAL extract were selected mainly based on preliminary studies of sensory evaluation. The dry VAL ethanol extract was dissolved in DDW (10%, v/w), added immediately to the minced pork samples, and mixed thoroughly. The same volume of DDW was added to the control minced pork sample. Portions of the treated minced pork (50 g) were formed into patties (model K450; Ningbo Heng Yue Plastic Factory, Yuyao, China), placed in laminated low oxygen-permeable packs (0.5 cm3/m2/atm/24 h; Danisco Flexible, Lyngby, Denmark), and then stored up to 14 d at 4°C (±1°C) before subsequent analysis. The experiment was repeated in triplicate with three observations per replication. The manufacturing process was repeated four times; thus, 20 batches of patties were produced.
Color evaluation of the pork patties
Color of the VAL-treated meat was measured with a spectrocolourimeter (CR 400; Minolta Co., Osaka, Japan) calibrated using a white plate and light trap supplied by the manufacturer. Color was expressed based on the Commission Internationale de l’Eclairage L*a*b* color system in which L* is lightness, a* redness, and b* yellowness. Measurements were made using the C illuminant and 2° standard observers. A total of six spectral readings were taken for each VAL-treated meat sample with different instrument orientations, and measurements were recorded on days 1, 3, 7, and 14 for all vacuum-packed samples. Each color parameter was evaluated in triplicate and the experiment was repeated three times.
Lipid oxidation of the pork patties
A 2-TBARS test was performed according to Tarladgis et al. (1960) to measure oxidative rancidity. A sample of VAL-treated meat (5 g) was homogenized with 2.5 μL of BHA (7.2% in ethanol) and 15 mL of DDW using a homogenizer (IKA model T-25 Basic, Selangor, Malaysia). Two mL of the homogenate were mixed with 4 mL of TBA in a TCA solution (20 mM TBA in 15% TCA), heated at 90°C for 15 min in a water bath, cooled on ice, and centrifuged 2,000 rpm for 15 min at 4°C (UNION 5KR; Hanil Science Industrial, Co., Ltd., Incheon, Korea). Absorbance of the supernatant was measured at 532 nm in a spectrophotometer (Spectronic model Genesys-5, San Francisco, CA, USA). The malonaldehyde concentration (mg/kg) was calculated based on a standard curve. TBARS values were measured on days 1, 3, 7, and 14 for all vacuum-packed samples. The TBARS values were measured in triplicate and the experiment was repeated three times.
Microbial analysis of the pork patties
Samples of VAL-treated meat (25 g) were taken aseptically, transferred to sterile plastic pouches (Seward, London, UK), and homogenized for 2 min at room temperature in 225 mL sterile 0.88% (w/v) NaCl solution in a stomacher Lab-Blender 78860 (ST-Nom; Interscience, St. Nam, France). Appropriate dilutions of the samples were prepared in sterile 0.88% (w/v) NaCl solution, plated in duplicate onto plate count agar (Difco Laboratory, Detroit, MI, USA), and incubated at 35°C for 48 h under aerobic conditions. Results are expressed as log10 colony forming units (CFU)/g VAL-treated pork after 1, 3, 7, and 14 d of storage. Each microbial count was performed in triplicate and the experiment was repeated three times.
Sensory evaluation of the pork patties
On day 3 of storage, the pork patties were analyzed by 12 experienced panel members. Twelve training sessions were held to familiarize the panelists with the attributes to be evaluated and the assessment scale. The samples were cut into pieces of 2.0×3.0×2.0 cm3 and served to each panelist separately under white illumination. The panelists were presented randomly coded samples. The color, aroma, and flavor (1 = extremely undesirable, 9 = extremely desirable), off-odor (1 = low intensity, 9 = very high intensity), and overall acceptability (1 = extremely undesirable, 9 = extremely desirable) of the samples were evaluated. The panelists were required to cleanse their palates with water between samples. Sensory evaluation was performed three times.
Statistical analysis
Data were analyzed using the general linear model of the Statistical Analysis System’s Procedures (SAS Institute Inc., 1999). Duncan’s multiple range tests were used to identify differences between mean values. Significance was set at 5%.
RESULTS AND DISCUSSION
Total phenolic contents of VAL extract
Phenolic compounds, such as quercetin, rutin, narigin, catechins, caffeic acid, gallic acid, and chlorogenic acid, are very important plant constituents due to their antioxidant activities (Paganga et al., 1999). The total phenolic contents of the VAL extract are presented as (+)-CE in Table 1. Results revealed that the total phenolic content was 60.46 mg/g dry weight. Varela et al. (2004) reported that the methanol extract of Phoradendron liga (Gill. ex H. et A.) Eichl. (Viscaceae) leaves is 29.6±4.2 mg/g dry weight and that of stems is 19.6±7.0 mg/g dry weight when expressed as tannin acid equivalents. The quantity of total phenolic contents was reported to be affected by molecular weight of the standard used such as catechin and gallic acid (290.27 and 170.12, respectively) for the same sample (Chen and Yen, 2007). The levels of total phenolic compounds in the VAL extract presented as (+)-CE were similar to those reported by Varela et al. (2004) who used gallic acid as a standard.
Table 1.
Total phenolic and flavonoid contents along with antioxidant activities of the mistletoe (Viscum album L.; VAL) ethanol extract
| VAL extract | BHA | Ascorbic acid | Quercetin | |
|---|---|---|---|---|
| Total phenolic contents (mg CE/g) | 60.46±3.38 | |||
| Total flavonoid contents (mg QE/g) | 36.38±1.89 | |||
| FRSA (1 mg/mL) | 34.01±2.68c | 64.11±1.23b | 81.38±2.25a | - |
| HRSA (1 mg/mL) | 40.19±1.61b | 47.58±2.28a | 35.51±1.90b | - |
| SRSA (0.5 mg/mL) | 30.05±4.11b | - | - | 73.98±0.75a |
n = 5.
BHA, butylated hydroxyanisole; CE, catechin equivalents; QE, quercetin equalents; FRSA, free radical scavenging activity; HRSA, hydroxyl radical scavenging activity; SRSA, superoxide anion radical scavenging activity.
Different letters in the same row indicate that mean values±standard deviation (SD) were significantly different (p<0.01).
Flavonoid content of VAL extract
Flavonoids are ubiquitous in plants and form a group of low molecular weight polyphenolic compounds. Antioxidant activity and capacity to inhibit the growth of cancer cells are among the important effects of these reagents in biological systems (Zolezzi et al., 2005). The stems and leaves of Argentine mistletoe (Ligaria cuneifolia [R. et P.] Tiegh. [Loranthaceae]) were reported to contain quercetin as the only flavonol, which was also found to occur freely and only monoglycosylated with xylose, rhamnose, and arabinose at the hydroxyl group in position 3 of the flavonol skeleton (Fernández et al., 1998). The concentration of flavonoids in the VAL extract was 36.38 mg QE/g dry weight as detected by 2% aluminum chloride (Table 1).
FRSA of VAL extract
The DPPH is a stable free radical. Researchers have used this compound to evaluate the efficiency of antioxidants and flavonoids (Brand-Williams et al., 1995). The DPPH radical has been widely used to measure the FRSA of various natural products and is accepted as a model compound of free radicals originating from lipids (Da Porto et al., 2000). Results of the FRSA analysis for the VAL extract are shown in Table 1. For this experiment, BHA and ascorbic acid served as references. The FRSA of BHA was 81.38% and 64.11% for ascorbic acid at equal concentrations (1 mg/mL). The VAL extract generally showed a high antioxidant capacity and significant (p<0.05) differences between samples were observed at the same concentration (1 mg/mL). These results clearly indicated that all tested samples exhibited different levels of antioxidant activity (p<0.05) in the following order: ascorbic acid>BHA>VAL extracts (34.01%).
HRSA of VAL extract
Among the reactive oxygen species, hydroxyl radicals are the most reactive and predominant radicals generated endogenously during aerobic metabolism that inflict cell damage in vivo (Mates and Sanchez-Jimenez, 2000). Inhibition of deoxyribose degradation is considered an indication of hydroxyl radical scavenging action (Halliwell et al., 1987; Lopes et al., 1999). The HRSA of VAL extracts at a concentration of 0.5 mg/mL relative to the same concentration of BHA and ascorbic acid is shown in Table 1. For this experiment, BHA and ascorbic acid were used as references. The HRSA was 47.58% for BHA and 35.51% for ascorbic acid at the same concentration (1 mg/mL). Significantly higher HRSA for BHA was observed. The HRSA of the VAL extract was similar to that of ascorbic acid.
SRSA of VAL extract
Superoxide anions are a reduced form of molecular oxygen created by the addition of one electron. Superoxide radicals have been observed to kill cells, inactivate enzymes; and degrade DNA, cell membranes, and polysaccharides (Halliwell, 1996). FRSA was, therefore, proposed to measure the comparative interceptive ability of antioxidant extracts to scavenge the superoxide radical (Vani et al., 1997). The SRSA of the VAL extract was compared to that of quercetin (0.5 mg/mL) at the same concentration as shown in Table 1. The SRSA of the VAL extract was lower than that of quercetin (73.98%; p<0.05). These data indicated that the VAL extract is a relatively good source of antioxidants in terms of phenolic and flavonoid contents. Thus, the effect of the extract on the quality of pork patties was evaluated.
pH of pork patties
In Table 2, pH of the uncooked pork patties mixed with the VAL extract and refrigerated is presented. The pH values significantly decreased (p<0.01) from day 1 to day 14 of storage for all samples, but this change was more drastic for the control. On the first day of storage, pH values of the VAL-treated patties were lower than those of the control (p<0.01). This could be due to the low pH of the VAL extract itself (3.63). The pH values measured on the third and seventh day of storage were not significantly (p>0.05) different when comparing the control and VAL-treated patties. However, the pH values of the VAL-treated patties were lower than those of the control (p<0.01) on the 14th day of storage. This difference could be attributed to the greater aerobic bacterial multiplication in the control compared to the VAL-treated samples (Table 3). These results were in agreement with data produced by Ahn et al. (2004) for ground beef and Juntachote et al. (2007) for ground pork.
Table 2.
Changes in pH of fresh pork patties mixed with Viscum album L. (VAL) extract
| Treatment1 | Storage period (d) | |||
|---|---|---|---|---|
|
| ||||
| 1 | 3 | 7 | 14 | |
| C | 5.75±0.09Aa | 5.81±0.04A | 5.47±0.10B | 5.44±0.06Aa |
| V | 5.61±0.03Abc | 5.65±0.06A | 5.43±0.02A | 4.75±0.02Bb |
| T1 | 5.65±0.01Ab | 5.69±0.06A | 5.43±0.06B | 4.78±0.02Cb |
| T2 | 5.54±0.03Bd | 5.64±0.07A | 5.44±0.06C | 4.75±0.07Db |
| T3 | 5.56±0.04Acd | 5.62±0.02A | 5.41±0.03A | 4.65±0.04Bc |
Values represent the mean of triplicate measurements.
C, uncooked pork patty without any added functional ingredients; V, uncooked pork patty mixed with 0.02% ascorbic acid; T1, T2, and T3, fresh patties mixed with 0.1%, 0.5%, and 1.0% VAL extract, respectively (dry base, w/w).
Mean values±standard deviation with different letters in the same row are significantly different (p<0.01).
Mean values±standard deviation with different letters in the same column are significantly different (p<0.01).
Table 3.
Changes in total plate counts (TPCs) expressed as log CFU/g of uncooked pork patties mixed with VAL ethanol extract
| Treatments1 | Storage period (d) | |||
|---|---|---|---|---|
|
| ||||
| 1 | 3 | 7 | 14 | |
| C | 4.19±0.66Ca | 5.29±0.07Ba | 5.98±0.27Aa | 6.51±0.33Aa |
| V | 3.04±0.21Bb | 3.06±0.34Bb | 5.03±0.23Ab | 5.27±0.41Abc |
| T1 | 2.73±0.12Cb | 2.37±0.04Cc | 4.45±0.38Bc | 5.67±0.40Ab |
| T2 | 2.73±0.55Bb | 2.58±0.34Bc | 4.84±0.43Abc | 5.04±0.24Ac |
| T3 | 2.83±0.26Bb | 2.67±0.04Bbc | 4.71±0.18Abc | 5.06±0.18Ac |
Values represent the mean of triplicate measurements.
CFU, colony-forming unit; VAL, Viscum album L.; SD, standard deviation.
C, uncooked pork patty without any added functional ingredients; V, uncooked pork patty mixed with 0.02% ascorbic acid; T1, T2, and T3, fresh patties mixed with 0.1%, 0.5%, and 1.0% VAL extract, respectively (dry base, w/w).
Mean values±SD with different letters in the same row are significantly different (p<0.01).
Mean values±SD with different letters in the same column are significantly different (p<0.01).
Color analysis of pork patties
Color plays an important role in both the quality and consumer acceptance of pork. Changes in color values of the uncooked pork patties mixed with VAL extract are presented in Table 4. On the first day of storage, the control exhibited the highest color value (L* value or lightness). Lightness was significantly different (p<0.05) among all samples on different storage days. This was clearly observed for the control in which lightness significantly decreased (p<0.05) after 14 d while at the same time lightness of samples T1 and T2 was significantly increased (p<0.05). Overall, lightness of the VAL extract-treated pork, except for sample T3, significantly increased (p<0.01) after 14 d of storage compared to the control. The L* value of raw pork treated with grape seed and bearberry extracts was found to not change over a 12-d period of storage (Carpenter et al., 2007). In contrast, a reduction in the L* value of chicken patties mixed with natural antioxidants was previously reported (Devatkal et al., 2010).
Table 4.
Changes in the color of uncooked pork patties mixed with VAL ethanol extract
| Index | Treatments1 | Storage period (d) | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 3 | 7 | 14 | |||
| L* | C | 65.83±0.91Aa | 66.00±1.35Aa | 65.87±1.76Aa | 62.30±0.78Bb | * |
| V | 64.07±0.59abc | 66.70±2.76a | 65.53±1.34ab | 68.00±1.31a | ||
| T1 | 65.00±1.32Bab | 63.50±1.70Bab | 65.63±0.40Bab | 68.57±1.80Aa | ** | |
| T2 | 63.10±0.92Bbc | 62.03±0.64Bb | 63.03±2.12Bbc | 69.53±1.75Aa | ** | |
| T3 | 62.43±1.65c | 61.60±1.37b | 62.67±0.91c | 63.50±1.47b | ||
| p-value | * | ** | * | ** | ||
| a* | C | 6.40±1.01AB | 7.30±0.53A | 7.04±0.69A | 5.54±0.31B | * |
| V | 7.73±0.31A | 6.73±1.03AB | 7.00±0.79A | 5.50±0.40B | * | |
| T1 | 7.73±0.90A | 8.23±0.64A | 7.60±0.30A | 4.57±0.46B | ** | |
| T2 | 7.70±1.04A | 7.77±0.29A | 7.93±0.47A | 4.43±0.58B | ** | |
| T3 | 6.80±0.78A | 6.90±0.56A | 6.43±0.75A | 4.63±0.80B | * | |
| p-value | ||||||
| b* | C | 13.03±1.02Ab | 12.97±0.21Ac | 12.47±0.61ABbc | 11.20±1.11Bc | * |
| V | 13.37±0.06b | 13.50±0.72bc | 13.01±0.40c | 13.10±0.10b | ||
| T1 | 13.33±1.00b | 13.57±0.29bc | 13.40±0.60b | 12.20±0.92bc | ||
| T2 | 14.27±1.62b | 14.70±0.40ab | 15.07±0.45a | 13.40±0.75b | ||
| T3 | 16.03±0.15a | 15.37±1.10a | 14.67±0.60a | 14.77±0.15Aa | ||
| p-value | * | ** | ** | ** | ||
Values represent the mean of triplicate measurements.
VAL, Viscum album L.; SD, standard deviation.
C, uncooked pork patty without any added functional ingredients; V, uncooked pork patty mixed with 0.02% ascorbic acid; T1, T2, and T3, fresh patties mixed with 0.1%, 0.5%, and 1.0% VAL extract, respectively (dry base, w/w).
Mean values±SD with different letters in the same row are significantly different (* p<0.05 and ** p<0.01).
Mean values±SD with different letters in the same column are significantly different (* p<0.05 and ** p<0.01).
The intensity and stability of meat color are important attributes to consumers with a preference for pork with more intense pink pigmentation (Brewer et al., 1998). The a* value (redness) is the most important color parameter for evaluating meat oxidation since decreased redness is associated with reduced consumer acceptance (Renerre, 2000). During storage, significant differences were found in the redness (a* values) between the control and VAL extract-treated pork although no significant differences were observed between any tested samples. Samples T1 and T2 had comparatively higher redness scores compared to the control on days 1, 3, and 7 of storage, but these values were not significantly different. By day 14 of storage, redness of all test samples had significantly decreased (p<0.05). These results agree with findings previously reported for other plant extracts of commercial origin (McCarthy et al., 2001b; Mason et al., 2005).
After day 1 of storage, T3 samples exhibited the highest b* value (yellowness), while samples T2 and T3 had significantly higher (p<0.01) yellowness compared to the control on days 3, 7, and 14 of storage. Yellowness of the control decreased significantly (p<0.05) after 14 d, but those of the VAL extract-treated samples were not significantly altered throughout the storage period. These results agreed with findings from a study by Biswas et al. (2012) who reported reduced L* values and increased b* values for raw ground pork mixed with natural antioxidants and refrigerated.
TBARS values of pork patties
In Figure 1, TBARS values of the refrigerated uncooked pork patties mixed with VAL extract are presented. As expected, the TBARS values of the control were significantly increased (p<0.01) between days 1 and 14 of storage, but those of T2 and T3 were significantly decreased during the same storage period. In contrast, the TBARS values of pork treated with 0.02% ascorbic acid (V) did not significantly changed throughout the storage period. In general, treatment with VAL extract (samples T1, T2, and T3) resulted in lower (p<0.05) TBARS values compared to the control and V samples on days 7 and 14 of storage. However, TBARS values of the samples treated with the 0.5% and 1.0% of the VAL extract (T2 and T3) were higher after 1 d of storage at 4°C compared to the other patties.
Figure 1.
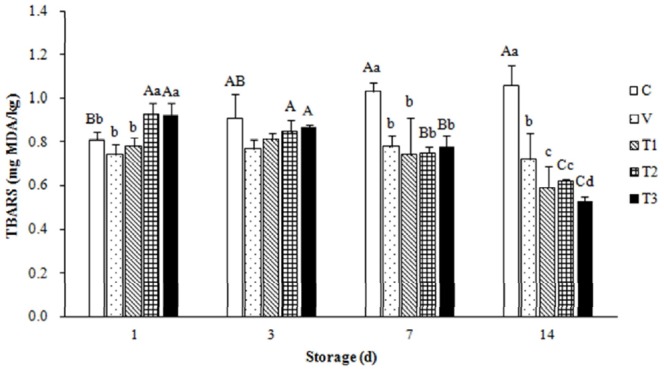
Thiobarbituric acid reactive substance (TBARS) values of fresh minced pork mixed with Viscum album L. (VAL) extract and subjected to refrigerated storage as described in Table 2. Values represent the mean of triplicate determinations. A-C Mean values±standard deviation (SD) with different letters for the same treatment during different storage periods are significantly different (p<0.01). a-d Mean values±SD with different letters for different treatments during the same storage period are significantly different (p<0.01).
The TBARS method has been widely used to determine the degree of lipid oxidation. TBARS are produced through the second stage of auto-oxidation during which peroxides are oxidized into aldehydes and ketones (e.g., malondialdehyde). High TBARS values of the T2 and T3 samples observed between the first and third day of storage could be a result of the dark brown pigments of the VAL extract. Ganhão et al. (2011) reported that unexpectedly high TBARS values could be ascribed to the presence of interfering compounds (mainly colored pigments of the tested extracts). Additionally, anthocyanins and other natural pigments that naturally produce red pigmentation similar to that of TBA-malondialdehyde (MDA) adduct should be considered responsible for lipid oxidation.
Numerous plant tissues contain endogenous pigments that absorb the 532 nm region of the spectrum or form such pigments under acidic conditions, and are necessary for MDA extraction and subsequent reaction with TBA (Draper et al., 1993). However, TBARS values of the VAL extract-treated uncooked pork patties were decreased by 19.03% and 33.37% for T2 and 14.81% and 42.45% for T3 after 7 and 14 d of storage, respectively. Recently, a positive correlation between the phenolic content of plant by-product extract and reduction of TBARS in cooked goat meat patties was reported (Devatkal et al., 2010). Phenolic compounds are known to inhibit free radical formation and the propagation of free radical reactions through chelation of transition metal ions such as iron (McBride et al., 2007). The antioxidant activity of phenolic compounds was reported to be associated with the hydroxyl group linked to the aromatic ring, which is capable of donating hydrogen atoms with electrons and neutralizing free radicals (Kim et al., 2013). This mechanism blocks further degradation into more active oxidizing forms such as MDA (Oliveira et al., 2012).
TPC of pork patties
Changes in the TPC of fresh pork patties mixed with the VAL extract and stored at 4°C are shown in Table 3. The relatively high initial TPC of fresh minced pork could be attributed to the mincing process itself, which contributes to increased total viable counts. Significant differences (p<0.01) in the mean TPC were observed between the control and VAL extract-treated pork patties during the entire storage period. TPC of the control increased significantly (p<0.01) throughout the storage period and reached an unacceptable level (almost 6 CFU/g) on day 14. On day 3, the TPCs all VAL extract-treated samples showed more than 2 log reductions compared to the control. The TPCs of V, T1, T2, and T3 samples were reduced by approximately 1.3 log on days 1, 7, and 14 compared to the control. The VAL extract reduced the TPC (p<0.01) throughout the storage period relative to the control values. A concentration of 0.1% VAL extract was sufficient while a dose of 1.0% provided no significant improvement. Thus, the addition of 0.1% VAL extract to uncooked pork patties significantly delayed bacterial proliferation and extended the product shelf life beyond 14 d versus 7 d for the control.
The shelf life of fresh meat is usually limited by microbial spoilage. Fresh pork patties have a shelf life of around 7 d in refrigeration and aerobiosis depending on hygiene and preservation conditions (Tang et al., 2005). The inhibitory effect of VAL ethanol extract on microflora in uncooked pork patties may be attributed to the presence of polyphenolic compounds (quercetin, catechins, lignans, and phenolic acids) that enter the circulatory system, and are distributed and retained in animal tissues (Raccach, 1984). These compounds interact with the cell membrane of microorganisms and cause leakage of cellular components, changes in fatty acid and phospholipid constituents, impaired energy metabolism, altered nutrient uptake and electron transport, and changes in genetic material synthesis (Nychas, 1995). Moreover, Fung et al. (1977) showed that these compounds could also interact with microorganism membrane proteins to produce structure deformation and disrupt functionality.
Sensory evaluation of pork patties
Sensory panel results for the VAL extract-treated pork patties are presented in Table 5. Comparison of the sensory evaluation data for the VAL extract-treated pork patties and control did not reveal any difference in color score, flavor score, or overall acceptability (p>0.05). However, odor scores of VAL extract-treated pork patties were lower than those of the control and 0.02% ascorbic acid-treated patties after 3 d of storage. This finding indicated that the VAL extract was able to improve the sensory qualities of uncooked patties, which could be attributed to the reduction of bacterial populations and lipid oxidation.
Table 5.
Sensory evaluation1 results for uncooked pork patties mixed with VAL ethanol extract on the third day of storage
| Treatments2 | |||||
|---|---|---|---|---|---|
|
| |||||
| C | V | T1 | T2 | T3 | |
| Color | 4.52±1.13 | 5.20±0.45 | 4.30±1.10 | 3.75±0.96 | 4.50±1.66 |
| Flavor | 4.70±0.84 | 4.70±1.86 | 4.50±0.22 | 4.40±1.29 | 5.00±1.58 |
| Off-odor | 7.40±1.14A | 6.00±1.87B | 5.60±1.92C | 5.25±2.22C | 5.40±2.41C |
| Acceptability | 3.45±1.13 | 5.43±1.66 | 5.73±0.79 | 5.70±1.14 | 5.80±1.55 |
Values represent the mean of triplicate determinations.
VAL, Viscum album L.; SD, standard deviation.
Scores were assigned based on a 9-point scale (color, flavor, and acceptability: 1 = very objectionable, 9 = very acceptable; odor: 1 = low intensity, 9 = very high intensity).
C, uncooked pork patty without any added functional ingredients; V, uncooked pork patty mixed with 0.02% ascorbic acid; T1, T2, and T3, fresh patties mixed with 0.1%, 0.5%, and 1.0% VAL extract, respectively (dry base, w/w).
Mean values±SD with different letters in the same row are significantly different (p<0.05).
In conclusion, the results of the present investigation demonstrated that the VAL ethanol extract (containing 60.46 of total phenolics–mg of CE/g and 36.38 of flavonoid-mg of QE/g, respectively) is a good source of antioxidants. In addition, in situ testing confirmed that 0.1% VAL ethanol extract is highly effective for maintain the quality of uncooked pork patties because this compound was able to inhibit lipid oxidation and consequently prevent odor development. The effects of this concentration of VAL extract were more potent than those of ascorbic acid. Data from this study strongly suggest that the VAL ethanol extract is a potential natural antioxidant source that can preserve the quality of meat and meat products.
ACKNOWLEDGMENTS
This research was supported by a Daegu University Research Grant (20140576).
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- Ahn J, Grun IU, Fernando LN. Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. J Food Sci. 2002;67:1364–1369. [Google Scholar]
- Ahn J, Grün IU, Mustapha A. Antimicrobial and antioxidant activities of natural extracts in vitro and in ground beef. J Food Prot. 2004;67:148–155. doi: 10.4315/0362-028x-67.1.148. [DOI] [PubMed] [Google Scholar]
- Bar-Sela G. White-berry mistletoe (Viscum album L.) as complementary treatment in cancer: Does it help? Eur J Integr Mad. 2011;3:e55–e62. [Google Scholar]
- Biswas AK, Chatli MK, Sahoo J. Antioxidant potential of curry (Murraya koenigii L.) and mint (Mentha spicata) leaf extracts and their effect on colour and oxidative stability of raw ground pork meat during refrigeration storage. Food Chem. 2012;133:467–472. doi: 10.1016/j.foodchem.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Brewer MS, Lan HY, McKeith FK. Consumer evaluation of pork appearance with differing physiological and packaging conditions. J Muscle Foods. 1998;9:173–183. [Google Scholar]
- Buxiang S, Fukuhara M. Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoid on the activation of mutagens and drug-metabolizing enzymes in mice. Toxicology. 1997;122:61–72. doi: 10.1016/s0300-483x(97)00078-4. [DOI] [PubMed] [Google Scholar]
- Carpenter R, O’Grady MN, O’Callaghan YC, O’Brien NM, Kerry JP. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 2007;76:604–610. doi: 10.1016/j.meatsci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Chen HY, Yen GC. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007;101:686–694. [Google Scholar]
- Da Porto C, Calligaris S, Celotti E, Nicoli MC. Antiradical properties of commercial cognacs assessed by the DPPH test. J Agric Food Chem. 2000;48:4241–4245. doi: 10.1021/jf000167b. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010;85:155–159. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med. 1993;15:353–363. doi: 10.1016/0891-5849(93)90035-s. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Marshall JD. Water relations. In: Press MC, Graves J, editors. Parasitic plants. Chapman and Hall; London, UK: 1995. pp. 125–214. [Google Scholar]
- Fernández T, Wagner ML, Varela BG, Ricco RA, Hajos SE, Gurni AA, Alvarez E. Study of an Argentine Mistletoe, the hemiparasite Ligaria cuneifolia (R. et P.) Tiegh. (Loranthaceae) J Ethnopharmacol. 1998;62:25–34. doi: 10.1016/s0378-8741(98)00030-0. [DOI] [PubMed] [Google Scholar]
- Franz H, Ziska P, Kindt A. Isolation and properties of three lectins from mistletoe (Viscum album L.) Biochem J. 1981;195:481–484. doi: 10.1042/bj1950481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung DYC, Taylor S, Kahan J. Effect of butylated hydroxyanisole (BHA) and buthylated hydroxytoluene (BHT) on growth and aflatoxin production of Aspergillus flavus. J Food Saf. 1997;1:39–51. [Google Scholar]
- Ganhão R, Estévez M, Morcuende D. Suitability of the TBA method for assessing lipid oxidation in a meat system with added phenolic-rich materials. Food Chem. 2011;126:772–778. [Google Scholar]
- Halliwell B. Ascorbic acid in the prevention and treatment of cancer. Altern Med Rev. 1996;3:174–186. [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Jung ML, Baudino S, Ribereau-Gayon G, Beck JP. Characterization of cytotoxic proteins from mistletoe (Viscum album L.) Cancer Lett. 1990;51:103–108. doi: 10.1016/0304-3835(90)90044-x. [DOI] [PubMed] [Google Scholar]
- Juntachote T, Berghofer E, Siebenhandl S, Bauer F. Antioxidative effect of added dried Holy basil and its ethanolic extracts on susceptibility of cooked ground pork to lipid oxidation. Food Chem. 2007;100:129–135. [Google Scholar]
- Khalil AH, Mansour EH. Control of lipid oxidation in cooked and uncooked refrigerated carp fillets by antioxidant and packaging combinations. J Agric Food Chem. 1998;46:1158–1162. [Google Scholar]
- Khwaja TA, Varven JC, Pentecost S, Pande H. Isolation of biologically active alkaloids from Korean mistletoe Visum album, colouratum. Experientia. 1980;36:599–600. doi: 10.1007/BF01965825. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Cho AR, Han JJ. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control. 2013;29:112–120. [Google Scholar]
- Liu F, Ooi VEC, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997;60:763–771. doi: 10.1016/s0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lopes GK, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta. 1999;1472:142–152. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- Mannel DN, Becker H, Gundt A, Kist A, Franz H. Induction of tumor necrosis factor expression by a lectin from Viscum album. Cancer Immunol Immunother. 1991;33:177–182. doi: 10.1007/BF01756139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason LM, Hogan SA, Lynch A, O’Sullivan K, Lawlor PG, Kerry JP. Effects of restricted feeding and antioxidant supplementation on pig performance and quality characteristics of longissimus dorsi muscle from Landrace and Duroc pigs. Meat Sci. 2005;70:307–317. doi: 10.1016/j.meatsci.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Mates JM, Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- McBride NTM, Hogan SA, Kerry JP. Comparative addition of rosemary extract and additives on sensory and antioxidant properties of retail packaged beef. Int J Food Sci Technol. 2007;42:1201–1207. [Google Scholar]
- McCarthy TL, Kerry JP, Kerry JF, Lynch PB, Buckley DJ. Evaluation of the antioxidant potential of natural food/plant extracts on compared with synthetic antixodants and vitamin E in raw and cooked pork patties. Meat Sci. 2001a;58:45–52. doi: 10.1016/s0309-1740(00)00129-7. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Kerry JP, Kerry JF, Lynch PB, Buckley DJ. Assessment of the antioxidant potential of natural food and plant extracts in fresh and previously frozen pork patties. Meat Sci. 2001b;57:177–184. doi: 10.1016/s0309-1740(00)00090-5. [DOI] [PubMed] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- Mueller EA, Anderer FA. Viscum album oligosaccharide activating human natural cytotoxicity is an interferon γ inducer. Cancer Immunol Immunother. 1990;32:221–227. doi: 10.1007/BF01741704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nychas GJE. Natural antimicrobials from plants. In: Gould GW, editor. New methods of food preservation. Blackie Academic Professional; London, UK: 1995. pp. 58–89. [Google Scholar]
- Oliveira TLC, Carvalho SM, Araújo Soares R, Andrade MA, Graças Cardoso M, Ramos EM. Antioxidant effects of Satureja montata L. essential oil on TBARS and color of mortadella-type sausages formulated with different levels of sodium nitrite. LWT – Food Sci Technol. 2012;45:204–212. [Google Scholar]
- Paganga G, Miller N, Rice-Evans CA. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Radic Res. 1999;30:153–162. doi: 10.1080/10715769900300161. [DOI] [PubMed] [Google Scholar]
- Raccach M. The antimicrobial activity of phenolic antioxidants in foods: A review. J Food Saf. 1984;6:141–170. [Google Scholar]
- Renerre M. Review – biochemical basis of fresh meat color. Proceedings of the 45th international congress of meat science and technology; Yokohama, Japan. 2000. pp. 344–352. [Google Scholar]
- Romagnoli S, Ugolini R, Fogolari F, Schaller G, Urech K, Giannattasio M, Ragona L, Molinari H. NMR structural determination of viscotoxin A3 from Viscum album L. Biochem J. 2000;350:569–577. [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JR. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Tang SZ, Ou SY, Huang XS, Li W, Kerry JP, Buckley DJ. Effects of added tea catechins on colour stability and lipid oxidation in minced beef patties held under aerobic and modified atmospheric packaging conditions. J Food Eng. 2005;77:248–253. [Google Scholar]
- Tarladgis BG, Watts BM, Younathan MT. A distillation method for the quantitative determination of malondialdehyde in rancid foods. J Am Oil Chem Soc. 1960;37:44–48. [Google Scholar]
- Vani T, Rajani M, Sarkar S, Shishoo CJ. Antioxidant properties of the ayurvedic formulation triphala and its constituents. Int J Pharmacogn. 1997;35:313–317. [Google Scholar]
- Varela BG, Fernández T, Ricco RA, Zolezzi PC, Hajos SE, Gurni AA, Alvarez E, Wagner ML. Phoradendron liga (Gill. ex H. et A.) Eichl. (Viscaceae) used in folk medicine: anatomical, phytochemical, and immunochemical studies. J Ethnopharmacol. 2004;94:109–116. doi: 10.1016/j.jep.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Zolezzi PC, Fernández T, Aulicino P, Cavaliere V, Greczanik S, Lopes EC, Wagner M, Ricco R, Gurni A, Hajos S, Álvarez E. Ligaria cuneifolia flavonoid fractions modulate cell growth of normal lymphocytes and tumor cells as well as multidrug resistant cells. Immunobiology. 2005;209:737–749. doi: 10.1016/j.imbio.2005.03.001. [DOI] [PubMed] [Google Scholar]


