Abstract
We performed this meta-analysis of epidemiological studies to comprehensively assess the association between parity and gastric cancer risk, because previous studies have shown conflicting results regarding this topic. Relevant prospective studies were identified by searching the following databases: PubMed, EMBASE, and Web of Science, and random-effects models were used to estimate summary relative risks (SRRs) and 95% confidence intervals (CIs). Our search yielded 10 prospective cohort studies involving a total of 6624 gastric cancer cases and 5,559,695 non-cases. The SRRs for ever parity vs. nulliparous and highest vs. lowest parity number were 0.96 (95%CI = 0.87–1.05, I2 = 0%) and 1.03 (95%CI = 0.94–1.13, I2 = 0%), respectively. Additionally, the SRR for an increment of one live birth was 1.00 (95%CI = 0.97–1.03, I2 = 18.6%). These non-significant associations were observed in all subgroups as stratified by the number of gastric cases, follow-up years, geographic location, menopausal status, anatomic subsite of gastric cancer, and adjustment for potential confounders, as well as in sensitivity analyses. Our meta-analysis found no significant association between parity and gastric cancer risk. However, further studies should be conducted to validate our findings and could provide more detailed results by stratifying their findings by Lauren’s subtype, histology, and anatomic site, as well as fully adjusting for potential confounding factors.
In 2012, gastric cancer was the fourth most common type of cancer and the third leading cause of cancer mortality among both men and women worldwide1. The age standardized incidence and mortality rate for gastric cancer were 17.4 and 12.8 per 100,000 individuals, respectively among both men and women2. Although the incidence of gastric cancer varies widely among different countries, its incidence is generally 2-fold higher in men compared to women1. Because this discrepancy in occurrence cannot be totally attributed to environmental factors (e.g., cigarette smoking and fruit and vegetable intake) or Helicobacter pylori (H. pylori) infection3, some investigators have proposed that sex hormonal factors (e.g., parity, hormone replacement therapy, and age at menarche), and especially estrogen levels, might play etiological roles in gastric cancer4,5,6,7.
Pregnant women have markedly elevated serum levels of certain hormones, including estrogens8. Results of our previous studies suggested that parity was significantly associated with the risk for kidney cancer, but not the risk for colorectal or pancreatic cancer9,10,11. Although several studies have suggested that parity might be associated with gastric cancer risk through its effects on steroid hormone metabolism and related pathways4,12,13,14, the published epidemiological evidence for this hypothesis is inconclusive; possible due to the limited number of gastric cancer cases investigated8,15,16,17,18,19,20,21,22,23. Some investigators have argued that pregnancy-related hormones may protect against gastric cancer, whereas others have argued that they have a stimulatory effect or no effect at all. Furthermore, the most recent meta-analysis (conducted in 2011) of 12 observational studies with mixed study designs and quality found limited evidence for the aforementioned associations, and a high degree of heterogeneity in the study results24. Notably, that previous meta-analysis did not include a dose-response analysis to evaluate associations regardless of their category. Hence, we conducted a systematic review and meta-analysis which included the most prospective studies to comprehensively and quantitatively evaluate the relationship between parity and the risk for developing gastric cancer.
Results
Search results, study characteristics, and quality assessment
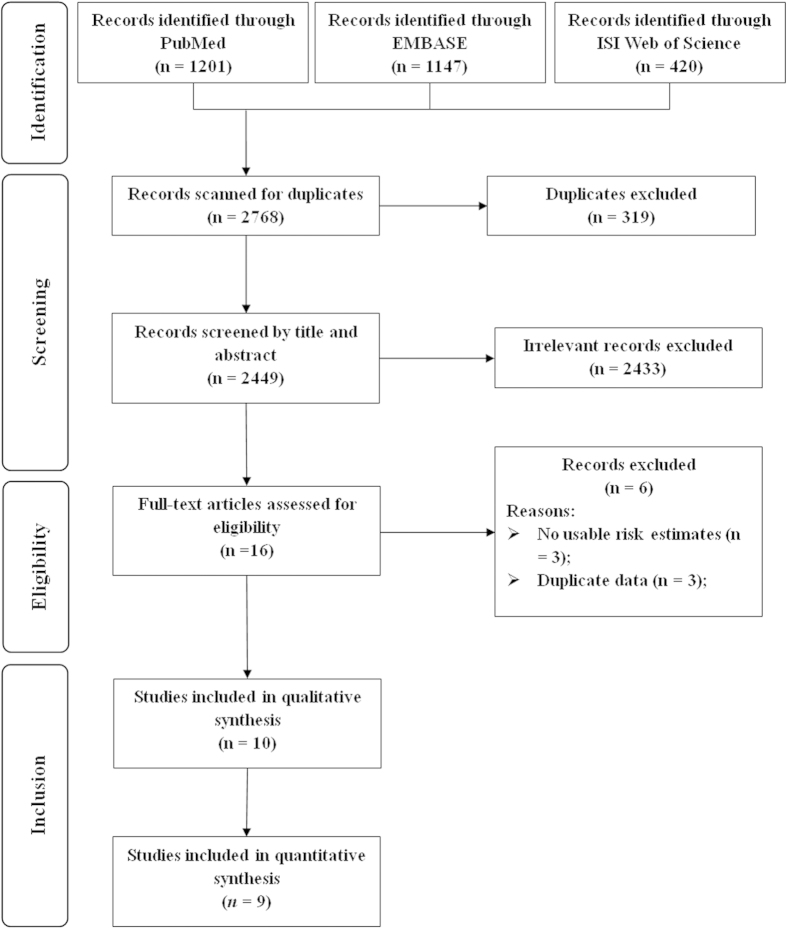
The detailed procedures used for searching and screening articles are outlined in Fig. 1. In brief, our search strategy retrieved 2449 unique reports after excluding the duplications: 1201 from PubMed, 1147 from EMBASE, and 420 from Web of Science. Of these, 2433 reports were excluded after the first screening based on their abstracts or titles, leaving 16 reports eligible for full-text review. Among these 16 reports, 6 were excluded due to i) a lack of usable risk estimates or 95% CIs or ii) study population duplication. Finally, a total of 10 prospective studies were included in the present meta-analysis8,15,16,17,18,19,20,21,22,23.
Figure 1. Selection of studies for inclusion in meta-analysis.
Characteristics of the 10 selected studies are shown in Table 1. These studies were published between 2000 and 2012 and involved a total of 6624 gastric cancer cases and 5,559,695 non-cases. Five of the 10 studies were conducted in Europe, four were conducted in Asia, and one was conducted in the United States. A majority of risk measures were adjusted for or stratified by age (7 studies); however, fewer studies were adjusted for cigarette smoking (5 studies), body mass index (4 studies), alcohol consumption (3 studies), and socioeconomic status (5 studies).
Table 1. Characteristics of included prospective studies of parity and gastric cancer risk.
| First author, publication year (reference), Country | Cases/subject (age), duration of follow up | Exposure categories (exposure/case assessment) | RR (95% CI) | Matched/Adjusted factors |
|---|---|---|---|---|
| Green et al.15,† 2012, United Kingdom | 1194/1,319,409 (50–64y), 9.1y | Parity: Ever parous vs. Nulliparous Parity: :≥3 vs. 2 (Self-questionnaire/Cancer registry) | 0.99 (0.82–1.19) 1.03 (0.90–1.19) | Stratified by age, region and socioeconomic status and adjusted for BMI, smoking, alcohol, strenuous exercise, use of OC and for all other reproductive factors and for use of hormone therapy for the menopause |
| Chang et al.16,† ‡ 2011, Taiwan, China | 1090/1,292,462 (40–69y), 31y | Parity: :≥3 vs. Nulliparous (Registration form/Cancer registry) | 1.32 (1.02–1.72) | Age, marital status, years of schooling, and birth place |
| Duell et al.17, 2010, Europe | 181/335,216 (35–70y), 8.7y | Parity: Ever parous vs. Nulliparous Parity: :≥4 vs. Nulliparous (Self-questionnaire/Cancer registry) | 0.97 (0.61–1.53) 1.19 (0.65–2.18) | Age, center, smoking status, education, BMI, and calorie-adjusted vegetable, fruit, red meat, and processed meat intakes |
| Freedman et al.18, 2010, USA | 97/201,506 (50–71y), 7.5y | Non-cardia gastric cancer Parity: Ever parous vs.Nulliparous Parity: :≥3 vs. Nulliparous (Self-questionnaire/Cancer registry) | 0.76 (0.44–1.31) 0.83 (0.47–1.47) | Age, BMI, fruit and vegetable consumption, smoking use, alcohol intake, physical activity, and total energy intake |
| Bahmanyar et al.8, 2008, Sweden | 2784/2,406,439 (≥30y), 34y | Cardia gastric cancer Parity: Ever parous vs. Nulliparous Parity: :≥4 vs. 1 Non-cardia gastric cancer Parity: Ever parous vs. Nulliparous Parity: :≥4 vs. 1 (Self-questionnaire/Cancer registry) | 0.70 (0.50–1.10) 0.90 (0.50–1.50) 1.01 (0.89–1.15) 0.96 (0.81–1.15) | Occupational class, education level, and age at first birth Age, family history of gastric cancer, and study area |
| Persson et al.19,† 2008, Japan | 368/44,453 (40–69y), 12.2y | Parity: Ever parous vs. Nulliparous Parity: :≥3 vs. Nulliparous (Self-questionnaire/Cancer registry) | 0.84 (0.56–1.27) 0.83 (0.54–1.26) | |
| Freedman et al.20,† 2007, China | 154/73,442 (40–70y), 5.7y | Parity: Ever parous vs. Nulliparous Parity: :≥4 vs. Nulliparous (Self-questionnaire/Cancer registry) | 1.11 (0.44–2.82) 1.06 (0.34–3.25) | Age, BMI, education, income, cigarette smoking status, and smoking dose |
| Koski-Rahikkala et al.21,‡ ¶2006, Finland | 28/12,002 (mean, 27.8y), 36y | Parity: :≥10 vs. 2–4 (Self-questionnaire/Cancer registry) | 2.42 (0.71–8.20) | N/A |
| Kaneko et al.22,† ‡ 2003, Japan | 156/120,000 (40–79y), 8.2y | Parity: Ever parous vs. Nulliparous Parity: :>3 vs. Nulliparous (Self-questionnaire/Cancer registry) | 0.58 (0.29–1.20) 0.60 (0.29–1.28) | Smoking status, family history, past history of peptic ulcer, alcohol intake, educational background, number of rice bowls, and dietary consumption |
| Heuch et al. 23,† 2000, Norway | 572/63,090 (32–74y), 29y | Parity: :≥5 vs. 1 (Self-questionnaire/Cancer registry) | 1.10 (0.80–1.50) | Age, birth cohort, urban/rural residence and county |
BMI: body mass windex; CI: confidence interval; N/A: not available; OC: oral contraceptive; RR: relative risk.
†Recalculate the RR by the method proposed by Hamling et al.49.
‡Using mortality data to calculate risk estimates.
¶Relative risks and w95% CIs were calculated from published data using EpiCalc 2000.
Information regarding the quality assessment of studies is provided in Supplementary Table S1. Briefly, when controlling for an important factor or an additional factor category, five of the included studies8,16,19,21,23 were not assigned two scores because results of their primary analyses were not adjusted for potential confounders. When testing for whether a study’s follow-up time was long enough for outcomes to occur, five of the included studies8,16,19,21,23 received a score because their mean follow-up period was >10 years.
Ever parity versus nulliparous
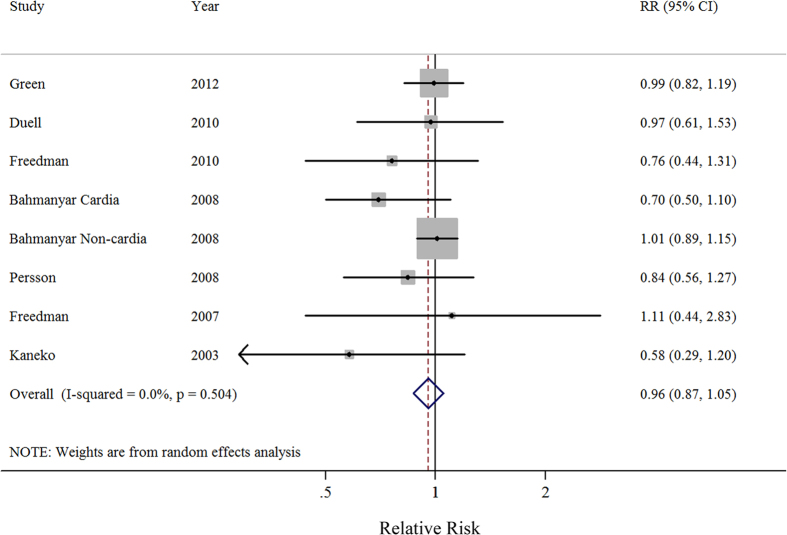
Seven prospective studies investigated the relationship between ever parity and the risk of gastric cancer. A comparison of ever parity vs. nulliparous yielded a SRR of 0.96 (95%CI = 0.87–1.05), without heterogeneity (I2 = 0%) (Fig. 2). Both a visual inspection of a funnel plot and Egger’s test (P = 0.159) failed to show evidence of publication bias, and similar non-significant results were observed in all stratified analyses performed based on study characteristics. Additionally, the results of a meta-regression showed no evidence of significant heterogeneity between subgroup analyses (Table 2).
Figure 2. Forest plot (random-effects model) of ever parity and gastric cancer risk.
The squares indicate study-specific relative risks (size of the square reflects the study specific statistical weight); the horizontal lines indicate 95% CIs; and the diamond indicates the summary relative risk estimate with its 95% CI.
Table 2. Summary risk estimates of the association between ever parity and gastric cancer risk.
| No. of Study | SRR | 95% CI | I2 (%) | Ph† | Ph‡ | |
|---|---|---|---|---|---|---|
| Overall | 7 | 0.96 | 0.87–1.05 | 0 | 0.504 | |
| Subgroup analyses | ||||||
| Number of cases | 0.383 | |||||
| ≥250 | 3 | 0.96 | 0.85–1.08 | 15.1 | 0.317 | |
| <250 | 4 | 0.84 | 0.62–1.13 | 0 | 0.594 | |
| Follow-up years | 0.974 | |||||
| ≥10 | 2 | 0.90 | 0.87–1.05 | 42.4 | 0.176 | |
| <10 | 5 | 0.94 | 0.81–1.11 | 0 | 0.593 | |
| Geographic location | 0.221 | |||||
| Europe | 3 | 0.98 | 0.89–1.08 | 0.8 | 0.388 | |
| Asia | 3 | 0.80 | 0.58–1.12 | 0 | 0.517 | |
| North America | 1 | 0.76 | 0.44–1.31 | N/A | N/A | |
| Menopausal status | 0.962 | |||||
| Pre-menopausal | 1 | 0.83 | 0.65–1.05 | N/A | N/A | |
| Post-menopausal | 3 | 0.83 | 0.61–1.13 | 66.7 | 0.029 | |
| Anatomic subsite | 0.279 | |||||
| Cardia | 2 | 0.81 | 0.61–1.07 | 1.2 | 0.314 | |
| Non-cardia | 3 | 0.99 | 0.88–1.12 | 0 | 0.589 | |
| Adjustment for potential confounders and risk factors | ||||||
| Age | 0.769 | |||||
| Yes | 5 | 0.95 | 0.82–1.10 | 0 | 0.862 | |
| No | 2 | 0.82 | 0.59–1.15 | 60.0 | 0.082 | |
| Cigarette smoking | 0.974 | |||||
| Yes | 5 | 0.95 | 0.81–1.11 | 0 | 0.593 | |
| No | 2 | 0.90 | 0.72–1.12 | 42.4 | 0.176 | |
| Body mass index | 0.617 | |||||
| Yes | 4 | 0.97 | 0.82–1.14 | 0 | 0.827 | |
| No | 3 | 0.85 | 0.68–1.08 | 44.3 | 0.146 | |
| Alcohol drinking | 0.844 | |||||
| Yes | 3 | 0.88 | 0.67–1.15 | 24.6 | 0.265 | |
| No | 4 | 0.97 | 0.86–1.08 | 0 | 0.468 | |
| Socioeconomic status | 0.340 | |||||
| Yes | 5 | 0.97 | 0.87–1.07 | 3 | 0.397 | |
| No | 2 | 0.81 | 0.58–1.12 | 0 | 0.774 | |
CI, confidence interval; N/A, not available; SRR, summarized relative risk.
†P-value for heterogeneity within each subgroup.
‡P-value for heterogeneity between subgroups with meta-regression analysis.
High parity number versus low parity number
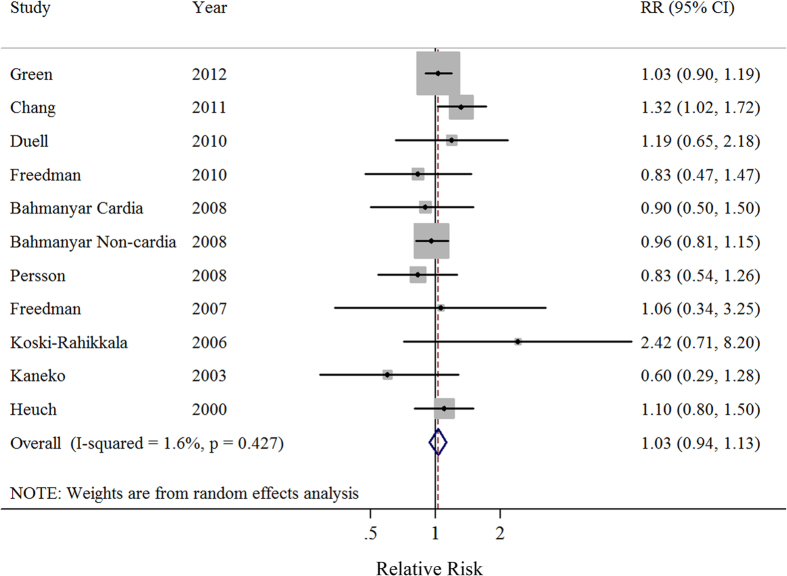
Ten prospective studies investigated the relationship between parity number and the risk for gastric cancer. A comparison of the highest vs. the lowest parity number yielded a SRR of 1.03 (95%CI = 0.94–1.13), without heterogeneity (I2 = 1.6%) (Fig. 3). Similar non-significant results were also observed in all of the stratified analyses performed based on study characteristics. Additionally, the results of a meta-regression showed no evidence of significant heterogeneity between these subgroup analyses (Table 3).
Figure 3. Forest plot (random-effects model) of parity number (highest vs. lowest) and gastric cancer risk.
The squares indicate study-specific relative risks (size of the square reflects the study specific statistical weight); the horizontal lines indicate 95% CIs; and the diamond indicates the summary relative risk estimate with its 95% CI.
Table 3. Summary risk estimates of the association between parity number and gastric cancer risk.
| Highest vs. lowest |
Dose-response analysis (per 1 live birth) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Study | SRR | 95% CI | I2 (%) | Ph† | Ph‡ | No. of Study | SRR | 95% CI | I2 (%) | Ph† | Ph‡ | |
| Overall | 10 | 1.03 | 0.94–1.13 | 1.6 | 0.427 | 9 | 1.00 | 0.97–1.03 | 18.6 | 0.272 | ||
| Subgroup analyses | ||||||||||||
| Number of cases | 0.693 | 0.826 | ||||||||||
| ≥250 | 5 | 1.04 | 0.94–1.14 | 8.7 | 0.361 | 4 | 1.00 | 0.96–1.04 | 45.7 | 0.118 | ||
| <250 | 5 | 0.96 | 0.67–1.38 | 11.1 | 0.343 | 5 | 1.00 | 0.94–1.05 | 0 | 0.454 | ||
| Follow-up years | 0.618 | 0.217 | ||||||||||
| ≥10 | 5 | 1.06 | 0.89–1.25 | 31.5 | 0.199 | 4 | 1.01 | 0.97–1.06 | 46.9 | 0.110 | ||
| <10 | 5 | 1.01 | 0.89–1.15 | 0 | 0.607 | 5 | 0.97 | 0.92–1.02 | 0 | 0.802 | ||
| Geographic location | 0.976 | 0.968 | ||||||||||
| Europe | 5 | 1.02 | 0.92–1.13 | 0 | 0.690 | 4 | 0.99 | 0.97–1.02 | 0 | 0.707 | ||
| Asia | 4 | 0.98 | 0.67–1.42 | 51.1 | 0.105 | 4 | 0.99 | 0.91–1.07 | 60.2 | 0.057 | ||
| North America | 1 | 0.83 | 0.47–1.47 | N/A | N/A | 1 | 0.96 | 0.81–1.13 | N/A | N/A | ||
| Menopausal status | 0.794 | 0.831 | ||||||||||
| Pre-menopausal | 1 | 0.80 | 0.46–1.40 | N/A | N/A | 1 | 0.93 | 0.77–1.12 | N/A | N/A | ||
| Post-menopausal | 3 | 0.90 | 0.76–1.07 | 0 | 0.468 | 3 | 0.97 | 0.94–1.00 | 0 | 0.773 | ||
| Anatomic subsite | 0.879 | 0.342 | ||||||||||
| Cardia | 2 | 1.04 | 0.80-1.34 | 0 | 0.566 | 2 | 0.93 | 0.82–1.05 | 49.4 | 0.160 | ||
| Non-cardia | 3 | 1.01 | 0.85–1.20 | 13.2 | 0.316 | 3 | 0.99 | 0.96–1.02 | 0 | 0.876 | ||
| Adjustment for potential confounders and risk factors | ||||||||||||
| Age | 0.276 | 0.939 | ||||||||||
| Yes | 7 | 1.06 | 0.96–1.18 | 0 | 0.538 | 6 | 0.99 | 0.94–1.05 | 43.1 | 0.118 | ||
| No | 3 | 0.94 | 0.72–1.22 | 20.4 | 0.288 | 3 | 0.99 | 0.97–1.02 | 0 | 0.626 | ||
| Cigarette smoking | 0.618 | 0.217 | ||||||||||
| Yes | 5 | 1.01 | 0.89–1.15 | 0 | 0.607 | 4 | 0.97 | 0.92–1.02 | 0 | 0.802 | ||
| No | 5 | 1.06 | 0.89–1.25 | 31.5 | 0.199 | 5 | 1.01 | 0.97–1.06 | 46.9 | 0.110 | ||
| Body mass index | 0.938 | 0.285 | ||||||||||
| Yes | 4 | 1.03 | 0.90–1.17 | 0 | 0.857 | 4 | 0.97 | 0.92–1.02 | 0 | 0.652 | ||
| No | 6 | 1.03 | 0.86–1.23 | 36.1 | 0.153 | 5 | 1.01 | 0.97–1.05 | 37.2 | 0.159 | ||
| Alcohol drinking | 0.475 | 0.323 | ||||||||||
| Yes | 3 | 0.96 | 0.77–1.20 | 16.7 | 0.301 | 3 | 0.97 | 0.92–1.03 | 0 | 0.993 | ||
| No | 7 | 1.06 | 0.93–1.20 | 6.4 | 0.381 | 6 | 1.01 | 0.97–1.05 | 38.2 | 0.137 | ||
| Socioeconomic status | 0.257 | 0.056 | ||||||||||
| Yes | 5 | 0.99 | 0.90–1.10 | 0 | 0.753 | 5 | 0.98 | 0.96–1.01 | 0 | 0.836 | ||
| No | 5 | 1.10 | 0.87–1.38 | 33.7 | 0.196 | 4 | 1.03 | 0.98–1.09 | 24.6 | 0.264 | ||
CI, confidence interval; N/A, not available; SRR, summarized relative risk.
†P-value for heterogeneity within each subgroup.
‡P-value for heterogeneity between subgroups with meta-regression analysis.
Dose-response analysis
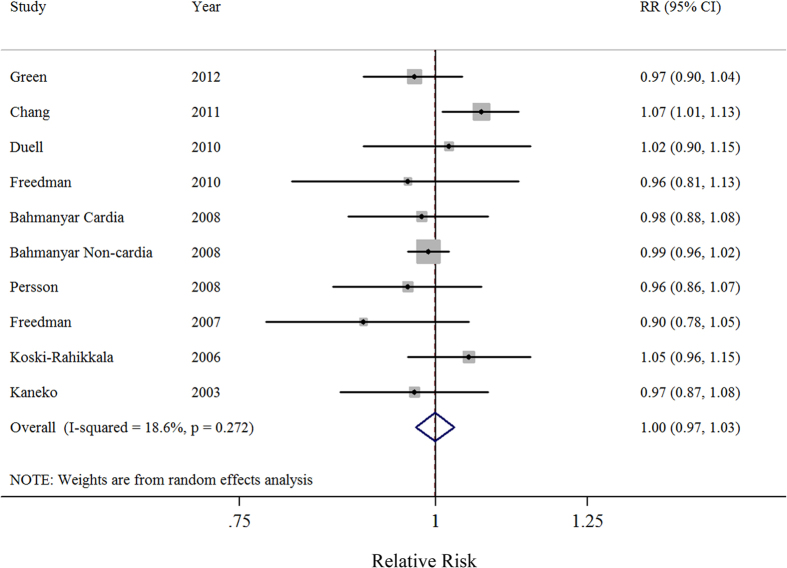
Nine prospective studies were included in the dose-response analysis. Results showed the summary relative risk per live birth was 1.00 (95%CI = 0.97–1.03), with low heterogeneity (I2 = 18.6%) (Fig. 4). There was no evidence for a nonlinear association between the parity number and gastric cancer risk (P for nonlinearity = 0.093) (Supplementary Figure S1). Similar non-significant results were also observed in all of the stratified analyses performed based on study characteristics. Additionally, the results of a meta-regression showed no evidence of significant heterogeneity between these subgroup analyses (Table 3).
Figure 4. Forest plot (random-effects model) of parity number (per 1 live birth) and gastric cancer risk.
The squares indicate study-specific relative risks (size of the square reflects the study specific statistical weight); the horizontal lines indicate 95% CIs; and the diamond indicates the summary relative risk estimate with its 95% CI.
Sensitivity analysis
Our sensitivity analysis of ever parity vs. never parity performed by excluding one study at a time showed that the SRR for gastric cancer ranged from 0.93 (95%CI = 0.80–1.08; I2 = 0%) when Bahmanyar et al.8 was excluded, to 0.96 (95%CI = 0.88–1.06; I2 = 0%), when Persson et al.19 was excluded. Similarly, in the sensitivity analysis of high parity number vs. low parity number, the SRR for gastric cancer ranged from 0.99 (95%CI = 0.91–1.09; I2 = 0%) when Chang et al.16 was excluded, to 1.06 (95%CI = 0.93–1.21; I2 = 11.7%) when Bahmanyar et al.8 was excluded. Additionally, in the sensitivity analysis of dose response, the SRR for gastric cancer ranged from 0.99 (95%CI = 0.96–1.01; I2 = 0%) when Chang et al.16 was excluded, to 1.00 (95%CI = 0.97–1.04; I2 = 22.7%) when Green et al.15 was excluded. When the analysis of highest vs. lowest parity number was restricted to studies included in the dose-response analysis of parity number, the SRR was 1.02 (95%CI = 0.92–1.14; I2 = 9.7%), which is similar to that in the original analysis. Furthermore, we excluded three studies which reported their risk estimates based on mortality resulting from gastric cancer. However, even after excluding those studies, the results remained statistically non-significant (data not shown).
Discussion
The findings from this meta-analysis of prospective studies only marginally suggested that parity might help protect against gastric cancer. To the best of our knowledge, this is the most comprehensive and quantitative assessment of the aforementioned association conducted using high quality evidence. Additionally, these non-significant results were consistent with those in subgroup analyses stratified by study characteristics and adjustments for potential confounders.
Increasing evidence has suggested that there are differences in risk factors associated with developing different Lauren’s subtypes of gastric cancer (intestinal vs. diffuse) and gastric cancers located at different anatomic sites (cardia vs. non-cardia)24,25. Furthermore, the biology and etiology is varied by histological type of this disease. However, only one and three of the included studies reported risk estimates stratified by Lauren’s subtype19 and the anatomic site of gastric cancer8,15,18 respectively. Notably, only three studies15,17,18 provided the aforementioned association on the basis of the adenocarcinomas of gastric cancer. Therefore, our meta-analysis was insufficient to draw conclusions concerning the risk for developing gastric cancer in relation to parity or based on cancer subtype, histology and location.
Previous studies demonstrated that hormones might help protect against gastric cancer during a woman’s fertile years, but their effect diminishes after menopause26,27. The results of several studies support this hypothesis, because they showed that rates of gastric cancer increase more slowly in women compared to men until the age of 60 years; after which, gastric cancer rates in women increase rapidly and become more similar to those in men20,26. However, until recently, similar to the previous discussion of anatomical subtypes of gastric cancer, only a limited number of prospective studies have included a subgroup analysis stratified by menopausal status. This is because the majority of studies had insufficient numbers of gastric cancers to allow a comparison of risks in pre- and postmenopausal women. Although the results of our meta-regression showed no difference regarding the gastric cancer risk in pre- and postmenopausal women, only three studies provided a risk estimate stratified by menopausal status8,19,22, and thus additional studies are warranted to examine the association between menopausal status and gastric cancer risk after eliminating the possibility of chance findings.
Although our present meta-analysis found limited evidence for a relationship between parity and the risk of developing gastric cancer, several potential biological mechanisms might cause such an association. Results from previous studies have suggested that sex hormones, including estrogens, might inhibit the development and progression of gastric cancer by increasing an individual’s resistance to inflammation and inhibiting production of gastric acid and gastrin. For example, estrogens are known to regulate numerous physiological processes primarily by binding to estrogen receptors, which are potent regulators of gene transcription47. As estrogen receptors are present in gastric epithelial tissue28,29, and have been shown to inhibit inflammation30,31,32, it is biologically plausible that estrogens might protect against gastric cancer. Furthermore, estrogens are known to directly affect production gastric acid and progesterone, and indirectly affect gastrin production via somatostatin33,34.
Our study has several strengths that should be mentioned. To the best of our knowledge, this is the most comprehensive and quantitative assessment of the relationship between parity and the risk for developing gastric cancer. Our current meta-analysis included results from 10 prospective studies which enrolled a total of 6624 gastric cancer cases and 5,559,695 non-cases, and thus had sufficient statistical power to detect an association between parity and gastric cancer. Furthermore, we divided parity into several categories (i.e., ever parity vs. nulliparous; high parity number vs. low parity number) instead of merely presenting results representing a summary of all different categories of parity (Table 2 and Table 3). Notably, the results of our numerous subgroup and sensitivity analyses were consistent with the main findings and without heterogeneity, albeit they revealed no statistically significant differences.
This study also has several limitations that should be mentioned. First, this meta-analysis was based on the observational designs of the included prospective studies, and confounding is a concern in all observational studies. All of the included studies except one21 reported their risk estimates based on multivariable models. However, several studies made adjustments for important potential confounders such as cigarette smoking, body mass index, alcohol consumption, and socioeconomic status in their primary analyses (Table 1). Thus it cannot be ruled out that the observed association between parity and gastric cancer risk might be explained by residual confounding. Furthermore, it is possible that unmeasured or unidentified risk factors may have affected the results even after controlling for potential confounders. Notably, all of the included studies lacked information concerning H. pylori, which is an important risk factor of gastric cancer. The lack of this information severely limited our ability to adjust for or conduct a proper subgroup analysis stratified by H. pylori infection. Several studies have indicated that an increased susceptibility to H. pylori infection during pregnancy might be related to an increased risk of gastric cancer35,36. In contrast, Freedman et al.18 mentioned that H. pylori infection did not confound an observed association between menstrual and reproductive factors and gastric cancer risk. Therefore, future studies are needed to analyze such important confounders and adjust for or stratify them along with other risk factors to rule out possible residual confounding. Second, in contrast to the previous register-based analyses, the parity information used in our analysis was collected from self-reports included in several prospective studies, and this might have introduced errors due to misclassification. Although parity information is generally well-reported and highly reliable when compared with reports concerning other reproductive factors, a non-differential misclassification of parity may have slightly shifted risk estimates towards the null value. However, our subgroup analyses stratified by the aforementioned variable produced results similar to the main findings, and showed no significant differences (data not shown). Third, although the outcomes, the incidence of gastric cancer, were identified via record linkage to the cancer registries, the criteria and procedure of diagnosis of this disease might be slightly different among these studies. For example, three included studies15,17,22 codes gastric cancer according to the 10th revision of the WHO International Classification of Diseases (ICD-10). Two included studies16,20 codes this disease by the ICD-9. By comparison, Bahmanyar et al.8 and Heuch et al.23 both used ICD-7 in their studies. The site and histology of gastric cancer cases were coded using the International Classification of Diseases for Oncology, Third Edition in study of Persson et al.19. Last, although levels of endogenous hormone obviously change throughout pregnancy, parity might be an imperfect marker of overall exposure to sex hormones, because when compared to the life-span of a woman, the exposure period is too short-lived or inadequately timed to have an appreciable effect gastric carcinogenesis8. Therefore, further analyses which include adjustments or stratification for other menstrual and reproductive factors need to be conducted in the future.
In summary, our meta-analysis of prospective studies found no association between parity and the risk for developing gastric cancer. The reliability of our finding is increased by the fact that evidence from prospective studies is generally considered to be more reliable than evidence from retrospective studies. However, with regards to public health recommendations, our findings are complicated by the fact that parity may have both beneficial and adverse effects with regards to other diseases. Further studies of parity in relation to other cancers as well as overall cancer risk and mortality are needed to better assess the risk-benefit of exposure to pregnancy-related hormones.
Material and Methods
Search strategy
Two independent investigators (T-TG and Q-JW) systematically searched PubMed (MEDLINE), EMBASE, and Web of Science for relevant prospective studies published starting from the time of each database’s inception to May 30, 2015. The following keywords were used for searching: (parity OR pregnancy OR livebirth OR reproductive OR reproduction) AND (stomach OR gastric) AND (cancer OR tumor OR carcinoma OR neoplasm). This search strategy was similar to that used in our previous studies9,10,11,25,37. Guidelines in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were followed when planning, conducting, and reporting this meta-analysis38.
Study selection and exclusion
Two independent investigators (JC and T-TG) performed the study selection and exclusion procedures. Studies included in this analysis were required to satisfy the following criteria: (i) utilized a prospective study design; (ii) evaluated the association between parity and the risk for developing gastric cancer; (iii) presented estimates of relative risk (RR) or hazard ratio (HR) with 95% confidence intervals (CIs) or data necessary to calculate those parameters. If several publications involved overlapping patients, the study with the most patients was included.
The following types of studies were excluded from our meta analysis: (i) randomized controlled trials, case-control studies, retrospective studies, reviews without original data, ecological studies, editorials, and case reports; (ii) studies reported with risk estimates that could not be summarized (e.g., studies reported without 95%CIs).
Data abstraction and quality assessment
Data were extracted using a data extraction form and entered into a database by a single investigator (Q-JW). Next, an independent investigator (JC) checked the data, and all differences were resolved by a third investigator (T-TG). The following information was extracted for each included study: last name of the first author, publication year, geographic location of the study, number of cases/size of cohort, follow-up years, exposure and outcome assessment, categories of exposure, and study-specific adjusted estimates with their 95%CIs (including adjusted confounder information if applicable). If there were multiple estimates for the association, we used the estimate adjusted for the most appropriate confounding variables. This was similar to the method used in other studies39,40,41,42,43.
The Newcastle-Ottawa Scale (NOS)25,39,40,41,42,43,44,45 uses three quality parameters (selection, comparability, and exposure/outcome), and was used to assess the methodological quality of the studies included in our meta-analysis. We used the NOS instead of a scoring system that divides studies into categories of high or low quality, because quality scoring might not only hide important information by combining disparate study features into a single score, but also introduce an arbitrary subjective element into the analysis46,47,48.
Statistical analysis
We used the counting method proposed by Hamling et al.49 to recalculate the relative risks in studies15,17,18,19,20,22 that did not provide results for ever parity vs. nulliparous, and studies15,20 that did not use the category with the lowest parity number as a reference. The results from one study8 that reported separate data for cardia and non-cardia gastric cancer were pooled with the results of other studies.
To examine associations between parity and gastric cancer risk, the summarized relative risks (SRRs) with their 95% CIs were estimated by summarizing the risk estimates of each study using random effect models that considered both within- and between-study variations50. Furthermore, we summarized the study-specific SRR for each one live birth increment in the parity number. A study-specific trend reflecting correlated log RRs across different categories of parity number was computed using the generalized least-squares trend estimation method developed by Greenland et al.51 and Orsini et al.52. Furthermore, a potential nonlinear dose-response relationship between parity number and gastric cancer risk was modeled by using restricted cubic splines with 3 knots at fixed percentiles (10%, 50%, and 90%) of the distribution of exposure. An overall P value was calculated by showed that these two regression coefficients simultaneously equaled zero. The P value for nonlinearity was calculated by showing that the coefficient of the second spline equaled zero. The details of this method for calculating P values have been previously described53,54.
The following information was required and used to conduct the dose-response meta-analysis: 1) the distribution of cases and non-cases, and the risk estimates with the variance estimates for at least three quantitative exposure categories; 2) the median or mean level of these exposures in each category (if reported by ranges, mean levels were calculated by averaging the lower and upper bound; if the lowest category was open ended, the lowest boundary was considered to be zero; if the highest category was open ended, the open-ended interval length was assumed to be the same as the adjacent interval)37. When using these techniques, nine studies8,15,16,17,18,19,20,21,22 met our specifications, and were included in the dose-response analysis of parity number and gastric cancer risk.
The I2 metric was used to evaluate between-study heterogeneity. Values for I2 range between 0% and 100%, and represent the ratio of between-study variance divided by the sum of the within-study and between-study variances55. Pre-specified subgroup analyses were conducted based on the number of gastric cases (≥250 vs. <250), number of follow-up years (≥10 vs. <10), geographic location (North America, Europe or Asia), patient menopausal status (pre-menopausal vs. post-menopausal), anatomic subsite of the gastric cancer (cardia vs. non-cardia), and adjustments made for potential confounders including body mass index, cigarette smoking, alcohol consumption, and socioeconomic status. Heterogeneity between subgroups was evaluated by meta-regression25,39,41,56,57. Egger’s regression asymmetry test43 was used to test for small study biases, such as publication bias, that can reflect genuine heterogeneity, chance, or other reasons for differences between small and large studies. A P-value of 0.05 was used to determine whether significant publication bias existed. Furthermore, sensitivity analyses were conducted by deleting each study in turn to examine the influence of individual data on the overall estimate. All statistical analyses were performed using Stata (version 12; StataCorp, College Station, TX, USA).
Additional Information
How to cite this article: Chen, J. et al. Parity and gastric cancer risk: a systematic review and dose-response meta-analysis of prospective cohort studies. Sci. Rep. 6, 18766; doi: 10.1038/srep18766 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Younger research fund of Shengjing Hospital (Grant 2014sj09 for Qi-Jun Wu). Qi-Jun Wu was supported by the Fogarty International Clinical Research Scholars and Fellows Support Center at the Vanderbilt Institute for Global Health, funded by the Fogarty International Center, NIH, through an R24 Training Grant (D43 TW008313 to Xiao-Ou Shu).
Footnotes
Author Contributions J.C., T.-T.G. and Q.-J.W. designed research; J.C., T.-T.G. and Q.-J.W. conducted research; J.C., T.-T.G. and Q.-J.W. analyzed data; J.C., T.-T.G. and Q.-J.W. wrote the draft; All authors read, reviewed and approved the final manuscript. Q.-J.W. had primary responsibility for final content.
References
- Torre L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015). [DOI] [PubMed] [Google Scholar]
- Ferlay J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. (Date of access: 17/November/2015).
- Karimi P. et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 23, 700–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandanos E. & Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer 44, 2397–403 (2008). [DOI] [PubMed] [Google Scholar]
- Sipponen P. & Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer 5, 213–9 (2002). [DOI] [PubMed] [Google Scholar]
- Furukawa H., Iwanaga T., Koyama H. & Taniguchi H. Effect of sex hormones on carcinogenesis in the stomachs of rats. Cancer Res 42, 5181–2 (1982). [PubMed] [Google Scholar]
- Furukawa H., Iwanaga T., Koyama H. & Taniguchi H. Effect of sex hormones on the experimental induction of cancer in rat stomach - a preliminary study. Digestion 23, 151–5 (1982). [DOI] [PubMed] [Google Scholar]
- Bahmanyar S. et al. Parity and risk of stomach cancer by sub-site: a national Swedish study. Br J Cancer 98, 1295–300 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H. B., Wu Q. J. & Gong T. T. Parity and kidney cancer risk: evidence from epidemiologic studies. Cancer Epidemiol Biomarkers Prev 22, 2345–53 (2013). [DOI] [PubMed] [Google Scholar]
- Guan H. B. et al. Parity and risk of colorectal cancer: a dose-response meta-analysis of prospective studies. PLoS One 8, e75279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H. B. et al. Parity and pancreatic cancer risk: a dose-response meta-analysis of epidemiologic studies. PLoS One 9, e92738 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Thompson M. L. Estrogen receptor alpha and beta expression in upper gastrointestinal tract with regulation of trefoil factor family 2 mRNA levels in ovariectomized rats. Biochem Biophys Res Commun 240, 478–83 (1997). [DOI] [PubMed] [Google Scholar]
- Katoh M. Trefoil factors and human gastric cancer (review). Int J Mol Med 12, 3–9 (2003). [PubMed] [Google Scholar]
- Oshima C. T. et al. Estrogen and progesterone receptors in gastric and colorectal cancer. Hepatogastroenterology 46, 3155–8 (1999). [PubMed] [Google Scholar]
- Green J. et al. Reproductive factors and risk of oesophageal and gastric cancer in the Million Women Study cohort. Br J Cancer 106, 210–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Chen C. C., Chiu H. F. & Yang C. Y. Higher parity associated with higher risk of death from gastric cancer. World J Gastroenterol 17, 784–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duell E. J. et al. Menstrual and reproductive factors, exogenous hormone use, and gastric cancer risk in a cohort of women from the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol 172, 1384–93 (2010). [DOI] [PubMed] [Google Scholar]
- Freedman N. D. et al. The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer 116, 1572–81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C. et al. Female reproductive factors and the risk of gastric cancer in a large-scale population-based cohort study in Japan (JPHC study). Eur J Cancer Prev 17, 345–53 (2008). [DOI] [PubMed] [Google Scholar]
- Freedman N. D. et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut 56, 1671–7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski-Rahikkala H., Pouta A., Pietilainen K. & Hartikainen A. L. Does parity affect mortality among parous women? J Epidemiol Community Health 60, 968–73 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S. et al. Menstrual and reproductive factors and the mortality risk of gastric cancer in Japanese menopausal females. Cancer Causes Control 14, 53–9 (2003). [DOI] [PubMed] [Google Scholar]
- Heuch I. & Kvale G. Menstrual and reproductive factors and risk of gastric cancer: a Norwegian cohort study. Cancer Causes Control 11, 869–74 (2000). [DOI] [PubMed] [Google Scholar]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64, 31–49 (1965). [DOI] [PubMed] [Google Scholar]
- Wu Q. J. et al. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci 104, 1067–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen P. & Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer 5, 213–9 (2002). [DOI] [PubMed] [Google Scholar]
- Camargo M. C. et al. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 21, 20–38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karat D. et al. Expression of oestrogen and progesterone receptors in gastric cancer: a flow cytometric study. Br J Cancer 80, 1271–4 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano N. et al. Expression of estrogen receptor-alpha and -beta mRNAs in human gastric cancer. Cancer Lett 176, 129–35 (2002). [DOI] [PubMed] [Google Scholar]
- De Bosscher K., Vanden B. W. & Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene 25, 6868–86 (2006). [DOI] [PubMed] [Google Scholar]
- Kalaitzidis D. & Gilmore T. D. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab 16, 46–52 (2005). [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Koditz R., Pfohl M. & Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 23, 90–119 (2002). [DOI] [PubMed] [Google Scholar]
- La Vecchia C., Negri E., Franceschi S. & Parazzini F. Long-term impact of reproductive factors on cancer risk. Int J Cancer 53, 215–9 (1993). [DOI] [PubMed] [Google Scholar]
- Watson S. A., Grabowska A. M., El-Zaatari M. & Takhar A. Gastrin - active participant or bystander in gastric carcinogenesis? Nat Rev Cancer 6, 936–46 (2006). [DOI] [PubMed] [Google Scholar]
- Lanciers S., Despinasse B., Mehta D. I. & Blecker U. Increased susceptibility to Helicobacter pylori infection in pregnancy. Infect Dis Obstet Gynecol 7, 195–8 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 61, 1–241 (1994). [PMC free article] [PubMed] [Google Scholar]
- Wu Q. J. et al. Parity and endometrial cancer risk: a meta-analysis of epidemiological studies. Sci Rep 5, 14243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. J. et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol 24, 1079–87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan N. N. et al. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies. Cancer Causes Control 26, 65–78 (2015). [DOI] [PubMed] [Google Scholar]
- Gong T. T., Wu Q. J., Wang Y. L. & Ma X. X. Circulating adiponectin, leptin and adiponectin-leptin ratio and endometrial cancer risk: Evidence from a meta-analysis of epidemiologic studies. Int J Cancer 137, 1967–78 (2015). [DOI] [PubMed] [Google Scholar]
- Wu Q. J., Gong T. T. & Wang Y. Z. Dietary fatty acids intake and endometrial cancer risk: A dose-response meta-analysis of epidemiological studies. Oncotarget 6, 36081–97 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R., Wu Q. J., Gong T. T. & Jiang L. Dietary fat and fatty acid intake and epithelial ovarian cancer risk: evidence from epidemiological studies. Oncotarget (2015) 10.18632/oncotarget.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Date of access: 17/November/2015).
- Wang Y. Z., Wu Q. J., Zhu J. & Wu L. Fish consumption and risk of myeloma: a meta-analysis of epidemiological studies. Cancer Causes Control 26, 1307–14 (2015). [DOI] [PubMed] [Google Scholar]
- Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol 140, 290–6 (1994). [DOI] [PubMed] [Google Scholar]
- Greenland S. & O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2, 463–71 (2001). [DOI] [PubMed] [Google Scholar]
- Greenland S. & O’ Rourke K. Meta-analysis. In: Modern Epidemiology, 3rd edn, (eds Rothman K. J. et al.) 652–682. (Lippincott Williams & Wilkins, 2008). [Google Scholar]
- Hamling J., Lee P., Weitkunat R. & Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27, 954–70 (2008). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–88 (1986). [DOI] [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–9 (1992). [DOI] [PubMed] [Google Scholar]
- Orsini N. et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnardi V., Zambon A., Quatto P. & Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol 159, 1077–86 (2004). [DOI] [PubMed] [Google Scholar]
- Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 19, 1831–47 (2000). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–58 (2002). [DOI] [PubMed] [Google Scholar]
- Gong T. T. et al. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int J Cancer 132, 2894–900 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan N. N. et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr 98, 1020–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.