Abstract
Genome-wide association studies have shown that variants in fat mass and obesity-associated (FTO) gene are robustly associated with body mass index and obesity. These FTO variants are also associated with end stage renal disease and all-cause mortality in chronic kidney diseases. However, the exact role of FTO in kidneys is currently unknown. Here we show that FTO expression is increased after ureteral obstruction and renal fibrosis. Deficiency of the FTO gene attenuates the fibrogenic responses induced by ureteral obstruction in the kidney. Renal tubular cells deficient of FTO produce less α-SMA after TGF-β stimulation. FTO is indispensable for the extracellular matrix synthesis after ureteral obstruction in kidneys. Indeed, global gene transcriptions amplitude is reduced in FTO deficient kidneys after ureteral obstruction. These data establish the importance of FTO in renal fibrosis, which may have potential therapeutic implications.
Genome-wide association studies have shown that variations in the first intron of the Fat mass and obesity associated (FTO) gene are associated with obesity and diabetes in global studies of different ethnicities1. The variation at FTO rs7202116 locus is shown to be associated with phenotypic variability in body mass index2. However, FTO has multiple functions other than associations with obesity. FTO transgenic mice displayed increased obesity3 and FTO deficient mice have increased prenatal mortality with multiple organ abnormalities4. Humans with FTO gene loss-of-function mutations also have facial dysmorphism, brain abnormalities, and heart diseases5. Recent findings that those FTO variants are associated with IRX3 further imply that FTO could have important functions other than controlling body weight or metabolism6.
FTO is a 2-oxoglutarate-dependent N6-methyladenosine RNA (m6A) demethylase7. FTO is expressed in most tissues and is abundant in the hippocampus, cerebellum, and hypothalamus8,9. Clinical studies have implied that FTO functions are linked to lipolysis10, telomere length11, food intake12, breast cancer13, and Alzheimer’s disease14. FTO levels are regulated by essential aminoacids and FTO-deficient cells have increased autophagy and reduced mammalian target of rapamycin signaling15. Furthermore, FTO regulates dopaminergic signaling in midbrain16, interacts with CaMKII/CREB pathway17, associates with ciliopathies through Wnt signaling18, and involves in leptin receptor/STAT3 in brain19. Moreover, FTO affects circadian rhythm through inhibiting the CLOCK-BMAL1-induced transcription20. These studies provide complex evidences of fundamental FTO functions in many organs or cells. However, the function of FTO in kidneys is still unknown.
Previous case-control clinical studies have suggested the associations between FTO polymorphism and risks of end-stage renal disease (ESRD). The FTO rs17817449 variants are associated with increased risks of chronic kidney diseases (CKD) and onset of ESRD21. The FTO polymorphism is also an independent predictor of all-cause mortality in patients with CKD. Meta-analysis for 1540 CKD patients cohorts showed that individuals with the A allele in rs708259 polymorphism on intron 8 of FTO had a 42% excess risk of death22. The underlying mechanisms of these associations and the role of FTO in kidneys are currently unknown. We hypothesize that FTO plays important role in kidneys and regulates kidney fibrogenic response, a critical underlying mechanism of CKD.
Results
FTO levels in kidneys are increased after ureteral obstruction
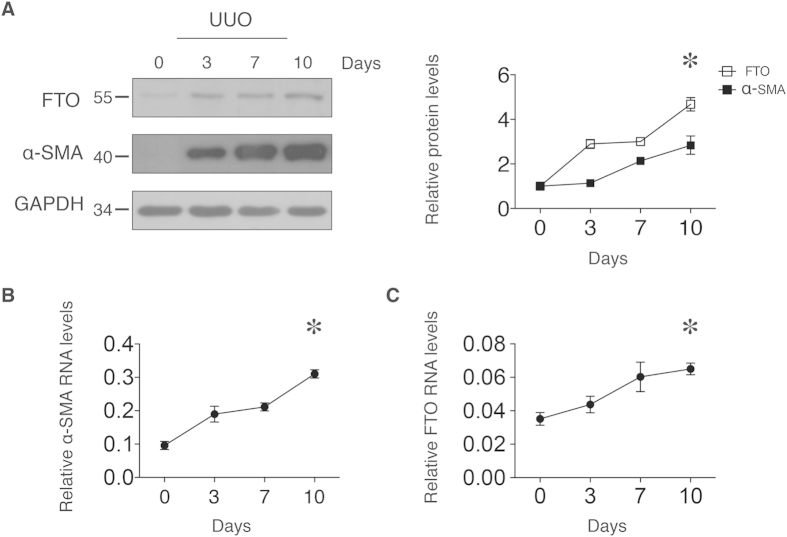
To investigate the role of FTO in kidneys, we first analyzed the FTO protein abundance and mRNA expression levels in the kidneys after unilateral ureteral obstruction (UUO). We first confirmed that there were significant kidney fibrosis and increases of mRNA and protein of alpha-smooth muscle actin (α–SMA) after UUO surgery (Fig. 1A,B). The α–SMA is the actin isoform characteristic of vascular smooth-muscle cells23 and a marker for kidney fibrosis24,25. After UUO, the FTO protein concentrations increased by 4.78-fold in the kidneys from day 3 to day 10 (Fig. 1A). The mRNA levels of FTO increased from day 3 to day 10 (Fig. 1C). The increases of FTO after UUO implied FTO plays certain role in the kidney. Based on this observation, we hypothesized that FTO regulates tubulointerstitial fibrosis after UUO in the kidney.
Figure 1. FTO levels in mouse kidney after ureteral obstruction.
Mice kidneys were sampled from 0 to 10 days after UUO and analyzed for mRNA and protein concentrations. (A) Representative western blot images and densitometry quantification of FTO and α-SMA protein levels from mice kidneys. (B) Kidney α-SMA and (C) FTO mRNAs were analyzed and normalized to 18S. Data represent means ± SD, n = 3 mice per time point. *P < 0.05 by one-way ANOVA (effect of time).
Tubulointerstitial Fibrosis is decreased in FTO deficient mice
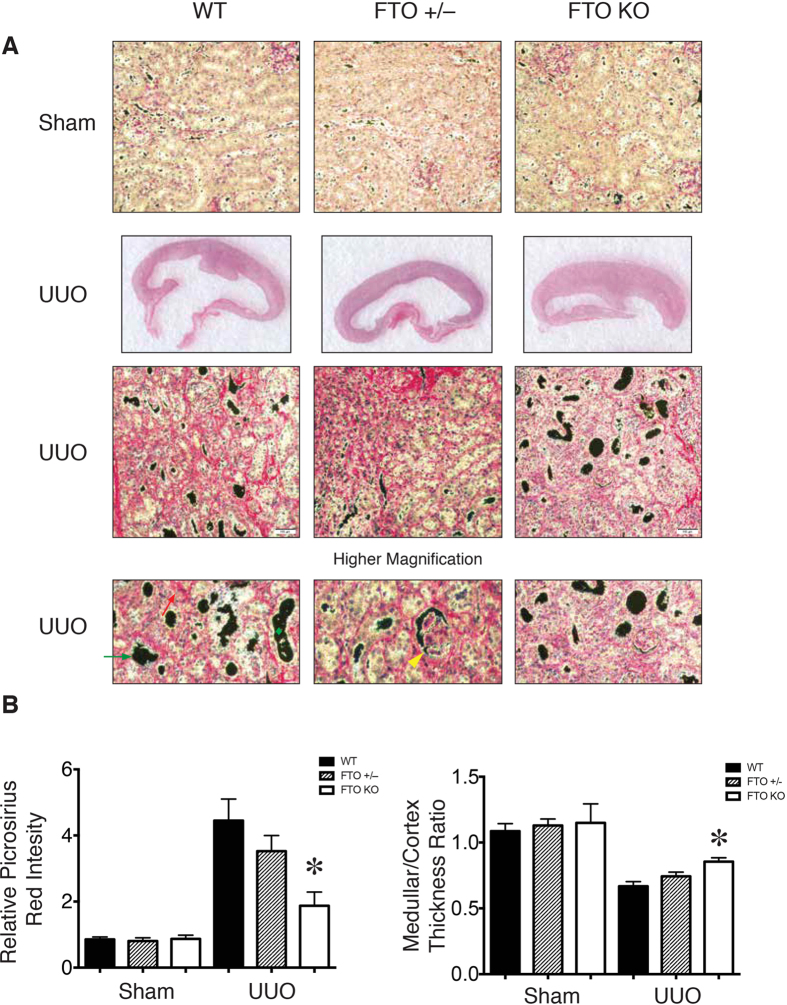
To study whether FTO indeed has critical role in tubulointerstitial fibrosis in the kidney, we performed UUO surgery in wild-type, FTO+/–, and FTO–/– mice and analyzed the severity of tubulointerstitial fibrosis at day 10. After UUO surgery, wild-type mice had renal tubulointerstitial fibrosis, tubular dilation, glomerular sclerosis, and flattened tubular epithelial cells as evidenced by picrosirius red staining (Fig. 2A). In comparison with wild-type kidneys, the FTO–/– kidneys exhibited less tubulointerstitial damage, better medullar/cortex thickness ratio, and less fibrosis after UUO surgery (Fig. 2A,B). This result indicated that FTO deficiency protected kidneys from UUO injury.
Figure 2. FTO deficiency attenuated renal fibrosis and extracellular matrix deposition.
(A) Representative picrosirius red staining and gross pathology images of wild-type, FTO+/–, and FTO–/– kidney sections for assessment of total collagen deposition and extent of fibrosis. Higher magnification images compared tubular dilation (Green diamond), flattened tubular epithelial cells (Green arrow), focal glomerular sclerosis (Yellow arrowhead), and extracellular matrix deposition (Red arrow) in the cortex of obstructed kidneys. (B) Quantitative analysis of collagen deposition and medullary/cortex thickness ratio in wild-type, FTO+/–, and FTO–/– mice. 10–12 weeks female wild-type (n = 10), FTO+/– (n = 10), and FTO–/– (n = 6) mice with body weight of 25.0±1.3, 24.3±1.1, and 21±1.5 g, respectively. Data represent means ± SD. *P < 0.05 by two-sample Mann-Whitney analysis, WT compared to FTO–/– mice.
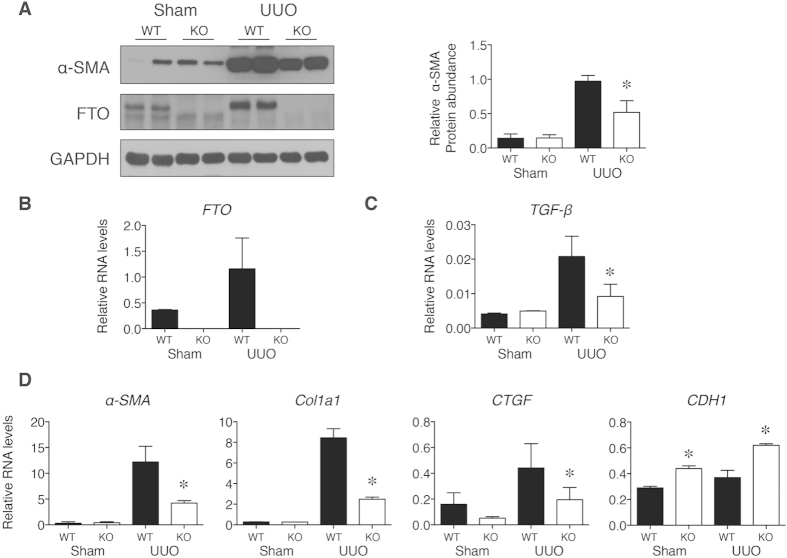
After UUO surgery, the α-SMA and FTO protein concentrations increased in wild-type kidneys (Fig. 3A,B). FTO–/– kidneys had significantly lower α-SMA protein concentrations after UUO surgery when compared with that in wild-type kidneys (Fig. 3A). The transforming growth factor-β (TGF-β) mRNA levels were significantly lower in FTO–/– kidneys after UUO compared with that in wild-type kidneys (Fig. 3C). Furthermore, the a-SMA, collagen, type I, alpha 1 (Col1a1), and connective tissue growth factor (CTGF) mRNA, which were markers for kidney fibrosis and downstream targets of TGF-β26,27, also exhibited significantly lower levels in FTO–/– kidneys after UUO (Fig. 3D). CDH1 (E-cadherin) is a downstream factor of TGF-β and serves as a marker for epithelial to mesenchymal transition (EMT) of kidney proximal tubular cells28. Initiation of EMT is associated with reduced expression of CDH129. After UUO surgery in FTO–/– mice, CDH1 mRNA levels were higher and implied a decreased EMT response with FTO deficiency (Fig. 3D). Taken together, these observations suggest that FTO plays an important role in fibrogenic response obstructive nephropathy.
Figure 3. Protein and RNA levels in WT and FTO deficient mice (KO) after UUO.
(A) Representative western blot images and densitometry quantification of α-SMA protein concentrations from mice kidneys. (B–D) Kidney mRNAs levels after UUO surgery were analyzed and normalized to 18S. Data represent means ± SD, n = 6 mice per time point. *P < 0.05 by two-sample Mann-Whitney analysis, WT UUO groups compared to KO UUO groups.
Deficiency of FTO inhibits TGF-β stimulated α-SMA protein expression
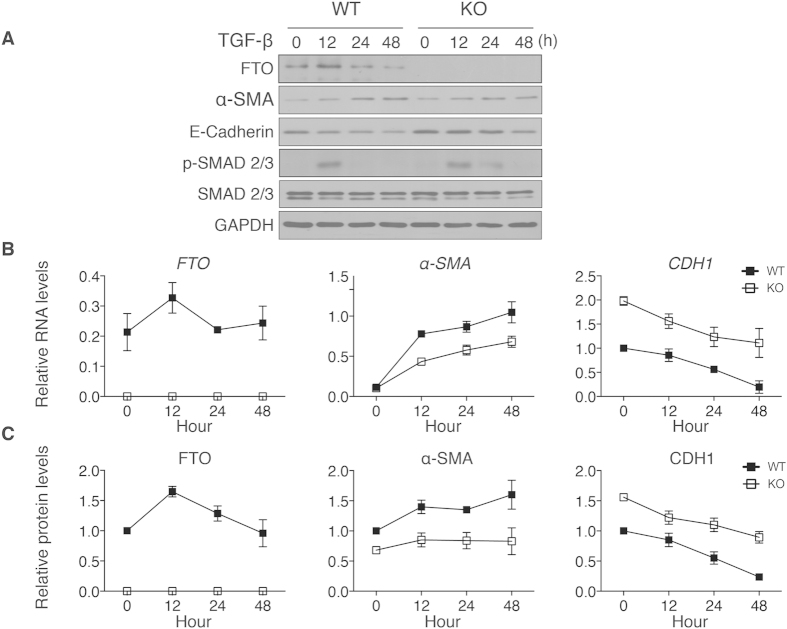
Previous studies have shown that TGF-β signaling is a key mediator in renal fibrosis after UUO30. FTO–/– kidneys had lower TGF-β expressions after UUO (Fig. 3C). We thought to examine whether deficiency of FTO inhibits TGF-β stimulation of α-SMA expression and other downstream signaling factors in isolated renal tubular cells. After TGF-β stimulation, FTO mRNA levels increased at 12 h and returned to baseline at 24 while FTO protein concentrations increased from 12 to 24 h and returned to baseline at 48h (Fig. 4A–C). The α-SMA mRNA levels and protein concentrations increased at 12, 24, and 48 h in wild-type renal tubular cells after TGF-β stimulations, (Fig. 4A–C). FTO deficient renal cells exhibited significant lower α-SMA mRNA and protein concentrations when compared with wild-type cells after stimulation (Fig. 4A–C). Consistent with the findings in UUO kidneys, CDH1 mRNA levels and protein concentrations were significantly higher in FTO deficient renal cells (Fig. 4A–C). There were no significant differences of phospho-SMAD 2/3 and SMAD 2/3 protein abundance between FTO deficient and wild-type cells (Fig. 4A). These data supported that FTO deficiency affects the downstream factors of TGF-β signaling, such as α-SMA and CDH1.
Figure 4. FTO deficiency attenuated α-SMA concentrations induced by TGF-β in renal tubular cells.
(A) Representative western blot images of FTO, α-SMA, E-Cadherin, phospho-SMAD 2/3, and SMAD 2/3 protein concentrations after TGF-β (1ng/mL) stimulation in wild-type and FTO–/– renal tubular cells. (B) Quantitative analysis of FTO, α-SMA, and CDH1 (E-Cadherin) mRNA levels in wild-type and FTO–/– renal tubular cells after TGF-β stimulation (n = 6). (C) Quantitative analysis of FTO, α-SMA, and CDH1 (E-Cadherin) protein concentrations in wild-type and FTO–/– renal tubular cells after TGF-β stimulation (n = 3). Data represent means ± SD. *P < 0.05 by two-way ANOVA.
FTO modulates UUO-dependent gene transcriptions
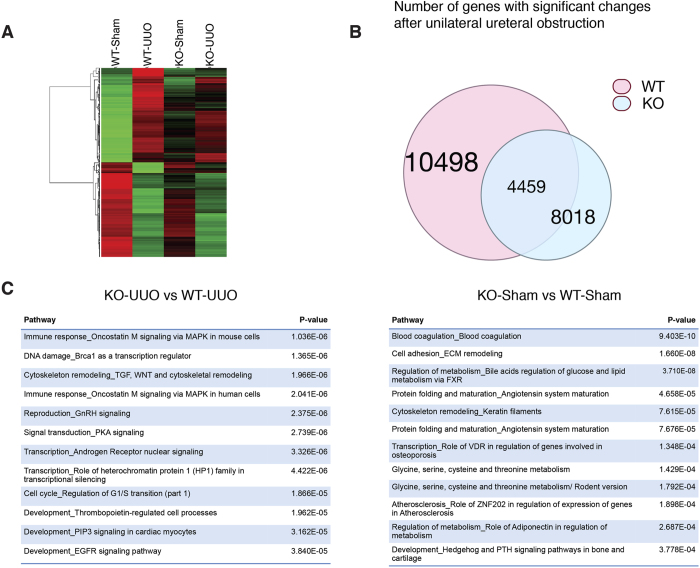
FTO levels affect RNA modification and transcriptome31. It can act as transcription co-activator and affect transcription processes20,32. Based on the findings that tubulointerstitial fibrosis and TGF-β was attenuated in FTO deficiency mice, we reasoned that FTO was critical in mediating global gene transcriptions after UUO surgery. To determine whether FTO modulates endogenous gene transcriptions after UUO, we analyzed the global gene expression changes of wild-type and FTO–/– mice after UUO (Fig. 5A). UUO are expected to result in both positive and negative transcriptional responses in kidneys33. If deficiency of FTO is independently capable of abrogating transcriptions by UUO, we would expect to see a global repressive shift in the transcriptional response to UUO. Indeed, we found that the transcriptional response after UUO in FTO deficient mice had significantly less amplitude when compared to wild-type mice. When comparing the top 200 activated or repressed genes in wild-type mice, genes in FTO deficient mice exhibited less activation or repression (Fig. 5B). FTO deficient mice had 1412 less genes activated and 1068 less genes repressed compared to wild-type mice. We analyzed the differential expressed genes between wild-type and FTO deficient mice with MetaCore package. Comparing between wild-type and FTO deficient mice after UUO or sham procedure, top 12 gene pathway maps affected by FTO were summarized in Fig. 5C. The immune response/MAPK, cytoskeleton/TGF, DNA damage/BRCA1, and blood coagulation pathway were the top ranked pathway affected by FTO deficiency after UUO. Taken together, these data concluded that FTO deficiency decreases fibrogenic responses and protects kidney from UUO associated fibrotic damages.
Figure 5. FTO modulates gene transcriptions after UUO.
(A) Heat map: color denoted UUO-induced top 200 increases or decreases in gene transcripts from WT or FTO deficient mice (KO). (B) Significant transcripts after UUO in wild-type (pink circle) or FTO deficient (blue circle) kidneys. (C) Pathway analysis between KO and WT mice after UUO or sham procedure by MetaCore.
Discussion
Our data provide a mechanistical insight into the association of FTO and chronic kidney diseases. FTO expression levels were altered after UUO and deficiency of FTO results in decreased fibrogenic responses. FTO deficiency resulted in decreased α-SMA synthesis in renal tubular epithelial cells after TGF-β stimulation. Indeed, global gene transcriptions amplitude was reduced in FTO deficient mice. Pathway analysis revealed that deficiency of FTO affects immune response, DNA damage, and cytoskeleton remodeling through TGF-β signaling.
This important role of FTO in kidneys implies a link between obesity and kidney. Overweight patients without diabetes or hypertension had increased risk for CKD34. However, in patients with ESRD, a higher body mass index is paradoxically associated with better survival35. The exact relationships between obesity and CKD are still unclear. Our results provide a direct link between obesity gene variant and kidney fibrogenic responses, which could be a possible key for future therapeutic choice. Although recent data suggested that Irx3 is a functional target of variants within introns of FTO36, the exact role of FTO or IRX3 in human obesity is still unclear37. The role of IRX3 in kidneys also remains unknown. Further studies will be needed to dissect the roles of FTO and IRX3 in kidney and CKD patients.
Our data showed that several downstream targets of TGF-β, such as α-SMA or CTGF, had decreased responses to stimulation by TGF-β or UUO with FTO deficiency. These results implied that FTO not only affected TGF-β levels (Fig. 3C) but also acted downstream of TGF-β. Previous studies have shown that FTO is able to act as a transcription co-activator17,20 and has a role in RNA methylation38. Our results showed that phosphorylation and protein abundance of SMAD, which transduce extracellular TGF-β signals to nucleus, were unaffected by FTO deficiency. These data indicated that FTO may act downstream of SMAD to affect TGF-β targets. Future study will need to investigate the exact mechanism how FTO modulates these TGF-β regulated genes. Moreover, our pathway analysis showed that FTO also affected immune response, DNA damage, and cytoskeleton remodeling pathways. It is also possible that FTO exerts broad influences upon several signaling pathways to affect kidney fibrosis besides TGF-β signaling.
FTO is implicated in several signaling pathways, including mTOR, CREB, Wnt, and STAT31. Our results proved that FTO also plays an important role in TGF-β signaling. Previous studies have shown that TGF-β and obesity are closely related39,40,41. Hypothalamic TGF-β is overproduced by astrocytes and proopiomelanocortin neurons under conditions such as obesity and aging40. It is then reasonable that deficiency of FTO also affects TGF-β signaling. TGF-β regulates multiple cellular functions including survival, proliferation, differentiation, and migration42. In kidneys, TGF-β governs a variety of pathophysiological function, such as inflammation, fibrogenesis, epithelial-to-mesenchymal transition, and metabolism30. Our observations that FTO modulates the fibrogenic response in kidneys and TGF-β signaling open several speculations whether FTO deficiency also affects epithelial-to-mesenchymal transition or inflammatory responses in the kidney. Future studies will answer these questions.
Materials and Methods
Cell culture and Antibody
Mouse proximal tubular epithelial cells were isolated from wild-type or FTO–/– mice. The kidneys were de-capsulated and the medulla removed. The cortices were finely dissected and digested with collagenase type-II. The kidney digests were filtered with 70μm sieve (BD) and cell pellets were resuspended in renal cell culture medium (DMEM-F12, 10% FBS, 5 μg/mL insulin, 5 μg/mL transferrin, 50 nM selenium, 5 nM T3, 50 mM hydrocortisone, and Penicillin/Streptomycin). Helper-dependent adenovectors (Microbix) were generated with mouse FTO cDNA in the shuttle vector pDC516 and Flp-FRT system. Antibodies used for immunoblotting and immunofluorescence included anti-FTO (Abnova, PAB11419), α-SMA (Sigma), GAPDH (Cell signaling), phosphor-SMAD 2/3 (Cell signaling), and SMAD2/3 (Cell signaling).
Animals and surgery
All animal experimental protocols were approved by Chang Gung University and Chang Gung Memorial Hospital Institutional Animal Care and Use Committee. All experiments were performed in accordance with the approved protocols, guidelines, and regulations.
Embryonic stem cells and mice with loxP sites surrounding FTO exon 3 were obtained from EUCOMM (Institute of Developmental Genetics) and Mouse Genetics Programme (Wellcome Trust Sanger Institute). FTO-deficient mice were generated from matings between FTOflox/flox mice and Ella-cre mice (Jackson Laboratory). Homozygous FTO-deficient, heterozygous FTO-deficient, and wild-type mice were generated from matings between two heterozygous mice. The unilateral ureteral obstruction procedure was performed under general anesthesia with ketamine and xylazine (80/6 μg/g intraperitoneally). The incision was from left flank area with scalpel. Ureters were then explored and ligated with 2-0 silk with double ligature. After ligation, the operative fields were rinsed with sterile PBS to prevent future adhesion. After 3 or 7 or 10 days, mice were sacrificed and kidneys were harvested for further analysis.
Protein and mRNA analysis
Total RNA was extracted using TRI reagent (Ambion) according to the manufacturer’s instructions. One microgram of total RNA was reverse-transcribed and analyzed using the Applied Biosystems Real-time PCR system. The relative gene expression method was used for analysis, and the expression of the target genes was normalized to that of 18S rRNA. The assay was repeated independently at least three times. Protein was isolated from homogenized frozen kidneys or cells with cell lysis buffer (Cell Signaling). The lysates were separated by electrophoresis, transferred to polyvinylidene fluoride membranes, and probed with specific antibodies. The results were normalized to GAPDH band and calculated with Image J (NIH).
Immunohistochemistry analysis
The kidney after UUO or sham operations were fixed with 4% PFA and processed for paraffin embedding. The sections were then deparaffinize/rehydrated and stained with or without Weigerts Hematoxylin. The staining was then proceeded with modified picrosirius staining kit (Polysciences, #24901) according to manufacturing protocol. The results were analyzed with Adobe Photoshop CS2 and ImageJ (NIH).
Microarray analysis
Microarray experiments were performed at Genomic Medicine Research Core Laboratory (GMRCL) of Chang Gung Memorial Hospital. The RNA samples from kidneys of wild-type and FTO–/– mice 10 days after sham or UUO surgery were hybridized using Affymetrix Mouse Genome 430A 2.0 Oligonucleotide Microarrays. All analysis was done in duplicate and dye swap experiments were used. The signals that were differentially expressed >2 or <0.75 were considered significant and further analyzed. Network and pathway analyses were performed with MetaCore (GeneCo).
Statistical analysis
Values were expressed as mean ± standard deviation. Data were compared using Student’s t-tests or analysis of variance (ANOVA), where appropriate. For data with small numbers and non-normal distribution, two-sample Mann-Whitney analysis was used. P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Wang, C.-Y. et al. FTO modulates fibrogenic responses in obstructive nephropathy. Sci. Rep. 6, 18874; doi: 10.1038/srep18874 (2016).
Acknowledgments
C.Y.W. received support from the National Health Research Institute (NHRI-EX100-9925SC), National Science Council (101-2314-B-182-100-MY3, 101-2314-B-182A-009), and Chang Gung Memorial Hospital (CMRPG3B1643, CMRPG3D1002, CMRPG3D0581, and CMRPG3C1763). M.S.W. received support from National Science Council and Chang Gung Memorial Hospital (103-2314-B-182A-092-MY3, CMRPG3E1851, and CMRPG3E1891). S.S.S. received support from Chang Gung Memorial Hospital (CMRPG3C0722).We appreciate the technical assistance from Mei-Hsiu Lin, Yu-Jung Hu, and Hui-Ting Su.
Footnotes
Author Contributions C.Y.W. conceived, designed, and supervised the project. M.L.T., C.H.Y., K.C.H., C.C.W. and I.C.H. analyzed the data, provided project concepts, and wrote the article. C.Y.W., S.S.S. and M.S.W. performed the experiments and wrote the article.
References
- Loos R. J. & Yeo G. S. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol 10, 51–61, 10.1038/nrendo.2013.227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. et al. FTO genotype is associated with phenotypic variability of body mass index. Nature 490, 267–272, 10.1038/nature11401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church C. et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 42, 1086–1092, 10.1038/ng.713 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. et al. Inactivation of the Fto gene protects from obesity. Nature 458, 894–898, 10.1038/nature07848 (2009). [DOI] [PubMed] [Google Scholar]
- Boissel S. et al. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet 85, 106–111, 10.1016/j.ajhg.2009.06.002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussnitzer M. et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N Engl J Med, 10.1056/NEJMoa1502214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken T. et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318, 1469–1472, 10.1126/science.1151710 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J., Jing W. & Jiang Y. Molecular characterization and expression analysis of fat mass and obesity-associated gene in rabbit. J Genet 92, 481–488 (2013). [DOI] [PubMed] [Google Scholar]
- McTaggart J. S. et al. FTO is expressed in neurones throughout the brain and its expression is unaltered by fasting. PLoS One 6, e27968, 10.1371/journal.pone.0027968 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlen K., Sjolin E. & Hoffstedt J. The common rs9939609 gene variant of the fat mass- and obesity-associated gene FTO is related to fat cell lipolysis. J Lipid Res 49, 607–611, 10.1194/jlr.M700448-JLR200 (2008). [DOI] [PubMed] [Google Scholar]
- Dlouha D., Pitha J., Lanska V. & Hubacek J. A. Association between FTO 1st intron tagging variant and telomere length in middle aged females. 3PMFs study. Clin Chim Acta 413, 1222–1225, 10.1016/j.cca.2012.03.025 (2012). [DOI] [PubMed] [Google Scholar]
- Speakman J. R. FTO effect on energy demand versus food intake. Nature 464, E1; discussion E2, 10.1038/nature08807 (2010). [DOI] [PubMed] [Google Scholar]
- da Cunha P. A. et al. Interaction between obesity-related genes, FTO and MC4R, associated to an increase of breast cancer risk. Mol Biol Rep 40, 6657–6664, 10.1007/s11033-013-2780-3 (2013). [DOI] [PubMed] [Google Scholar]
- Keller L. et al. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. J Alzheimers Dis 23, 461–469, 10.3233/JAD-2010-101068 (2011). [DOI] [PubMed] [Google Scholar]
- Gulati P. et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci USA 110, 2557–2562, 10.1073/pnas.1222796110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess M. E. et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci 16, 1042–1048, 10.1038/nn.3449 (2013). [DOI] [PubMed] [Google Scholar]
- Lin L., Hales C. M., Garber K. & Jin P. Fat mass and obesity-associated (FTO) protein interacts with CaMKII and modulates the activity of CREB signaling pathway. Hum Mol Genet 23, 3299–3306, 10.1093/hmg/ddu043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn D. P. et al. Loss of FTO antagonises Wnt signaling and leads to developmental defects associated with ciliopathies. PLoS One 9, e87662, 10.1371/journal.pone.0087662 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. et al. Involvement of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in brain fat mass- and obesity-associated (FTO) downregulation during energy restriction. Mol Med 17, 523–532, 10.2119/molmed.2010.00134 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Shie S. S., Hsieh I. C., Tsai M. L. & Wen M. S. FTO modulates circadian rhythms and inhibits the CLOCK-BMAL1-induced transcription. Biochem Biophys Res Commun 464, 826–832, 10.1016/j.bbrc.2015.07.046 (2015). [DOI] [PubMed] [Google Scholar]
- Hubacek J. A. et al. The FTO gene polymorphism is associated with end-stage renal disease: two large independent case-control studies in a general population. Nephrol Dial Transplant 27, 1030–1035, 10.1093/ndt/gfr418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoto B. et al. The fat-mass and obesity-associated gene (FTO) predicts mortality in chronic kidney disease of various severity. Nephrol Dial Transplant 27 Suppl 4, iv58–62, 10.1093/ndt/gfs550 (2012). [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O. & Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63, 21–29 (1990). [PubMed] [Google Scholar]
- Yuan Y., Zhang F., Wu J., Shao C. & Gao Y. Urinary candidate biomarker discovery in a rat unilateral ureteral obstruction model. Sci Rep 5, 9314, 10.1038/srep09314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. D. et al. Circadian CLOCK Mediates Activation of Transforming Growth Factor-beta Signaling and Renal Fibrosis through Cyclooxygenase 2. Am J Pathol, 10.1016/j.ajpath.2015.08.003 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Inhibition of Mitochondrial Complex-1 Prevents the Downregulation of NKCC2 and ENaCalpha in Obstructive Kidney Disease. Sci Rep 5, 12480, 10.1038/srep12480 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. et al. Elevated serum 1,25(OH)2-vitamin D3 level attenuates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction in kl/kl mice. Sci Rep 4, 6563, 10.1038/srep06563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns W. C. & Thomas M. C. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev Mol Med 12, e17, 10.1017/S1462399410001481 (2010). [DOI] [PubMed] [Google Scholar]
- Xiong M. et al. The miR-200 family regulates TGF-beta1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol 302, F369–379, 10.1152/ajprenal.00268.2011 (2012). [DOI] [PubMed] [Google Scholar]
- Bottinger E. P. TGF-beta in renal injury and disease. Semin Nephrol 27, 309–320, 10.1016/j.semnephrol.2007.02.009 (2007). [DOI] [PubMed] [Google Scholar]
- Berulava T. et al. FTO levels affect RNA modification and the transcriptome. Eur J Hum Genet 21, 317–323, 10.1038/ejhg.2012.168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Saunders R. A., Szkudlarek-Mikho M., Serna Ide L. & Chin K. V. The obesity-associated Fto gene is a transcriptional coactivator. Biochem Biophys Res Commun 401, 390–395, 10.1016/j.bbrc.2010.09.064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. F. et al. DNA oligonucleotide microarray technology identifies fisp-12 among other potential fibrogenic genes following murine unilateral ureteral obstruction (UUO): modulation during epithelial-mesenchymal transition. Kidney Int 64, 2079–2091, 10.1046/j.1523-1755.2003.00306.x (2003). [DOI] [PubMed] [Google Scholar]
- Ejerblad E. et al. Obesity and risk for chronic renal failure. J Am Soc Nephrol 17, 1695–1702, 10.1681/ASN.2005060638 (2006). [DOI] [PubMed] [Google Scholar]
- Park J. et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 56, 415–425, 10.1016/j.pcad.2013.10.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smemo S. et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507, 371–375, 10.1038/nature13138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedernaes J. & Benedict C. Human obesity: FTO, IRX3, or both ? Mol Metab 3, 505–506, 10.1016/j.molmet.2014.05.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G. et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7, 885–887, 10.1038/nchembio.687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. R. et al. APOE and TGF-beta1 genes are associated with obesity phenotypes. J Med Genet 40, 918–924 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J. et al. Obesity- and aging-induced excess of central transforming growth factor-beta potentiates diabetic development via an RNA stress response. Nat Med 20, 1001–1008, 10.1038/nm.3616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurutani Y. et al. The roles of transforming growth factor-beta and Smad3 signaling in adipocyte differentiation and obesity. Biochem Biophys Res Commun 407, 68–73, 10.1016/j.bbrc.2011.02.106 (2011). [DOI] [PubMed] [Google Scholar]
- Schmierer B. & Hill C. S. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8, 970–982, 10.1038/nrm2297 (2007). [DOI] [PubMed] [Google Scholar]