Abstract
T helper (Th) 17 cells are a subset of Th cells expressing interleukin- (IL-) 17 and initiating an inflammatory response in autoimmune diseases. Graft-versus-host disease (GVHD) is an immune inflammatory disease caused by interactions between the adaptive immunity of donor and recipient. The Th17 lineage exhibits proinflammatory activity and is believed to be a central player in GVHD. IL-1 performs a key function in immune responses and induces development of Th17 cells. Here, we show that blockade of IL-1 signaling suppresses Th17 cell differentiation and alleviates GVHD severity. We hypothesized that the IL-1 receptor antagonist (IL-1Ra) would suppress Th17 cell differentiation in vitro via inhibition of glycolysis-related genes. Blockade of IL-1 using IL-1Ra downregulated Th17 cell differentiation, an alloreactive T cell response, and expression of genes of the glycolysis pathway. Severity of GVHD was reduced in mice with a transplant of IL-Ra-treated cells, in comparison with control mice. To clarify the mechanisms via which IL-1Ra exerts the therapeutic effect, we demonstrated in vivo that IL-1Ra decreased the proportion of Th17 cells, increased the proportion of FoxP3-expressing T regulatory (Treg) cells, and inhibited expression of glycolysis-related genes and suppressed Th17 cell development and B-cell activation. These results suggest that blockade of IL-1 signaling ameliorates GVHD via suppression of excessive T cell-related inflammation.
1. Introduction
Interleukin- (IL-) 1 is a proinflammatory cytokine that drives an inflammatory response through IL-1 receptor signaling. For example, IL-1 is known to play an important role in the pathogenesis of metabolic inflammatory disorders [1]. Moreover, IL-1 triggers a self-amplifying cytokine network. IL-1 induces expression of inflammatory cytokines, and IL-1 signaling enhances differentiation into Th17 cells [2, 3]. Thus, IL-1 receptor antagonist (IL-1Ra) may be useful as an anti-inflammatory agent in inflammatory T cell-mediated diseases. Additionally, IL-1 is involved in the glycolysis pathway; various studies have shown that IL-1 is an important factor for upregulation of glucose uptake and glycolysis [4, 5].
Graft-versus-host disease (GVHD), the leading cause of morbidity and mortality associated with an allogeneic hematopoietic cell transplant, is a complex illness involving dysregulation of inflammatory cytokine cascades and distortion of the donor's cellular response to host alloantigens. Activation of alloreactive donor T cells is initiated by host antigen-presenting cells (APCs) including dendritic cells. Thus, T cells have been suggested as immunocompetent cells that cause GVHD [6], especially because Th17 cells contribute to the development of GVHD [7]. In addition, APCs play a significant role in the pathogenesis of GVHD; evidence shows that inactivation of APCs alleviates GVHD [8–10].
Th17 cells produce IL-17 and can lead to an autoimmune disease by activating an inflammatory response and innate immunity. There is a general consensus that Th17 cells control inflammation status and autoimmune diseases [11, 12]. Th17 cells are also involved in glucose and amino acid metabolism; the latter processes require Th17 cells [13], and hypoxia-induced factor-1α-dependent glycolysis activates differentiation into Th17 cells [14].
Blockade of IL-1 signaling is an effective therapeutic strategy against inflammatory disorders; it has been suggested that the inhibition of IL-1 signaling suppresses inflammation, and IL-1 antagonists are used as therapeutic agents in autoimmune diseases [15–17]. Nevertheless, there is a controversy regarding the therapeutic effects of IL-1 antagonists in GVHD [18, 19]. The aim of the present study was to determine the efficacy and mechanism of action of IL-1Ra treatment in acute GVHD. In this study, we performed in vivo and in vitro experiments to identify the effects and mechanisms of IL-1Ra activity during the development of acute GVHD in a mouse model.
2. Methods
2.1. Animals
Eight- to 10-week-old C57BL/6 (H-2kb, termed B6) and BALB/c (H-2kd) mice were purchased from Orient Bio (Sungnam, Korea). Foxp3-GFP knock-in mice (C57BL/6 strain) were purchased from Jackson Laboratories. The mice were maintained under specific pathogen-free (SPF) conditions at an animal facility with controlled humidity (55 ± 5%), light (12/12 h light/dark), and temperature (22 ± 1°C). The air at the facility was passed through a high-efficiency particulate arrestance (HEPA) filter system designed to exclude bacteria and viruses. The animals were fed standard mouse chow and tap water ad libitum. The protocols used in this study were approved by the Animal Care and Use Committee of the Catholic University of Korea.
2.2. The Bone Marrow Transplant (BMT) Model and Histopathological Analysis
After lethal irradiation (800 cGy), recipient (BALB/c) mice were injected intravenously (i.v.) with total bone marrow cells from donor mice. To induce acute GVHD, we isolated splenocytes from the donor mice, and then the splenocytes (1 × 107) from MHC major and minor antigen-disparate B6 donors were incubated with IL-1Ra (anakinra, 50 ng/mL) or with vehicle (control) for 2 h at 37°C before adoptive transfer into the recipient mice. The clinical severity of GVHD was assessed twice a week using a scoring system consisting of five clinical parameters: weight loss, posture, activity, fur texture, and skin integrity [20]. The mice were euthanized on day 14 after the BMT for blinded histopathological analysis of GVHD-affected organs (skin and small intestine). The organs were harvested, cryoembedded, and sectioned on a cryotome. The tissue slices were fixed in 10% buffered formalin and stained with hematoxylin and eosin (H&E) for histological examination.
2.3. Cell Culture and Experimental Treatment
CD4+T cells were isolated from the spleen using CD4+T cell isolation kits according to the manufacturer's instructions. The purity of the isolated CD4+T cells was >95%. These cells were stimulated with a plate-immobilized anti-CD3 antibody (0.5 μg/mL) and a soluble anti-CD28 antibody (1 μg/mL) for 72 h in 24-well plates. Th17 cell development was induced by treatment with anti-interferon- (IFN-) γ (4 μg/mL) and anti-IL-4 (4 μg/mL) antibodies, TGF-β (2 ng/mL), and IL-6 (20 ng/mL) for 72 h. Aliquots of 105 CD4+T cells (responders) were cultured with 105 irradiated (2500 cGy) APCs in 96-well plates containing 200 μL of the complete medium at 37°C in a humidified atmosphere containing 5% CO2 and were then pulsed with 1 μCi of [3H]TdR for 18 h before harvesting and counted using an automated harvester.
2.4. Flow Cytometry
To analyze intracellular cytokines, we stained splenocytes with PerCP-conjugated anti-CD4, APC-conjugated anti-CD25, FITC-conjugated anti-IL-17, and PE-conjugated anti-Foxp3 antibodies (eBiosciences), followed by fixation and permeabilization using a Foxp3 Staining Buffer Kit (BD Bioscience). Four hours before the staining, the cells were stimulated with phorbol myristate acetate (25 ng/mL) and ionomycin (250 ng/mL) (all from Sigma-Aldrich) and then treated with GolgiStop (BD Bioscience). All data were analyzed in the FlowJo software (Tree Star, Ashland, OR, USA).
2.5. Real-Time Quantitative PCR
The mRNA expression levels were estimated using a LightCycler 2.0 instrument (Roche Diagnostic, Mannheim, Germany) with version 4.0 software. All reactions were performed using the LightCyclerFastStart DNA Master SYBR Green I Kit (Takara, Shiga, Japan). The mRNA expression was normalized to that of β-actin. The primer sequences are shown in Table 1.
Table 1.
PCR primers used in this study.
| Gene | Sense primer (5′→3′) | Antisense primer (3′→5′) | PCR product size (bp) |
|---|---|---|---|
| IL-4 | CGA GTA ATC CAT TTG CAT GAT GC | ACG GAG ATG GAT GTG CCA AAC GTC | 279 |
| IL-17 | CCT CAA AGC TCA GCG TGT CC | GAG CTC ACT TTT GCG CCA AG | 101 |
| Glut1 | CAGTTCGGCTATAACACTGGTG | GCCCCCGACAGAGAAGATG | 156 |
| HK2 | TGATCGCCTGCTTATTCACGG | AACCGCCTAGAAATCTCCAGA | 112 |
| GPI | TCAAGCTGCGCGAACTTTTTG | GGTTCTTGGAGTAGTCCACCAG | 105 |
| Eno1 | TGCGTCCACTGGCATCTAC | CAGAGCAGGCGCAATAGTTTTA | 118 |
| RORgt | TGT CCT GGG CTA CCC TAC TG | GTG CAG GAG TAG GCC ACA TT | 188 |
| IL-21 | CCC TTG TCT GTC TGG TAG TCA TC | ATC ACA GGA AGG GCA TTT AGC | 347 |
| Rnux1 | TAC CTG GGA TCC ATC ACC TC | GAC GGC AGA GTA GGG AAC TG | 164 |
| TPI | CCAGGAAGTTCTTCGTTGGGG | CAAAGTCGATGTAAGCGGTGG | 144 |
| PGK1 | ATGTCG CTTTCCAACAAGCTG | GCTCCATTGTCCAA GCAGAAT | 164 |
| PGAM | TCTGTGCAGAAGAGAGCAATCC | CTGTCA GACCGCCATAGTGT | 118 |
| PKM2 | GCCGCCTGGACATTGACTC | CCATGAGAGAAATTCAGCCGAG | 145 |
| LDHα | CATTGTCAAGTACAGTCCACACT | TTCCAATTACTCGGTTTTTGGGA | 113 |
2.6. The Enzyme-Linked Immunosorbent Assay (ELISA)
The concentrations of IL-17, IFN-γ, TGF-β, and IL-10 were measured using sandwich ELISA (R&D Systems). Serum levels of IgG and IgG1 antibodies were measured using a commercially available ELISA kit (Bethyl Laboratories, Montgomery, TX, USA).
2.7. Statistical Analysis
All data were expressed as mean ± standard deviation (SD). The experimental data are presented as mean ± SD of triplicate cell culture experiments and are representative of the three independent experiments (not simultaneous). Statistical significance was assessed using the Mann-Whitney U test or analysis of variance (ANOVA) with Bonferroni's post hoc test using the GraphPad Prism software (v.5.01). P < 0.05 was assumed to denote statistical significance.
3. Results
3.1. Regulation of Th17 Cell Development and Expression of Genes Related to Glycolysis
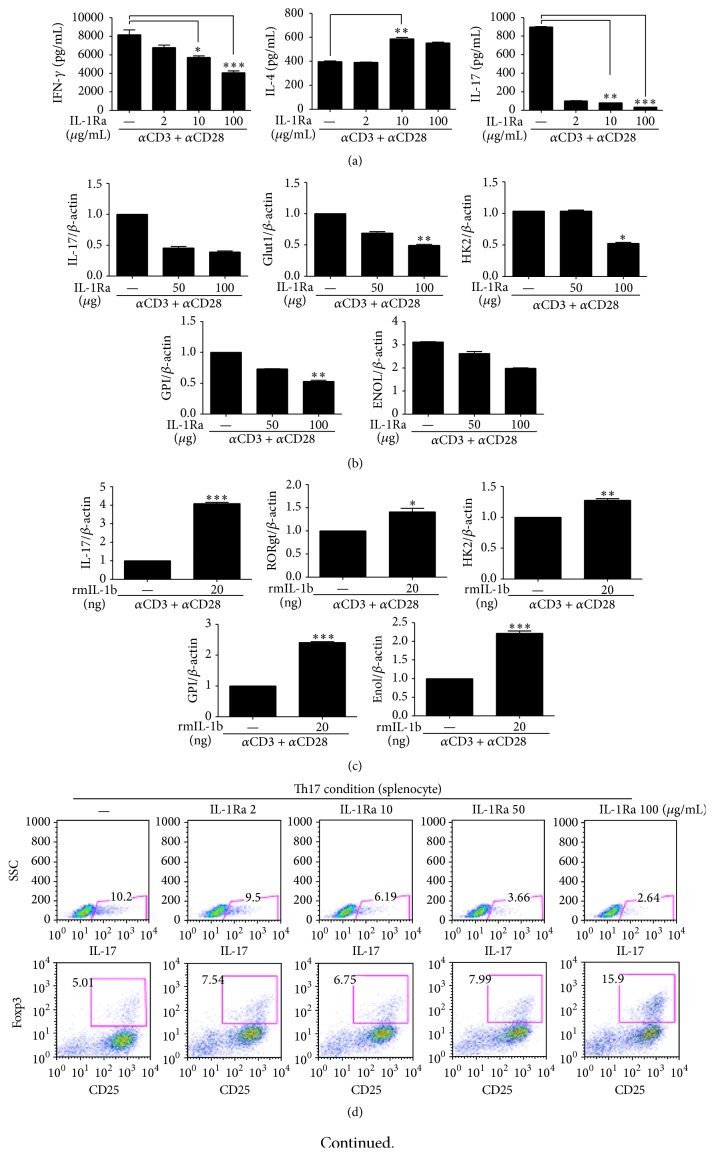
Total splenocytes from normal C57BL/6 mice were cultured with anti-CD28 and anti-CD3 antibodies in the presence or absence of IL-1Ra. This molecule inhibited differentiation into Th17 cells in a dose-dependent manner; IL-17 concentration in the culture supernatant was significantly decreased by the IL-1Ra treatment (Figure 1(a)). IL-1Ra also inhibited secretion of IFN-γ into the culture supernatant in the Th0 condition. On the other hand, IL-4 secretion into the culture medium was enhanced significantly (Figure 1(a)). IL-1Ra inhibited expression of IL-17- and glycolysis-associated genes (Figure 1(b)). On the other hand, IL-1β treatment induced mRNA expression of IL-17, RORγt, and genes involved in glycolysis such as HK2 (Figure 1(c)). To determine whether IL-1Ra inhibits differentiation into Th17 cells in vitro, we cultured the spleen cells under Th17-polarizing conditions in the presence of IL-1Ra. Differentiation into IL-17-expressing CD4+T cells, mainly Th17 cells, was suppressed significantly, whereas differentiation into CD4+CD25+Foxp3+Treg cells was enhanced in a dose-dependent manner by IL-1Ra (Figure 1(d)). In addition, IL-1Ra inhibited expression of the Th17- and glycolysis-associated genes and of IL-17, IL-21, and RORγt genes (Figure 1(e)).
Figure 1.
IL-1β controls the glycolysis pathway and differentiation into T helper (Th) 17 cells. Splenocytes from C57BL/6 mice were activated in the Th0 condition in the presence or absence of either IL-1 receptor antagonist (IL-1Ra) or recombinant IL-1β for 3 days. (a) The concentrations of interferon- (IFN-) γ, IL-4, and IL-17 in culture supernatants were measured by means of enzyme-linked immunosorbent assays (ELISAs). ((b) and (c)) The mRNA levels of IL-17, RORγt, and glycolysis-related factors were quantified using real-time PCR. Splenocytes from C57BL/6 mice were cultured under Th17-polarizing conditions for 3 days in the presence or absence of IL-1Ra. (d) The proportion of CD4+IL-17+ cells or CD4+CD25+Foxp3+ cells was determined using intracellular flow cytometric analysis. (e) The mRNA expression of IL-17, IL-21, Runx1, RORγt, Glut1, HK2, GPI, and MNOL was quantified using real-time PCR. ∗ P < 0.05, ∗∗ P < 0.01, and ∗∗∗ P < 0.001. Data are representative of 2 independent experiments.
3.2. Inhibition of Th17 Cell Development Reduced Expression of Genes Involved in Glycolysis
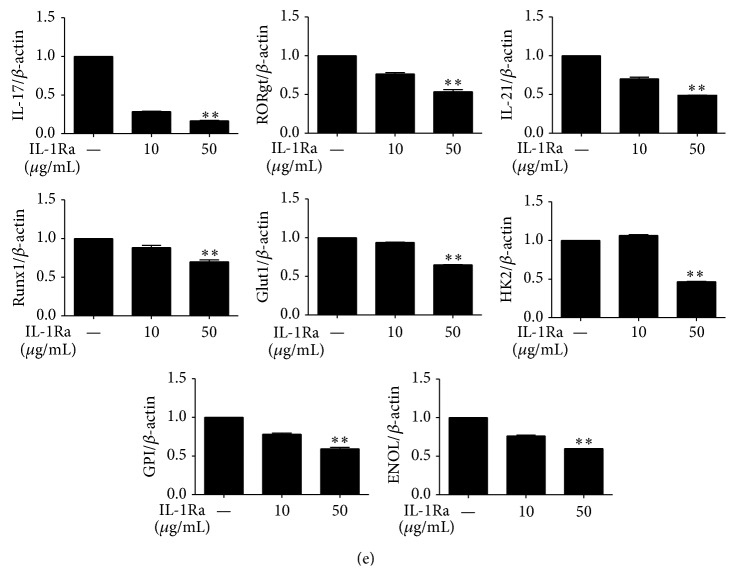
To determine whether blockade of IL-1 signaling in T cells can inhibit differentiation into Th17 cells, we cultured CD4+T cells (that were isolated from the spleen) under Th17-polarizing conditions in the presence of IL-1Ra. IL-1Ra dose-dependently inhibited Th17 cell development and promoted differentiation into Treg cells in the culture of mouse splenocytes under Th17 induction conditions (Figure 2(a)). In addition, IL-1Ra inhibited the production of IL-17 but enhanced TGF-β production (Figure 2(b)). The mRNA levels of IL-17 and glycolysis-associated genes were reduced dose-dependently by the IL-1Ra treatment (Figure 2(c)). Gene expression of glycolysis factors including triosephosphate isomerase (TPI) and lactate dehydrogenase A (LDLα) was also decreased significantly by IL-1Ra treatment (Figure 2(d)). These results suggested that the blockade of IL-1 receptor drove the development of Th17 cells and Treg cells in opposite directions.
Figure 2.
Treatment with IL-1Ra inhibits Th17 cell development via downregulation of the glycolysis pathway. Splenic CD4+T cells from C57BL6 mice were cultured under Th17-polarizing conditions in the presence or absence of IL-1Ra for 3 days. (a) The proportion of Th17 cells or Treg cells was determined using flow cytometric analysis. (b) The supernatants were collected, and an enzyme-linked immunosorbent assay (ELISA) was performed to quantify the production of TGF-β and IL-17. ((c) and (d)) The mRNA levels of IL-17 and glycolysis-related factors and enzymes were measured using real-time PCR. ∗ P < 0.05, ∗∗ P < 0.01, and ∗∗∗ P < 0.001. Data are representative of 2 independent experiments.
3.3. Attenuation of the Alloreactive T Cell Response
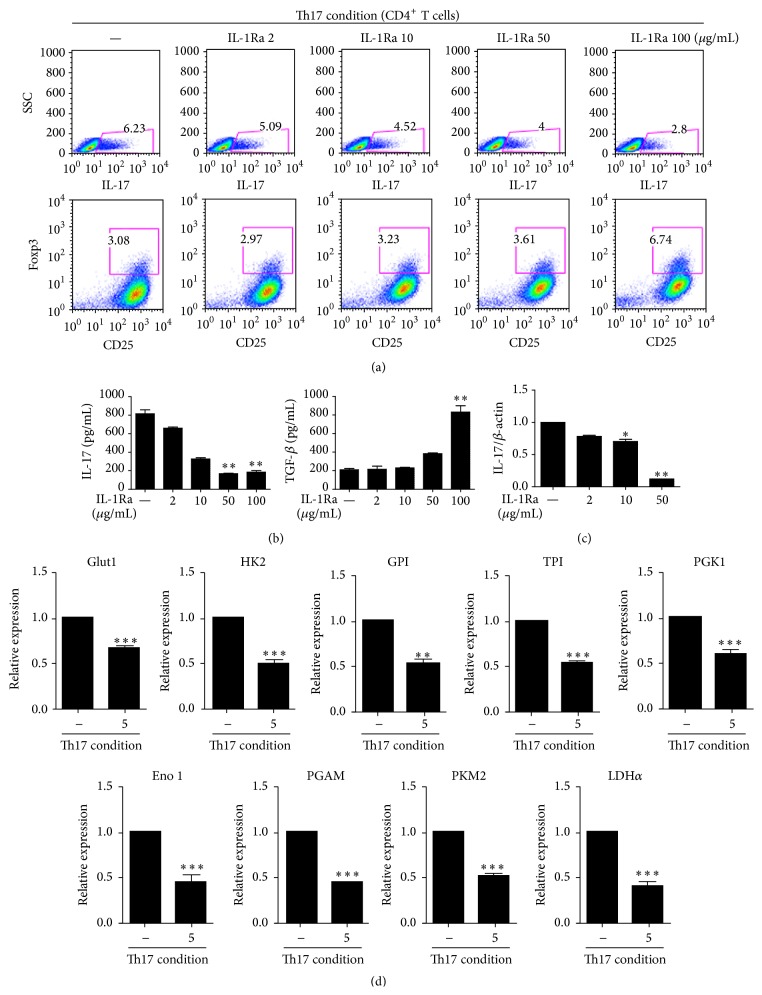
To determine the impact of IL-1 receptor blockade on the proliferative capacity of donor CD4+T cells in response to alloantigens, we measured T cell alloreactivity after treatment with IL-1Ra by means of [3H]thymidine incorporation. After 3 days, CD4+T cells proliferated excessively in response to allogeneic APCs. In contrast, treatment with IL-1Ra resulted in a potent dose-dependent inhibition of the proliferation of the alloreactive T cells (Figure 3(a)). The elevation of IFN-γ and IL-17 concentrations in the culture supernatant was also attenuated by the IL-1Ra treatment in a dose-dependent manner (Figure 3(b)). In addition, the IL-1Ra treatment reduced the population of Th1 cells and Th17 cells in a dose-dependent manner (Figure 3(c)). These data showed that IL-1Ra was an effective regulator of the alloreactive-CD4+T cell response.
Figure 3.
Treatment with IL-1 receptor antagonist (IL-1Ra) reduces the alloreactive T cell response. Antigen-presenting cells (APCs) from C57BL/6 mice (an allogeneic stimulator) were cocultured with T cells from BALB/c mice (responder cells) and subjected to the indicated stimuli for 3 days. (a) The proliferation of alloreactive T cells was quantified by MLR. (b) The concentrations of IL-17 and interferon- (IFN-) γ in the culture supernatants were measured using enzyme-linked immunosorbent assays (ELISAs). (c) Flow cytometric assessment of the numbers of Th1 cells and Th17 cells. ∗ P < 0.05, ∗∗ P < 0.01, and ∗∗∗ P < 0.001. Data are representative of 2 independent experiments.
3.4. Alleviation of GVHD by a Transplant of Donor Cells with Blocked IL-1 Signaling
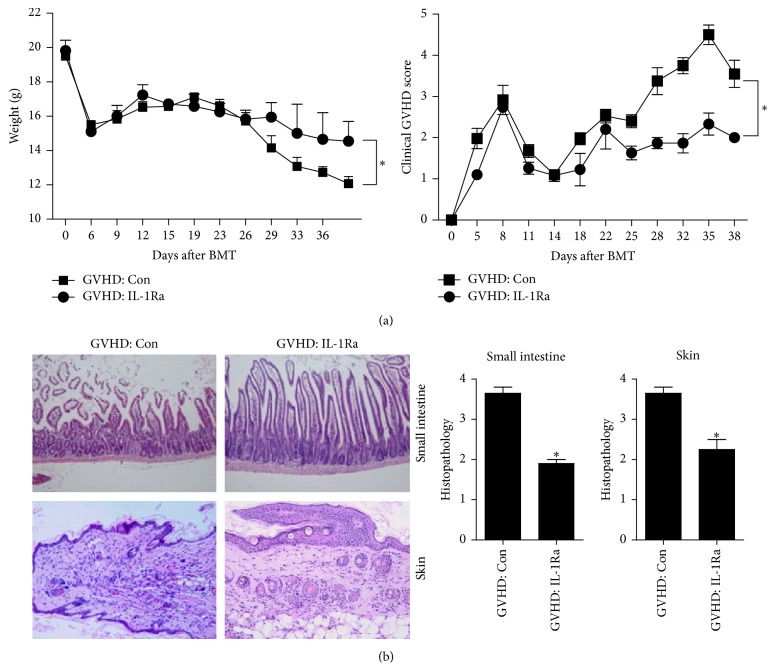
To test whether blockade of IL-1 signaling has therapeutic effects on GVHD, a BMT was performed using splenocyte culture in the presence or absence of IL-1Ra. Animals with acute GVHD who received a transplant of IL-1Ra-treated splenocytes showed a reduction of severity of acute GVHD and attenuation of weight loss, in comparison with the animals in the control GVHD group (Figure 4(a)). In addition, we compared the histopathological features of GVHD-affected organs. In recipient mice that received a transplant of IL-1Ra-treated donor cells, the clinical severity of acute GVHD affecting the skin and small intestine was reduced. These findings were suggestive of decreased lymphocyte infiltration, inflammation, and fibrosis in comparison with control mice (Figure 4(b)).
Figure 4.
Blockade of IL-1 signaling in donor cells inhibits severity of acute graft-versus-host disease (GVHD). (a) Splenocytes (from C57BL/6 mice) that were cultured with or without IL-1 receptor antagonist (IL-1Ra) for 2 h were transplanted into recipient mice (BALB/c), and the clinical features of acute GVHD were then monitored (n = 5 per group). (b) Twelve days after the bone marrow transplant (BMT), histopathological analysis was performed on the skin and small intestine. ∗ P < 0.05. Data are representative of 2 independent experiments.
3.5. Analysis of B Cells and CD4+T Cells in IL-1Ra-Treated Mice with GVHD
To elucidate the in vivo mechanism of action of IL-1Ra in the murine model of acute GVHD, we used fluorescence-activated cell sorting (FACS) to count the Th1, Th2, Th17, and Treg cells in spleens isolated from each group of mice. The percentages of IFN-γ-producing CD4+T cells and IL-17-producing CD4+T cells in spleens were lower in IL-1Ra-treated GVHD animals than in the GVHD control group (Figure 5(a)). In contrast, the numbers of IL-4-producing CD4+T cells and CD4+CD8+Foxp3+Treg cells were significantly higher in the IL-1Ra-treated group. To determine whether there was a change in B cell subpopulations after the IL-1Ra treatment, we analyzed splenocytes from the mice with acute GVHD. Differentiation into mature B cells in GVHD was suppressed significantly in the IL-1Ra group compared to controls. On the other hand, differentiation into immature B cells was increased significantly in the GVHD IL-1Ra group compared to controls (Figure 5(c)). Concentrations of IgG and IgG1 decreased in the serum of IL-1Ra-treated animals compared to the GVHD control group (Figure 5(d)).
Figure 5.
Analysis of B cells and CD4+T cells in mice with acute graft-versus-host disease (GVHD), in whom splenocytes were treated with IL-1 receptor antagonist (IL-1Ra): (a) to elucidate the in vivo mechanism of action of the blockade of IL-1 signaling in amurine model of acute GVHD, we analyzed the proportion of Th1, Th2, and Th17 and (b) shows Treg cells among ex vivo splenocytes from each group by means of flow cytometry. (c) The share of the B-cell subset was analyzed using flow cytometry. B220+ B cells included IgMhighIgDlow (immature B cells) and IgMlowIgDlow (mature B cells). (d) shows The level of IgG in serum. ∗ P < 0.05, ∗∗ P < 0.01. Data are representative of 2 independent experiments.
4. Discussion
Blockade of IL-1 signaling can reduce inflammation, which makes IL-1Ra an important target of research in inflammatory diseases. Nonetheless, the effects of IL-1Ra in GVHD are not clear [18, 19]. However, IL-1Ra is involved in GVHD pathogenesis. Indeed, IL-1Ra expression in saliva of GVHD patients was decreased significantly compared to normal controls [21]. Recently, IL-1 receptor deficiency in dendritic cells and T cells ameliorates acute GVHD enhancing survival [22]. It is also documented that IL-1 blockade could be effective in reducing GVHD development [23]. Our study shows that IL-1Ra inhibits Th17 cell development and the alloreactive T cell response through inhibition of the glycolysis pathway. Additionally, we confirmed alleviation of severity and the immune response in GVHD by a transplant with IL-1Ra-treated splenocytes. This therapeutic effect and the apparent mechanism of the IL-1Ra treatment are the most substantial findings of our study.
Each subset of T cells plays a specific role in adaptive immunity. It is well known that Th1 cells and Th17 cells activate immunity and inflammation, whereas Treg cells inhibit the development of Th1 cells and Th17 cells, thereby limiting redundant inflammatory responses [24]. Moreover, the Th17/Treg ratio plays an important role in GVHD. There is evidence that the Th17/Treg ratio in the peripheral blood of patients with GVHD is significantly higher in comparison with healthy controls, suggesting that the Th17/Treg ratio can be used as a sensitive and specific biomarker of GVHD [25]. According to our data, IL-1Ra treatment reduces differentiation into Th1 and Th17 cells while inducing differentiation into Treg cells. These results indicate that IL-1Ra may have a therapeutic value in GVHD.
The Th17 lineage has been recognized as the activator of proinflammatory responses; in particular, Th17 cells perform a pivotal function in inflammation in many autoimmune diseases including GVHD [26, 27]. IL-4 that is produced by Th2 cells exerts a coordinated anti-inflammatory activity by inhibiting IL-1 expression and by upregulating IL-1Ra [28, 29]. In the present study, Th17 cell development is stimulated by IL-1β treatment. On the other hand, IL-1Ra inhibits differentiation into Th1 cells and Th17 cells while inducing Th2 cell development in our mouse model of GVHD. Thus, IL-Ra may be used to inhibit T cell-related inflammation and to enhance an anti-inflammatory response.
GVHD is characterized by weight loss and selective damage to several organs including the skin and gastrointestinal tract. In GVHD patients, these organs are damaged predominantly [30]. It is also known that weight loss usually occurs in patients with GVHD [31]. Moreover, a skin biopsy is necessary for diagnosis of GVHD after an intestinal transplant [32]. We demonstrate here that IL-Ra treatment suppresses weight loss and decreases tissue damage in a mouse model of GVHD. Therefore, IL-1Ra may be a promising therapeutic agent for GVHD.
Alloreactive T cells take part in the pathogenesis of GVHD. It has been suggested that expansion and development of alloreactive T cells contribute to the development of GVHD [33]. Inhibition of differentiation into alloreactive T cells suppresses preexisting GVHD; for example, an inhibitor of proliferation of alloreactive T cells (inducing apoptosis) slows down the development of GVHD [34]. Our study shows that IL-1Ra inhibits the alloreactive T cell response and the production of IFN-γ and IL-17 in vitro. These results indicate that IL-1Ra may stop the progression of GVHD.
Glycolysis is known to be involved in Th17 cell development. Because suitable energy precursors and synthetic precursors are necessary for activation of T cells, during this process, glucose uptake and glycolysis are enhanced, as are amino acid transport and glutaminolysis [35–40]. Specific metabolic pathways are needed to activate different T cell subsets in order to utilize their unique activities in immunity and an inflammatory response. For example, lipid oxidation enhances Treg cell development and reduces the activity and endurance of Th17 cells [41]. In addition, glucose uptake and expression of genes that are involved in glycolysis (such as Glut1) are induced in Th17 cells compared to Treg cells [41]. It is also known that aerobic glycolysis in response to hypoxia induces differentiation into Th17 cells, thus regulating the Th17/Treg balance [14].
Although IL-1 blockade revealed nontherapeutic effect in GVHD patients [18], IL-1 exacerbated the severity of GVHD in murine model [23]. Additionally, GVHD related mortality was decreased by receptor antagonism or depletion of IL-1β [22]. Thus, clinical trial in GVHD patients will be needed to confirm the therapeutic effect of IL-1 blockade.
In the present study, IL-1β stimulates Th17 cell development by upregulating the glycolysis pathway. On the other hand, IL-1Ra suppresses differentiation into Th17 cells, while upregulating Treg cells through inhibition of the glycolysis pathway. The therapeutic properties of IL-1Ra can be explained by downregulation of Th17 cells via inhibition of glycolysis.
The observations pointing to the anti-inflammatory effects of IL-1Ra open up new possibilities with respect to treatment of GVHD. We believe that IL-1Ra induces Treg cell development and downregulates the Th17 cells, thereby reducing an inflammatory response through inhibition of the glycolysis pathway in Th17 cells. This observational evidence demonstrates that IL-1Ra is a strong candidate for a new therapeutic agent against GVHD.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Grant no. 2013R1A1A2064849).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Min-Jung Park, Seung Hoon Lee, and Sung-Hee Lee contributed equally to this work.
References
- 1.Gabay C., Lamacchia C., Palmer G. IL-1 pathways in inflammation and human diseases. Nature Reviews Rheumatology. 2010;6(4):232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 2.Acosta-Rodriguez E. V., Napolitani G., Lanzavecchia A., Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nature Immunology. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello C. A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Shlomo I., Kol S., Roeder L. M., et al. Interleukin (IL)-1β increases glucose uptake and induces glycolysis in aerobically cultured rat ovarian cells: evidence that IL-1β may mediate the gonadotropin-induced midcycle metabolic shift. Endocrinology. 1997;138(7):2680–2688. doi: 10.1210/en.138.7.2680. [DOI] [PubMed] [Google Scholar]
- 5.Taylor D. J., Faragher E. B., Evanson J. M. Inflammatory cytokines stimulate glucose uptake and glycolysis but reduce glucose oxidation in human dermal fibroblasts in vitro. Circulatory Shock. 1992;37(2):105–110. [PubMed] [Google Scholar]
- 6.Korngold R., Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. Journal of Experimental Medicine. 1978;148(6):1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malard F., Bossard C., Brissot E., et al. Increased Th17/Treg ratio in chronic liver GVHD. Bone Marrow Transplantation. 2014;49(4):539–544. doi: 10.1038/bmt.2013.215. [DOI] [PubMed] [Google Scholar]
- 8.Ruggeri L., Capanni M., Urbani E., et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 9.Shlomchik W. D., Couzens M. S., Tang C. B., et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285(5426):412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 10.Teshima T., Reddy P., Lowler K. P., et al. Flt3 ligand therapy for recipients of allogeneic bone marrow transplants expands host CD8α + dendritic cells and reduces experimental acute graft-versus-host disease. Blood. 2002;99(5):1825–1832. doi: 10.1182/blood.v99.5.1825. [DOI] [PubMed] [Google Scholar]
- 11.Cooke A. Th17 cells in inflammatory conditions. The Review of Diabetic Studies. 2006;3(2):72–75. doi: 10.1900/rds.2006.3.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maddur M. S., Miossec P., Kaveri S. V., Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. The American Journal of Pathology. 2012;181(1):8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 13.Shi L. Z., Wang R., Huang G., et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of Experimental Medicine. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang E. V., Barbi J., Yang H.-Y., et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello C. A., Simon A., van der Meer J. W. M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature Reviews Drug Discovery. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello C. A. Blocking IL-1 in systemic inflammation. The Journal of Experimental Medicine. 2005;201(9):1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldbach-Mansky R. Blocking interleukin-1 in rheumatic diseases: its initial disappointments and recent successes in the treatment of autoinflammatory diseases. Annals of the New York Academy of Sciences. 2009;1182:111–123. doi: 10.1111/j.1749-6632.2009.05159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antin J. H., Weisdorf D., Neuberg D., et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100(10):3479–3482. doi: 10.1182/blood-2002-03-0985. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy P. L., Jr., Abhyankar S., Neben S., et al. Inhibition of interleukin-1 by an interleukin-1 receptor antagonist prevents graft-versus-host disease. Blood. 1991;78(8):1915–1918. [PubMed] [Google Scholar]
- 20.Fukui J., Inaba M., Ueda Y., et al. Prevention of graft-versus-host disease by intra-bone marrow injection of donor T cells. STEM CELLS. 2007;25(6):1595–1601. doi: 10.1634/stemcells.2006-0234. [DOI] [PubMed] [Google Scholar]
- 21.Devic I., Shi M., Schubert M. M., et al. Proteomic analysis of saliva from patients with oral chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2014;20(7):1048–1055. doi: 10.1016/j.bbmt.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankovic D., Ganesan J., Bscheider M., et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. The Journal of Experimental Medicine. 2013;210(10):1899–1910. doi: 10.1084/jem.20130084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Y., Ma S., Zhang Y., et al. IL-1β and TLR4 signaling are involved in the aggravated murine acute graft-versus-host disease caused by delayed bortezomib administration. Journal of Immunology. 2014;192(3):1277–1285. doi: 10.4049/jimmunol.1203428. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J., Yamane H., Paul W. E. Differentiation of effector CD4 T cell populations. Annual Review of Immunology. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratajczak P., Janin A., De Latour R. P., et al. Th17/Treg ratio in human graft-versus-host disease. Blood. 2010;116(7):1165–1171. doi: 10.1182/blood-2009-12-255810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yen D., Cheung J., Scheerens H., et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. The Journal of Clinical Investigation. 2006;116(5):1310–1316. doi: 10.1172/jci21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serody J. S., Hill G. R. The IL-17 differentiation pathway and its role in transplant outcome. Biology of Blood and Marrow Transplantation. 2012;18(supplement 1):S56–S61. doi: 10.1016/j.bbmt.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss L., Haeffner-Cavaillon N., Laude M., Cavaillon J.-M., Kazatchkine M. D. Human T cells and interleukin 4 inhibit the release of interleukin 1 induced by lipopolysaccharide in serum-free cultures of autologous monocytes. European Journal of Immunology. 1989;19(7):1347–1350. doi: 10.1002/eji.1830190731. [DOI] [PubMed] [Google Scholar]
- 29.Vannier E., Miller L. C., Dinarello C. A. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(9):4076–4080. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratanatharathorn V., Nash R. A., Przepiorka D., et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 31.Jacobsohn D. A., Margolis J., Doherty J., Anders V., Vogelsang G. B. Weight loss and malnutrition in patients with chronic graft-versus-host disease. Bone Marrow Transplantation. 2002;29(3):231–236. doi: 10.1038/sj/bmt/1703352. [DOI] [PubMed] [Google Scholar]
- 32.Pintar T., Zorc-Pleskovič R., Alessiani M., Milutinović A., Pleskovič A. Prognostic value of skin histology in GVHD after intestinal transplantation. European Journal of Pediatric Surgery. 2007;17(6):412–415. doi: 10.1055/s-2007-965812. [DOI] [PubMed] [Google Scholar]
- 33.Socié G., Blazar B. R. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatza E., Wahl D. R., Opipari A. W., et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Science Translational Medicine. 2011;3(67) doi: 10.1126/scitranslmed.3001975.67ra8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox C. J., Hammerman P. S., Thompson C. B. Fuel feeds function: energy metabolism and the T-cell response. Nature Reviews Immunology. 2005;5(11):844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 36.Heiden M. G. V., Cantley L. C., Thompson C. B. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frauwirth K. A., Riley J. L., Harris M. H., et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 38.Maciver N. J., Jacobs S. R., Wieman H. L., Wofford J. A., Coloff J. L., Rathmell J. C. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. Journal of Leukocyte Biology. 2008;84(4):949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinclair L. V., Rolf J., Emslie E., Shi Y.-B., Taylor P. M., Cantrell D. A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nature Immunology. 2013;14(5):500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R., Dillon C. P., Shi L. Z., et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michalek R. D., Gerriets V. A., Jacobs S. R., et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of Immunology. 2011;186(6):3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]