Abstract
Context
Changes in reimbursements for clinical laboratory testing may help us assess the effect of various variables, such as testing recommendations, market forces, changes in testing technology, and changes in clinical or laboratory practices, and provide information that can influence health care and public health policy decisions. To date, however, there has been no report, to our knowledge, of longitudinal trends in national laboratory test use.
Objective
To evaluate Medicare Part B–reimbursed volumes of selected laboratory tests per 10 000 enrollees from 2000 through 2010.
Design
Laboratory test reimbursement volumes per 10 000 enrollees in Medicare Part B were obtained from the Centers for Medicare & Medicaid Services (Baltimore, Maryland). The ratio of the most recent (2010) reimbursed test volume per 10 000 Medicare enrollees, divided by the oldest data (usually 2000) during this decade, called the volume ratio, was used to measure trends in test reimbursement. Laboratory tests with a reimbursement claim frequency of at least 10 per 10 000 Medicare enrollees in 2010 were selected, provided there was more than a 50% change in test reimbursement volume during the 2000–2010 decade. We combined the reimbursed test volumes for the few tests that were listed under more than one code in the Current Procedural Terminology (American Medical Association, Chicago, Illinois). A 2-sided Poisson regression, adjusted for potential overdispersion, was used to determine P values for the trend; trends were considered significant at P < .05.
Results
Tests with the greatest decrease in reimbursement volumes were electrolytes, digoxin, carbamazepine, phenytoin, and lithium, with volume ratios ranging from 0.27 to 0.64 (P < .001). Tests with the greatest increase in reimbursement volumes were meprobamate, opiates, methadone, phencyclidine, amphetamines, cocaine, and vitamin D, with volume ratios ranging from 83 to 1510 (P < .001).
Conclusions
Although reimbursement volumes increased for most of the selected tests, other tests exhibited statistically significant downward trends in annual reimbursement volumes. The observed changes in reimbursement volumes may be explained by disease prevalence and severity, patterns of drug use, clinical or laboratory practices, and testing recommendations and guidelines, among others. These data may be useful to policy makers, health systems researchers, laboratory directors, and industry scientists to understand, address, and anticipate trends in laboratory testing in the Medicare population.
Recognizing changes in reimbursement volume for clinical laboratory tests may help us assess the effect of test recommendations, inform new laboratory practice guidelines and recommendations or modify existing ones, and provide information for health care and public health policy decisions. In 1996, the US Centers for Disease Control and Prevention (CDC) evaluated laboratory test use in a random, stratified sample of US clinical laboratories to estimate clinical laboratory test volumes of the most commonly ordered analytes.1 That inventory of laboratory services provided a baseline for tracking changes in the access to laboratory tests and the effect of changes in the health care system for laboratory services, which may lead public regulatory and private accreditation systems toward any needed changes. However, the full value of that study was not realized because it was not repeated after 1996, and hence, the collected data are no longer relevant to current laboratory testing practices. There have been reports of longitudinal testing trends in specific areas of laboratory medicine, such as testing for influenza virus.2 However, there has been no report, to our knowledge, of longitudinal trends in the more common tests in various areas of laboratory medicine.
We present the reimbursement volumes per 10 000 enrollees in Medicare Part B for the most common laboratory tests and test panels from 2000 through 2010. These data, consequently, are derived primarily from testing of outpatients who are 65 years or older. We focused on analytes that had high reimbursement volumes with volumes that changed more than 50% during this decade. Many factors affect reimbursement trends, including evolving knowledge, national availability of testing systems and their ease of use as mediated by changes in laboratory technology, costs of testing, changing prevalence or incidence rates of diseases, changing disease severity, changing rates for screening and diagnostic workup of specific diseases, code use and revisions of the Current Procedural Terminology (CPT; American Medical Association, Chicago, Illinois), and changes in therapies that use existing or new tests, in addition to revised or new clinical and laboratory practice guidelines and recommendations. We examined the published literature for evidence of changes in laboratory practice guidelines and recommendations or other variables that might explain the observed changes in reimbursement volumes. During the study period, many publications provided guidance on the use of specific analytes, which may have affected their use. Many of the selected analytes, some included in one or more test panels, showed significant trends in their reimbursement volumes, and those specifically discussed in this report include fibrinogen3; high-sensitivity C-reactive protein (hs-CRP)4–6; brain natriuretic peptide (BNP)7–9; cardiac troponins7–13; thyroid-stimulating hormone (TSH)14–17; testosterone18,19; human papillomavirus (HPV) DNA20–22; carbohydrate antigen (CA) 19-923–27; vitamin D28–30; hepatitis B virus (HBV) surface antigen31,32; hepatitis C virus (HCV) antibody33–35; Borrelia burgdorferi36; Clostridium difficile37,38; influenza virus2; and drugs of abuse, such as cocaine39; tacrolimus40,41; lithium42–45; antiepileptic drugs46; digoxin47; psychotropic drugs48; carbamazepine49; and phenytoin.50,51
MATERIALS AND METHODS
Reimbursement volumes for laboratory tests per 10 000 enrollees in Medicare Part B (defined as the normalized reimbursement volume and called simply reimbursement volume throughout this article) were obtained from the US Centers for Medicare & Medicaid Services. The ratio of the most recent (2010) reimbursement volume divided by the earliest volumes (usually 2000), called the volume ratio (VR), during this decade, was used as a measure of trends in test reimbursement volume. Laboratory tests were selected based on reimbursement claim frequency (at least 10 in 2010 per 10 000 Medicare enrollees). Another inclusion criterion was that there be a greater than 50% incremental change (increase or decrease) in test reimbursement volume during this decade. A few tests were listed under more than one CPT code; therefore, in those cases, reimbursement could be requested with more than one CPT code, and they were combined in our study. The CPT codes for reimbursement claims were dynamic during the decade, with some new codes added and others eliminated or changed. A 2-sided Poisson regression adjusted for potential overdispersion was used to determine the P value for trends; trends were considered significant at P < .05.
RESULTS
Enrollees in Medicare Part B, obtained from the US Centers for Medicare & Medicaid Services, increased steadily every year, from 37.4 million in 2000 to 44.0 million in 2010. The percentage of annual increase in the number of enrollees in Medicare Part B also increased from 0.9% in 2000–2001 (resulting from an increase from 37.4 million to 37.7 million enrollees) to 2.3% in 2009–2010 (due to the population of Medicare Part B enrollees increasing from 43.0 million to 44.0 million enrollees). All the data have been adjusted for more Medicare Part B enrollees (approximately 18% from 2000 through 2010) by determining reimbursement per 10 000 enrollees. However, reimbursed laboratory tests per 10 000 enrollees also increased by approximately 35% during the decade (from 64 200 in 2000 to 86 700 in 2010).
Laboratory Test Panels
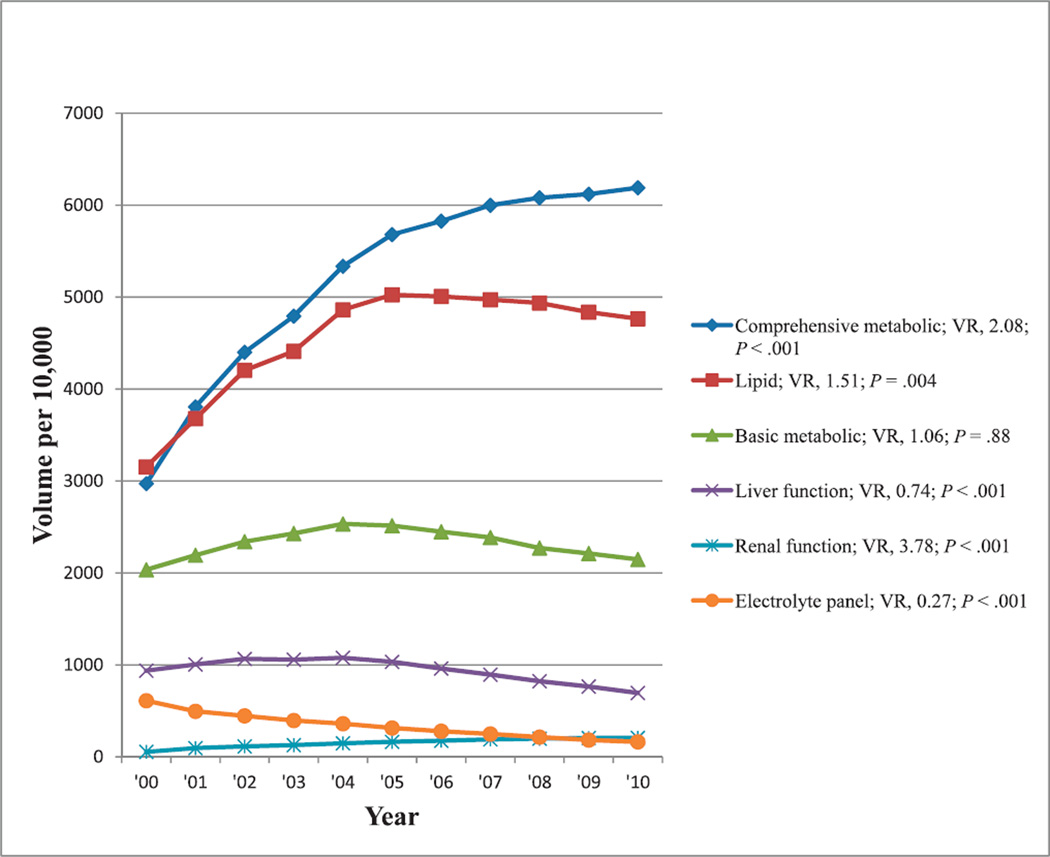
Reimbursement volumes per 10 000 enrollees in Medicare Part B for the most commonly reimbursed laboratory test panels, which also showed at least a 50% change in reimbursement volume during the past decade, are shown in Figure 1. Although there is a general downward trend in the reimbursement volume for liver function and electrolyte test panels, there is a general upward trend in the reimbursement volume for basic metabolic, lipid, comprehensive metabolic, and renal function tests when comparing the numbers of tests at the beginning and end of the decade. The caption to Figure 1 contains the list of laboratory tests each panel comprised. The trend in the reimbursement volumes of these test panels may be considered in view of the test composition of each panel. For example, the comprehensive metabolic panel includes all 8 tests in the basic metabolic panel and all 4 tests in the electrolyte panel, while also containing 6 of the 7 tests in hepatic function and 9 of the 10 tests in renal function panels; and the 4 tests in the electrolyte panel are also included in comprehensive metabolic, basic metabolic, and renal function panels. Except for the basic metabolic panel, all test panels showed significant reimbursement volume trends with time (P ≤ .004).
Figure 1.
Reimbursement volumes for common laboratory test panels. Comprehensive metabolic panel: glucose, calcium, albumin, total protein, sodium, potassium, CO2, chloride, urea nitrogen, creatinine, ALP, ALT, AST, bilirubin. Lipid panel: total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides. Basic metabolic panel: glucose, calcium, sodium, potassium, CO2, chloride, urea nitrogen, bilirubin. Liver function panel: ALT, AST, ALP, bilirubin, direct bilirubin, albumin, total protein. Renal function panel: creatinine, urea nitrogen, albumin, calcium, CO2, chloride, glucose, phosphorus, potassium, sodium. Electrolyte panel: sodium, potassium, CO2, chloride. Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; VR, volume ratio (see “Materials and Methods”).
Common Cancer, Cardiovascular, Coagulation, Diabetes, Hematology, and Renal Tests
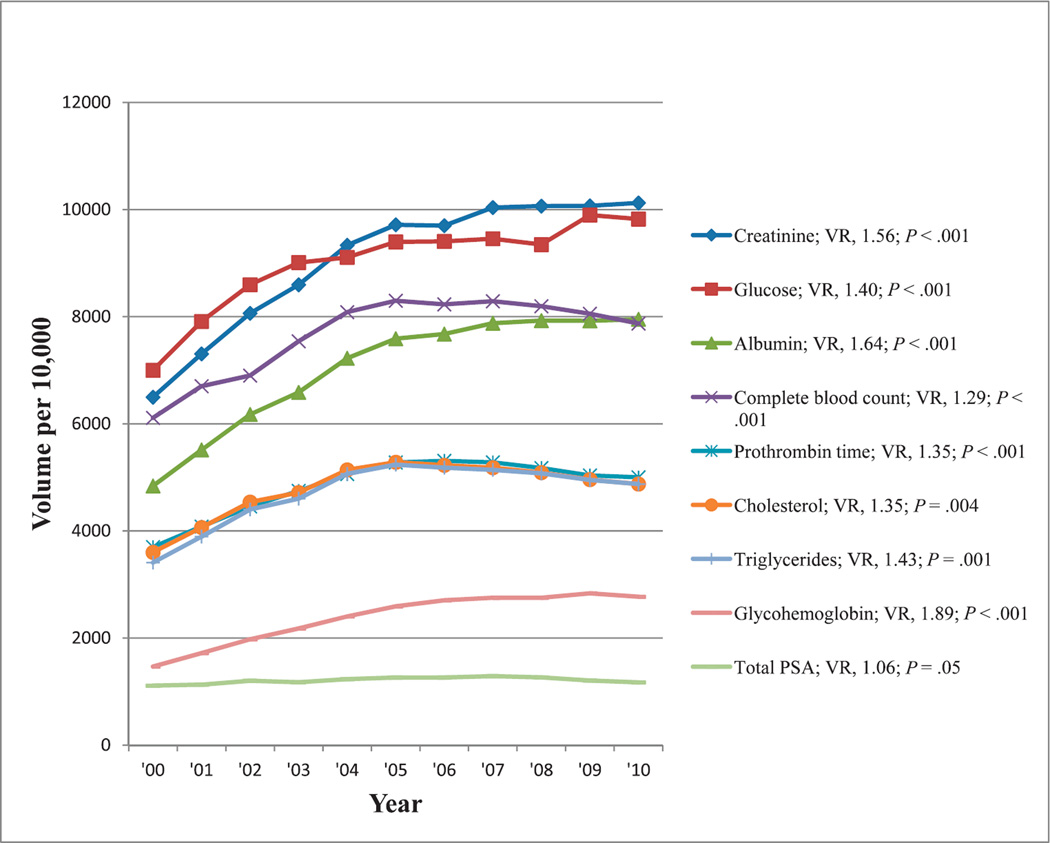
In the general categories of cancer, cardiovascular, coagulation, diabetes, hematology, and renal tests, the most commonly reimbursed tests that were associated with at least a 50% change in reimbursement volume in 2000 through 2010 were tests for total prostate-specific antigen (PSA), total cholesterol, triglycerides, prothrombin time (reported either in seconds or as an international normalized ratio), glucose, glycohemoglobin, complete blood cell count, creatinine, and albumin levels. Reimbursement volumes per 10 000 enrollees for these tests in 2000 through 2010 are shown in Figure 2. All tests except PSA had significantly increasing trends in reimbursement volume (P ≤ .004). The trend for total PSA reimbursement volume during the past decade approached significance (P = .05).
Figure 2.
Reimbursement volumes for the most common cancer, cardiovascular, coagulation, diabetes, hematology and renal tests. Abbreviations: PSA, prostate-specific antigen; VR, volume ratio (see “Materials and Methods”) .
Tests Used for Triage and Risk Assessment of Cardiovascular Diseases
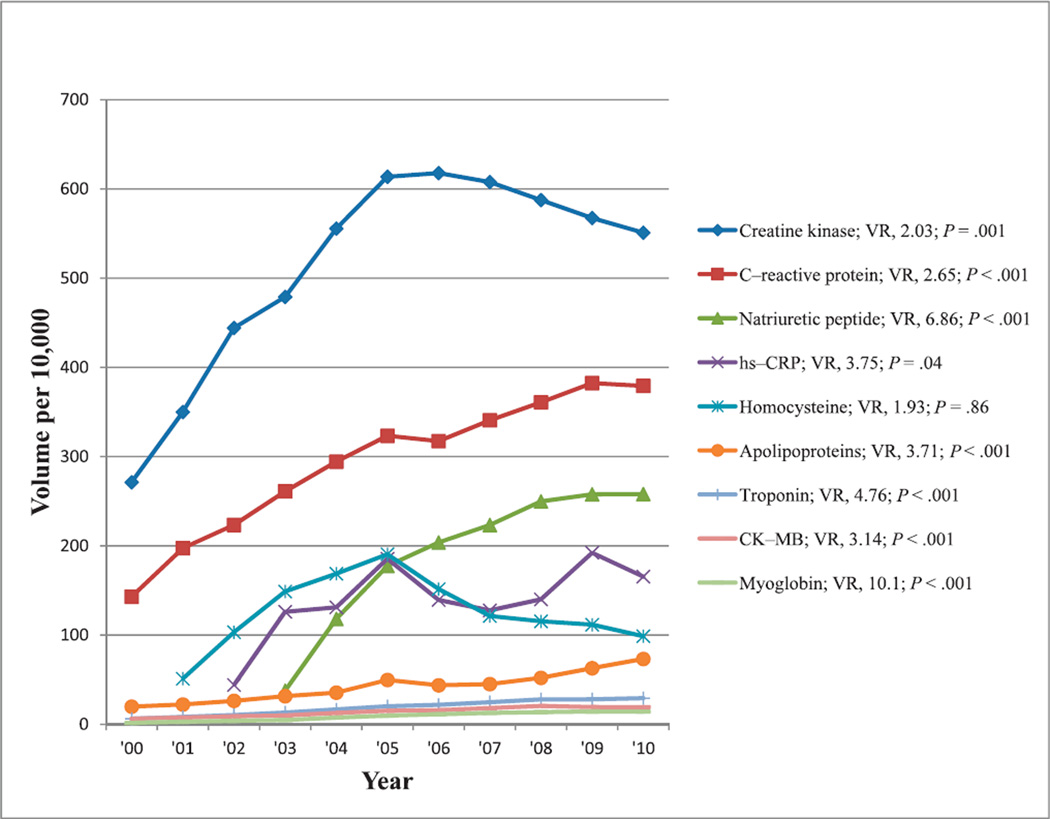
Total cholesterol and triglycerides were the most commonly reimbursed tests for assessing risk of cardiovascular diseases, demonstrating at least a 50% change in reimbursement volume during the past decade. Commonly ordered tests, such as HDL-cholesterol and LDL-cholesterol, showed less than a 50% incremental change during the past decade, and hence, they were not included, even though lipid panel, including both of these tests, met the inclusion criteria. Other commonly reimbursed tests with at least 50% change in reimbursement volume used for triage and evaluation of risk for cardiovascular diseases were creatine kinase, C-reactive protein, BNP, hs-CRP, homocysteine, apolipoproteins, cardiac troponin, creatine kinase isoenzyme MB (CK-MB), and myoglobin tests. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 3. Except for homocysteine, all of these tests had increasing reimbursement volumes and statistically significant trends (P ≤ .04). The increased reimbursement volumes were most pronounced for myoglobin, BNP, cardiac troponin, hs-CRP, and apolipoproteins (VR, 4–10).
Figure 3.
Reimbursement volumes for tests used for triage and risk assessment of cardiovascular diseases. Abbreviations: CK-MB, creatine kinase MB isoenzyme; hs-CRP, high-sensitivity C-reactive protein; VR, volume ratio (see “Materials and Methods”).
Cancer Monitoring or Screening Tests
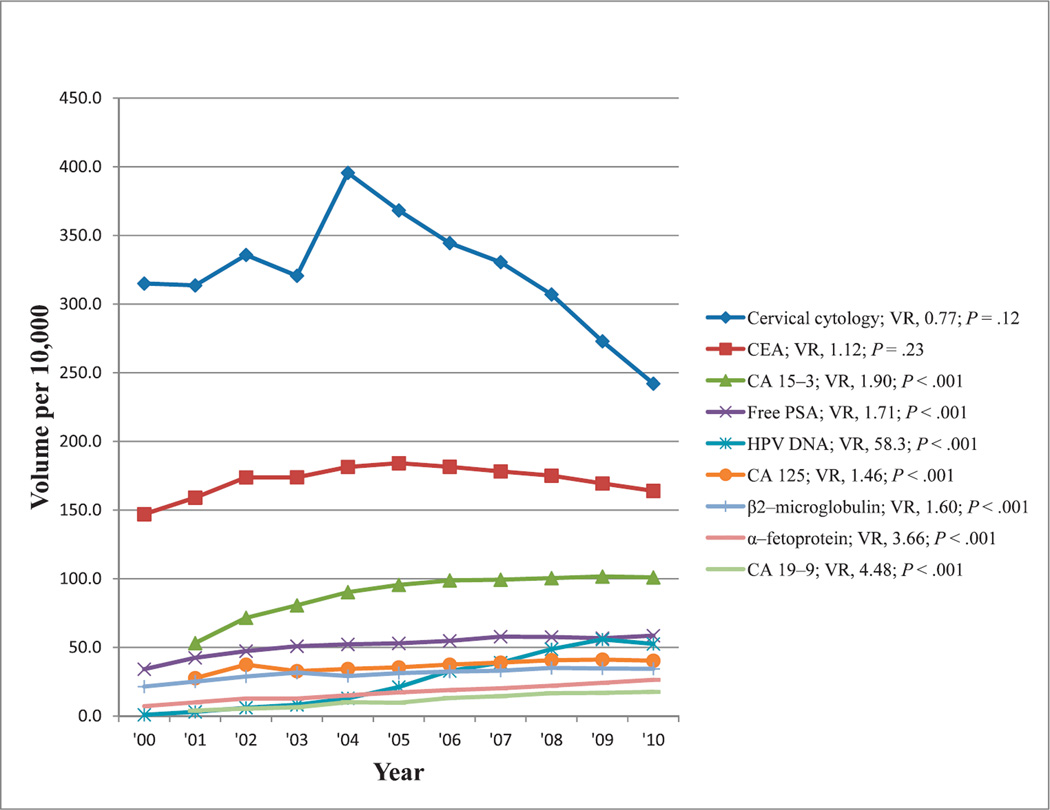
Following total PSA tests (Figure 2), the most commonly reimbursed tests for cancer monitoring or screening showing at least a 50% change in reimbursement volume during the past decade were those for cervicovaginal cytology, carcinoembryonic antigen, CA 15-3, free PSA, HPV DNA, CA 125, β2-microglubulin, α-fetoprotein, and CA 19-9. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 4. All of these tests, except cervical cytology, showed increases in reimbursement volumes over time, and all trends were significant (P < .001). Most notable was an approximately 60-fold increase in reimbursement volumes for HPV DNA testing from 2000 through 2010.
Figure 4.
Reimbursement volumes for tests used for cancer monitoring or screening. Abbreviations: CA, carbohydrate antigen; CEA, carcinoembryonic antigen; HPV, human papillomavirus; PSA, prostate specific antigen; VR, volume ratio (see “Materials and Methods”).
Hematology and Coagulation Tests
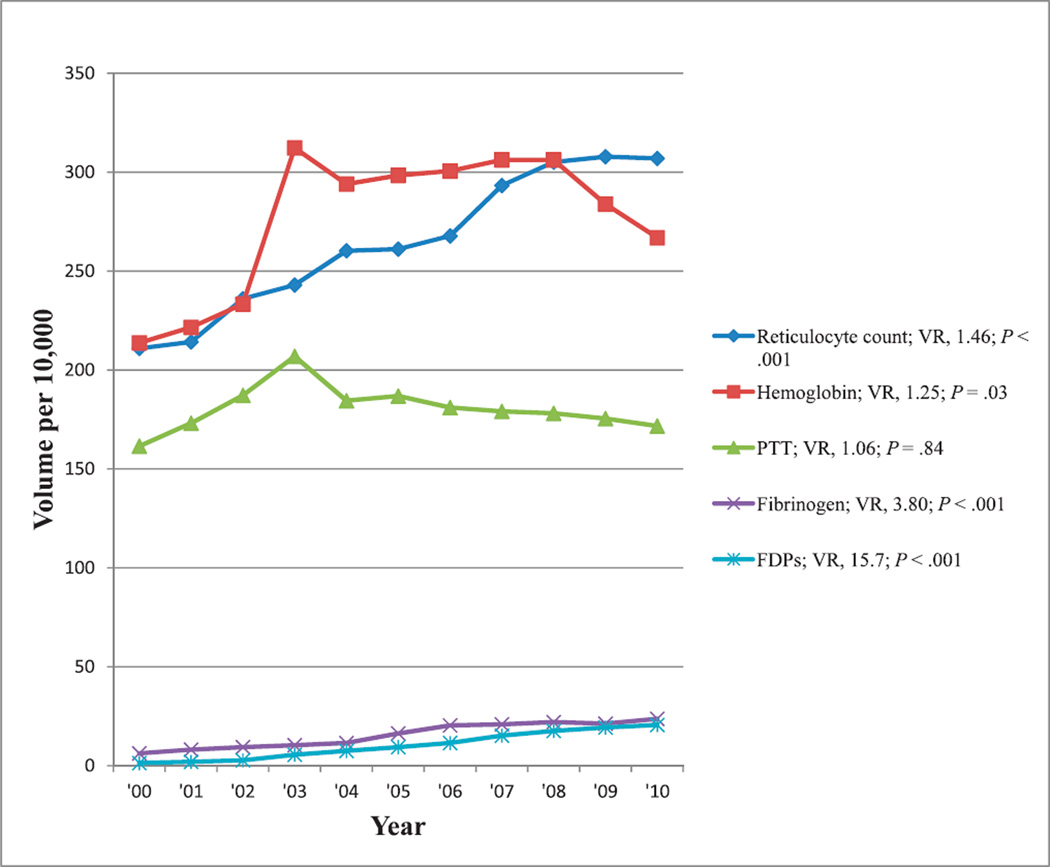
Excluding complete blood cell count and prothrombin time tests (Figure 2), the most reimbursed hematology and coagulation tests that also showed at least a 50% change in reimbursement volume during the past decade were tests for reticulocyte count, hemoglobin, partial thromboplastin time, fibrinogen, and fibrin degradation products. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 5. There were increasing reimbursement volumes for all of these tests (up to 16-fold for fibrin degradation products), and all showed significant trends (P ≤ .03) with the exception of partial thromboplastin time.
Figure 5.
Reimbursement volumes for hematology and coagulation tests. Abbreviations: FDPs, fibrin degradation products; PTT, partial thromboplastin time; VR, volume ratio (see “Materials and Methods”).
Hormone Tests
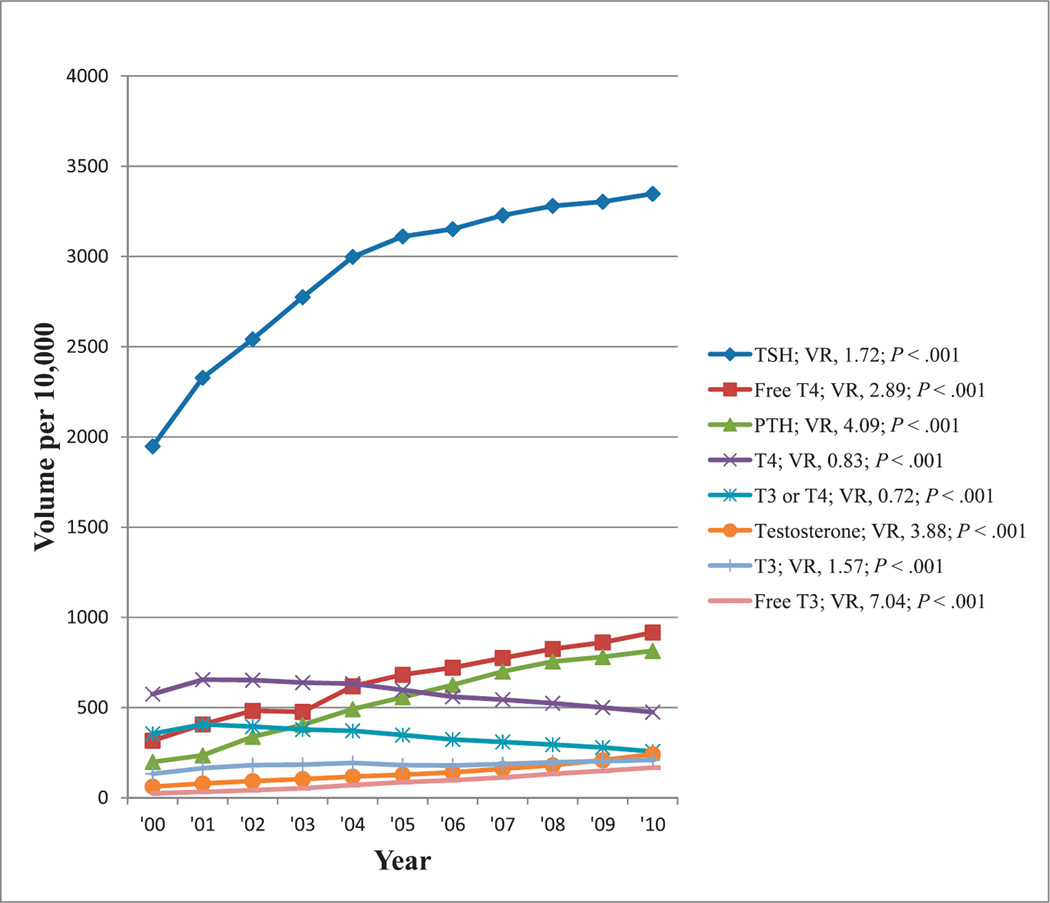
The most commonly reimbursed hormone test showing at least a 50% change in reimbursement volume from 2000 through 2010 was TSH, showing increasing reimbursement volumes during this period, appearing to replace thyroxin alone or in combination with triiodothyronine. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 6. There were increasing reimbursement volumes for other thyroid-related tests with a 7-fold change noted for free triiodothyronine. The most reimbursed hormone tests unrelated to the thyroid gland were tests for parathyroid hormone and testosterone, each showing an approximately 4-fold increase in reimbursement volumes from 2000 through 2010. All trends were significant (P < .001).
Figure 6.
Reimbursement volumes for hormone tests. Abbreviations: PTH, parathyroid hormone; T4, thyroxin, T3, triiodothyronine; TSH, thyroid-stimulating hormone; VR, volume ratio (see “Materials and Methods”).
Hepatobiliary Tests
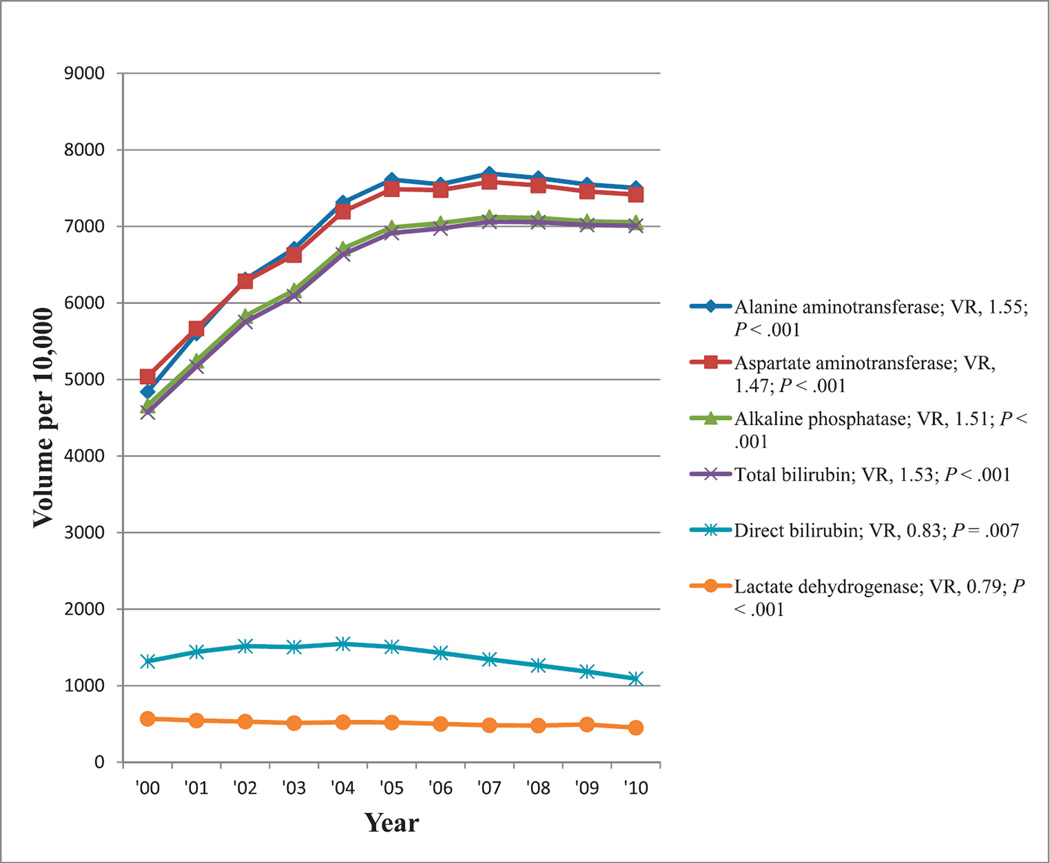
The most commonly reimbursed hepatobiliary tests showing at least a 50% change in reimbursement volumes from 2000 to 2010 were alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, direct bilirubin, and lactate dehydrogenase. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 7. In contrast to decreasing reimbursement volumes for the liver function panel, there were increasing reimbursement volumes for the liver enzyme tests and for total bilirubin. However, tests for direct bilirubin and lactate dehydrogenase both showed decreasing reimbursement trends. All trends were significant (P ≤ .007).
Figure 7.
Reimbursement volumes for hepatobiliary tests. Abbreviation: VR, volume ratio (see “Materials and Methods”).
Immunology and Nutrition Tests
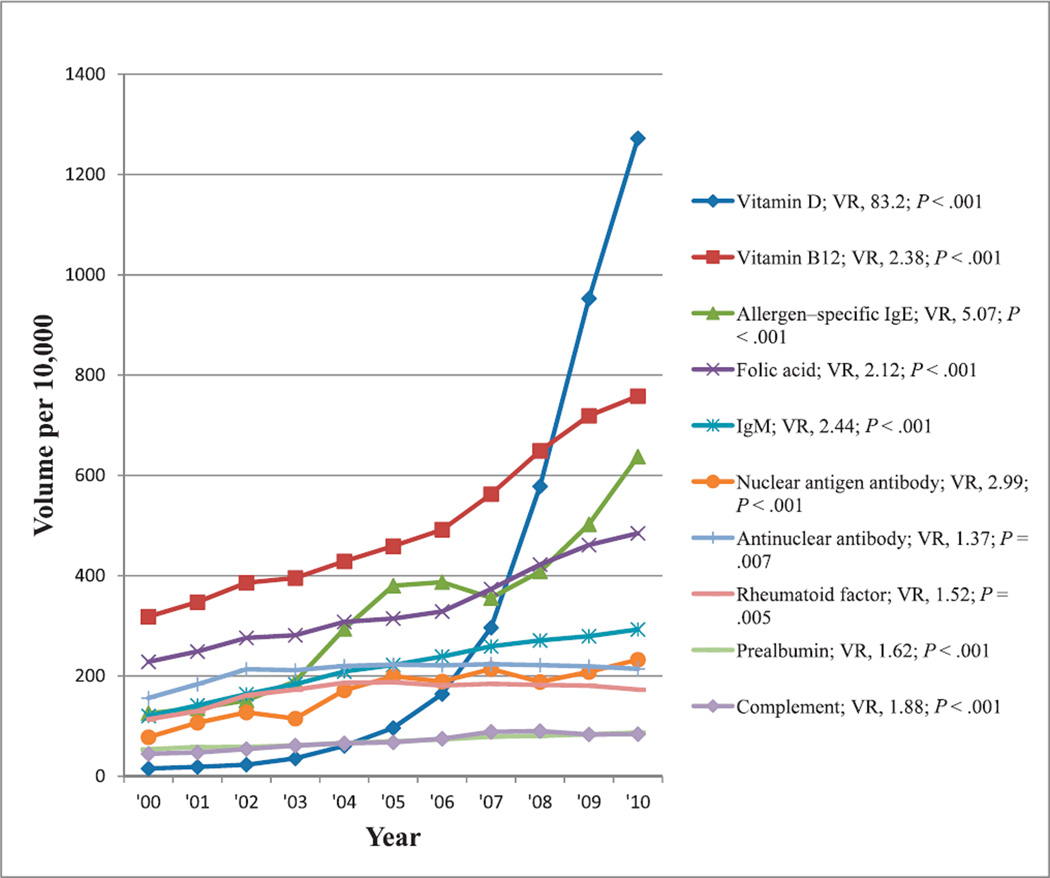
Aside from albumin, the most commonly reimbursed nutrition and immunology tests showing at least a 50% change in reimbursement volumes from 2000 through 2010 were those tests for vitamin D, vitamin B12, allergen-specific immunoglobulin (Ig) E, folic acid, IgM, nuclear antigen antibody (antibodies against various nuclear antigens), antinuclear antibody, rheumatoid factor, prealbumin, and complement. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 8. There was increasing reimbursement for all of these tests, particularly for vitamin D, which showed greater than an 80-fold increase in test reimbursement volumes from 2000 through 2010. All trends were significant (P ≤ .007).
Figure 8.
Reimbursement volumes for immunology and nutrition tests. Abbreviations: IgM, immunoglobulin M; VR, volume ratio (see “Materials and Methods”).
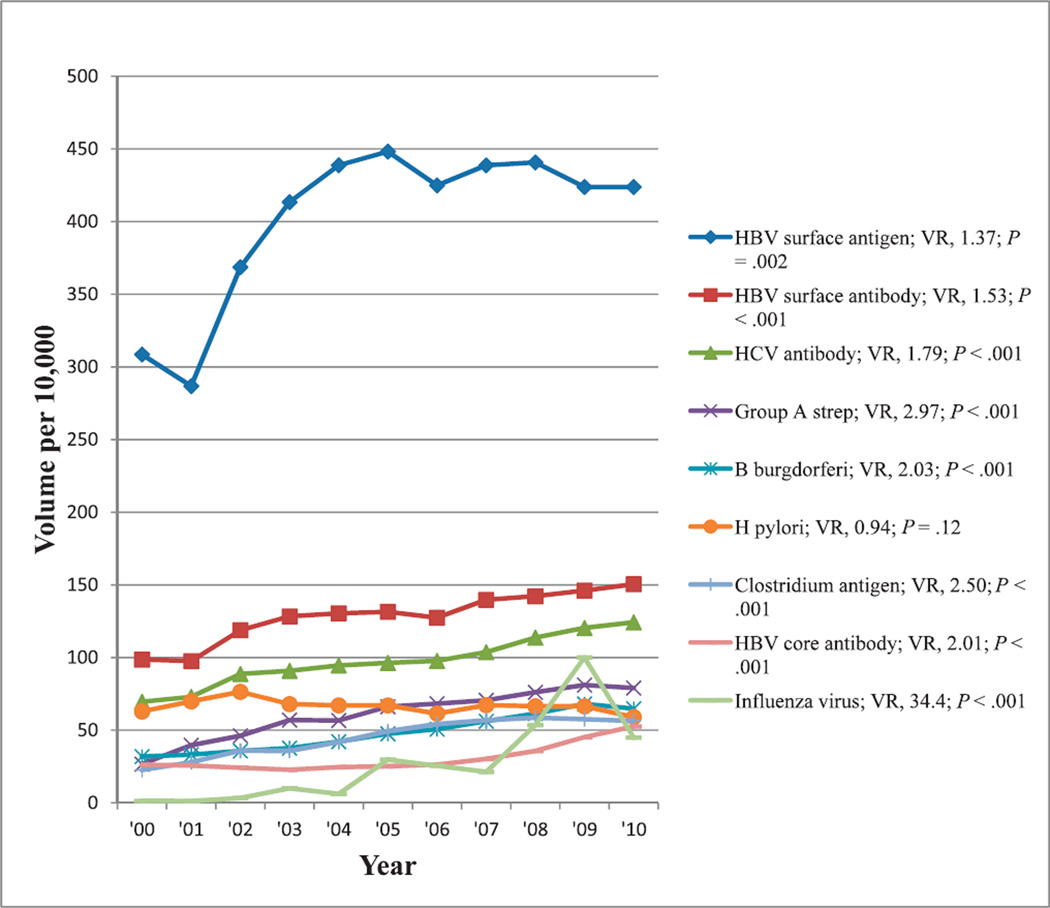
Infectious Disease Tests
The most commonly reimbursed infectious disease tests showing at least a 50% change in reimbursement volume from 2000 through 2010 in decreasing order of reimbursement volumes were those tests for HBV surface antigen, HBV surface antibody, HCV antibody, group A streptococcus antigen, Borrelia burgdorferi antibody, Helicobacter pylori (both antibody and antigen tests), Clostridium difficile antigen, HBV core antibody, and influenza virus antigen and antibody. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 9. Excluding H pylori, there was increasing reimbursement for all tests, particularly for influenza virus, which showed more than a 30-fold increase in test reimbursement volume from 2000 through 2010 with significant trends (P ≤ .002).
Figure 9.
Reimbursement volumes for infectious disease tests. Abbreviations: B burgdorferi, Borrelia burgdorferi; HBV, hepatitis B virus; HCV, hepatitis C virus; H pylori, Helicobacter pylori; strep, streptococcus bacteria; VR, volume ratio (see “Materials and Methods”).
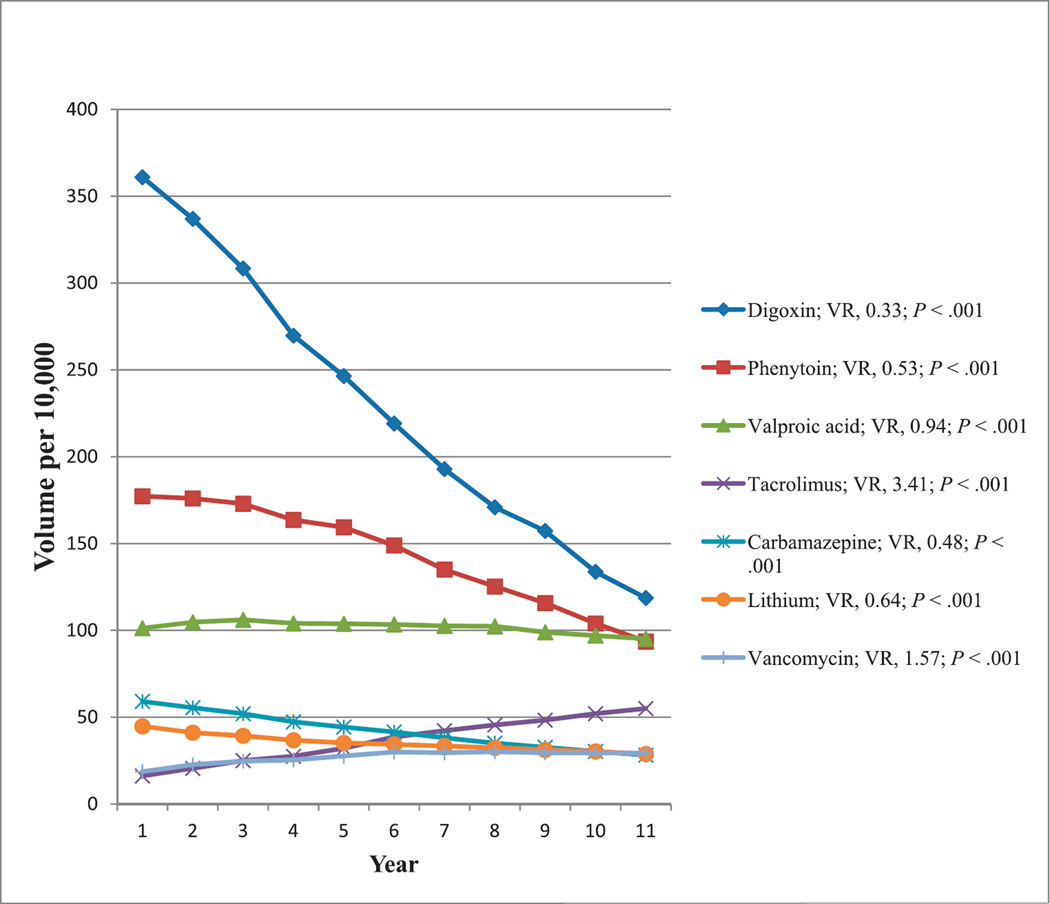
Tests for Monitoring of Therapeutic Drugs Not Generally Abused
Many therapeutic drugs have minimal potential for abuse. Among these, the most commonly reimbursed tests for therapeutic drug monitoring showing at least a 50% change in reimbursement volumes from 2000 through 2010 were those for digoxin, phenytoin, valproic acid, tacrolimus, meprobamate, carbamazepine, lithium, and vancomycin. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 10. Decreasing reimbursement volumes were noted for digoxin, carbamazepine, phenytoin, lithium, and valproic acid. Increasing reimbursement volumes were observed for vancomycin and tacrolimus. All tests showed significant trends (P < .001).
Figure 10.
Reimbursement volumes for tests used in therapeutic drug monitoring. Abbreviations: VR, volume ratio (see “Materials and Methods”).
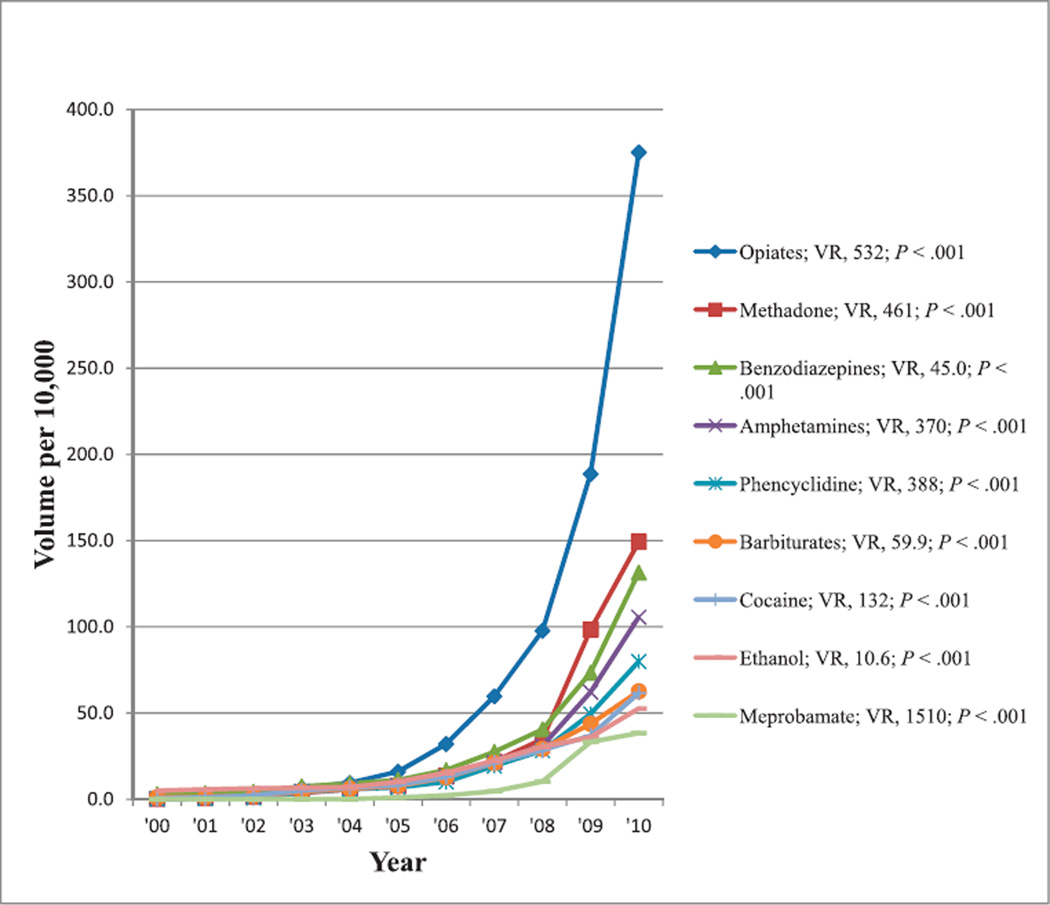
Tests for Monitoring of Drugs Used Therapeutically or Misused
The most commonly reimbursed tests showing at least a 50% change in reimbursement volume from 2000 to 2010 that were used either for monitoring of therapeutic drugs with potential for overuse or abuse or for determining use of illicit drugs, including those for opiates, methadone, benzodiazepine, amphetamine, phencyclidine, barbiturates, cocaine, ethanol, and meprobamate. Reimbursement volumes per 10 000 enrollees for these tests from 2000 through 2010 are shown in Figure 11. For these drugs, the same CPT code could be used for reimbursement, regardless of whether testing was performed to assure that drugs were within therapeutic range or to determine whether they were being misused. There were increasing reimbursement volumes for all of these tests, and all trends were significant (P < .001).
Figure 11.
Reimbursement volumes for tests to monitor drugs used therapeutically, overused, or abused. Some of these are urine-based tests. Abbreviation: VR, volume ratio (see “Materials and Methods”).
Reimbursement Volume Trends
Of the 76 laboratory tests and the 6 test panels that met the inclusion criteria, 11 laboratory tests (14%) and 2 test panels (33%) exhibited decreased reimbursements volumes during the past decade (VR, 0.27–0.94), whereas 65 laboratory tests (86%) and 4 test panels (67%) showed increased reimbursement volumes (VR, 1.06–1510). The 5 laboratory tests and test panel (5 of 13; 38%) with the greatest decrease in reimbursement volumes from 2000 to 2010 were electrolyte panel (VR, 0.27), digoxin (VR, 0.33), carbamazepine (VR, 0.48), phenytoin (VR, 0.53), and lithium (VR, 0.64). Of the 65 laboratory tests with increased reimbursement volumes, the top 14 (22%) were meprobamate (VR, 1510), opiates (VR, 532), methadone (VR, 461), phencyclidine (VR, 388), amphetamines (VR, 370), cocaine (VR, 132), vitamin D (VR, 83), barbiturates (VR, 60), HPV DNA (VR, 58), benzodiazepines (VR, 45), influenza virus (VR, 34), fibrin degradation products (VR, 16), ethanol (VR, 11), and myoglobin (VR, 10).
COMMENT
Many variables may have contributed to the laboratory reimbursement trends we have observed, including evolving knowledge of testing recommendations and guidelines; national availability, the cost of testing systems, and their ease of use; changing prevalence, incidence, or severity of diseases; changing rates of screening and diagnostic workup of specific diseases; changing reimbursement rates, which may result from CPT code revisions and rules for using the codes; changes in treatments modalities; and revision or introduction of practice guidelines and recommendations.
Tests Used for Triage and Risk Assessment of Cardiovascular Diseases
Comments relevant to selected tests with observed changes in reimbursement volumes used for triage and risk assessment in cardiovascular diseases (Figures 3 and 5) are discussed below.
Fibrinogen
Use of the fibrinogen test in coagulation panels tripled during the 6 years from 2000 to 2006 (Figure 5).3 Increasingly, the fibrinogen test is used with other cardiac risk markers to determine overall risk for cardiovascular disease. As an acute-phase reactant, fibrinogen, as well as CRP, provide added information that may lead to more-aggressive treatment to prevent cardiovascular diseases.3 The reimbursement volume for this test increased 3.8-fold from 2000 through 2010.
High-Sensitivity CRP
Myeloperoxidase and hs-CRP predict the risk of coronary heart disease in otherwise healthy individuals, allowing clinicians to initiate early preventive treatment.52 The predictive value of hs-CRP has been shown to be independent of traditional cardiovascular risk factors.53–55 The National Academy of Clinical Biochemistry convened a multidisciplinary, expert panel to develop laboratory medicine practice guidelines for a selected subset of emerging risk factors in the primary prevention of heart disease and stroke that included (apo)lipoproteins, hs-CRP, fibrinogen, homocysteine, and BNP. In 2009, that group concluded that only hs-CRP met all of the stated criteria required for acceptance as a biomarker for risk assessment in primary prevention.6 However, the US Preventive Services Task Force announced that same year that there was insufficient evidence to assess the balance of benefits and harms of hs-CRP screening to prevent cardiac events in asymptomatic individuals with no history of congestive heart disease.5 Data regarding the association of hs-CRP with cardiovascular disease are extensive and consistent; however, absent information on clinical relevance or cost-effectiveness, the role of hs-CRP in clinical practice remains unclear.4,56,57 This risk marker’s reimbursement began in 2002, peaked in 2009, and declined in 2010 (Figure 3; P = .04).
Brain Natriuretic Peptide
Published literature supporting the use of BNP began to appear in 2003,58–60 and that was the first year this test was reimbursed under Medicare Part B. There was a rapid increase in the reimbursement volume from 2003 until 2005, which continued, but more slowly, until 2008, when reimbursement volume began to plateau (Figure 3). When BNP was first introduced, associated data indicated clinical utility comparable to other methods for evaluating patients with heart failure.58–60 However, subsequent reports noted limited evidence supporting the use of the BNP test for diagnosis of cardiac dysfunction or heart failure in the elderly of 75 years and older.7 Current findings justify neither use nor confident rejection of BNP levels to inform the prognosis or diagnosis of heart failure,61 with recommendations for clinicians to cautiously interpret concentrations of BNP to predict the outcome of persons with coronary artery disease.62 Several markers identified in patients with heart failure have direct clinical relevance in aiding diagnosis, risk stratification, monitoring therapy, and treatment to improve clinical outcomes, including both BNP and hs-CRP.63 Preoperative BNP levels have considerable diagnostic value when used in addition to signs and symptoms, especially in patients younger than 75 years who are suspected of having heart failure in primary care.7,64 Elevated BNP levels have been helpful in diagnosing heart failure and in screening for left ventricular systolic dysfunction.65 For diagnosis in primary care, low BNP values may be used to rule out heart failure, but because of poor specificity, high values cannot be used to “rule in” the condition.66 Levels of BNP may help assess heart failure in a patient with dyspnea as well as providing information for making both triage and management decisions.58,60 Brain natriuretic peptide is a consistent, independent predictor of mortality in patient populations with risk of coronary artery disease, diagnosed coronary artery disease, and diagnosed heart failure.67 However, there is insufficient evidence to determine the value of BNP in screening for preclinical ventricular remodeling or dysfunction in the general population.59 There is insufficient evidence to demonstrate that BNP levels change in response to therapies that manage patients with stable chronic heart failure.67
Cardiac Troponin
In 2007, a joint task force of the European Society of Cardiology; American College of Cardiology Foundation; American Heart Association, Inc; and the World Heart Federation proposed a new definition for acute myocardial infarction based on detection of cardiac troponin and associated clinical evidence of myocardial ischemia.10 Small increases in cardiac troponin concentration within reference limits are associated with increased odds of acute coronary syndrome.11 Meta-analysis provided evidence for an association between postoperative cardiac troponin release with midterm and short-term, all-cause mortality after adult cardiac surgery.12 The National Academy of Clinical Biochemistry reported on the use of cardiac troponin and BNP for diagnosing etiologies other than acute coronary syndromes and heart failure,9 as well as on clinical characteristics and the use of biochemical markers in acute coronary syndromes.13 In patients with acute myocardial infarction, as well as in patients suffering from stable and unstable angina, measurement of cardiac troponin alone or combined with other biochemical markers is of practical value for the diagnosis, prognosis, and selection of the most effective therapeutic treatment, as well as for risk assessment. Figure 3 shows that there was an approximately 4-fold increase in the reimbursement volume for cardiac troponin from 2000 through 2010, reflecting the increasing advocacy for this test as the marker of myocardial ischemia.
Tests Used to Evaluate Endocrinologic Diseases and Conditions
Comments relevant to changes in reimbursement volumes for 2 tests used to evaluate endocrinologic diseases and conditions (Figure 6) are discussed below.
Thyroid-Stimulating Hormone
Laboratory tests are the most commonly used aids in the diagnosis and monitoring individuals with thyroid disease.17 Testing for TSH is a first-line diagnostic procedure for assessment of thyroid function and, in some cases, may be the only diagnostic measure indicated.68 With increasing assay sensitivity and specificity, several programs in the United States and worldwide have switched to the use of TSH testing as a primary screening strategy for thyroid diseases,14 even though it is unknown whether that treatment is likely to improve the quality of life in otherwise healthy patients who have abnormal TSH and whose thyroxin levels are within reference range.15,69 There is evidence that mild thyroid dysfunction has adverse consequences and should not merely be regarded as a prognostic indicator; however, evidence is insufficient to support population-based screening by using either TSH or the thyroxin test.70 Nevertheless, it may be appropriate to screen pregnant women, women older than 60 years, and others at higher risk, such as patients receiving psychotropic drugs (lithium, phenothiazines, and tricyclic antidepressants),71 for thyroid dysfunction because subclinical hyperthyroidism is associated with adverse effects on the skeleton and the heart, and it is best assessed by measuring serum TSH.16 However, there is no consensus on whether there is a causal relationship between mild thyroid failure and dyslipidemia.72 From 2000 to 2004, there was an approximately 1.5-fold increase in the reimbursement volume for TSH in the Medicare population, which has since been leveling off (VR, 1.72; see Figure 6), reflecting increasing use of the TSH test in the evaluation of, and screening for, thyroid diseases as promoted in some publications in the past decade and cited earlier.
Testosterone
Total testosterone should be measured in all men with erectile dysfunction in accordance with contemporary guidelines and particularly in those who have a chronic illness associated with low testosterone.18 Testosterone, the predominant androgen in men, when deficient, leads to a multiplicity of symptoms and signs that are corrected with physiologic substitution. When testosterone replacement is initiated, close monitoring for efficacy and safety is advised.19 From 2000 through 2010, there was an approximately 4-fold increase in the reimbursement volume for testosterone testing, reflecting the increasing use of testosterone replacement therapy and monitoring promoted in media and in published literature (see Figure 6).18,19
Tests Used for Cancer Monitoring or Screening
We discuss below the changes observed in the reimbursement volume for 2 tests used to screen for or monitor malignant diseases (Figure 4).
Human Papillomavirus DNA
Testing for HPV DNA in cervical specimens increased in the past decade as a useful option for triaging women with equivocal diagnoses from Papanicolaou tests for follow-up colposcopy.73 Testing for HPV DNA improves diagnostic accuracy by limiting unnecessary colposcopy in patients with borderline or mildly abnormal cytologic test results.74 A strategy of combined cytology and HPV screening every 3 years for women with dually negative results is more effective than cytology alone when colposcopies quantify the disease burden.20 However, a sensitivity analysis suggested that a strategy of HPV testing followed by cytology (for women with HPV+ test results) warranted further study.20 Current evidence has shown superiority of HPV testing over cervical cytology as a more-sensitive screening method and for follow-up of women treated for cervical intraepithelial neoplasia.75 Detection of HPV DNA has become an established tool for diagnosis and monitoring of HPV-related disease,76 given that persistent infection with HPV has been recognized as a significant risk factor for most precancerous lesions and cancers of the cervix.77 Although testing for HPV DNA in cervical specimens is more sensitive than the Papanicolaou test in detecting high-grade cervical lesions, HPV testing is less specific than cervical cytology,78 and it should be reserved for the more labor-efficient task of triaging patients with HPV+ test results because most HPV+ tests contain relevant abnormalities that can be evaluated by cervical cytology with sufficient accuracy.79 The 58-fold increase in the reimbursement of HPV DNA testing from 2000 through 2010 (Figure 4) seems to reflect the increasing advocacy for the use of HPV DNA testing for cervical cancer screening.
Carbohydrate Antigen 19-9
In the 2006 recommendations by the American Society for Clinical Oncology on the use of tumor markers in gastrointestinal cancer, measuring CA 19-9 was advocated every 1 to 3 months for patients with locally advanced or metastatic pancreatic cancer receiving active therapy, noting that elevations in serial CA 19-9 levels suggested progressive disease.26 The CA 19-9 levels provide critical information regarding survival that can help guide treatment of individuals diagnosed with pancreatic adenocarcinoma,80 and in patients with resected adenocarcinoma of the pancreas, high preoperative CA 19-9 levels are associated with adverse pathologic features and poorer survival,23 whereas low preoperative CA 19-9 levels are positively related to survival after resection of the pancreas.81 In advanced pancreatic cancer, pretreatment CA 19-9 levels have a prognostic effect regarding overall survival, and CA 19-9 decline under chemotherapy can provide prognostic information for median survival.82 Although several promising candidates have been identified as markers of pancreatic cancer, none has yet been convincingly proven to be better than CA 19-9,83 and it remains the most useful serologic marker for the diagnosis and follow-up of pancreatic cancer.84 The 1.4% increase in the incidence of pancreatic cancer between 2000 and 200985 may explain a small part of the increasing reimbursement rate for CA 19-9. Reported incidence rates refer to the entire US population, however, whereas the population in this study was composed of individuals who are 65 years and older. Testing for CA 19-9 was first reimbursed in 2001, when it was deemed to be of value in monitoring patients with pancreatic cancer. This test was increasingly used in the Medicare population between 2001 and 2010 (VR, 4.5; see Figure 4), reaching a plateau in 2008. The increasing popularity of this test in monitoring for pancreatic cancer and the increase in the incidence rate of this cancer85 may explain part of the increasing trend in this test’s reimbursement.
Nutritional Assessment Test
We discuss below the dramatic increase in the reimbursement volume for vitamin D (Figure 4).
Vitamin D
Testing for Vitamin D deficiency and supplementation has been increasingly advocated in the past decade. Vitamin D deficiency is increasingly implicated in many diseases, and its deficiency is common in all age groups because of lack of sun exposure and the few foods containing sufficient amounts of this vitamin.29,86 Older adults pose a particular challenge, not only because vitamin D deficiency results in abnormal metabolism of calcium and causes diseases such as osteoporosis, osteomalacia, and osteopenia, which become more prevalent with aging but also because there are often complications with other comorbidities.29 Accumulating evidence from experimental, clinical, and epidemiologic studies suggests that vitamin D may be associated with several indices of vascular function, including the development and progression of atherosclerotic cardiovascular disease, and several recent epidemiologic studies has implicated low vitamin D status in the pathogenesis of cardiovascular disease.30 The Endocrine Society Task Force recommended supplementation at tolerable upper-limit levels depending on age and clinical circumstances, and further recommended measurement of serum 25-hydroxyvitamin D levels as the initial diagnostic test in patients at risk for deficiency.28 However, evidence-based consensus guidelines are not available for use of laboratory tests for vitamin D status for medical management and screening of individuals, and observational studies of correlations between 25-hydroxyvitamin D and clinical outcomes are subject to confounding results and do not prove causation.86 Nevertheless, from 2000 through 2010, there was an 83-fold increase in the reimbursement volume for vitamin D, part of which may be explained by the numerous publications advocating increasing use and measurement of this vitamin, as well as by the increasing coverage in the media triggered by those publications (Figure 8).28–30,86
Infectious Disease Tests
Comments relevant to selected tests with observed changes in reimbursement volumes used in the evaluation of infectious diseases (Figure 9) are discussed below.
Hepatitis B Virus
Early identification of chronic HBV infection enables treatment interventions to prevent or delay onset of liver disease by identifying and vaccinating susceptible individuals (household contacts and sex partners of HBV-infected persons, pregnant women, persons born in countries with hepatitis B surface antigen prevalence .8%, and persons who are the source of blood or body fluid exposures that might warrant postexposure prophylaxis) and, hence, interrupting ongoing transmission.87 In 2008, the CDC published new recommendations for routine testing of several additional populations: those with hepatitis B surface antigen prevalence of at least 2%, those born in geographic regions with hepatitis B surface antigen prevalence of at least 2%, men who have sex with men, and injection-drug users.31 Serologic and nucleic acid testing are critical to disease prevention and treatment objectives,32 and information from such testing helps to determine patients’ infectivity and immune status, appropriate monitoring strategies, efficacy of treatment, and provision of data that may contribute to a better understanding of the natural history and epidemiology of this infection. These screening recommendations31,87 and disease prevention strategies32 may have contributed to the increase in the reimbursement volume for hepatitis B surface antigen by approximately 1.4-fold between 2000 and 2010 (Figure 9). However, this increase in reimbursement volume occurred in 2000 through 2005 in response to earlier screening87 and disease prevention32 recommendations, and the effect of the CDC’s latest recommendation in expanding the screening population31 seems not to have been realized yet.
Hepatitis C Virus
The HCV infection is a complex public health problem, characterized by a high prevalence of chronic infection, an increasing burden of HCV-associated disease, low rates of testing and treatment, and the prospect of increasing incidence associated with the epidemic of injection drug use.88 Testing for HCV antibody has been recommended for at-risk populations, including confirmatory quantification by polymerase chain reaction, if positive.89 Although antiviral treatment may successfully eradicate HCV, the available data in 2004 on long-term outcomes in populations identified by screening were lacking.35 Although targeted screening, particularly among intravenous drug users, may identify substantially higher incidence than found in the general population, data have been inadequate to accurately weigh the overall benefits and harms of screening in otherwise healthy, asymptomatic adults. Recommendations to screen for HCV infection in populations overlapping those of Medicare Part B enrollees33,35,88,89 may explain, at least in part, the increase in reimbursement volume for HCV antibody test of approximately 1.8-fold from 2000 through 2010 (Figure 9).
Lyme Disease
Serologic testing for Lyme disease should be used judiciously because it may produce misdiagnosis when performed on patients with a low prior probability of disease or nonspecific symptoms, such as fatigue or arthralgia, without objective signs of infection.36 The purpose of laboratory testing for Borrelia burgdorferi infection is to confirm a clinician’s judgment of possible Lyme disease. Detection of antibodies to B. burgdorferi is a practical and common approach for evaluating a patient with suspected Lyme disease; however, serologic testing is recommended only when there is at least 1 in 5 chance (20%), in the clinician’s estimation, that the patient has active Lyme disease.36 Comparing 2 recent 5-year intervals, the incidence of reported Lyme disease in the United States increased 34% from approximately 81 000 (1997–2001) to approximately 108 000 (2002–2006).90 Increasing incidence of Lyme disease during the past decade,90 coupled with greater utility of serologic testing when this disease becomes more prevalent,36 may explain the increasing reimbursement volume for Lyme disease testing by approximately 2-fold from 2000 through 2010 (Figure 9).
Clostridium difficile
Laboratory diagnosis of Clostridium difficile infections has increased because of the numbers of people and severity of this infection.37 During the past 20 years, the prevalence of health care–associated C. difficile disease has increased. Repeat testing, although of limited utility, is common in medical practice.38 In 5% of cases during outbreak settings, testing for C. difficile is repeated at least once. Repeat C. difficile testing for hospitalized patients has low clinical utility but may be considered in outbreak settings or when the pretest probability of disease is high.38 Increasing numbers of people with, and the severity of, this infection,37 coupled with the common practice of repeat testing,38 may have contributed to the increasing reimbursement volume for C. difficile antigen testing by approximately 2.5-fold from 2000 through 2010 (Figure 9).
Influenza Virus
Diagnostic tests for influenza virus have become increasingly important because some emerging strains pose pandemic potential. Influenza testing is critical for directing global influenza prevention and control activities2 and is increasing, as evidenced by reimbursement volume increasing by approximately 5-fold from the 2000– 2001 to the 2009–2010 season.91 There has been an even more dramatic increase in reimbursement volume in the Medicare population by approximately 34-fold from 2000 through 2010, and that increase appears to relate to recent pandemics (see the spike in 2009 in Figure 9).
Drug Testing
Comments relevant to observed changes in the reimbursement volumes for the tests used in detecting and monitoring of selected drugs are discussed below (see Figures 10 and 11).
Tacrolimus
Tacrolimus is superior to cyclosporine in improving survival from transplantation grafts and in preventing acute rejection after kidney transplantation; however, its use increases posttransplant diabetes with demonstrated neurologic and gastrointestinal side effects.92 Although reimbursement volume for tacrolimus increased by approximately 3.4-fold from 2000 through 2010, the reimbursement volume for cyclosporine decreased by 0.46-fold (data not shown). In 2000, the ratio of reimbursement volume for cyclosporine over tacrolimus was approximately 1.5. That ratio decreased consistently during the past decade until it reached 0.19 in 2010. Treating 100 recipients with tacrolimus instead of cyclosporine would cause 12 patients to avoid acute rejection and 2 to avoid losing their grafts, but cause an extra 5 patients to become insulin-dependent diabetics.40,92 After renal transplantation, immunosuppression with tacrolimus significantly reduced acute rejection compared with cyclosporine.41 Follow-up studies of high methodologic quality are needed to determine whether tacrolimus indeed improves long-term renal graft survival. With the increasing popularity of tacrolimus as an immunosuppressant for patients having transplants, there has been an increase in the reimbursement volume for tacrolimus test by approximately 3.4-fold from 2000 through 2010 (Figure 10).
Lithium
Lithium is a first-line drug for treating patients with major depression who do not respond adequately to standard antidepressants.42 Lithium reduces suicidal acts and completed suicide; however, there are significant side-effect burdens, narrow therapeutic indices, and prolonged treatment requirements.43 As an antidepressive, lithium is not recommended as monotherapy because newer antidepressants and anticonvulsants are better tolerated and more effective.44 Lithium use for bipolar disorder has decreased in the United States,45 and that may be a contributing factor to the decrease in the reimbursement volume for lithium monitoring in the Medicare population by approximately 0.6-fold from 2000 through 2010 (Figure 10).
Digoxin
The use and dosage of digoxin have declined, with an accompanied decline in hospitalizations for digoxin toxicity in the United States, calling into question whether digoxin is increasingly underused, given that guidelines continue to recommend this drug for both heart failure and atrial fibrillation.47 The number of prescriptions written for at least 250 µg of digoxin has decreased, and the public health burden of digoxin toxicity has declined dramatically from 1991 to 2004, with its use in atrial fibrillation decreasing over time.47 In the United States, digoxin use declined from 76% (191 of 252) in patients with atrial fibrillation in 1980–1981 to 37% (105 of 285) in such patients in 1999–2000, reflecting reports that digoxin is less effective than beta-blockers or calcium channel blockers in controlling tachycardia, despite no concomitant increase in the use of other agents used to control cardiac arrhythmia.93 In agreement with earlier observations that seem to reflect an increasing concern about digoxin toxicity and its use, and given availability of other agents for control of heart rate, there has been a decrease in the reimbursement volume for digoxin monitoring in the Medicare population by approximately 0.3-fold from 2000 through 2010 (Figure 10).
Carbamazepine
Antiepileptic drug use has changed gradually from 1993 to 2006.49 More individuals are prescribed lamotrigine than carbamazepine, and that mirrors a corresponding decrease in carbamazepine monitoring. There has been a decrease in the reimbursement volume for total carbamazepine monitoring in the Medicare population by approximately 0.5-fold from 2000 through 2010 (Figure 10), which reflects decreasing prescription rate for this drug.
Phenytoin
For those older than 65 years, there was a small reduction in the use of phenytoin (71% (1300 of 1840) to 66% (1160 of 1760) from 1998 to 2004 as an antiepileptic drug. Despite a growing list of clinical recommendations and guidelines for newer antiepileptic drugs, phenytoin was the most commonly used drug in this category, and there was little change in its use for elderly patients during those 6 years.50 However, in another study,51 phenytoin accounted for 40% (111 420 of 282 080) of treated person-years in 1993 declining to 18% (51 620 of 282 080) by 2008. There has been a decrease in the reimbursement volume for total phenytoin monitoring in the Medicare population by approximately 0.5-fold from 2000 to 2010 (Figure 10), reflecting increasing use of other newer antiepileptic drugs.
Other Drugs
Department of Health and Human Services’ guidelines for the workplace require testing for drugs of abuse, including amphetamines, cannabinoids, cocaine, opiates, and phencyclidine.94,95 Testing may determine whether a therapeutic level has been maintained, to detect potential abuse in a patient being treated with the drug, or to detect abuse in an individual not using a drug for legitimate treatment. However, the same CPT code in each case is used regardless of the indications for testing. These drugs, shown in Figure 11, include analgesics, tranquilizers, and depressants (opiates, methadone, benzodiazepines, barbiturates, phencyclidine, ethanol, and meprobamate) as well as stimulants (amphetamine and cocaine). From 2000 through 2010, reimbursement volume for these tests increased from approximately 11-fold for ethanol to approximately 1500-fold for meprobamate. The change in CPT coding that occurred just before 2000 allowed for reimbursement of testing for single drugs in either therapeutic drug monitoring or chemistry sections of the clinical laboratory, which may have contributed to a large increase in the volume of drug testing, further aggravated by the increase in drug abusers as well as patients taking various pain medications.94
Demands for drug testing to document adherence to, or avoidance of, prescribed drugs with both therapeutic and abuse potential has increased as reflected by the US Food and Drug Administration’s risk evaluation and mitigation strategies proposed for opioids, as well as clinical practice guidelines that have been published by entities such as the American Society of Interventional Pain Physicians96 and the State of Washington,97 and provided information98 and initiatives99 by the CDC. Clinicians are more interested in testing for pain relievers, reflected by more pain management laboratories profiting from rapid, automated, lessexpensive, and noninvasive test methods.100 Urine drug testing has greatly increased during recent years101 because urine is a specimen of choice for method of collection, method of analysis, and results interpretation. Adherence monitoring, including controlled substance agreements and various periodic measures of compliance, has been associated with a 49% reduction in opioid abuse.102 Drug screening tests are typically competitive immunoassays with antibodies directed against drug groups or classes. Therefore, individual drugs, such as morphine, codeine, or heroin, cannot be identified or quantitated, requiring a more-specific test, such as gas chromatography combined with mass spectrometry, to confirm the presence and quantity of each specific analyte. Urine drug testing of patients taking pain medications, however, can be an effective means to augment pharmacotherapy and to assist with complex medical and legal aspects of the current health care environment.103 The demand for clinical testing of drugs in urine for pain management has increased dramatically as clinicians, regulatory agencies, and payers seek objective measures to regulate compliance and support clinical diagnoses.
The growth of specialty laboratories and the marketing efforts targeting physician office practices, may partly explain the increased reimbursement trend data seen in Figure 11 because the pain management testing industry has evolved into a multimillion dollar business with laboratories specializing in this type of testing and because more clinicians are looking for guidance, feeling they are constantly faced with the difficult decision to prescribe medications, particularly to their older patients who experience pain more often for controlling their pain and improving their daily activities, whereas, at the same time, realizing the high abuse and addiction rates associated with the use of these medications.101 There has been an increasing trend in emergency department visits that has involved nonmedical use of various pharmaceuticals between 2004 and 2009.104 In 2009, 48% (approximately 516 000 of 1 079 700) of such visits were due to the use of pain relievers; and emergency department visits due to pain reliever use increased by 114% from 241 600 in 2004 to 516 000 in 2009. This included increases of 141% in visits (from approximately 172 700 to 416 500) due to use of opiates, 137% (from approximately 144 600 to 342 600) due to narcotic pain relievers (including 71% increase in emergency department visits (from approximately 36 800 to 63 000) due to use of methadone), and 118% (from approximately 143 500 to 312 900) due to benzodiazepines (see Table 19 of the 2011 report by the Substance Abuse and Mental Health Services Administration).104 Emergency department visits due to nonmedical use of stimulants increased by 122% (from approximately 9800 to 21 740) for the same period, including 276% increase (from 2303 to 8656) in visits due to use of amphetamines.
Concluding Remarks
The relative reimbursement volumes of tests in this study generally agree with the relative volumes estimated for the most commonly ordered tests in the 1996 CDC survey of US laboratories conducted 17 years ago.1 Our study presents the paid reimbursement volume per 10 000 enrollees in Medicare Part B for tests or panels of tests, as opposed to requests for reimbursement, but only for enrollees in Medicare Part B. These results cannot be equated to actual laboratory test volumes performed. In addition, Part B reimbursements cover only outpatient, not inpatient, testing. Nevertheless, to our knowledge, this is the only published report of longitudinal trends in test reimbursement volume for a broad group of laboratory tests that relate mostly to the US population age 65 years or older. Trends shown here can be expected to reflect the overall laboratory use rates in that population. The presented data cannot be generalized to the population younger than 65 years, and even for the population cohort age 65 years and older, the numbers for each year include only reimbursement under Medicare Part B. Despite these limitations, the data presented in this report may be useful to policy makers, health systems researchers, laboratory managers, and industry scientists in addressing and anticipating trends in the use of laboratory tests in the evaluation of the health of the Medicare-eligible US population.
Reimbursement volumes for all laboratory tests or panels can be obtained by going to a US Centers for Medicare & Medicaid Services Web site,105 which also provides volume data for all nonlaboratory tests or procedures.
Acknowledgments
We thank Catherine Hammett-Stabler, PhD, of the University of North Carolina (Chapel Hill); Paul Jannetto, PhD, of Mayo Clinic (Rochester, Minnesota); Gwen McMillin, PhD, of ARUP Laboratories (Salt Lake City, Utah); and Steven Steindel, PhD (Atlanta, Georgia) for their comments after reviewing this manuscript. We also thank the staff at the US Centers for Medicare & Medicaid Services for providing us the laboratory reimbursement data for Medicare Part B enrollees from 2000 through 2010.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
Presented in part as a poster at the annual meeting of the American Association for Clinical Chemistry; July 27, 2011; Atlanta, Georgia.
References
- 1.Steindel SJ, Rauch WJ, Simon MK, Handsfield J. National Inventory of Clinical Laboratory Testing Services (NICLTS): development and test distribution for 1996. Arch Pathol Lab Med. 2000;124(8):1201–1208. doi: 10.5858/2000-124-1201-NIOCLT. [DOI] [PubMed] [Google Scholar]
- 2.Jernigan DB, Lindstrom SL, Johnson JR, et al. Detecting 2009 pandemic influenza A (H1N1) virus infection: availability of diagnostic testing led to rapid pandemic response. Clin Infect Dis. 2011;52(suppl 1):S36–S43. doi: 10.1093/cid/ciq020. [DOI] [PubMed] [Google Scholar]
- 3.Amukele TK, Baird GS, Chandler WL. Reducing the use of coagulation test panels. Blood Coagul Fibrinolysis. 2011;22(8):688–695. doi: 10.1097/MBC.0b013e32834b8246. [DOI] [PubMed] [Google Scholar]
- 4.Schnell-Inderst P, Schwarzer R, Göhler A, et al. Prognostic value, clinical effectiveness, and cost-effectiveness of high-sensitivity C-reactive protein as a marker for major cardiac events in asymptomatic individuals: a health technology assessment report. Int J Technol Assess Health Care. 2010;26(1):30–39. doi: 10.1017/S0266462309990870. [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(7):474–482. doi: 10.7326/0003-4819-151-7-200910060-00008. [DOI] [PubMed] [Google Scholar]
- 6.Myers GL, Christenson RH, Cushman M, et al. NACB LMPG Committee Members. National Academy of Clinical Biochemistry Laboratory Medicine Practice guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem. 2009;55(2):378–384. doi: 10.1373/clinchem.2008.115899. [DOI] [PubMed] [Google Scholar]
- 7.Vaes B, de Ruijter W, Gussekloo J, Degryse J. The accuracy of plasma natriuretic peptide levels for diagnosis of cardiac dysfunction and chronic heart failure in community-dwelling elderly: a systematic review. Age Ageing. 2009;38(6):655–662. doi: 10.1093/ageing/afp157. [DOI] [PubMed] [Google Scholar]
- 8.Doust JA, Glasziou PP, Pietrzak E, Dobson AJ. A systematic review of the diagnostic accuracy of natriuretic peptides for heart failure. Arch Intern Med. 2004;164(18):1978–1984. doi: 10.1001/archinte.164.18.1978. [DOI] [PubMed] [Google Scholar]
- 9.Wu AH, Jaffe AS, Apple FS, et al. NACB Writing Group, NACB Committee. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53(12):2086–2096. doi: 10.1373/clinchem.2007.095679. [DOI] [PubMed] [Google Scholar]
- 10.Patil H, Vaidya O, Bogart D. A review of causes and systemic approach to cardiac troponin elevation. Clin Cardiol. 2011;34(12):723–728. doi: 10.1002/clc.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMullin N, Lindsell CJ, Lei L, et al. EMCREG i*trACS Investigators. Outcomes associated with small changes in normal-range cardiac markers. Am J Emerg Med. 2011;29(2):162–167. doi: 10.1016/j.ajem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Lurati Buse GA, Koller MT, Grapow M, Bolliger D, Seeberger M, Filipovic M. The prognostic value of troponin release after adult cardiac surgery—a meta-analysis. Eur J Cardiothorac Surg. 2010;37(2):399–406. doi: 10.1016/j.ejcts.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 13.Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 14.LaFranchi SH. Newborn screening strategies for congenital hypothyroidism: an update. J Inherit Metab Dis. 2010;33(suppl 2):S225–S233. doi: 10.1007/s10545-010-9062-1. [DOI] [PubMed] [Google Scholar]
- 15.Helfand M US Preventative Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(2):128–141. doi: 10.7326/0003-4819-140-2-200401200-00015. [DOI] [PubMed] [Google Scholar]
- 16.Ross DS. Serum thyroid-stimulating hormone measurement for assessment of thyroid function and disease. Endocrinol Metab Clin North Am. 2001;30(2):245–264. doi: 10.1016/s0889-8529(05)70186-9. vii. [DOI] [PubMed] [Google Scholar]
- 17.Dufour DR. Laboratory tests of thyroid function: uses and limitations. Endocrinol Metab Clin North Am. 2007;36(3):579–594. doi: 10.1016/j.ecl.2007.04.003. v. [DOI] [PubMed] [Google Scholar]
- 18.Jackson G, Boon N, Eardley I, et al. Erectile dysfunction and coronary artery disease prediction: evidence-based guidance and consensus. Int J Clin Pract. 2010;64(7):848–857. doi: 10.1111/j.1742-1241.2010.02410.x. [DOI] [PubMed] [Google Scholar]
- 19.Theodoraki A, Bouloux PM. Testosterone therapy in men. Menopause Int. 2009;15(2):87–92. doi: 10.1258/mi.2009.009025. [DOI] [PubMed] [Google Scholar]
- 20.Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. [Accessed January 8, 2014];Screening for cervical cancer: a decision analysis for the U.S. Preventive Services Task Force. 2011 http://www.ncbi.nlm.nih.gov/books/nbk92546/pdf/toc.pdf. [PubMed]
- 21.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103(5):368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agnantis NJ, Sotiriadis A, Paraskevaidis E. The current status of HPV DNA testing. Eur J Gynaecol Oncol. 2003;24(5):351–356. [PubMed] [Google Scholar]
- 23.Hallemeier CL, Botros M, Corsini MM, Haddock MG, Gunderson LL, Miller RC. Preoperative CA 19-9 level is an important prognostic factor in patients with pancreatic adenocarcinoma treated with surgical resection and adjuvant concurrent chemoradiotherapy. Am J Clin Oncol. 2011;34(6):567–572. doi: 10.1097/COC.0b013e3181f946fc. [DOI] [PubMed] [Google Scholar]
- 24.Sturgeon CM, Duffy MJ, Hofmann BR, et al. National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory medicine practice guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem. 2010;56(6):e1–e48. doi: 10.1373/clinchem.2009.133124. [DOI] [PubMed] [Google Scholar]
- 25.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Locker GY, Hamilton S, Harris J, et al. ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24(33):5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 27.Michl P, Pauls S, Gress TM. Evidence-based diagnosis and staging of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20(2):227–251. doi: 10.1016/j.bpg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 29.Chu MP, Alagiakrishnan K, Sadowski C. The cure of ageing: vitamin D— magic or myth? Postgrad Med J. 2010;86(1020):608–616. doi: 10.1136/pgmj.2010.101121. [DOI] [PubMed] [Google Scholar]
- 30.Brewer LC, Michos ED, Reis JP. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr Drug Targets. 2011;12(1):54–60. doi: 10.2174/138945011793591617. [DOI] [PubMed] [Google Scholar]
- 31.Weinbaum CM, Williams I, Mast EE, et al. Centers for Disease Control and Prevention. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1–20. [PubMed] [Google Scholar]
- 32.Gish RG, Locarnini SA. Chronic hepatitis B: current testing strategies. Clin Gastroenterol Hepatol. 2006;4(6):666–676. doi: 10.1016/j.cgh.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 34.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 35.Chou R, Clark EC, Helfand M. Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(6):465–479. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 36.Murray TS, Shapiro ED. Lyme disease. Clin Lab Med. 2010;30(1):311–328. doi: 10.1016/j.cll.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt ML, Gilligan PH. Clostridium difficile testing algorithms: what is practical and feasible? Anaerobe. 2009;15(6):270–273. doi: 10.1016/j.anaerobe.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Garimella PS, Agarwal R, Katz A. The utility of repeat enzyme immunoassay testing for the diagnosis of Clostridium difficile infection: a systematic review of the literature. J Postgrad Med. 2012;58(3):194–198. doi: 10.4103/0022-3859.101392. [DOI] [PubMed] [Google Scholar]
- 39.Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66–76. doi: 10.4065/83.1.66. [DOI] [PubMed] [Google Scholar]
- 40.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331(7520):810. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knoll GA, Bell RC. Tacrolimus versus cyclosporin for immunosuppression in renal transplantation: meta-analysis of randomised trials. BMJ. 1999;318(7191):1104–1107. doi: 10.1136/bmj.318.7191.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young AH, Newham JI. Lithium in maintenance therapy for bipolar disorder. J Psychopharmacol. 2006;20(suppl 2):17–22. doi: 10.1177/1359786806063072. [DOI] [PubMed] [Google Scholar]
- 43.Bauer M, Forsthoff A, Baethge C, et al. Lithium augmentation therapy in refractory depression-update 2002. Eur Arch Psychiatry Clin Neurosci. 2003;253(3):132–139. doi: 10.1007/s00406-003-0430-9. [DOI] [PubMed] [Google Scholar]
- 44.Grunze H. Lithium in the acute treatment of bipolar disorders—a stocktaking. Eur Arch Psychiatry Clin Neurosci. 2003;253(3):115–119. doi: 10.1007/s00406-003-0427-4. [DOI] [PubMed] [Google Scholar]
- 45.Castells X, Vallano A, Rigau D, Perez J, Casas M, Capella D. Trends in lithium prescription in Spain from 1985 to 2003. J Affect Disord. 2006;91(2–3):273–276. doi: 10.1016/j.jad.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Tsiropoulos I, Gichangi A, Andersen M, Bjerrum L, Gaist D, Hallas J. Trends in utilization of antiepileptic drugs in Denmark. Acta Neurol Scand. 2006;113(6):405–411. doi: 10.1111/j.1600-0404.2006.00639.x. [DOI] [PubMed] [Google Scholar]
- 47.Haynes K, Heitjan D, Kanetsky P, Hennessy S. Declining public health burden of digoxin toxicity from 1991 to 2004. Clin Pharmacol Ther. 2008;84(1):90–94. doi: 10.1038/sj.clpt.6100458. [DOI] [PubMed] [Google Scholar]
- 48.Comer JS, Olfson M, Mojtabai R. National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996–2007. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1001–1010. doi: 10.1016/j.jaac.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackers R, Besag FM, Wade A, Murray ML, Wong IC. Changing trends in antiepileptic drug prescribing in girls of child-bearing potential. Arch Dis Child. 2009;94(6):443–447. doi: 10.1136/adc.2008.144386. [DOI] [PubMed] [Google Scholar]
- 50.Pugh MJ, Van Cott AC, Cramer JA, et al. Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy: 2000–2004. Neurology. 2008;70(22, pt 2):2171–2178. doi: 10.1212/01.wnl.0000313157.15089.e6. [DOI] [PubMed] [Google Scholar]
- 51.Nicholas JM, Ridsdale L, Richardson MP, Ashworth M, Gulliford MC. Trends in antiepileptic drug utilisation in UK primary care 1993–2008: cohort study using the General Practice Research Database. Seizure. 2012;21(6):466–470. doi: 10.1016/j.seizure.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med. 2010;8:34. doi: 10.1186/1741-7015-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53(3):317–333. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Kones R. The Jupiter study, CRP screening, and aggressive statin therapy-implications for the primary prevention of cardiovascular disease. Ther Adv Cardiovasc Dis. 2009;3(4):309–315. doi: 10.1177/1753944709337056. [DOI] [PubMed] [Google Scholar]
- 55.de Ferranti SD, Rifai N. C-reactive protein: a nontraditional serum marker of cardiovascular risk. Cardiovasc Pathol. 2007;16(1):14–21. doi: 10.1016/j.carpath.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Abd TT, Eapen DJ, Bajpai A, Goyal A, Dollar A, Sperling L. The role of C-reactive protein as a risk predictor of coronary atherosclerosis: implications from the JUPITER trial. Curr Atheroscler Rep. 2011;13(2):154–161. doi: 10.1007/s11883-011-0164-5. [DOI] [PubMed] [Google Scholar]
- 57.Bajpai A, Goyal A, Sperling L. Should we measure C-reactive protein on earth or just on JUPITER? Clin Cardiol. 2010;33(4):190–198. doi: 10.1002/clc.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howie JN, Caldwell MA, Dracup K. The measurement of brain natriuretic peptide in heart failure: precision, accuracy, and implications for practice. AACN Clin Issues. 2003;14(4):520–531. doi: 10.1097/00044067-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Dyrbye LN, Redfield MM. The role of brain natriuretic peptide in population screening. Heart Fail Rev. 2003;8(4):349–354. doi: 10.1023/a:1026143231117. [DOI] [PubMed] [Google Scholar]
- 60.Maisel AS. The diagnosis of acute congestive heart failure: role of BNP measurements. Heart Fail Rev. 2003;8(4):327–334. doi: 10.1023/a:1026135029299. [DOI] [PubMed] [Google Scholar]
- 61.Busse JW, Bassler D, Guyatt GH. Evaluating the evidence for assessing BNP and NT-proBNP levels. Clin Biochem. 2008;41(4–5):227–330. doi: 10.1016/j.clinbiochem.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 62.Oremus M, Raina PS, Santaguida P, et al. A systematic review of BNPas a predictor of prognosis in persons with coronary artery disease. Clin Biochem. 2008;41(4–5):260–265. doi: 10.1016/j.clinbiochem.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Bohm M, Voors AA, Ketelslegers JM, et al. Biomarkers: optimizing treatment guidance in heart failure. Clin Res Cardiol. 2011;100(11):973–981. doi: 10.1007/s00392-011-0341-0. [DOI] [PubMed] [Google Scholar]
- 64.Kelder JC, Cowie MR, McDonagh TA, et al. Quantifying the added value of BNP in suspected heart failure in general practice: an individual patient data meta-analysis. Heart. 2011;97(12):959–963. doi: 10.1136/hrt.2010.220426. [DOI] [PubMed] [Google Scholar]
- 65.Ewald B, Ewald D, Thakkinstian A, Attia J. Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008;38(2):101–113. doi: 10.1111/j.1445-5994.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- 66.Hill SA, Balion CM, Santaguida P, et al. Evidence for the use of B-type natriuretic peptides for screening asymptomatic populations and for diagnosis in primary care. Clin Biochem. 2008;41(4–5):240–249. doi: 10.1016/j.clinbiochem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 67.Balion C, Santaguida PL, Hill S, et al. Testing for BNP and NT-proBNP in the diagnosis and prognosis of heart failure. Evid Rep Technol Assess (Full Rep) 2006;(142):1–147. [PMC free article] [PubMed] [Google Scholar]
- 68.Larson J, Anderson EH, Koslawy M. Thyroid disease: a review for primary care. J Am Acad Nurse Pract. 2000;12(6):226–232. doi: 10.1111/j.1745-7599.2000.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 69.Helfand M. Screening for thyroid disease. [Accessed January 8, 2014];Syst Evid Rev. 2004 :23. http://www.ncbi.nlm.nih.gov/books/nbk42812.
- 70.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 71.Bou Khalil R, Richa S. Thyroid adverse effects of psychotropic drugs: a review. Clin Neuropharmacol. 2011;34(6):248–255. doi: 10.1097/WNF.0b013e31823429a7. [DOI] [PubMed] [Google Scholar]
- 72.Stockigt JR. Case finding and screening strategies for thyroid dysfunction. Clin Chim Acta. 2002;315(1–2):111–124. doi: 10.1016/s0009-8981(01)00715-x. [DOI] [PubMed] [Google Scholar]
- 73.Jin XW, Xu H. Cervical cancer screening from Pap smear to human papillomavirus DNA testing. Compr Ther. 2001;27(3):202–208. doi: 10.1007/s12019-001-0015-3. [DOI] [PubMed] [Google Scholar]
- 74.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26(2):209–220. doi: 10.1016/j.bpobgyn.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Kroupis C, Vourlidis N. Human papilloma virus (HPV) molecular diagnostics. Clin Chem Lab Med. 2011;49(11):1783–1799. doi: 10.1515/CCLM.2011.685. [DOI] [PubMed] [Google Scholar]
- 77.Nishino HT, Tambouret RH, Wilbur DC. Testing for human papillomavirus in cervical cancer screening: a review of indications and methodology. Cancer Cytopathol. 2011;119(4):219–227. doi: 10.1002/cncy.20161. [DOI] [PubMed] [Google Scholar]
- 78.Rebolj M, Njor SH, Lynge E. Restriction of human papillomavirus DNA testing in primary cervical screening to women above age 30: systematic review. Eur J Cancer Prev. 2012;21(1):73–81. doi: 10.1097/CEJ.0b013e3283498dbe. [DOI] [PubMed] [Google Scholar]
- 79.Tota J, Mahmud SM, Ferenczy A, Coutlee F, Franco EL. Promising strategies for cervical cancer screening in the post-human papillomavirus vaccination era. Sex Health. 2010;7(3):376–382. doi: 10.1071/SH10022. [DOI] [PubMed] [Google Scholar]
- 80.Greer JB, Brand RE. New developments in pancreatic cancer. Curr Gastroenterol Rep. 2011;13(2):131–139. doi: 10.1007/s11894-011-0175-y. [DOI] [PubMed] [Google Scholar]
- 81.Mossner J. What’s new in therapy of pancreatic cancer? Dig Dis. 2010;28(4–5):679–683. doi: 10.1159/000320096. [DOI] [PubMed] [Google Scholar]
- 82.Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70(4):255–264. doi: 10.1159/000094888. [DOI] [PubMed] [Google Scholar]
- 83.Buxbaum JL, Eloubeidi MA. Molecular and clinical markers of pancreas cancer. JOP. 2010;11(6):536–544. [PubMed] [Google Scholar]
- 84.Fry LC, Monkemuller K, Malfertheiner P. Molecular markers of pancreatic cancer: development and clinical relevance. Langenbecks Arch Surg. 2008;393(6):883–890. doi: 10.1007/s00423-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 85.Surveillance, epidemiology and end results: cancer of pancreas; National Cancer Institute; 2012. [Accessed January 8, 2014]. http://seer.cancer.gov/statfacts/html/pancreas.html#prevalence. [Google Scholar]
- 86.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weinbaum CM, Mast EE, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology. 2009;49(suppl 5):S35–S44. doi: 10.1002/hep.22882. [DOI] [PubMed] [Google Scholar]
- 88.Smith BD, Jorgensen C, Zibbell JE, Beckett GA. Centers for Disease Control and Prevention initiatives to prevent hepatitis C virus infection: a selective update. Clin Infect Dis. 2012;55(suppl 1):S49–S53. doi: 10.1093/cid/cis363. [DOI] [PubMed] [Google Scholar]
- 89.Wilkins T, Malcolm JK, Raina D, Schade RR. Hepatitis C: diagnosis and treatment. Am Fam Physician. 2010;81(11):1351–1357. [PubMed] [Google Scholar]
- 90.Centers for Disease Control and Prevention. [Accessed January 8, 2014];Lyme disease data. 2012 http://www.cdc.gov/lyme/stats/index.html?utm_source=publish2&utm_medium=referral&utm_campaign=www.kpbs.org.
- 91.Centers for Disease Control and Prevention. [Accessed January 8, 2014];Influenza—United States surveillance data: 1997–1998 through 2009–2010 seasons. 2010 http://www.cdc.gov/flu/weekly/ussurvdata.htm.
- 92.Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005;(4) doi: 10.1002/14651858.CD003961.pub2. CD003961. [DOI] [PubMed] [Google Scholar]
- 93.Fang MC, Stafford RS, Ruskin JN, Singer DE. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164(1):55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 94.Department of Health and Human Services. Mandatory guidelines and proposed revisions to mandatory guidelines for federal workplace drug testing programs; notices. Fed Regist. 2004;69(71):19644–19673. [Google Scholar]
- 95.Department of Health and Human Services. Mandatory guidelines for federal workplace drug testing programs. Fed Regist. 2010;75(83):22809–22810. [Google Scholar]
- 96. [Accessed January 8, 2014];American Society of Interventional Pain Physicians. Guidelines. 2012 http://www.asipp.org/Guidelines.htm.
- 97.Agency Medical Doctors Group. [Accessed January 8, 2014];Interagency guideline on opioid dosing for non-cancer pain: an educational aid to improve care and safety with opioid therapy. 2010 http://www.agencymeddirectors.wa.gov/files/opioidgdline.pdf.
- 98.Centers for Disease Control and Prevention. [Accessed January 8, 2014];Prescription painkiller overdoses. http://www.cdc.gov/vitalsigns/methadoneoverdoses.
- 99.Centers for Disease Control and Prevention. National Center for Injury Prevention and Control program report. 2012 [Google Scholar]
- 100.Lusky K. Probing the nuances of pain management. [Accessed January 8, 2014];CAP Today. 2009 Apr; http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt%7BactionForm.contentReference%7D=cap_today%2F0409%2F0409b_pain_management.html&_state=maximized&_pageLabel=cntvwr.
- 101.Heit HA, Gourlay DL. Urine drug testing in pain medicine. J Pain Symptom Manage. 2004;27(3):260–267. doi: 10.1016/j.jpainsymman.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Manchikanti L, Manchukonda R, Damron KS, Brandon D, McManus CD, Cash K. Does adherence monitoring reduce controlled substance abuse in chronic pain patients? Pain Physician. 2006;9(1):57–60. [PubMed] [Google Scholar]
- 103.Fornari FA, Siwicki DM, Bauer GB. [Accessed January 8, 2014];Urine drug testing and monitoring in pain management. 2006 https://www.dominiondiagnostics.com/docs/pubs/0607DominionPub_UDTinPainManagement.pdf.
- 104.Substance Abuse and Mental Health Services Administration. [Accessed January 8, 2014];Drug abuse warning network, 2009: national estimates of drug-related emergency department visits. 2011 http://www.samhsa.gov/data/2k11/dawn/2k9dawned/html/dawn2k9ed.htm. [PubMed]
- 105.Centers for Medicare & Medicaid Services. [Accessed January 8, 2014];Part B: national summary data file (previously known as BESS) 2012 http://www.cms.gov/research-statistics-data-and-systems/files-for-order/nonIdentifiabledatafiles/partbnationalsummarydatafile.html.