Abstract
Eradication of HIV-1 from an infected individual requires a means of inducing production of virus from latently infected cells and stimulating an immune response against the infected cells. We report the development of lentiviral vectors that transduce dendritic cells (DCs) to both induce production of virus from latently infected cells and stimulate antigen-specific CTLs. The vectors package Vpx, a lentiviral accessory protein that counteracts the SAMHD1-mediated block to DC transduction, allowing for long-term expression of vector-encoded proteins. The vectors encode influenza or HIV-1-derived epitopes fused via a self-cleaving peptide to CD40L that releases the peptide into the endoplasmic reticulum for entry into the antigen presentation pathway. Expression of CD40L caused transduced DCs to mature and produce Th1-skewing cytokines. The DCs presented antigen to CD8 T cells, enhancing antigen-specific CTLs. Coculture of the transduced DCs with latently infected cells induced high level virus production, an effect that was mediated by TNF-α. The ability of a DC vaccine to reactivate latent HIV-1 and stimulate an adaptive immune response provides a means to reduce the size of the latent reservoir in patients. This strategy can also be applied to develop DC vaccines for other diseases.
Introduction
Therapeutic dendritic cell (DC) vaccines take advantage of the ability of this critical cell-type to capture, process, and present antigens to T cells to stimulate an adaptive immune response.1, 2 DC vaccination strategies generally involve leukapheresis after which monocytes are isolated and differentiated with cytokines to monocyte-derived dendritic cells (MDDCs). These are then pulsed with antigen and re-infused. Alternatively, antigen coupled to a DC-targeting moiety can be directly injected. Vaccination strategies are also under development in which DCs are transduced ex vivo with an antigen-expressing viral vector, providing endogenous production of antigen that results in more effective presentation on class I MHC and sustained production of antigen.
The use of lentiviral vectors as DC vaccine vectors has the advantage that they integrate into the target cell genomic DNA, resulting in long-term expression and do not encode viral proteins.3, 4 However, the development of lentiviral vectors as DC vaccines has been limited by the low efficiency with which the cells are transduced. DCs express SAMHD1, a phosphohydrolase that depletes the cell of deoxynucleotide triphosphates, causing their concentration to fall below what is required to support reverse transcription of the viral genome and resulting in low titers of HIV-1-based vectors.5 HIV-2 and some SIV isolates, encode the accessory protein Vpx that counteracts SAMHD1-mediated restriction. Vpx is packaged into virions and upon infection, binds to SAMHD1. The complex then recruits the E3 ubiquitin ligase CRL4 that induces the proteasomal degradation of SAMHD1 and relieves the block to infection.6, 7
HIV-1-based lentiviral vectors do not encode Vpx and Vpx cannot be packaged into HIV-1 virions. Vpx is packaged into HIV-2 and SIV virions by a 10 amino acid packaging motif in the P6 protein of the respective Gag precursor polyprotein, a motif that is absent from HIV-1 Gag. To improve the ability of lentiviral vectors to transduce DCs, we generated a lentiviral packaging system in which the Vpx packaging motif was introduced into P6 of the HIV-1 Gag/Pol packaging vector to allow for the production of HIV-1 virions that contain packaged Vpx.8 Using this vector, virus stock is produced by cotransfection of 293T cells with lentiviral vector plasmid and Vpx expression vector. The resulting virus contains a high copy number of Vpx molecules and infects DCs with a two-log increase in titer allowing for the stable expression of transgenes or shRNA knock-down of target genes.9
We report here the development of Vpx-containing lentiviral DC vaccine vectors that express the DC stimulatory protein CD40L together with an immunodominant epitope derived from influenza virus or HIV-1. DCs transduced with Vpx-containing lentiviral vectors stimulated antigen-specific CTLs and induced the production of Th1-skewing and proinflammatory cytokines. Coculture of CD40L-expressing transduced DCs with latently infected T cells induced provirus expression. The ability of the transduced DCs to induce virus production from latently infected cells and to boost anti-HIV-1 T cell responses may provide a means of decreasing the size of the latent reservoir in patients on combination antiretroviral therapy (cART), a strategy that has been the focus of attempts to achieve a functional cure for HIV-1 infection. Such vectors may also be useful in the development of therapeutic vaccines against other diseases including cancer, where antigenic targets have been identified.
Results
Lentiviral vectors expressing CD40L-antigen fusion proteins
The ability to efficiently transduce DCs with Vpx-containing lentiviral vectors provides a means of continuous, endogenous production of antigen and coexpression of immunomodulatory proteins that enhance the adaptive immune response. To test this concept, we generated lentiviral vectors encoding influenza A matrix protein amino acids 58-66 (termed here M1), an immunodominant, HLA-A*0201-restricted epitope to which most donor CD8 T cells generate a strong memory response in vitro.10 To enhance the ability of the transduced DCs to stimulate a strong response to the antigen, we introduced the sequence encoding CD40L. CD40L expressed on activated T cells binds to CD40 on DCs to induce their maturation and activate antigen presentation pathways.11-14 CD40L binding to DC CD40 also induces the production of the Th1-skewing and proinflammatory cytokines IL-12 and TNF-α and promotes B cell activation and immunoglobulin class switch recombination.15-17 Because CD40L is a type II transmembrane protein such that its carboxy-terminus faces the endoplasmic reticulum (ER) lumen during biosynthesis, we reasoned that fusion of the antigenic peptide to the carboxy-terminus of CD40L, separated by a P2A self-cleaving peptide, would provide a means of enhancing the immunogenicity of the antigen. Cleavage of the P2A sequence upon biosynthesis would release the peptide into the ER, providing it access to the MHC class I antigen presentation pathway. As controls, we generated vectors that expressed GFP (Control LV), CD40L alone (CD40L), and a dual promoter vector that expressed GFP and M1 with an amino-terminal signal peptide (ER-M1) (Fig. 1a).
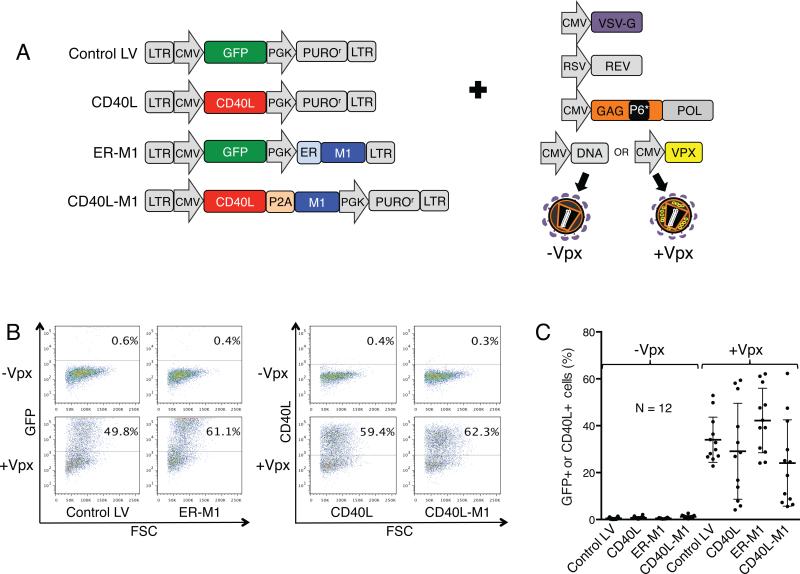
Figure 1. Lentiviral vector virions that contain SIVmac Vpx infect DCs with high efficiency.
(a) Lentiviral vectors that express CD40L and influenza epitopes are diagrammed. The vectors are derived from pLenti.CMV.GFP.puro (658-5 Addgene), a dual promoter HIV-1-based lentiviral vector that has a 5’ cytomegalovirus (CMV) promoter and 3’ phosphoglycerate kinase promoter (PGK).42 The vectors express GFP (Control LV), CD40L (CD40L), GFP and influenza A matrix protein amino acids 58-66, M1,10 with an amino-terminal signal peptide (ER-M1), or CD40L joined to the M1 coding sequence (CD40L-M1). CD40L fusion proteins contained an intervening P2A picornavirus self-cleaving sequence.44 The vectors, except for ER-M1, contained a PGK promoter-driven puromycin resistance gene that served as a spacer and was not used. Lentiviral vector stocks containing or lacking packaged Vpx were produced by cotransfecting 293T cells with lentiviral vector plasmid, the pMDL-X Gag/Pol packaging vector that contains the SIVmac P6 Vpx packaging motif,8 and vectors encoding HIV-1 Rev, VSV-G, and SIVmac Vpx8, 45 or pcDNA6 empty vector. (b) DCs were transduced with Control LV, ER-M1, CD40L, and CD40L-M1 viruses at MOI=2. After 72 hours, GFP+ and CD40L+ cells were quantified by flow cytometry. Representative results from one donor are shown. (c) Pooled results from 12 donors are shown with error bars indicating mean +/− SD. Some of the lentiviral vectors were tested in eight additional donors and showed similar trends (data not shown).
CD40L-M1 fusion protein induces DC maturation and T cell proliferation
To determine the ability of the vectors to induce DC maturation, we produced virus stocks for the lentiviral vectors such that they contained or lacked packaged Vpx.8 For this, we cotransfected 293T cells with the HIV-1 Gag/Pol expression vector pMDL-X that contains the Vpx packaging motif and SIVmac Vpx or empty expression vector control plasmid and normalized their infectivity on 293T cells (Fig. S1). We then infected HLA-A*02+ DCs and after three days quantified the GFP+ and CD40L+ cells by flow cytometry. Representative flow cytometry plots are shown for one donor (Fig. 1b). On average, Vpx-containing viruses infected DCs with a 55-fold higher titer compared to those that lacked Vpx (Fig. 1c). Despite having been normalized for infectivity on 293T cells, on some donor DCs the titer of the CD40L-expressing lentiviral vectors appeared over 50% lower than that of the GFP-expressing viruses. This apparent reduction in titer may be caused by blocking the accessibility of the anti-CD40L staining antibody to CD40L by endogenous CD40 on the DCs, preventing detection of the transduced DCs by flow cytometry.
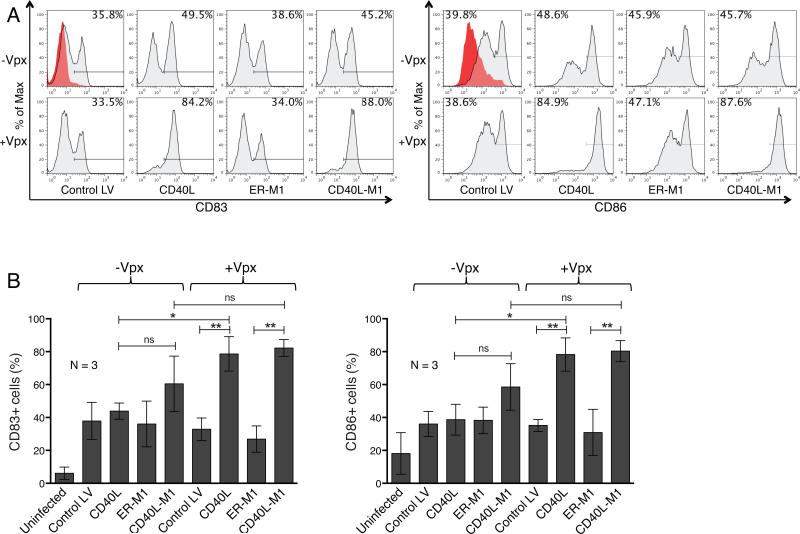
To determine the effect of CD40L on DC maturation, we transduced DCs with the lentiviral vectors and after three days determined how many cells expressed CD83 and CD86. The results showed that the Control LV induced one third of the cells to express CD83 and this was independent of Vpx (Fig. 2a). CD40L-expressing lentiviral vectors that lacked or contained Vpx resulted in 49.5% and 84.2% CD83+ cells, respectively. Lentiviral vectors expressing ER-M1 induced maturation similar to that of Control LV, and vectors expressing the CD40L-M1 fusion protein induced maturation similar to that of CD40L, indicating that the fusion protein was biologically active. In the case of CD86, vectors that lacked Vpx induced 40-49% of the DCs to express CD86 while those transduced with CD40L-expressing vectors containing Vpx were nearly all matured. These results were further supported by an analysis of three donor DC populations (Fig. 2b).
Figure 2. Transduction with CD40L lentiviral expression vectors induces DC maturation.
DCs were transduced with lentiviral vectors that contained or lacked Vpx and after 72 hours CD83 and CD86 were quantified by flow cytometry. (a) Representative flow cytometry analyses of untransduced DCs (red) and DCs transduced with Control LV, CD40L, ER-M1, and CD40L-M1 Vpx-containing or lacking lentiviral vectors (gray) are shown for both CD83 and CD86 expression. (b) CD83 and CD86 on the transduced DCs of three donors were analyzed by flow cytometry. The average number of positive cells is shown with error bars indicating mean +/− SD. *p<0.05 and **p<0.01 as determined by paired two-tailed t test.
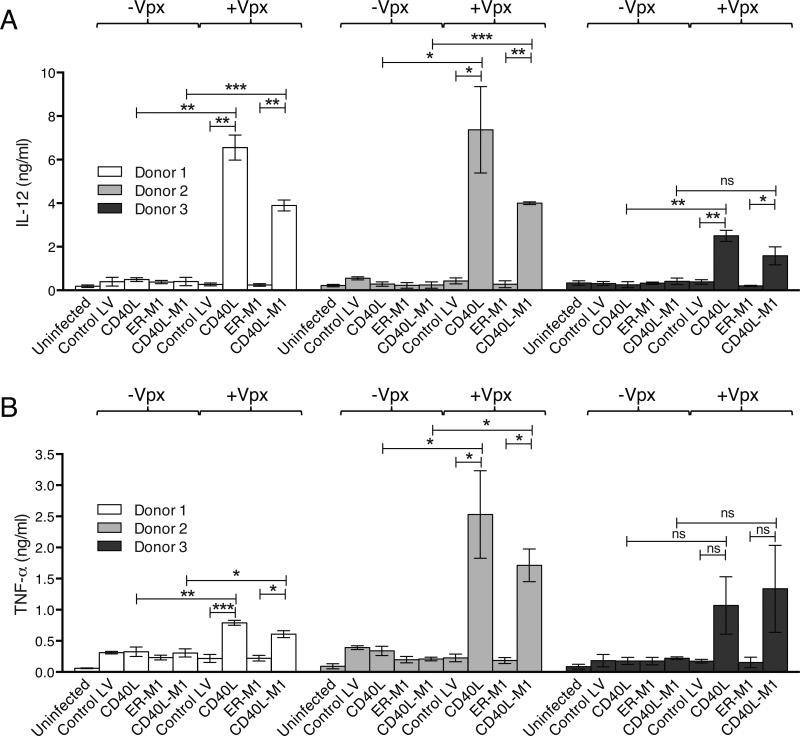
To determine the ability of the vectors to induce Th1-skewing and proinflammatory cytokines, we transduced DCs and quantified supernatant IL-12p70, TNF-α, and IL-6. Lentiviral vectors that contained Vpx and expressed CD40L or CD40L-M1 induced IL-12 32-fold and 18-fold more than control vectors (Fig. 3a). TNF-α (Fig. 3b) and IL-6 (not shown) were similarly induced.
Figure 3. Transduction with CD40L lentiviral expression vectors induces DCs to produce Th1-skewing and proinflammatory cytokines.
DCs were transduced with lentiviral vectors that contained or lacked Vpx and after 72 hours cytokine production was measured in the culture supernatant. (a) The quantification of IL-12 is shown. (b) The quantification of TNF-α is shown. The results are the average data from three donors assayed in triplicate with error bars indicating mean +/− SD. *p<0.05, **p<0.01, and ***p<0.001 as determined by paired two-tailed t test.
To understand how the Vpx-lacking vectors induced DC maturation although they infected only a small number of cells, we prepared control supernatants from untransfected 293T cells or from 293T cells transfected with the lentiviral vector plasmids but without pcVSV-G or the pMDL Gag/Pol packaging construct and prepared viruses from 293T cells treated with nevirapine or raltegravir. The results showed that 293T-conditioned medium itself induced a low level of maturation (Fig. S2a). Supernatant produced from 293T cells transfected with the CD40L lentiviral vector (in the absence of Gag/Pol) induced about 60% of the DCs to mature, suggesting that it was mediated by a low level of soluble form of CD40L present in the supernatant. Infection of DCs with Vpx-containing lentiviral vectors encoding CD40L increased the extent of maturation to nearly 100% and this increase was prevented by the antiretroviral drugs suggesting that it was caused by productive infection. Control supernatants and viruses did not induce the production of proinflammatory cytokines and their production was induced only by infection with CD40L-expressing lentiviral vectors (Fig. S2b). Taken together, these results suggest that CD40L vector stocks contain some soluble CD40L that induces DCs to mature but that full maturation requires productive infection and expression of the lentiviral vector encoded CD40L.
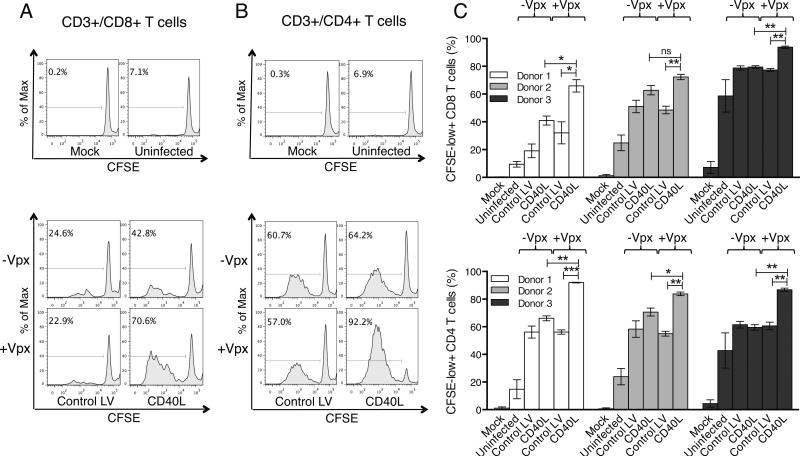
To determine whether CD40L-expressing DCs would induce antigen-independent T cell proliferation, we transduced DCs with the lentiviral vectors and after two days, added autologous CFSE-labeled, monocyte-depleted PBMCs. After seven days, the extent to which the CFSE fluorescence decreased as a result of cell division was analyzed in the CD8 (CD3+/CD8+) and CD4 (CD3+/CD4+) T cell populations. Analysis of a representative donor showed that the CD8 T cells did not proliferate when cultured alone (mock) or with untransduced DCs (Fig. 4a, top). Transduction of DCs with virus that lacked Vpx induced only a small amount of T cell proliferation (Fig. 4a, bottom). In contrast, DCs transduced with Vpx-containing CD40L-expressing lentiviral vector strongly induced CD8 T cell proliferation. The CD4 T cells behaved similarly but were more sensitive overall in their proliferative response to both the control vectors and CD40L-expressing virus (Fig. 4b, top and bottom). Analysis of the CD8 and CD4 T cells in three donors was consistent with these results (Fig. 4c, top and bottom).
Figure 4. DCs transduced with CD40L lentiviral expression vectors induce T cells to proliferate.
DCs were transduced with Control LV or CD40L vectors that contained or lacked Vpx and after 48 hours, cocultured with CFSE-labeled leukocytes. Seven days later, the cells were stained with fluorescent anti-CD3, CD4, and CD8 mAbs and analyzed by flow cytometry. As controls, CFSE-labeled leukocytes were cultured alone (Mock) or with uninfected DCs. (a) CFSE staining of the CD3+/CD8+ T cells is shown. (b) CSFE staining of the CD3+/CD4+ T cells is shown. The mock and uninfected controls are above and cocultures of DCs transduced with Control LV or CD40L vectors are below. (c) CFSE staining of the CD3+/CD8+ and CD3/CD4+ T cells of three donors is shown. The average number of CFSE-low+ cells is shown with error bars indicating mean +/− SD. *p<0.05, **p<0.01, and ***p<0.001 as determined by paired two-tailed t test.
Transduced DCs present antigen to CTLs
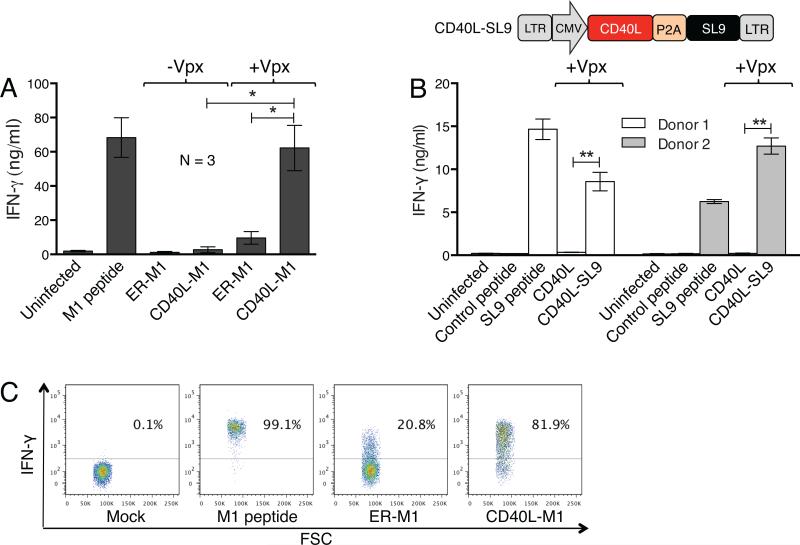
DCs expressing the CD40L-M1 fusion protein should present antigen to T cells bearing the relevant T cell receptor. To determine whether this was the case, we cocultured the transduced DCs with an HLA-A*0201-restricted M1-specific CTL clone18 and after 24 hours, quantified supernatant IFN-γ as a measure of CTL activation. To control for maximal CTL activation, the DCs were pulsed with M1 peptide. DCs transduced with lentiviral vectors that lacked Vpx weakly activated the CTLs as compared to the peptide-pulsed DCs. DCs expressing ER-M1 moderately activated the CTLs. In contrast, DCs transduced with Vpx-containing CD40L-M1 fusion protein vector strongly activated the CTLs, approaching the level of peptide-pulsed DCs (Fig. 5a). These findings suggested that the CD40L-peptide fusion protein was efficiently presented on MHC class I by the transduced DCs.
Figure 5. DCs transduced with CD40L-antigen fusion lentiviral expression vectors activate antigen-specific CTLs and induce IFN-γ production.
(a) HLA-A*02+ DCs were mock infected or transduced with the M1 lentiviral expression vectors containing or lacking Vpx and after three days, cocultured with an HLA-A*0201-restricted M1-specific CTL clone at a ratio of 1:1. Uninfected DCs cocultured with the CTL in the presence or absence of M1 peptide were used as controls. After 24 hours, supernatant IFN-γ was quantified. Average data from three donors is shown with error bars indicating mean +/− SD. *p<0.05 as determined by paired two-tailed t test. (b) The lentiviral vector that expresses CD40L and an HIV-1 peptide epitope is diagrammed. The vector expresses CD40L fused to the HIV-1 MA epitope SL9 coding sequence19 with an intervening P2A self-cleaving sequence (CD40L-SL9). IFN-γ assay was performed similar to (a), except that the DCs were pulsed with SL9 peptide or transduced with the Vpx-containing CD40L or CD40L-SL9 lentiviral expression vectors and cultured with an HLA-A*0201-restricted SL9-specific CTL clone. **p<0.01 as determined by paired two-tailed t test. (c) DCs from two donors were mock-infected, pulsed with M1 peptide or transduced with the Vpx-containing lentiviral vectors expressing M1 and then cocultured with the M1-specific CTL clone. After eight hours, CTL cells were analyzed for intracellular IFN-γ by flow cytometry. Representative results from one donor are shown.
To determine whether the vectors would allow for efficient presentation of an antigen relevant to HIV-1 infection, we generated a vector that expressed CD40L fused to SL9, an HLA-A*0201-restricted HIV-1 CTL epitope (Fig. 5b, top).19 To test the ability of the DCs transduced with SL9 lentiviral vector to present antigen to T cells, we cocultured the HLA-A*0201-restricted SL9-specific CTL clone S1-SL9-3.23T20 with Vpx-containing lentiviral vector-transduced DCs and measured IFN-γ production by the CTL. The results showed that the CTL clone was activated by CD40L-SL9-transduced DCs but not by the control M1 peptide, reaching levels comparable to SL9 peptide-pulsed DCs (Fig. 5b, bottom). The activation was specific for expression of the SL9 epitope.
To evaluate the CTL response at the single cell level, we analyzed intracellular IFN-γ in the cocultured CTLs. DCs pulsed with M1 peptide induced nearly all of the CTLs to produce IFN-γ, while DCs transduced with M1-expressing lentiviral vectors that lacked Vpx had no effect (data not shown). In contrast, lentiviral vectors that contained Vpx and expressed ER-M1 or CD40L-M1 activated 21% and 82% of the CTLs, respectively (Fig. 5c). Moreover, of the cells that responded, CD40L-M1 induced >two-fold more IFN-γ per cell than the lentiviral vector that expressed ER-M1, suggesting that the CD40L-M1 fusion protein was more efficiently presented than the signal peptide-linked epitope. To determine the number CD40L-M1-expressing DCs required to activate the CTL clone, we titrated the number of DCs in the coculture. As little as 1:20 DC per CTLs caused CTL activation, far fewer than what was required for DCs expressing ER-M1 (Fig. S3). These results supported the increased potency with which the CD40L-M1 activated the CTLs.
CD40L-M1-transduced DCs induce CTL proliferation
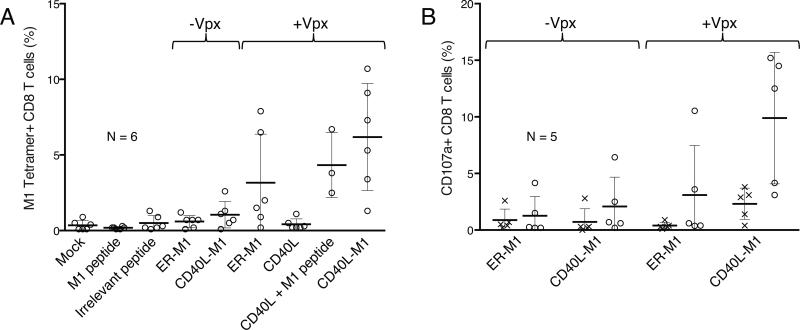
To determine whether the CD40L-M1-transduced DCs would induce the proliferation of antigen-specific CD8 T cells, we transduced DCs with the lentiviral vectors and/or pulsed them with M1 peptide and then cocultured the cells with autologous CD8 T cells in the absence of added cytokines. After nine days, we quantified the number of M1-specific CD8 T cells by staining with M1 tetramer and CD8. We found that M1 peptide-pulsed DCs induced few antigen-specific T cells, while Vpx CD40L-transduced DCs pulsed with peptide induced a clear response (Fig. 6a). DCs transduced with vectors that expressed M1 but lacked Vpx induced few antigen specific T cells. DCs transduced with the Vpx-containing vector that encoded M1 without CD40L were inefficient at expanding antigen-specific T cells, inducing tetramer-staining T cells in only 2 of 6 donors. In contrast, the Vpx-containing vector encoding CD40L and M1 induced a T cell response in 5 of 6 donors. The response was as strong, or stronger, than the response to DCs transduced with CD40L vector pulsed with pure peptide.
Figure 6. DCs transduced with CD40L-antigen fusion lentiviral expression vectors expand antigen-specific primary CTLs.
(a) DCs were mock infected (treated with DMSO), pulsed with M1 or irrelevant peptide, transduced with the Vpx CD40L expressing vector with or without M1 peptide, or transduced with the M1 expressing lentiviral vectors containing or lacking Vpx. After two days, the DCs were cocultured with autologous CD8+ T cells. Nine days later the cells were stained for viability, anti-CD8 mAb, and M1 or irrelevant peptide tetramers (not shown) and analyzed by flow cytometry. Pooled results from six donors with are shown with error bars indicating mean +/− SD. CD40L + M1 peptide was tested in three of six donors. (b) DC-CD8 T cell cocultures were maintained for ten days and then restimulated with M1 or irrelevant peptide in the presence of monensin and anti-CD107a mAb for six hours after which the cells were stained with for viability and CD8 and analyzed by flow cytometry. Pooled results from five donors are shown with error bars indicating mean +/− SD.
To measure the cytolytic potential of activated CD8 T cells, we stained the cells for intracellular CD107a, a marker of antigen-specific degranulation.21 For this, we transduced the DCs and two days later added autologous CD8 T cells. After ten days, M1 or irrelevant peptide was added and after six hours the cultures were stained for intracellular CD107a and costained for CD8. Analysis of the cells by flow cytometry showed that viruses lacking Vpx induced little degranulation. In cultures in which the DCs were transduced with the Vpx-containing CD40L-M1 fusion protein expression vector, about 10% of the CD8 T cells had degranulated (Fig. 6b). Vectors lacking CD40L induced significantly fewer T cells. Taken together these results show that the antigen-specific T cells expanded and were likely to have cytolytic activity.
CD40L-transduced DCs activate quiescent HIV-1
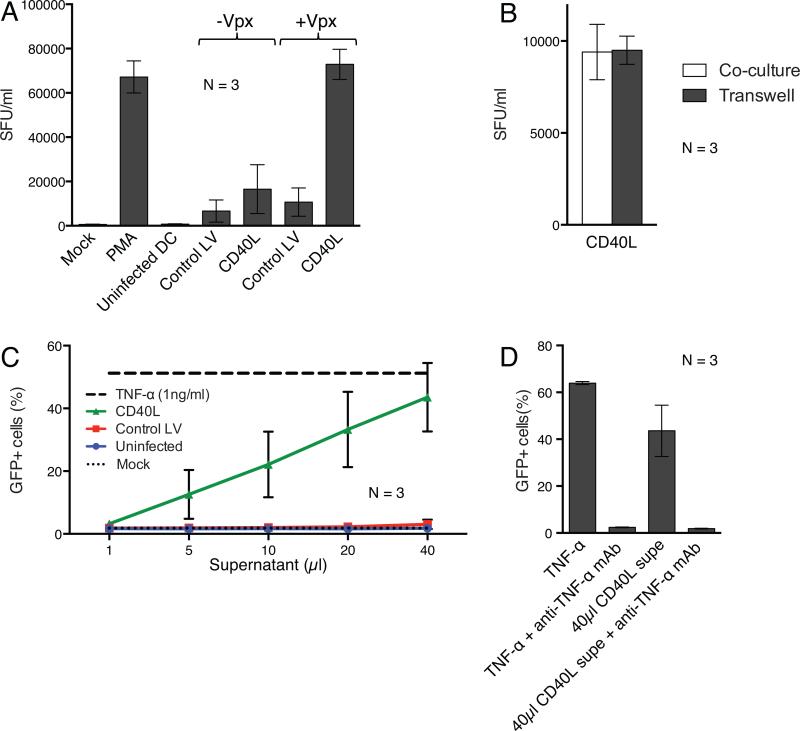
The ability of the CD40L-expressing DCs to activate T cells might provide a means of inducing latent proviruses. To test this possibility, we determined whether CD40L-transduced DCs would activate HIV-1 production from ACH-2 cells, a cell-line that contains a latent HIV-1 provirus.22 Transduced DCs were cultured with ACH-2 cells for two days after which we quantified infectious HIV-1 in the culture supernatant on TZM-bl reporter cells. As a measure of maximal provirus reactivation, we treated the ACH-2 cells with PMA. The results showed that DCs transduced with virions lacking Vpx induced low level virus production while DCs transduced with Vpx-containing CD40L-expressing lentiviral vector induced virus production comparable to that induced by PMA (Fig. 7a). To test whether the reactivation required cell contact, we cultured the transduced DCs and ACH-2 cells separately in a transwell membrane filter plate or together in a single well. The DCs separated by the transwell membrane filter activated HIV-1 production from the ACH-2 cells to an extent equivalent to the coculture. This finding suggested that reactivation could be mediated by a soluble factor (Fig. 7b).
Figure 7. DCs transduced with CD40L lentiviral expression vectors reactivate latent HIV-1 provirus expression.
(a) DCs from three donors were mock infected or transduced with lentiviral vectors containing or lacking Vpx. 24 hours later, the cells were cocultured with infected ACH-2 cells and after two days, infectious virus in the culture supernatant was quantified on TZM-bl reporter cells as the number of spot forming units per milliliter (SFU)/ml. As controls, ACH-2 cells were cultured alone (Mock) or treated with PMA. The data are the average of three donors with error bars indicating mean +/− SD. (b) DCs transduced with Vpx-containing lentiviral vectors expressing CD40L were cocultured with ACH-2 cells or in separate chambers of a transwell plate. The data are the average of three donors with error bars indicating mean +/− SD. (c) DCs from three donors were mock infected or transduced with Vpx-containing lentiviral vectors. After 72 hours, serial dilutions of culture supernatant were assayed by adding increasing amounts of supernatant to J-Lat cells. Control J-Lat cells were untreated or treated with TNF-α. 24 hours later, the number of GFP+ cells was quantified by flow cytometry. The data are the average of three donors with error bars indicating mean +/− SD. (d) Similar to (c), except that TNF-α supernatant from CD40L transduced DCs was pretreated with an anti-TNF-α mAb prior to incubation with the J-Lat cells.
To further test the ability of the CD40L-transduced DCs to reactivate latent provirus, we incubated supernatants of the lentiviral vector-transduced DCs with J-Lat cells. J-Lat cells are Jurkat cells that harbor a latent full-length HIV.GFP provirus that can be reactivated by various stimuli.23 After 24 hours, the cells were harvested and the GFP+ cells were quantified (Fig. 7c). As a measure of maximal virus production, we treated the cells with TNF-α. Supernatant from CD40L-transduced DCs strongly induced provirus reactivation, causing detectable induction at a 1:100 dilution. Virus production increased linearly with increasing amounts of supernatant, reaching a level comparable to that induced by TNF-α. To determine whether the active factor produced by the transduced DCs was TNF-α, we treated the supernatant with an anti-TNF-α monoclonal antibody (Fig. 7d). As a control, we added the antibody to TNF-α. The anti-TNF-α antibody blocked virus induction in response to TNF-α, demonstrating its blocking activity. Addition of anti-TNF-α antibody to the CD40L-transduced DC culture supernatant prevented virus induction from the J-Lat cells, thereby demonstrating that TNF-α present in the CD40L-tranduced DCs was required for induction from latency in J-Lat cells.
Discussion
We report here the development of Vpx-containing lentiviral vectors that transduce DCs to induce CTL responses to encoded peptide antigens. The Vpx-containing vectors transduced DCs at high titers and the vector-encoded CD40L-peptide antigen caused the cells to mature and present antigen to MHC class I-restricted CD8 T cells. Expression of the antigen as a fusion to CD40L resulted in efficient antigen presentation of the peptide to CD8 T cells. Because CD40L is a type-II transmembrane protein, fusion of the peptide to its carboxy-terminus allows for release of the peptide into the ER lumen during biosynthesis for assembly with MHC class I protein molecules. CD40L caused the DCs to mature and secrete high levels of TNF-α and IL-12, proinflammatory cytokines that promote the differentiation of Th1 T cells.24 The transduced DCs activated CTLs against influenza and HIV-1-derived peptide antigens and expanded antigen-specific primary CD8 T cells.
Transduction of DCs with CD40L-encoding lentiviral vectors induced nearly all of the cells to mature and produce proinflammatory cytokines. This occurred even though not all of the cells were transduced, suggesting a bystander effect. This effect could have been mediated by cell:cell contact of transduced and untransduced cells or by the presence of soluble factors produced by the transduced DCs. In addition, it appeared that the virus stocks of vectors the encoded CD40L contained a small amount of a soluble factor produced by the transfected 293T cells that caused some maturation of the DCs. We did not identify the active factor but it was present in the supernatant of 293T cells transfected with the CD40L vector alone (without Gag/Pol packaging vector), suggesting that it was a soluble form of CD40L produced by cleavage of the protein from the cell surface. The presence of a small amount of soluble CD40L in the virus stock was unlikely to have influenced the experimental results, as it did not induce the DCs to produce proinflammatory cytokines.
DCs transduced with lentiviral vectors encoding antigen and CD40L induced the proliferation of CD8 T cells with receptors specific for the antigen. For most donors, antigen-specific T cell proliferation was dependent upon the expression of both the antigen and CD40L. For these donors, CD40L caused DC maturation and activation, leading to a robust CD8 T cell response. In 2/6 donors, antigen-specific T cells proliferated without a need for CD40L, either because their T cells were highly sensitive to activation signals from the DCs or because their DCs had been activated by some other stimulus.
CD40L induced a considerable amount of non-specific T cell proliferation as reflected by the T cell proliferation induced by CD40L in the absence of peptide antigen. The proliferation was detected as dilution of CFSE staining and could be readily observed microscopically (not shown). The nonspecific T cell proliferation may mask the extent of antigen-specific T cell activation, causing an under-estimate in the absolute number of antigen-specific T cells. For vaccine purposes, CD40L-induced nonspecific T cell proliferation would be problematic; however, whether this will occur in vivo is not clear. In vivo the transduced DCs will be rapidly diluted such that systemic increases in cytokines would be much smaller. In individuals on in cART, some level of generalized T cell proliferation could in fact help to restore the number of circulating CD4 T cells.
DCs transduced to express CD40L induced the production of latent HIV-1 in two widely used cell-line models, J-Lat and ACH-2 cells. The induction did not require cell-to-cell contact and in the J-Lat model was mediated by TNF-α. While the cell-lines do not fully model the latent reservoir, TNF-α reactivates latently infected cells isolated from the peripheral blood of HIV-infected individuals on cART, indicating that this feature is accurately modeled25. CD40L also induces the expression other pro-inflammatory cytokines that may contribute to disruption of the latent reservoir in vivo, including IL-6 and IL-713, 26, 27. The ability of the transduced DCs to induce latent virus by producing soluble factors maybe advantageous in vivo where it would allow for the induction of T cells without a need for cell contact. Moreover, the ability of DCs to migrate to lymph nodes and intestinal mucosa may provide a means to stimulate hard to reach sanctuaries of latently infected cells. To further evaluate the ability of the vaccine to induce latent provirus expression, we used a Bcl-2 transformed primary T cell latency model. However, in this model CD40L was not active and that the cells did not respond to TNF-α (not shown).
Previous reports have described lentiviral vectors for transduction of DCs. Durand, et al., developed lentiviral vectors that package Vpx through the attachment of a c-Src membrane-targeting domain28 and Negri, et al., used this Vpx-packaging system to generate non-integrating lentiviral vectors to prime antigen-specific CD8 T cells.29 Tareen, et al., reported on integration-deficient lentiviral vectors that target DCs through a modified Sindbis virus envelope glycoprotein and package Vpx without any modifications to the HIV-1 capsid.30 Non-integrating vectors avoid the problem of insertional mutagenesis as occurred in a clinical trial of γ-retrovirus-based vector.31 However, integration-competent HIV-1-based vectors have not encountered this problem and have the advantage that they provide long-term, higher level protein production.29 The vectors we have developed package Vpx at high copy number and binding to the packaging motif in Gag P6 places the protein in its native location in the virion. In the absence of the Vpx packaging motif, HIV-1-based lentiviral vectors package only trace quantities of Vpx and the virus has much lower titers on DCs.8, 9
Several studies support the utility of CD40L for enhancing immune responses and inducing latent virus production. In a prime-boost vaccination of mice, CD40L expression vector was found to stimulate polyfunctional T cell responses.32 In another study, the treatment of HIV-infected individuals with soluble CD40L was found to augment CD4 T cell proliferative responses to HIV-1 capsid.33 In addition, virus-like particles produced by insect cells together with CD40L were found to enhance immune responses to the immunogen in mice.34 The soluble CD40L that is produced by transduced DCs may also be advantageous as trimeric recombinant soluble CD40L was found to induce virus production from a latently infected macrophage cell-line.35 Moreover, soluble CD40L has been shown to be safe in humans.36
A consideration in the development of a therapeutic DC vaccine for HIV-1 is that the DCs from infected individuals may be functionally impaired.37 While this appears to be the case prior to therapy, DC function is at least partially restored by antiretroviral therapy.38 The data we show here was derived using DCs from healthy donors. However, we were able to analyze DCs from a single donor on cART. We found that upon transduction with the CD40L-M1 expressing lentiviral vector, the DCs produced proinflammatory cytokines and induced antigen-specific CD8 T cells at a level comparable to healthy donors (Fig. S4).
The inability of cART to cure HIV-1 infection has led to attempts to attack the long-lived reservoir of latently infected quiescent T cells.39 HDAC inhibitors have been used to activate latent virus but because these do not clear the infected cells40 stimulation of an immune response may be required. We showed here that DCs transduced to express CD40L and SL9 peptide can both reactivate quiescent HIV-1 production from latently infected cells and stimulate antigen-specific CTLs. To enhance the ability of the vectors to induce an anti-HIV immune response the vectors could be modified to express an HIV-1 polyepitope in which several peptide epitopes are linked in single polyprotein or full-length protein.41 In light of the central role of DCs in innate and adaptive immune responses, Vpx-containing lentiviral vector vaccines have potential for the treatment of diseases for which antigens have been defined in addition to HIV, including cancers, infections, and autoimmune diseases.
Materials & Methods
Plasmid construction
To construct pLenti.CD40L, CD40L cDNA with 5’ and 3’ BamH-I and Sal-I sites was generated by reverse transcriptase PCR from a human lymphocyte RNA template. The fragment was cleaved with BamH-I and Sal-I and ligated to similarly cleaved pLenti.CMV.GFP.puro (658-5 Addgene, Cambridge, MA).42 To construct pLenti.GFP.M1, an amplicon was generated in which the ER retention signal MRYMILGLLALAAVCSAA43 was fused in-frame to the influenza virus matrix protein peptide GILGFVFTL,10 termed here ER-M1, and flanked by Pst-I and Xho-I sites. The fragment was cleaved with Pst-I and Xho-I and ligated to similarly cleaved pLenti.CMV.GFP. To construct pLenti.CD40L-M1, an amplicon was generated in which the CD40L coding sequence was fused to the M1 coding sequence via the self-cleaving picornavirus 2A (P2A) sequence GSGATNFSLLKQAGDVEENPGP44 by overlapping PCR. The amplicon was cleaved with BamH-I and Sal-I and ligated to similarly cleaved pLenti.CMV.GFP.puro. pLenti.CD40L-SL9 was constructed the same way except that the overlapping PCR generated the CD40L-SL9 amplicon where SL9 encodes the Gag MA peptide SL9, SLYNTVATL. The pMDL-X and pcVpx plasmids have been previously described.8, 9
Cell culture
MDDCs were cultured in RPMI supplemented with 10 mM HEPES, gentamicin, and 5% heat inactivated pooled human serum (PHS; GemCell, Sacramento, CA). T cells were cultured in RPMI supplemented with HEPES, L-glutamine, penicillin/streptomycin, MEM nonessential amino acids, and 5% heat-inactivated PHS. J-Lat cells were cultured in RPMI supplemented with L-glutamine, penicillin/streptomycin, and 10% fetal bovine serum (FBS). ACH-2 cells were cultured in RPMI supplemented with HEPES, L-glutamine, and 10% FBS. TZM-bl and 293T cells were cultured in DMEM supplemented with 10% FBS and penicillin/streptomycin. PBMCs of anonymous healthy donors were purchased from the New York Blood Center. Buffy coats were prepared by Ficoll density gradient centrifugation and typed for class I HLA-A*0201 by flow cytometry with an allophycocyanin (APC)-conjugated anti-A2 monoclonal antibody (mAb) (Abcam, Cambridge, MA). Monocytes were purified by plastic adherence and cultured for 4 days in medium supplemented with 110 U/ml granulocyte-macrophage colony stimulating factor (GM-CSF; Invitrogen Inc., Grand Island, NY) and 282 U/ml IL-4 (R&D Systems, Minneapolis, MN) to generate MDDCs. The medium was replenished every two days with fresh cytokines. Autologous CD8 T cells were purified from the monocyte-depleted fraction using the EasySep Human CD8+ T Cell Enrichment Kit (Stem Cell Technologies, Vancouver, Canada) and frozen for later use. The cells were >95% CD3+/CD8+ by flow cytometry.
Virus preparation and infections
To prepare lentiviral vector stocks, 293T cells were cotransfected by the calcium phosphate coprecipitation method with lentiviral vector plasmid, the p6-modified HIV-1 Gag/Pol expression vector pMDL-X,8 pcRev,45 pcVSV-G,45 and pcVpx8 or pcDNA6/myc-His A (Invitrogen) at a mass ratio of 28:10:7:5:2. For virions produced without Gag/Pol or without VSV-G, pcDNA6 was substituted to maintain the mass ratios. After 48 hours, virus-containing supernatant was harvested, passed through a 0.45-μm filter and concentrated on an Amicon filter (Millipore, Darmstadt, Germany) or pelleted through a 20% sucrose cushion. Amicon filter concentrated virus was used for all of the experiments except for the primary CD8 T cell assays for which the pelleted virus was used. GFP and CD40L-expressing viruses were titered on 293T cells by flow cytometry to determine the number of GFP+ or CD40L+ cells/ml and frozen in aliquots at −80°C.
Lentiviral vector-induced DC maturation and activation
DCs (2.0 × 105) were plated in a 96 well dish and infected with titered lentiviral vector stock at a multiplicity of infection (MOI) of 2 in the presence or absence of 10 μM raltegravir (Fisher, Waltham, MA) or 1 μM nevirapine. After 72 hours, cells were stained with PE-conjugated anti-CD40L mAb (BD Biosciences, San Jose, CA) and the GFP+ and CD40L+ cells were quantified by flow cytometry. The differentiation state of the DCs was determined 72 hours post-infection by staining with APC-conjugated anti-CD83 and phycoerythrin (PE)-conjugated anti-CD86 mAbs (BioLegend, San Diego, CA) and measuring supernatant IL-12p70, TNF-α, IL-6, IL-1β, and IL-10 using the Human Inflammatory Cytokine Cytometric Bead Array (BD Biosciences).
T cell proliferation responses to lentiviral vector-transduced DCs
Autologous DCs (2.0 × 105) were plated in a 96 well dish and infected with lentiviral vectors at MOI=2. After 48 hours, 2.5 × 104 transduced DC were cocultured with 2.0 × 105 autologous monocyte-depleted leukocytes that had been labeled for 10 minutes with 10 μM CFSE (Invitrogen) in PBS/0.1%BSA. Cocultures were maintained for one week without cytokines and then stained with APC-conjugated anti-CD4, PE-conjugated anti-CD3, and peridinin chlorophyll protein complex (PerCP)-conjugated anti-CD8 mAbs (BioLegend) and analyzed by flow cytometry.
CTL activation assays
HLA-A*0201+ DCs (2.0 × 105) were plated in a 96 well dish and transduced with lentiviral vectors at MOI=2. After 72 hours, 5.0 × 104 DCs were harvested and transferred to a new plate to containing an equal number of HLA-A*0201-restricted CTL clone specific for M118 or SL9 (S1-SL9-3.23T).20 Maximal CTL clone stimulation was determined by pulsing the DCs with 1 μg/ml M1 or SL9 peptide (ProImmune, Oxford, England). After 24 hours, supernatant IFN-γ was measured by Th1/Th2 cytokine cytometric bead array (BD Biosciences) or Human IFN-γ Flex Set (BD Biosciences). For intracellular staining, 5.0 × 104 transduced DCs were cocultured with the 5.0 × 104 M1-specific CTLs. After one hour, 10 ng/ml brefeldin A (Sigma) was added and 7 hours later, the cells were harvested, stained with fluorescent-conjugated anti-CD3, CD8 mAbs, fixed, permeabilized, stained with PE-conjugated anti-IFN-γ (BioLegend), and analyzed by flow cytometry.
For primary CD8 T cell assays, DCs were transduced with lentiviral vectors concentrated by ultracentrifugation through a 20% sucrose cushion. After two days, 2.0 × 104 were cocultured in a 96 well dish with 2.0 × 105 autologous CD8 T cells. Cocultures were maintained for 9 days in T cell medium that was replenished every 2-3 days with fresh medium devoid of cytokines, after which the cells were harvested and stained with eFluor 450-Viability dye (eBioscience, San Diego, CA), PerCP-conjugated anti-CD8 mAb, and APC-conjugated M1 or SL9 tetramers (prepared by the NYULMC Vaccine and Cell Therapy Laboratory) and analyzed by flow cytometry. For primary CD8 T cell cytotoxicity assays, the cocultured cells were restimulated with 5 μg/ml of M1 peptide or SL9 peptide (irrelevant peptide) in the presence of monensin (BD Biosciences), brefeldin A, and APC-conjugated CD107a (BD Biosciences). Six hours post-stimulation, the cells were harvested, washed, surface stained with eFluor 450-Viability dye and PerCP-conjugated anti-CD8 mAbs and analyzed by flow cytometry.
Latent virus reactivation assay
For assays with ACH-2 cells,22, 46 5.0 × 104 lentiviral vector-transduced DCs were cocultured with 2.0 × 105 ACH-2 cells in a 96 well plate or were plated at an equivalent ratio in the upper and lower chambers of a 0.4 μM transwell plate (Corning, Corning, NY). 1 μM PMA (Sigma, St. Louis, MO) was added to one well as a positive control. After 48 hours, the culture supernatant was harvested and infectious virus was plated on 1 × 104 TZM-bl reporter cells47-49 in a 96 well plate. After two days, the plate was stained for β-galactosidase, and the spots were quantified with an ImmunoSpot S6 Micro Analyzer Elispot Reader (Cellular Technology, Ltd.). For assays with J-Lat cells,23 the supernatant from cultures of 2.0 × 105 lentiviral vector-transduced DCs was collected 72 hours post-infection, serially diluted and applied to 2.0 × 105 J-Lat cells (full-length clone 10.6) in a 96 well dish. TNF-α (R&D Systems) was added to 1 ng/ml to one well as a positive control. After 24 hours, the GFP+ cells were quantified by flow cytometry. For select assays, 40 μl TNF-α (10 ng/ml) or 40 μl of culture supernatant from lentiviral vector-transduced DCs were pretreated with 2 mg/ml anti-TNF-α mAb for one hour and then added to the J-Lat cells.
Statistical Analysis
Statistical significance was determined using two-tailed parametric t tests calculated with Graph Pad Prism 6.0e. Significance was attributed to p values as follows: *p<0.05, **p<0.01, and ***p<0.001.
Supplementary Material
Acknowledgements
The following reagents were obtained through the AIDS Reagent Program, Division of AIDS, NIAID, NIH: Nevirapine, ACH-2 cells from Thomas Folks; J-Lat cells from Eric Verdin; TZM-bl cells from John Kappes, Xiaoyun Wu, and Tranzyme Inc. We thank Otto Yang (UCLA) for CTL clone S1-SL9-3.23T, Shane Boley (NYU) for anti-TNF-α mAb, Mengling Liu (NYU) for assistance with statistical analysis, and David Raulet (U. C. Berkeley) and Megan Schultz (NYU) for critical reading of the manuscript. This work was supported by grants from the NIH (UL1TR000038, AI058864, AI067059, AI84578, AI044628, AI067854, AI43222), the Bill and Melinda Gates Foundation (38645) and NYU School of Medicine Saul Farber Scholar Fund, Grunebaum AIDS Research Scholarship, and Physician Scientist Training Program Awards to E. A. M. and T. D. N.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Material
Supplementary information is available at Gene Therapy's website.
References
- 1.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39(1):38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 3.Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Current opinion in biotechnology. 1998;9(5):457–63. doi: 10.1016/s0958-1669(98)80029-3. [DOI] [PubMed] [Google Scholar]
- 4.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–7. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 5.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nature immunology. 2012;13(3):223–8. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474(7353):658–61. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474(7353):654–7. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunseri N, O'Brien M, Bhardwaj N, Landau NR. Human immunodeficiency virus type 1 modified to package Simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells. Journal of virology. 2011;85(13):6263–74. doi: 10.1128/JVI.00346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobadilla S, Sunseri N, Landau NR. Efficient transduction of myeloid cells by an HIV-1-derived lentiviral vector that packages the Vpx accessory protein. Gene therapy. 2012 doi: 10.1038/gt.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bednarek MA, Sauma SY, Gammon MC, Porter G, Tamhankar S, Williamson AR. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. Journal of immunology. 1991;147(12):4047–53. [PubMed] [Google Scholar]
- 11.Koya RC, Kasahara N, Favaro PM, Lau R, Ta HQ, Weber JS, et al. Potent maturation of monocyte-derived dendritic cells after CD40L lentiviral gene delivery. Journal of immunotherapy. 2003;26(5):451–60. doi: 10.1097/00002371-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ., Jr. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. Journal of immunology. 1998;161(5):2094–8. [PubMed] [Google Scholar]
- 13.Tureci O, Bian H, Nestle FO, Raddrizzani L, Rosinski JA, Tassis A, et al. Cascades of transcriptional induction during dendritic cell maturation revealed by genome-wide expression analysis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(8):836–47. doi: 10.1096/fj.02-0724com. [DOI] [PubMed] [Google Scholar]
- 14.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of leukocyte biology. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 15.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, et al. Activation of human dendritic cells through CD40 cross-linking. The Journal of experimental medicine. 1994;180(4):1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160(4):1666–76. [PubMed] [Google Scholar]
- 17.Gray D, Siepmann K, Wohlleben G. CD40 ligation in B cell activation, isotype switching and memory development. Seminars in immunology. 1994;6(5):303–10. doi: 10.1006/smim.1994.1039. [DOI] [PubMed] [Google Scholar]
- 18.Fonteneau JF, Larsson M, Somersan S, Sanders C, Munz C, Kwok WW, et al. Generation of high quantities of viral and tumor-specific human CD4+ and CD8+ T-cell clones using peptide pulsed mature dendritic cells. Journal of immunological methods. 2001;258(1-2):111–26. doi: 10.1016/s0022-1759(01)00477-x. [DOI] [PubMed] [Google Scholar]
- 19.Tsomides TJ, Aldovini A, Johnson RP, Walker BD, Young RA, Eisen HN. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. The Journal of experimental medicine. 1994;180(4):1283–93. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adnan S, Balamurugan A, Trocha A, Bennett MS, Ng HL, Ali A, et al. Nef interference with HIV-1-specific CTL antiviral activity is epitope specific. Blood. 2006;108(10):3414–9. doi: 10.1182/blood-2006-06-030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. Journal of immunological methods. 2003;281(1-2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 22.Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(7):2365–8. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. The EMBO journal. 2003;22(8):1868–77. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature reviews. Immunology. 2003;3(2):133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 25.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, et al. An In-Depth Comparison of Latent HIV-1 Reactivation in Multiple Cell Model Systems and Resting CD4+ T Cells from Aviremic Patients. PLoS pathogens. 2013;9(12):e1003834. doi: 10.1371/journal.ppat.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poli G, Bressler P, Kinter A, Duh E, Timmer WC, Rabson A, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. The Journal of experimental medicine. 1990;172(1):151–8. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang FX, Xu Y, Sullivan J, Souder E, Argyris EG, Acheampong EA, et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. The Journal of clinical investigation. 2005;115(1):128–37. doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durand S, Nguyen XN, Turpin J, Cordeil S, Nazaret N, Croze S, et al. Tailored HIV-1 vectors for genetic modification of primary human dendritic cells and monocytes. Journal of virology. 2013;87(1):234–42. doi: 10.1128/JVI.01459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negri DR, Rossi A, Blasi M, Michelini Z, Leone P, Chiantore MV, et al. Simian immunodeficiency virus-Vpx for improving integrase defective lentiviral vector-based vaccines. Retrovirology. 2012;9:69. doi: 10.1186/1742-4690-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tareen SU, Kelley-Clarke B, Nicolai CJ, Cassiano LA, Nelson LT, Slough MM, et al. Design of a Novel Integration-deficient Lentivector Technology That Incorporates Genetic and Posttranslational Elements to Target Human Dendritic Cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2013 doi: 10.1038/mt.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCIDX1. The Journal of clinical investigation. 2008;118(9):3132–42. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auten MW, Huang W, Dai G, Ramsay AJ. CD40 ligand enhances immunogenicity of vector-based vaccines in immunocompetent and CD4+ T cell deficient individuals. Vaccine. 2012;30(17):2768–77. doi: 10.1016/j.vaccine.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dybul M, Mercier G, Belson M, Hallahan CW, Liu S, Perry C, et al. CD40 ligand trimer and IL-12 enhance peripheral blood mononuclear cells and CD4+ T cell proliferation and production of IFN-gamma in response to p24 antigen in HIV-infected individuals: potential contribution of anergy to HIV-specific unresponsiveness. J Immunol. 2000;165(3):1685–91. doi: 10.4049/jimmunol.165.3.1685. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Zhang S, Li M, Chen C, Yao Q. Incorporation of CD40 ligand into SHIV virus-like particles (VLP) enhances SHIV-VLP-induced dendritic cell activation and boosts immune responses against HIV. Vaccine. 2010;28(31):5114–27. doi: 10.1016/j.vaccine.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kutsch O, Levy DN, Kosloff BR, Shaw GM, Benveniste EN. CD154-CD40-induced reactivation of latent HIV-1 infection. Virology. 2003;314(1):261–70. doi: 10.1016/s0042-6822(03)00413-6. [DOI] [PubMed] [Google Scholar]
- 36.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, et al. Phase I study of recombinant human CD40 ligand in cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(13):3280–7. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 37.Miller E, Bhardwaj N. Dendritic cell dysregulation during HIV-1 infection. Immunological reviews. 2013;254(1):170–89. doi: 10.1111/imr.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller EA, Spadaccia MR, O'Brien MP, Rolnitzky L, Sabado R, Manches O, et al. Plasma factors during chronic HIV-1 infection impair IL-12 secretion by myeloid dendritic cells via a virus-independent pathway. J Acquir Immune Defic Syndr. 2012;61(5):535–44. doi: 10.1097/QAI.0b013e31826afbce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J. Rouzioux Cet al. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet. 2013 doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firat H, Tourdot S, Ureta-Vidal A, Scardino A, Suhrbier A, Buseyne F, et al. Design of a polyepitope construct for the induction of HLA-A0201-restricted HIV 1-specific CTL responses using HLA-A*0201 transgenic, H-2 class I KO mice. European journal of immunology. 2001;31(10):3064–74. doi: 10.1002/1521-4141(2001010)31:10<3064::aid-immu3064>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PloS one. 2009;4(8):e6529. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. The Journal of experimental medicine. 1991;174(2):489–92. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, et al. Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector. Nature biotechnology. 2004;22(5):589–94. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 45.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. Journal of virology. 1998;72(11):8463–71. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clouse KA, Powell D, Washington I, Poli G, Strebel K, Farrar W, et al. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142(2):431–8. [PubMed] [Google Scholar]
- 47.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, et al. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. Journal of virology. 2000;74(18):8358–67. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. Journal of virology. 2009;83(16):8289–92. doi: 10.1128/JVI.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrobial agents and chemotherapy. 2002;46(6):1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.