Abstract
Purpose
Diabetes mellitus (DM) is a group of debilitating and costly diseases with multiple serious complications. Lower urinary tract complications or Diabetic Uropathy (DU) are among the most common complications of DM, surpassing the widely recognized complications such as neuropathy and nephropathy1. DU develops in both type 1 and type 2 diabetic humans and very little is known about the natural history of these common and troublesome complications. Animal models have the potential to reveal mechanisms and aid in the development of treatment strategies.
Methods
We present a review of available animal models of DM relative to their use in the study of DU.
Results
Both large animal and small animal models of DM are available. While large animals such as dogs and swine may closely mirror the human disease in size and phenotype, the length of time between onset and development of diabetic complications and associated husbandry expenditures can make the acquisition of data from statistically valid sample sizes prohibitively expensive. In contrast small animal models (rats and mice) have much lower expenditures for larger numbers of animals, compressed observation time due to shorter lifespan and mice are readily manipulated genetically to facilitate the isolation of the effect of single genes (transgenic and knockout mice). Type 1 DM can be induced chemically using streptozotocin which is selectively toxic to pancreatic beta cells. Type 2 DM models have been developed by selective breeding for hyperglycemia with or without associated obesity. There are several well characterized and predictable animal models of DM in which the presence of DU has been demonstrated.
Conclusions
Diabetic Uropathy, including diabetic bladder dysfunction have been more frequently studied among small animals of type I diabetes. Recent availability of transgenic models provides a new opportunity for further studies of DU among mice models of both types I and II DM.
Keywords: Animal Models, Diabetes, Bladder Dysfunction, Uropathy
INTRODUCTION
Diabetes mellitus (DM) is a common and serious disease with increasing prevelance. According to the Centers for Disease Control about one of every 14 Americans, including almost one of every seven African-Americans and one of every five seniors (≥65 years old), has diabetes mellitus (DM), with roughly 30% of those undiagnosed2. Furthermore, type 1 DM, characterized by immune destruction of the pancreatic β-cells, usually strikes children and young adults and accounts for 5% to 10% of all diagnosed cases. About one of every 500 children and adolescents has Type 1 DM2. The more prevalent Type 2 DM is commonly associated with visceral obesity, increasing insulin insensitivity, and declining beta cell function. The incidence of Type 2 DM increased by 33% between 1990 and 1998 and by 75% among those 30 to 39 years of age3. Continuation of this trend is expected due to the continuing rise in obesity, a major risk factor for type 2 DM.
Both Type 1 and Type 2 diabetics live decades with the disease and are susceptible to numerous burdensome and costly complications. According to leading experts, it is indeed the complications of DM that render it a debilitating and devastating disease4. The total medical and indirect costs (work loss, disability, etc.) of DM and its complications were estimated to be $132 billion in the U.S. in 2005, accounting for about 10% of total health care costs 2.
DIABETIC UROPATHY
Diabetic Uropathy (DU) is found in more than 80% of diabetic individuals, a higher rate than that of widely recognized complications such as neuropathy and nephropathy, which affect less than 60% and 50% of patients, respectively 1. DU includes diabetic cystopathy or diabetic bladder dysfunction (DBD); sexual or erectile dysfunction (ED) and urinary tract infection (UTI) 5. Lower urinary tract symptoms (LUTS) as seen in Benign Prostatic Hyperplasia (BPH) and DM have also been considered as a part of DU6. Although DU manifestations are not life threatening, they affect quality of life substantially. Yet, little is known about the natural history, clinical presentation or pathophysiology of DU, in comparison to other also common complications of DM such as diabetic retinopathy, nephropathy and cardiovascular complications. The NIH-NIDDK Bladder Research Progress Review Group's August 2002 report notes, “Because diabetes significantly alters the urinary tract, a large portion of people who have this disease will develop costly and debilitating urologic complications” and “Unfortunately, the mechanisms involved are poorly understood. The paucity of knowledge has been a barrier to developing the best methods of prevention and treatment of urologic complications” 5.
Extensive review of clinical and laboratory status of knowledge related to DU manifestations are discussed in other articles of this issue of the Journal of Urology. Herein we focus our attention to discussion of available animal models that have and could be used for translational studies of DU.
Animal Models are a critical translational tool in the research hierarchy
The nature of DU in humans does not allow for easy access to the affected organs and tissues for experimental investigation and a creditable animal model is sorely needed. Availability of animal models in which the essential phenotypical characterization of the disease can be replicated is of outmost value. Dietary, environmental, genetic, and surgical manipulation of animals permits isolation of the many biological influences on the development and course of DM and DU. For example polyuria can be induced to study specific influence of increased urine volume without hyperglycemia and the resulting protein glycosylation and tissue damage. The natural history of the disease is also greatly compressed in time in short lived animals compared to the development of complications over decades in humans. While several species have been used in the past, the laboratory mouse is becoming a preferred model because of lower cost and the ready availability of strains precisely modified to investigate the influence of specific genes
CHALLENGES FOR PHENOTYPIC CHARACTERIZATION OF ANIMAL MODELS OF DU
The challenge in selection of an animal model for any type of human disease relates to how closely the model represents the human phenotype, and therefore the clinical applicability of the findings. Ideally, animal models of diabetes should follow the clinical situation in both the mechanisms by which diabetes is created (pathogenesis) and the resulting organ specific complications, such as DU.
The primary challenge for phenotypic characterization of animal models of DU stems from a lack of clarity on the `human phenotype' or details of manifestations of various components of DU in humans. For example, DBD has been described traditionally as a triad of decreased sensation, increased capacity and poor emptying7, but many inconsistencies with those “classic” findings have been found. In most of the asymptomatic diabetic patients they studied, Ueda et al. found increased bladder volume at first sensation to void and a decrease in detrusor contractility, with resultant increased post void residual urine volume, but they also found a 25% incidence of detrusor overactivity8. A review by Kaplan and coworkers of urodynamic findings in 182 diabetic patients revealed 55% with detrusor overactivity but only 23% with impaired contractility, with 10% of patients areflexic and 11% “indeterminate”9. The mixed clinical picture of DBD has also been revealed in recent large-scale studies, in which DM was associated with a 40–80% increased risk of urge incontinence (storage dysfunction) and a 30–80% increased risk for overflow incontinence (voiding dysfunction) in controlled multivariate analyses10. So, it is now clear that DBD manifestations are a combination of storage and voiding bladder problems. Emergence of recent experimental data allows us to contemplate the role of the following issues in phenotypic characterization of DU and DBD:
Polyuria/Diuresis
Given the plausible differences in mechanisms of pathogenesis of DU between Type I DM (T1D), and Type II DM (T2D), it is critically important to address some of those key mechanistic differences, prior to discussion of types of animal models. For example, the experimental data have shown the important role of diuresis or polyuria in initiation and progression of bladder remodeling in T1D11,12. Whereas, the presence and role of polyuria as a key mechanistic element during the early phase of bladder remodeling in T2D is unknown, as the onset and progression of polyuria in patients with T2D is unknown. The review of the published literature on clinical manifestations of T2D DBD reveals that the patients with T2D have predominantly bladder storage problems including increased frequency of urination and nocturia, bladder over activity in up to 61%, and that the establishment of T2D DBD is at least 8–9 years after the diagnosis of T2D13. In an intriguing combined clinical and experimental report, Spira et al14 explored the association between polyuria and hyperglycemia in human subjects and two experimental groups of Sprague Dawley rats. Their report was triggered by admission of a 69 year old man with non-insulin dependent DM who despite having a high serum glucose of 880 mg/DL, did not have any polyuria. In a review of 29 other diabetic patients and in animal experiments the authors' concluded that the polyuria is the result of interaction between serum and urine osmolality, and the kidney's excretory abilities, and hence in polyuria caused by hyperglycemia/diabetes urine glucose should be 300–400 mmol/L with normal renal function. The BB rat model of autoimmune type 1 diabetes was used15 (described below) and high glucose concentration was infused to normal and BB rats while the serum and urine osmolality were measured, the authors' concluded that the polyuria is the result of interaction between serum and urine osmolality, and the kidney's excretory abilities, and hence in polyuria caused by hyperglycemia/diabetes urine glucose should be 300–400 mmol/L with normal renal function. Therefore, it is plausible that the wide variability in serum glucose levels, kidney function and variation in insulin levels of patients with T2D may make the role of polyuria different from that of T1D-BD.
Obesity
Another key mechanistic difference between T1D and T2D is the presence of obesity among patients with T2D. The association between obesity and DU and particularly urinary incontinence has recently received notable attention. Obesity, quantified as high body mass index (BMI) or weight, was a positive and statistically significant correlate of urinary incontinence (UI) in eight of 12 studies examining the relationship in a MEDLINE survey of the 1980–2002 English language literature on UI prevalence worldwide. More recent studies16–20 have further validated this relationship. Of particular interest are demonstration of a cross-sectional relationship in identical twins, among whom women with BMI > 30 had a UI odds ratio (OR) of 3.1 (95% CI 1.50–6.56, p=0.02) relative to women with BMI < 2521, and substantial reductions in incontinence symptoms over 3–6 months in a small clinical trial of a liquid diet weight reduction intervention22.
Separate from the association between obesity and UI, obesity is an integral part of T2D and metabolic syndrome in humans, and thus its combined or independent role on T2DDBD needs further clarification. Thankfully, a number of investigators interested in creation of animal models of T2D have distinguished between the tendencies of the animal models for `adiposity' vs. `diabesity' phenotypes23–25.
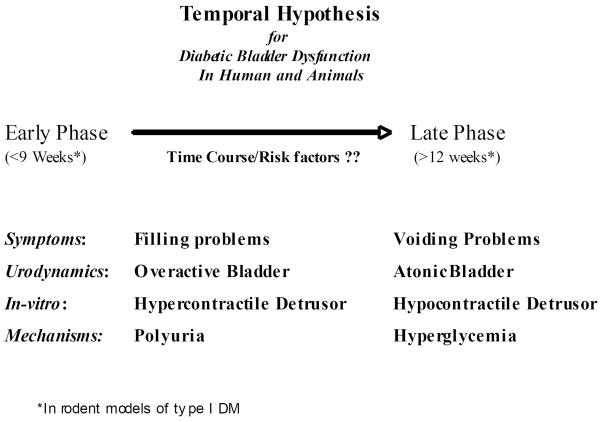
Temporal Effects of DM- another factor in consideration of phenotype of DU manifestations in animal models of DM relates to the time course of alterations in the DU manifestations (DBD, ED or UTI), as several investigators have observed that morphological and functional manifestations of DU at least in streptozotocin (STZ)-induced DM are time-dependent. We have observed that bladder hypertrophy and remodeling, increased contractility and associated neurogenic changes (expressed as bladder storage problems) occur soon after the onset of DM12,26, while bladder voiding problems, associated with a marked drop in the cystometric measure of peak voiding pressure, develop only at a later stage of DM11,27. Some of the early changes, but not the later changes, were observed after diuresis alone. Those time-dependent manifestations of DBD served as the basis for the `temporal hypothesis of DBD'27 (Figure 1) in which we have proposed that DM causes the bladder to undergo two phases of alterations via two main mechanisms: In the early phase, hyperglycemia-induced osmotic polyuria is the main mechanistic factor that causes compensatory bladder hypertrophy and associated myogenic and neurogenic alterations, which manifest as storage problems. In the later phase, accumulation of oxidative stress products during prolonged hyperglycemia causes decompensation of the bladder tissues and function, which manifest as bladder emptying problems. In DBD, the temporal hypothesis of the pathophysiology of DBD provides a potentially unifying theory under which the complex interaction between seemingly confusing bladder storage and voiding problems can be explained. Further, it provides a scientific road map under which the timing and specific roles of various components such as detrusor, urothelium, autonomic nerves and urethra can be explored. Presence or importance of temporal effects of DM on other DU manifestations has to be addressed in phenotype characterization of animal models of DM, and most importantly in translation of laboratory to clinical understanding of DU.
Figure 1.
Temporal hypothesis of DBD.
Animal Models of Types 1–2 DM
A number of animal models of DM have been used. These models could be divided to small (rodent) or large (rabbit, swine, monkeys); spontaneously occurring DM, or induced DM with STZ or alloxan induction28. While large animals may closely mirror the human disease in size and phenotype, the length of time between onset and development of diabetic complications and associated husbandry expenditures can make the acquisition of data from statistically valid sample sizes prohibitively expensive. The most reported use of large animals such as swine has been in experimental therapeutic areas including islet transplantation29. Using a rabbit model of alloxan-induced diabetes, investigators have reported evidence of DU including decrease in the contractility of the detrusor smooth muscle (DSM)30.
Small animal models of DM
Small rodent models (mouse and rat) present a convenient and yet scientifically valid hierarchical step in experimental studies of DM. The advantages of small rodent models include relatively lower husbandry costs, compression of time requirements due to comparatively short lifespans and age of onset, and the ease of genetic manipulation (transgenic and knockout mice)
T1D models
Following the generally accepted pathogenesis of pancreatic β-cell destruction leading to hyperglycemia in T1D, several methods of chemically inducing damage to the β-cells have been used successfully to create mouse models of T1D, with the toxins streptozotocin (STZ) and alloxan being the most commonly used inducers. STZ is an alkylating agent with anti-neoplastic activity that has been shown to interfere with glucose transport and, at high doses, induce multiple DNA strand breaks31. However, injection of multiple small doses of STZ in male mice gives the advantage of causing hypoinsulinemia primarily by direct destruction of the pancreatic β-cells. Creation of T1D by injection of STZ has been widely used and accepted as a valid model for T1D complications, as it causes within days a reproducible and sustainable hyperglycemia.
The three most well known genetic models of T1D are the Non Obese Diabetic (NOD) mouse and the Akita mouse and the diabetes-prone BioBreeding rat (BB-DP) another spontaneously developing autoimmune T1D model15. NOD mice develop severe, autoimmune T1D. However, the onset of DM is variable, typically between 12 and 30 weeks of age, and it develops in only about 60% of males32. A mouse model of insulin dependent diabetes without autoimmunity is provided by mice carrying a dominant mutation in the insulin Z gene (so-called “Akita” mice). Akita mice contain an inactivating missense mutation in the insulin 2 gene that results in reduced pancreatic β-cell mass and function.
T2D models
A variety of rodent models of T2D and obesity are available, involving unspecified polygenic causes of DM and either polygenic or monogenic obesity23,25,33,34. In the monogenic models, obesity results from homozygous deficiency of either leptin (ob/ob mice) or its receptor (db/db mice and Zucker diabetic fatty (ZDF) rats)23. Those animals develop obesity and thereafter follow a variable course in development of hyperglycemia and severity of other metabolic aspects of T2D, depending on genetic background. In general, either the ob/ob or db/db mutation in C57BLKS, FVB/NJ and C57BL/6 mice results in severe DM, moderate DM, or transient hyperglycemia, respectively.
ZDF rats35 are homozygous for an inactivating leptin receptor mutation and exhibit a physiological and metabolic profile similar to what is seen in human T2D36,37. However, unlike ob/ob and db/db mice, ZDF rats do not exhibit obesity due to a severe defect in glucose utilization38. Peterson has recently created a polygenic model of T2D (ZDSD) with obesity by cross breeding homozygous lean (leptin receptor +/+) ZDF rats with diet-induced obese rats39.
Several investigators have been working to create improved models of T2D in mice by breeding polygenic models that could mimic the likely polygenic pathogenesis of T2D in humans. The polygenic models are thought to be more representative of the human condition of T2D in both pathogenic pathways and clinical/metabolic manifestations. The most promising of these new mouse models is one of obesity-induced diabetes generated recently by combining independent diabetes risk-conferring quantitative trait loci from two unrelated parental strains of New Zealand Obese (NZO/HILt) and Non Obese Non Diabetic (NON/Lt) mice33. Among the various recombinant congenic strains, one, NONcNZO10/LtJ, contains the greatest number of “diabesity” contributions from both parental backgrounds wherein male mice develop a maturity-onset obesity and hyperglycemia, with more than 90% of the mice exhibiting a T2D phenotype by 12 – 16 weeks of age when fed a diet with 11% fat. Another congenic strain, NONcNZO5/LtJ, develops non-diabetogenic obesity, providing an ideal control model for distinguishing non-diabetogenic from diabetogenic obesity 24,33. However, problems in breeding the latter strain preclude distribution in large numbers. Hence, the parental NON/LtJ male fed a low fat diet is currently suggested as a control. Table 1 summarizes the available polygenic and monogenic mouse models of T2D according to route of induction (with or without diet), presence or absence of adiposity phenotype, onset of hyperglycemia or polyuria, status of Beta cells and animals lifespan. These selection criteria are used in order to identify the natural history of T2D in these mice.
Table 1.
Comparison of mouse models of obesity and diabetes (data are for male mice)
| Strain, % fat diet | Background | Obesity | Hyperglycemia/Onset | Polyuria/Onset | Beta cells | Lifespan |
|---|---|---|---|---|---|---|
| NON, NZO & crosses (JAX, Leiter) – polygenic obesity, normal leptin receptor: | ||||||
| NONcNZO10/LtJ, 6% | NON/ShiLtJ | ~42g@20wks | >85%/24wks | ?/? | atrophy, >24wks | normal |
| NONcNZO10/LtJ, 11% | NON/ShiLtJ | ~45g@20wks | 90%/8wks | ?/? | atrophy, time ? | ? |
| NONcNZO5/LtJ, 6% | NON/ShiLtJ | ~43g@20wks | no | ?/? | possible defect | normal |
| NONcNZO5/LtJ, 11% | NON/ShiLtJ | ~45g@20wks | ~10%/>20wks | ?/? | possible defect | normal? |
| NON/ShiLtJ, 6% | ↓ | no | no | ?/? | possible defect | normal |
| NON/ShiLtJ, 11% | ↓ | ~43g@20wks | no | ?/? | possible defect | normal? |
| NZO/HlLtJ, 4% | ↓ | ~54g@16wks | ~50%/20–24wks | ?/? | defect | ? |
| ob/ob (leptin mutation) & db/db (leptin receptor mutation) – monogenic obesity: | ||||||
| B6 ob/ob, 5% | C57BL/6J | ~58g@20wks | transient | yes/? | hyperplasia | 18–20mo |
| BKS ob/ob, 5% | C57BLKS/J | ~54g@20wks | 100%/2wks | probably /? | atrophy | 3–7mo |
| FVB ob/ob, 5% | FVB/NJ | ~60g@10wks | ~ 100%@10wks | probably/? | hyperplasia | normal? |
| B6 db/db, 5% | C57BL/6J | ~60g@20wks | transient | probably /? | hyperplasia | normal? |
| BKS db/db, 5% | C57BLKS/J | ~50g@10wks | 100%/4wks | yes/? | atrophy | 5–8mo |
| FVB db/db, 9% | FVB/NJ | ~60g@10wks | ~ 100%@4wks | probably/? | hyperplasia | normal? |
| Diet induced: | ||||||
| C57BL/6J, 60% | ↓ | ~50g@24wks | ~100%(250 mg/dl)/4wks | ?/? | 2nd phase defect | normal? |
| TSOD spontaneous, polygenic DM, polyuria precedes hyperglycemia, normal leptin receptor: | ||||||
| TSOD, 5% | ddY | ~65g@16wks | 100%/~20wks | yes/8wks | hyperplasia | normal |
| TSNO, 5% | ddY | ~36g@16wks | no | no | normal | normal |
Conclusions
There are a number of large and small animal models of diabetes mellitus. Diabetic Uropathy, including diabetic bladder dysfunction have been more frequently studied among small animals including mice and rat models of type I diabetes. Recent availability of transgenic models provides a new opportunity for further studies of diabetic uropathy among mice models of both types I and II DM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Daneshgari F, Moore C. Diabetic uropathy. Semin Nephrol. 2006;26:182. doi: 10.1016/j.semnephrol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . National diabetes fact sheet: general information and national estimates on diabetes in the United States. 2005. [Google Scholar]
- 3.Bladder Research Progress Review Group . Urologic complications of diabetes mellitus in overcoming bladder disease: a strategic plan for research. A report of the NIH-NIDDK bladder research progress review group; 2004. [Google Scholar]
- 4.Jozwik M, Jozwik M. The physiological basis of pelvic floor exercises in the treatment of stress urinary incontinence. Br J Obstet Gynaecol. 1998;105:1046. [PubMed] [Google Scholar]
- 5.Bladder Research Progress Review Group . Urologic complications of diabetes mellitus. 2002. p. 133. [Google Scholar]
- 6.Sarma AV, Parsons JK. Diabetes and benign prostatic hyperplasia: emerging clinical connections. Curr Urol Rep. 2009;10 doi: 10.1007/s11934-009-0044-5. In press. [DOI] [PubMed] [Google Scholar]
- 7.Frimodt-Moller C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med. 1980;92:318. doi: 10.7326/0003-4819-92-2-318. [DOI] [PubMed] [Google Scholar]
- 8.Ueda T, Tamaki M, Kageyama S, Yoshimura N, Yoshida O. Urinary incontinence among community-dwelling people aged 40 years or older in Japan: prevalence, risk factors, knowledge and self-perception. Int J Urol. 2000;7:95. doi: 10.1046/j.1442-2042.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995;153:342. doi: 10.1097/00005392-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Brown JS, Nyberg LM, Kusek JW, Burgio KL, Diokno AC, Foldspang A, et al. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases International Symposium on Epidemiologic Issues in Urinary Incontinence in Women. Am J Obstet Gynecol. 2003;188:S77. doi: 10.1067/mob.2003.353. [DOI] [PubMed] [Google Scholar]
- 11.Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006;176:380. doi: 10.1016/S0022-5347(06)00582-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R837. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- 13.Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn. 2007;26:814. doi: 10.1002/nau.20422. [DOI] [PubMed] [Google Scholar]
- 14.Spira A, Gowrishankar M, Halperin ML. Factors contributing to the degree of polyuria in a patient with poorly controlled diabetes mellitus. Am J Kidney Dis. 1997;30:829. doi: 10.1016/s0272-6386(97)90089-5. [DOI] [PubMed] [Google Scholar]
- 15.Mordes JP, Desemone J, Rossini AA. The BB rat. Diabetes/Metabolism Rev. 1987;3:725. doi: 10.1002/dmr.5610030307. [DOI] [PubMed] [Google Scholar]
- 16.Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537. doi: 10.1001/archinte.165.5.537. [DOI] [PubMed] [Google Scholar]
- 17.Uustal Fornell E, Wingren G, Kjolhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand. 2004;83:383. doi: 10.1111/j.0001-6349.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 18.Santaniello F, Giannantoni A, Cochetti G, Zucchi A, Costantini E. Body mass index and lower urinary tract symptoms in women. Arch Ital Urol Androl. 2007;79:17. [PubMed] [Google Scholar]
- 19.Han MO, Lee NY, Park HS. Abdominal obesity is associated with stress urinary incontinence in Korean women. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:35. doi: 10.1007/s00192-005-1356-8. [DOI] [PubMed] [Google Scholar]
- 20.Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339. doi: 10.1016/j.ajog.2005.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg RP, Abramov Y, Botros S, Miller JJ, Gandhi S, Nickolov A, et al. Delivery mode is a major environmental determinant of stress urinary incontinence: results of the Evanston-Northwestern Twin Sisters Study. Am J Obstet Gynecol. 2005;193:2149. doi: 10.1016/j.ajog.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 22.Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol. 2005;174:190. doi: 10.1097/01.ju.0000162056.30326.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen D, Wang MW. Development and application of rodent models for type 2 diabetes. Diabetes Obes Metab. 2005;7:307. doi: 10.1111/j.1463-1326.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- 24.Leiter EH, Reifsnyder PC, Xiao Q, Mistry J. Adipokine and insulin profiles distinguish diabetogenic and non-diabetogenic obesities in mice. Obesity. 2007;15:1961. doi: 10.1038/oby.2007.234. [DOI] [PubMed] [Google Scholar]
- 25.Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Daneshgari F. Alterations in neurogenically mediated contractile responses of urinary bladder in rats with diabetes. Am J Physiol Renal Physiol. 2005;288:F1220. doi: 10.1152/ajprenal.00449.2004. [DOI] [PubMed] [Google Scholar]
- 27.Daneshgari F, Huang X, Liu G, Bena J, Saffore L, Powell CT. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1728. doi: 10.1152/ajpregu.00654.2005. [DOI] [PubMed] [Google Scholar]
- 28.Jensen-Waern M, Andersson M, Kruse R, Nilsson B, Larsson R, Korsgren O, et al. Effects of streptozotocin-induced diabetes in domestic pigs with focus on the amino acid metabolism. Lab Anim. 2009 doi: 10.1258/la.2008.008069. [DOI] [PubMed] [Google Scholar]
- 28.Wszola M, Berman A, Fabisiak M, Domagala P, Zmudzka M, Kieszek R, et al. TransEndoscopic Gastric SubMucosa Islet Transplantation (eGSM-ITx) in pigs with streptozotocine induced diabetes - technical aspects of the procedure - preliminary report. Ann Transplant. 2009;14:45. [PubMed] [Google Scholar]
- 30.Changolkar AK, Hypolite JA, Disanto M, Oates PJ, Wein AJ, Chacko S. Diabetes induced decrease in detrusor smooth muscle force is associated with oxidative stress and overactivity of aldose reductase. J Urol. 2005;173:309. doi: 10.1097/01.ju.0000141583.31183.7a. [DOI] [PubMed] [Google Scholar]
- 31.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22:359. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 33.Leiter EH, Reifsnyder PC. Differential levels of diabetogenic stress in two new mouse models of obesity and type 2 diabetes. Diabetes. 2004;53:S4. doi: 10.2337/diabetes.53.2007.s4. [DOI] [PubMed] [Google Scholar]
- 34.Diabetes and obesity. 2008:1. http://jaxmice.jax.org/strain/001290.html.
- 35.Wijekoon EP, Hall B, Ratnam S, Brosnan ME, Zeisel SH, Brosnan JT. Homocysteine metabolism in ZDF (type 2) diabetic rats. Diabetes. 2005;54:3245. doi: 10.2337/diabetes.54.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizuno M, Sada T, Kato M, Koike H. Renoprotective effects of blockade of angiotensin II AT1 receptors in an animal model of type 2 diabetes. Hypertens Res. 2002;25:271. doi: 10.1291/hypres.25.271. [DOI] [PubMed] [Google Scholar]
- 37.Hoshi S, Shu Y, Yoshida F, Inagaki T, Sonoda J, Watanabe T, et al. Podocyte injury promotes progressive nephropathy in zucker diabetic fatty rats. Lab Invest. 2002;82:25. doi: 10.1038/labinvest.3780392. [DOI] [PubMed] [Google Scholar]
- 38.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;291:H1780. doi: 10.1152/ajpheart.01297.2005. [DOI] [PubMed] [Google Scholar]
- 39.Peterson R. Personal communication. Feb, 2008.