Abstract
Background:
A number of biochemical predictors of preeclampsia have been reported, but little is known about their possible relationship with maternal and fetal outcomes. This study determined serum copeptin in pregnant women with preeclampsia and assessed its relationship with pregnancy outcomes.
Materials and Methods:
Thirty women with severe preeclampsia (SP), 30 with mild preeclampsia (MP), and 30 with uncomplicated pregnancy were enrolled into this study. Serum copeptin, creatinine, and liver function were determined using enzyme-linked immunosorbent assay and colorimetry as appropriate. Pregnancy outcomes, both maternal and fetal, were taken using standard methods.
Results:
Copeptin was significantly elevated in preeclampsia subjects compared with controls and in SP compared with MP. Assessing the diagnostic property of copeptin for preeclampsia, the area under the curve for copeptin was 0.99. Nine (30%) and 3 (10%) of SP and MP, respectively had abruptio placenta while 6 (20%), 2 (6.7%), and 1 (3.3%) still births were recorded in SP, MP, and controls, respectively. Neonates of mothers with preeclampsia had significantly lower birth weight, infant length, ponderal index, and head circumference compared with neonates of the controls. Copeptin had a significant inverse relationship with birth weight, ponderal index, head circumference, Apgar score, and infant length in neonates of mothers with preeclampsia.
Conclusion:
Serum copeptin level in the third trimester could predict preeclampsia and its elevation is associated with adverse perinatal outcome.
Keywords: Abruptio placenta, Apgar score, copeptin, perinatal outcome, ponderal index, preeclampsia
INTRODUCTION
Preeclampsia is a complex pregnancy disorder that has been associated with severe maternal, fetal, and neonatal complications. It is characterized by increased blood pressure (BP), proteinuria, vasospasm, increased peripheral vascular resistance, and reduced organ perfusion. Usually, it is associated with primigravid patients during the last trimester.1,2
The incidence of preeclampsia varies worldwide. The World Health Organization estimated that the incidence of preeclampsia is 7 times higher in developing countries (2.8% of live births) than in developed countries (0.4%).
Preeclampsia continues to be a leading cause of maternal mortality in both developed and developing countries.3 It is associated with a five-fold increase in mortality, which can increase several folds when necessary intervention is delayed especially when it has progressed to eclampsia.4,5 Previous reports in Nigeria showed that preeclampsia and eclampsia are among the most common causes of maternal mortality. This has been attributed to poor health seeking behavior, availability of health care facilities, and trained personnel.6,7
Preeclampsia is diagnosed with BP of >140/90 mmHg, in combination with proteinuria (300 mg/24 h or ≥1+ by dipstick) on at least 2 occasions measured at least 4 h apart.8 It is classified as mild by the presence of proteinuria of 1+, but without evidence of end-organ damage in the patient. However, it is classified as severe by the presence of proteinuria of more than 5 g in 24 h, or ≥3+ on 2 random urine samples. Other indicators of severe preeclampsia (SP) includes oliguria of <500 ml in 24 h, pulmonary edema or cyanosis, visual or cerebral disturbance, impaired liver function, thrombocytopenia, hyperuricemia, epigastria or right upper quadrant pain and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.9,10
Although age, parity, social status, race, genetic factors, twin gestation, hydatidiform mole, obesity, smoking, hypolumberlordosis, chronic hypertension, diabetes mellitus (DM), and chronic renal disease are among several risk factors identified for preeclampsia, the mechanism by which preeclampsia develops is still not well understood.11 It has been shown that abnormal placentation, oxidative stress, vasoconstriction and thickening of vascular media, reduced uteroplacental perfusion, numerous maternal immunologic intolerance, and genetics are involved in the pathogenesis of preeclampsia.
There has been intense research on reliable biological markers that could predict the onset and even the severity of preeclampsia with a view to provide early medical intervention.12 One of such markers that have received much attention is copeptin.
Copeptin is a peptide containing 39 amino acids. It is co-secreted (in an equimolar ratio) with arginine vasopressin (AVP) from the neurohypophysis upon hemodynamic or osmotic stimuli.13 Compared to AVP, copeptin is stable both in serum and plasma at room temperature and can easily be measured as AVP surrogate.14 Earlier reports showed that copeptin is a potent predictor of preeclampsia and may be involved in its pathogenesis.10,15 Furthermore, Santillan et al.16 recently showed that elevated maternal plasma copeptin is a highly significant predictor of preeclampsia as early as the 6th week of gestation. Despite these available reports on the ability of copeptin to predict possible development of preeclampsia, there is a dearth of information on its relationship with pregnancy outcomes such as abruptio placenta, maternal death, ponderal index, Apgar score, and even fetal/neonatal death in women with preeclampsia. Thus, this serves as the basis for this study.
MATERIALS AND METHODS
Subjects
A total of 90 pregnant women were consecutively recruited into this cohort study from the Antenatal Clinic and Labor Ward of the Department of Obstetrics and Gynecology, University College Hospital (UCH), Ibadan, Nigeria. They comprise 30 women with mild preeclampsia (MP), 30 with SP, and 30 normotensive pregnant women. All the participants were drug-naïve and were enrolled in the third trimester. The participants were followed up until discharge from the hospital.
Ethical consideration
All participants were enrolled into this study after approval from the University of Ibadan/UCH (UI/UCH) Joint Ethics Committee (UI/13/0081). Written informed consent was also obtained from each participant.
Diagnosis of preeclampsia
Preeclampsia was diagnosed as defined by the National High Blood Pressure Education Program working Group on High Blood Pressure in Pregnancy.2 Briefly, it is defined as a BP of >140 mmHg systolic or >90 mmHg diastolic in a woman, who was normotensive before 20 weeks gestation, accompanied by proteinuria (0.3 g/24 h, which correlates with ≥30 mg/dl or ≥1+ reading on dipstick in a random urine determination with no evidence of urinary tract infection). Participants with BP of 160/110 mmHg or more and proteinuria of 3 + or more were classified as having SP whereas participants with BP of 140/90 mmHg or more and proteinuria of 1 + were classified as having MP. Participants considered as controls had no proteinuria and were normotensive.
Exclusion criteria
Pregnant women with human immunodeficiency virus, urinary tract infection, renal disease, DM, gestational DM, chronic hypertension, pregnancy induced hypertension multiple gestation, and those in active labor were excluded from this study. In addition, smokers, alcohol consumers, rhesus negative mothers, and pregnant women who had had any medical/surgical intervention or who had been placed on any medication to manage preeclampsia before enrolment were also excluded.
Data collection
A short structured questionnaire was administered on each participant to obtain information on demography, alcohol use, drug use, smoking habits, medications, and established diseases. In addition, the presence of seizure, duration of hospital stay due to preeclampsia, abruptio placenta, maternal death, birth weight, head circumference, Apgar score (at 5 min), ponderal index (infant weight divided by tripled length in kg/m3), and perinatal/fetal death were also documented.
Blood pressure and anthropometric measurement
Height (m) was taken using a stadiometer. Body mass index (BMI) was calculated as the ratio of prepregnancy body weight (kg) to the square of height (m2). After at least 10 min of rest, BP was obtained with the patient in supine position using a mercury sphygmomanometer. Korotkoff phases 1 and 5 were used as recommended.2
Sample collection
About 10 ml of venous blood was collected from each participant and dispensed into lithium heparin and plain bottles to obtain plasma and serum, which were stored at −20°C until analyzed. In addition, 5 ml of random urine (midstream) was collected for urinalysis using dip stick method. All samples were collected at recruitment.
Assay methodology
Serum copeptin level was measured using enzyme-linked immunosorbent assay (Glory Biosciences, USA). Levels of total protein, albumin, bilirubin, creatinine, and activities of aspartate (AST) and alanine aminotransferases (ALT) were measured colorimetrically using Randox reagents (Randox Laboratories, UK).
Statistical analysis
Data analysis was done using SPSS, version 17.0 (IBM, Armonk, NY, USA). Comparison of variables between groups was done using one-way analysis of variance (ANOVA) followed by a post-hoc test. Prediction of the diagnostic property of copeptin was done by determining the area under the receiver operating characteristic curve while Pearson's correlation was used to test the association between variables. The relationship between qualitative variables was determined using Fisher's exact test. All tests were two-tailed, and P < 0.05 was considered to be statistically significant.
RESULTS
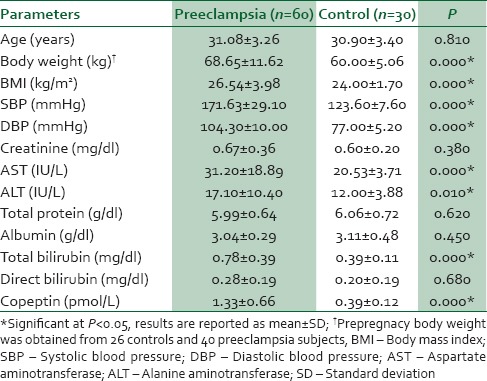
The anthropometric, clinical, and biochemical parameters of the study participants are summarized in Table 1. The mean prepregnancy body weight, BMI, systolic BP (SBP), diastolic BP (DBP), mean levels of total bilirubin and copeptin, and mean activities of AST, ALT were significantly higher in women with preeclampsia compared with the controls. The mean copeptin level was more than 3 times higher in women with preeclampsia compared with the controls.
Table 1.
Characteristics of the study participants
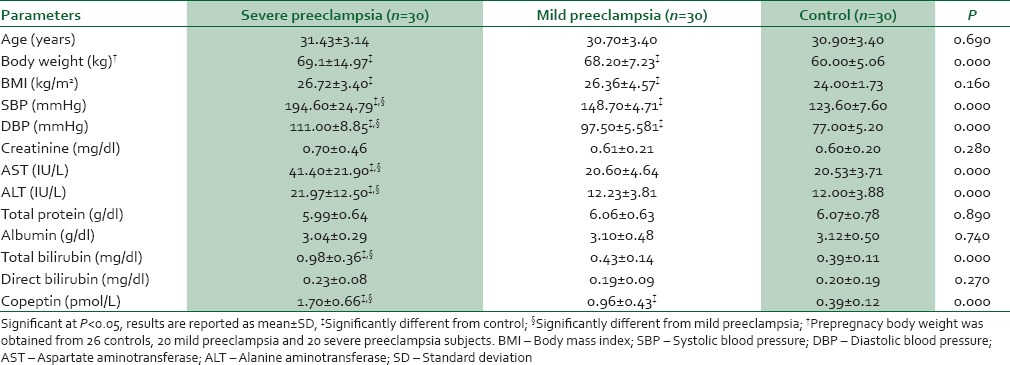
In Table 2, women with MP, SP, and controls were compared using ANOVA. It was observed that SBP, DBP, total bilirubin, copeptin, and activities of AST and ALT progressively increased from the controls through SP. Women with MP had significantly higher body weight, BMI, SBP, DBP, and copeptin level compared with controls, while body weight, BMI, SBP, DBP, mean levels of total bilirubin and copeptin, and mean activities of AST, ALT were higher in SP compared with the controls. However, SBP, DBP, mean levels of total bilirubin and copeptin, and mean activities of AST and ALT were higher in SP compared with MP.
Table 2.
Anthropometric, clinical, and biochemical data in women with severe and mild preeclampsia and controls
Assessing the diagnostic property of copeptin for preeclampsia, it was observed that the area under the curve for copeptin was 0.99 (P = 0.000) [Figure 1].
Figure 1.
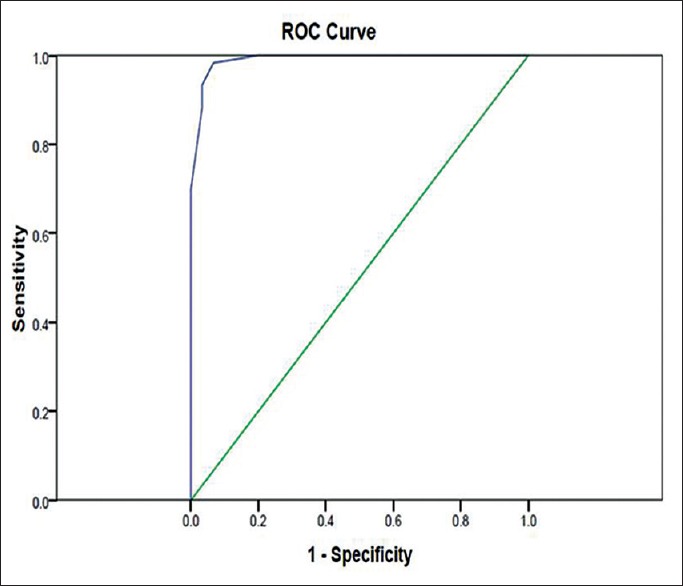
Receiver operating characteristics curve for copeptin
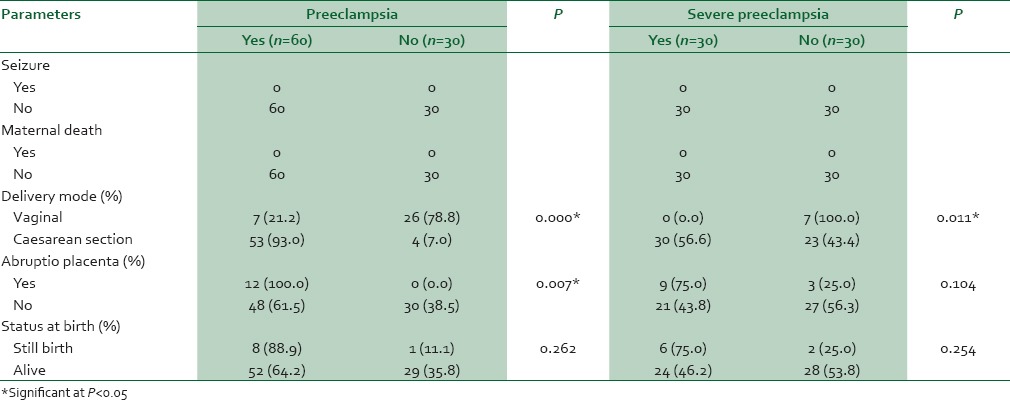
Selected outcomes are shown in Table 3. No maternal death or seizures were recorded in all the groups. None of the controls had abruptio placenta, but 9 SP and 3 MP had. In addition, 6, 2, and 1 still births were recorded in SP, MP, and controls, respectively [Table 3].
Table 3.
Maternal outcomes and fetal status at birth in women with preeclampsia and controls
In Table 4, birth weight, infant length, Apgar score, and head circumference were significantly lower in neonates of women with preeclampsia when compared with the controls. Similarly, birth weight, infant length, Apgar score, and head circumference were significantly lower in neonates of SP compared with MP [Table 5].
Table 4.
Fetal and neonatal outcomes in women with preeclampsia and controls
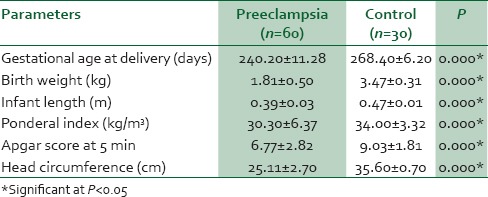
Table 5.
Fetal and neonatal outcomes in women with severe and mild preeclampsia, and controls

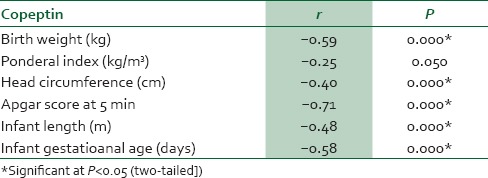
In women with preeclampsia, copeptin had significant positive correlation with SBP, DBP, proteinuria, creatinine, AST, ALT, total bilirubin, and length of hospital stay [Table 6]. However, in neonates of women with preeclampsia, copeptin had significant negative correlation with birth weight, head circumference, Apgar score, infant length, and infant gestational age [Table 7].
Table 6.
Correlation between copeptin and selected clinical and biochemical parameters, in mothers with preeclampsia
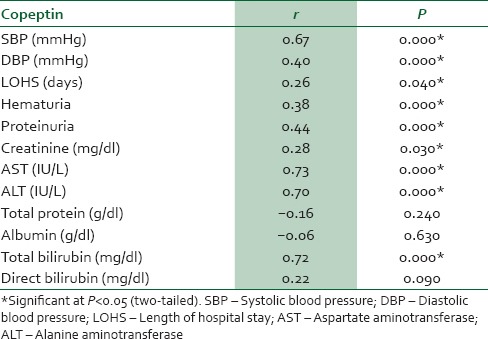
Table 7.
Correlation between copeptin and fetal/neonatal outcome from neonates of mothers with preeclampsia
DISCUSSION
Preeclampsia continues to be a major cause of maternal mortality, acute and long-term morbidities, perinatal deaths, preterm birth, and intrauterine growth restriction.5 In this study, prepregnancy body weight and BMI were elevated in women with preeclampsia compared with controls. This observation is in agreement with the report of Hauger et al.,17 which showed that high prepregnancy body weight increases the risk of developing preeclampsia.
The observed elevated BP (SBP and DBP) in women with preeclampsia compared with normotensive women and in SP compared with MP is not a novel finding. Preeclampsia has been identified as a hypertensive disorder, and hypertension is an important criterion in the diagnosis of preeclampsia. In normal pregnancy, there is a decrease in both SBP and DBP due to a decrease in systemic vascular resistance secondary to vasodilation. In preeclampsia, however, there is elevated BP due to increased level of angiotensin II accompanied by the exaggerated sensitivity of the blood vessels to the increased angiotensin II.18 Furthermore, derangement of endothelial derived vasoactive factors is thought to result in the predominance of substances that are vasoconstrictors over vasodilators in preeclampsia.
Elevated liver enzymes occur in 20–30% of pregnancies complicated by preeclampsia, and it is a component of HELLP counts syndrome which signifies SP.9 In this study, activities of AST and ALT were significantly higher in the preeclampsia group than the control group and in SP compared with MP. This observation, which could be attributed to periportal hemorrhagic lesion, indicates that the more the severity of preeclampsia, the higher the elevation in the plasma activities of the liver enzymes. Our observed higher bilirubin level in the preeclampsia group compared with control group and in SP compared with MP corroborates the report of Jaleel et al.19 This observation could be due to hemolysis, which is a component of HELLP syndrome.
The observed elevated serum copeptin level in women with preeclampsia compared with the controls and in SP compared with MP supports the report of Zulfikaroglu et al.10 They showed that plasma copeptin level was higher in women with preeclampsia compared to normotensive women and that a marked increase was observed with the severity of the disease. This observation could be due to activation of the neuroendocrine pathway involving the hypothalamic-pituitary-adrenal (HPA). Activation of the HPA axis by chronic psychosocial stress has been suggested as one of the mediators of the association between copeptin and preeclampsia.10 In addition, the observed elevated copeptin level was found to be diagnostic of preeclampsia. This observation is further supported by our observed positive correlation between copeptin and important components of preeclampsia such as SBP, DBP, proteinuria, creatinine, total bilirubin, and activities of AST and ALT in women with preeclampsia. Our observations indicate that copeptin could reliably predict preeclampsia and might be able to differentiate between MP and SP, as its concentration was higher in SP than MP. Earlier reports by Zulfikaroglu et al.10 and Santillan et al.16 showed that copeptin is a predictive biomarker for preeclampsia, as early as the first trimester, and it might be useful in the assessment of the severity of the disease.
Preeclampsia is a major obstetric problem causing substantial maternal and perinatal morbidity and mortality worldwide, especially in developing countries.2,5 Out of the 90 women recruited for this study, 9 SP and 3 MP had abruptio placenta, but no maternal death or seizures were recorded. This suggests that abruptio placenta is more associated with SP. This has been attributed to uteroplacental insufficiency.20
Low birth weight and length have been associated with increased risk of chronic diseases, mortality, and hospitalizations.21 Similarly, 5 min Apgar score <7 has been shown to have a consistent association with neurologic disability and low cognitive function in early adulthood.22 In this study, there was a higher fetal death in women with preeclampsia compared with controls. A similar observation has been reported 21 and this was attributed to the degree of hypoxia, which accompanies preeclampsia, especially when there is placental abruption which deprives the fetus of oxygen and nourishment and as a consequence, the fetus dies. The observed low birth weight, low gestational age, small infant length, low ponderal index, and small head circumference in infants of women with preeclampsia is in line with the report of Onyiriuka and Okolo.23 In addition, the observed significantly low Apgar score at 5 min in infants of women with preeclampsia supports the report of Kishwara et al.24 These poor neonatal outcomes have been attributed to uteroplacental insufficiency and inadequate transport of nutrients. These effects become more pronounced on the fetus as the pregnancy progresses and with the severity of preeclampsia, due to the inability of the uterine vasculature to keep up with the increased amount of blood and nutrients necessary for fetal development.25 Perhaps, this explains our observed poorer fetal/neonatal outcomes in infants of SP compared with MP. Therefore, the more SP is, the poorer the fetal/neonatal outcomes.
The observed significant negative correlation between maternal serum copeptin and some fetal/neonatal outcomes suggests that the higher the maternal serum level of copeptin, the lower the birth weight, ponderal index, head circumference, Apgar score, infant length, and infant gestational age. This inverse relationship could be due to stress-mediated HPA axis activation (regulated by AVP or copeptin), which may precipitate or induce other known humoral, vascular, immune, and morphological mechanisms of preeclampsia.16
It must be noted that small sample size was a major limitation of this study. Studies with a larger population are thus desirable to confirm our findings.
It could be concluded from this study that there is elevated maternal copeptin level in preeclampsia, which increases with severity. Furthermore, copeptin level in the third trimester could predict preeclampsia and its elevation is associated with adverse perinatal outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The contribution and co-operation of all the study participants and the Resident Doctors of the Departments of Obstetrics and Gynaecology and Paediatrics, University College Hospital, Ibadan, is highly appreciated.
REFERENCES
- 1.McLaughlin MK, Roberts JM. Hemodynamic changes. In: Lindheimer MD, Roberts JM, Cunningham FG, editors. Chesley's Hypertensive Disorders in Pregnancy. 2nd ed. Stamford, Conn: Appleton and Lange; 1999. pp. 69–102. [Google Scholar]
- 2.Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–22. [PubMed] [Google Scholar]
- 3.Vanderjagt DJ, Patel RJ, El-Nafaty AU, Melah GS, Crossey MJ, Glew RH. High-density lipoprotein and homocysteine levels correlate inversely in preeclamptic women in northern Nigeria. Acta Obstet Gynecol Scand. 2004;83:536–42. doi: 10.1111/j.1600-0412.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham F, Leveno K, Bloom S, Hauth J, Gilstrap L, Wenstrom K. Williams Obstetrics. 22nd ed. New York: McGraw-Hill; 2005. [Google Scholar]
- 5.Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402–10. doi: 10.1097/01.AOG.0000152351.13671.99. [DOI] [PubMed] [Google Scholar]
- 6.Adamu YM, Salihu HM, Sathiakumar N, Alexander GR. Maternal mortality in Northern Nigeria: A population-based study. Eur J Obstet Gynecol Reprod Biol. 2003;109:153–9. doi: 10.1016/s0301-2115(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 7.Tukur J, Umar BA, Rabi'u A. Pattern of eclampsia in a tertiary health facility situated in a semi-rural town in Northern Nigeria. Ann Afr Med. 2007;6:164–7. doi: 10.4103/1596-3519.55703. [DOI] [PubMed] [Google Scholar]
- 8.Lim KH. Preeclampsia. Medscape. [Last updated on 2014 Aug 05; Last cited on 2014 Sep 18]. Available from: http://www.emedicine.medscape.com/article/1476919.overview#aw2aab6b3 .
- 9.Zamorski MA, Green LA. NHBPEP report on high blood pressure in pregnancy: A summary for family physicians. Am Fam Physician. 2001;64:263–71. [PubMed] [Google Scholar]
- 10.Zulfikaroglu E, Islimye M, Tonguc EA, Payasli A, Isman F, Var T, et al. Circulating levels of copeptin, a novel biomarker in pre-eclampsia. J Obstet Gynaecol Res. 2011;37:1198–202. doi: 10.1111/j.1447-0756.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 11.Kanayama N, el Maradny E, Kajiwara Y, Maehara K, Tokunaga N, Terao T. Hypolumbarlordosis: A predisposing factor for preeclampsia. Eur J Obstet Gynecol Reprod Biol. 1997;75:115–21. doi: 10.1016/s0301-2115(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 12.Singhal SR, Deepika, Anshu, Nanda S. Maternal and perinatal outcome in severe pre-eclampsia and eclampsia. South Asian Fed Obstet Gynecol. 2009;1:25–8. [Google Scholar]
- 13.Morgenthaler NG, Struck J, Jochberger S, Dünser MW. Copeptin: Clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–9. doi: 10.1016/j.tem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Katan M, Morgenthaler N, Widmer I, Puder JJ, König C, Müller B, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29:341–6. [PubMed] [Google Scholar]
- 15.Wellmann S, Benzing J, Fleischlin S, Morgenthaler N, Fouzas S, Bührer CA, et al. Cardiovascular biomarkers in preeclampsia at triage. Fetal Diagn Ther. 2014;36:202–7. doi: 10.1159/000361016. [DOI] [PubMed] [Google Scholar]
- 16.Santillan M, Santillan D, Scroggins S, Min J, Leslie K, Hunter S, et al. Is preeclampsia all in the head? First-trimester prediction of preeclampsia via maternal plasma levels of the vasopressin pro-segment copeptin (860.20) The FASEB Journal. 2014;25(Suppl 860.20) [Google Scholar]
- 17.Hauger MS, Gibbons L, Vik T, Belizán JM. Prepregnancy weight status and the risk of adverse pregnancy outcome. Acta Obstet Gynecol Scand. 2008;87:953–9. doi: 10.1080/00016340802303349. [DOI] [PubMed] [Google Scholar]
- 18.Kim YH, Hwang HS, Kim YT, Kim HS, Park YW. Modulation of matrix metalloproteinase secretion by adenosine A3 receptor in preeclamptic villous explants. Reprod Sci. 2008;15:939–49. doi: 10.1177/1933719108322431. [DOI] [PubMed] [Google Scholar]
- 19.Jaleel A, Baseer A, Aamir S. Biochemical parameters for detection of haemolysis in pregnancy induced hypertensive women. J Coll Physicians Surg Pak. 1999;9:41–2. [Google Scholar]
- 20.Benirschke K, Kaufmann P. The Pathology of the Human Placenta. 4th ed. New York: Springer-Verlag; 2000. [Google Scholar]
- 21.Morris SS, Victora CG, Barros FC, Halpern R, Menezes AM, César JA, et al. Length and ponderal index at birth: Associations with mortality, hospitalizations, development and post-natal growth in Brazilian infants. Int J Epidemiol. 1998;27:242–7. doi: 10.1093/ije/27.2.242. [DOI] [PubMed] [Google Scholar]
- 22.Ehrenstein V, Pedersen L, Grijota M, Nielsen GL, Rothman KJ, Sørensen HT. Association of Apgar score at five minutes with long-term neurologic disability and cognitive function in a prevalence study of Danish conscripts. BMC Pregnancy Childbirth. 2009;9:14. doi: 10.1186/1471-2393-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onyiriuka AN, Okolo AA. Peri-natal outcome in patients with preeclampsia in Benin City, Nigeria. Trop J Obstet Gynaecol. 2004;21:148–52. [Google Scholar]
- 24.Kishwara S, Tanira S, Omar E, Wazed F, Ara S. Effects of preeclampsia on perinatal outcome. Bangladesh Med J. 2011;40:33–6. [Google Scholar]
- 25.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]


