Abstract
The aim of the present study was to evaluate hypnotic effect of Coriandrum sativum, Ziziphus jujuba, Lavandula angustifolia and Melissa officinalis hydroalcoholic extracts in mice to select the most effective ones for a combination formula. Three doses of the extracts (250, 500 and 1000 mg/kg of C. sativum and Z. jujuba and 200, 400 and 800 mg/kg of L. angustifolia and M. officinalis) were orally administered to male Swiss mice (20-25 g) and one hour later pentobarbital (50 mg/kg, i.p.) was injected to induce sleep. Onset of sleep and its duration were measured and compared. Control animals and reference group received vehicle (10 ml/kg, p.o.) and diazepam (3 mg/kg, i.p.), respectively. C. sativum and Z. jujuba failed to change sleep parameters. L. angustifolia at doses of 200, 400 and 800 mg/kg shortened sleep onset by 7.6%, 50% and 51.5% and prolonged sleep duration by 9.9%, 43.1% and 80.2%, respectively. Compared with control group the same doses of M. officinalis also decreased sleep onset by 24.7%, 27.5% and 51.2% and prolonged sleep duration by 37.9%, 68.7% and 131.7% respectively. Combinations of L. angustifolia and M. officinalis extracts showed additive effect and it is suggested that a preparation containing both extracts may be useful for insomnia.
Keywords: Hypnotic, Sleep, Coriandrum sativum, Ziziphus jujuba, Lavandula angustifolia, Melissa officinalis, Mice
INTRODUCTION
Sleep has a significant role in regaining mental and physical health (1). Adequate sleeping is indispensable to cognitive behaviors and accomplishes awakening (2). Insomnia is the most prevalent chief complaint in adults with sleep disorder and it means deficiency in quality or quantity of sleep relates to inadequate sleep-latency, frequent awakening, early waking or decreased sleep-efficacy (3,4). Short-term insomnia is often caused by anxiety. Usually it does not need any treatment plan but sometimes prescribing sedative or hypnotic drugs is required (1).
Some of the most popular insomnia medications include benzodiazepines, barbiturates, certain antidepressants and some of the first generation antihistamines (5). Drug dependence or drowsiness is evident as the adverse effects of these drugs. Although most of antihistamines have sedative effect, but next day-weakness and anticholinergic complications and other side effects limit their usage (6).
Hypnotic drugs can depress the central nervous system up to varying degrees. Increasing dose lead to anesthesia and greater amounts cause medulla oblongata life centers suppression and also death can be followed. So the effects are dose-related and respectively provide comfort, sleep, unconsciousness, coma and death (7).
Benzodiazepines used to induce sleep can cause some hangover effects, such as decreased ability to do certain things that requires alertness. Sensitivity to the effects of benzodiazepines increases by increasing age. Confusion and losing muscle adaptation in older adults take these drugs may lead to increased risk of hip fractures and driving accidents (8). Severe withdrawal symptoms can appear when benzodiazepines are suddenly stopped after chronic use (9).
Sedative effects of herbs have been considered traditionally and the efficacy of some of these plants has been demonstrated in recent studies.
Coriandrum sativum is used traditionally as a remedy for insomnia. Animal studies have shown that aqueous extract of C. sativum at doses of 200, 400 and 600 mg/kg and its hydroalcoholic extract at doses of 400 and 600 mg/kg increased pentobarbital-induced sleeping time compared to saline-treated group (10,11). Linalool is the major coriander mono-terpene which has shown sedative and calmative effects in animal studies and it has proven its anxiolytic effects in human ones. Hypnotic effects of other C. sativum monoterpenes like myrsine and limonene has been demonstrated in mice. Anxiolytic effects of coriander extract (100 mg/kg) in mice have been demonstrated (10,11).
Moreover, it is believed that Ziziphus spinosa also called Z. jujuba has sedative effect. In vitro studies have shown anxiolytic and sedative effect of Z. jujuba extract which is due to affinity to serotonin, benzodiazepine, dopamine and GABA receptors (12). Spinosine which is found in Z. jujuba has decreased sleep latency and increased sleep duration. Evaluation of various compounds of Jujuba extract has proved that saponins have more hypnotic effects compared with flavonoids and polysaccharides. Studies have shown that the Z. jujuba extract has sedative effects by inhibiting glutamate-mediated pathway in hippocampus (12).
Lavandula angustifolia has shown analgesic effects as aromatherapy in mice (13). It has been reported that methanol extract of L. officinalis has hypnotic effect at doses of 800 and 1000 mg/kg and sedative effect at doses of 200, 400 and 600 mg in mice (14).
Melissa officinalis is widely used in insomnia disorders (alone or combined with other herbs). Animal studies have shown that rosmarinic acid in M. officinalis has hypnotic effect (15,16).
We know that herbal drugs are usually less effective in comparison with chemical ones, and they are used generally in combination with other herbs to treat insomnia all around the world. Moreover the ecological and climate factors profoundly affect the chemical composition of herbs, so this study was conducted to find out the hypnotic effect of above plants grown in Iran alone or in combination to elucidate the possible additive or synergistic effect of a selected combination.
MATERIALS AND METHODS
Animals
Male Swiss mice weighting 20–25 g bred in animal house of School of Pharmacy (Isfahan, Iran) were used in this study. Animals were kept in standard environmental conditions of temperature and light/dark cycles and allowed free access to pelleted rodent chow and tap water. All animal experiments were approved by the Ethics Committee of Isfahan University of Medical Science and performed in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals.
Plant extracts
Hydroalcoholic extracts of the plants were provided by Barij Essence Pharmaceutical Company (Kashan, Iran). Pentobarbital was purchased from Sigma Chemical Company (USA) and diazepam was a gift from Sobhan Pharmaceutical Company (Rasht, Iran).
Sleep induction
Plant extracts were administered orally to the animals and one hour later, animals received an intraperitoneal (i.p.) injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (1% tween 80 in saline at a dose of 10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to a group of animals which served as reference group. Sleep latency characterized by losing of righting reflex was observed and recorded (10). Afterward, sleep duration of each animal was measured in a calm and quiet environment. Each group of animals contained 6-8 mice. Three doses of each extract (250, 500 and 1000 mg/kg for C. sativum and Z. jujube and 200, 400 and 800 mg/kg for L. angustifolia and M. officinalis) were checked and based on the obtained results; the combined doses were selected to consider additive or synergism effects.
Statistics
Data are presented as Mean ± SEM. Comparisons between the results of the treatment groups were done by one-way ANOVA followed by Duncan's test as Post Hoc. P<0.05 was considered as a significant difference.
RESULTS
Hypnotic effect of Coriandrum sativum
As it is seen in Fig. 1, C. sativum extract at doses of 250, 500 and 1000 mg/kg did not produce any significant change in sleep onset. Diazepam (3 mg/kg, i.p.) significantly (P<0.05) reduced pentobarbital-induced sleep onset (3.5 ± 0.9 min in diazepam group vs. 5.9 ± 0.6 min in control group). Results of the effect of C. sativum on sleep duration are demonstrated in Fig. 2. Again the three administered doses of C. sativum could not change the duration of sleep, while diazepam as the reference drug significantly (P<0.001) increased the sleep duration. The percent increase in sleep duration for diazepam was 101.8% compared with control group (Fig. 2).
Fig. 1.
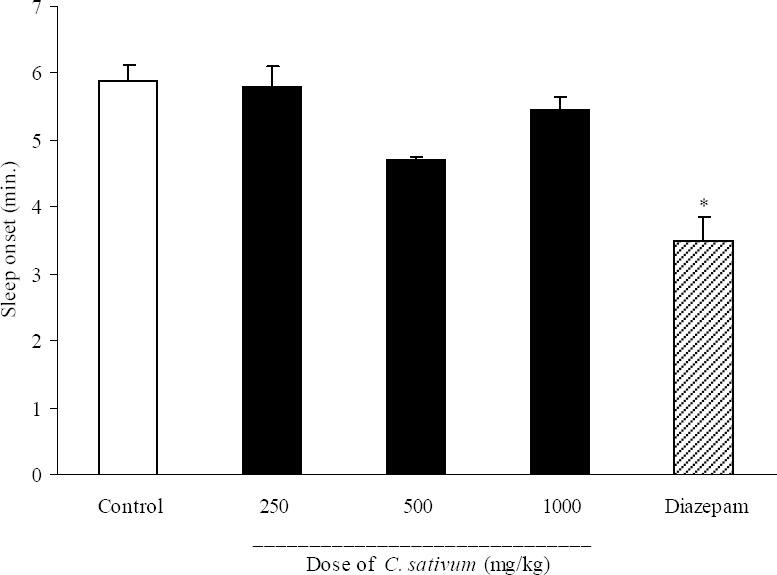
Effect of different doses of Coriandrum sativum seed extract on sleep onset in mice. Coriandrum sativum seed extract (250, 500 and 1000 mg/kg, p.o.) was administered to animals and one hour later, animals received an intraperitoneal injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to the reference group. Sleep latency characterized by losing of righting reflex was observed and recorded. Data show mean ± S.E.M. of 6-8 mice in each group. *P<0.05 compared with control group.
Fig. 2.
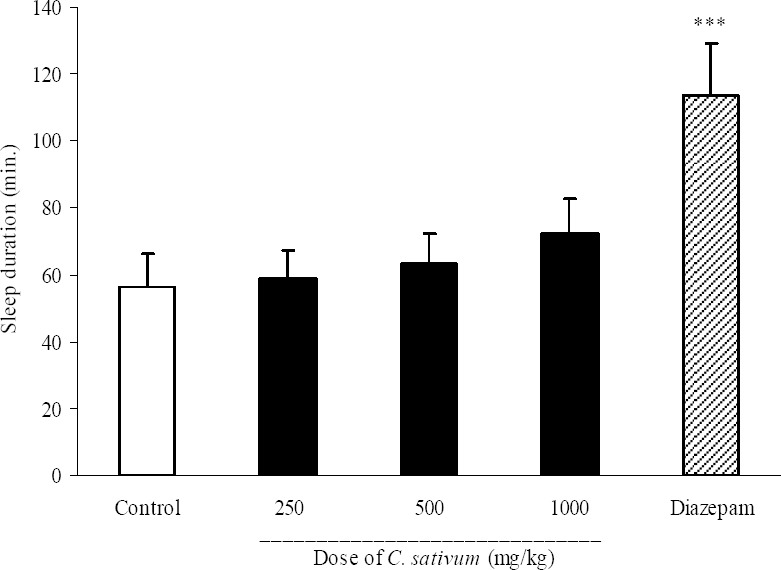
Effect of different doses of Coriandrum sativum seed extract on sleep duration in mice. Coriandrum sativum seed extract (250, 500 and 1000 mg/kg, p.o.) was administered to animals and one hour later, animals received an intraperitoneal injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to reference group. Data show mean ± S.E.M. of sleep duration of 6-8 mice in each group. ***P<0.001 compared with control group.
Hypnotic effect of Ziziphus jujuba
Results of Z. jujuba effect on sleep onset have been shown in Fig. 3. Z. jujuba extract at doses of 250, 500 and 1000 mg/kg did not make any significant change in sleep onset. Diazepam (3 mg/kg, i.p.) significantly (P<0.05) reduced pentobarbital-induced sleep onset (3.5 ± 0.9 min. in diazepam group and 5.9 ± 0.6 min. in control group). Also the three administered doses of Z. jujuba could not change the duration of sleep (Fig. 4).
Fig. 3.
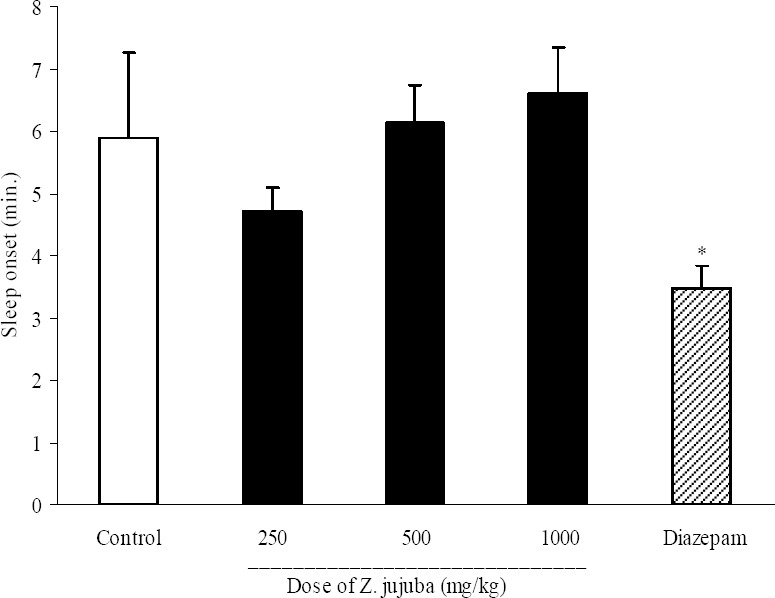
Effect of different doses of Ziziphus jujuba extract on sleep onset in mice. Ziziphus jujuba extract (250, 500 and 1000 mg/kg, p.o.) was administered to animals and one hour later, animals received an intraperitoneal injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to reference group. Sleep latency characterized by losing of righting reflex was observed and recorded. Data show mean ± S.E.M. of 6-8 mice in each group. *P<0.05 compared with control group.
Fig. 4.
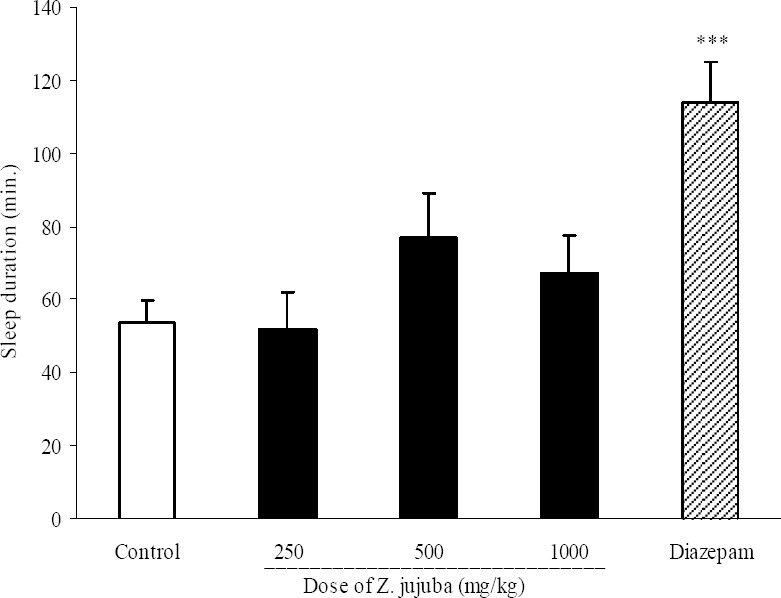
Effect of different doses of Ziziphus jujuba extract on sleep duration in mice. Ziziphus jujube extract (250, 500 and 1000 mg/kg, p.o.) was administered to animals and one hour later, animals received an intraperitoneal injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to reference group. Data show mean ± S.E.M. of sleep duration of 6-8 mice in each group. ***P<0.001 compared with control group.
Hypnotic effect of Lavandula angustifolia
As it is shown in Fig. 5, L. angustifolia extract at doses of 400 and 800 mg/kg significantly (P<0.05) reduced sleep onset latency (3.4 ± 0.9 min. in 400 mg/kg administered group, 3.2 ± 1.5 min in 800 mg/kg administered group and 5.9 ± 0.6 min in control group). Sleep duration results are demonstrated in Fig. 6. Doses of 400, 800 mg/kg and also diazepam (3 mg/kg) could change significantly the duration of sleep.
Fig. 5.
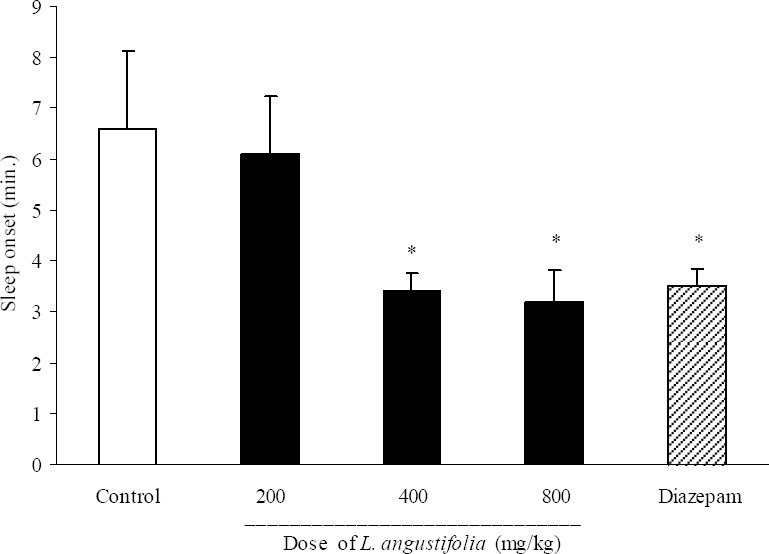
Effect of different doses of Lavandula angustifolia extract on sleep onset in mice. Lavandula angustifolia extract (200, 400 and 800 mg/kg, p.o.) was administered to animals and one hour later, animals received an intraperitoneal injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to reference group. Sleep latency characterized by losing of righting reflex was observed and recorded. Data show mean ± S.E.M. of 6-8 mice in each group. *P<0.05 compared with control group.
Fig. 6.
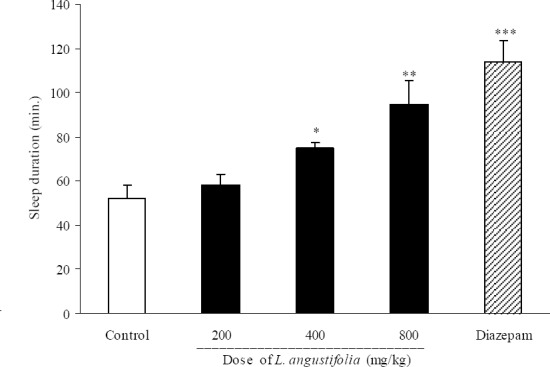
Effect of different doses of Lavandula angustifolia extract on sleep duration in mice. Lavandula angustifolia extract (200, 400 and 800 mg/kg, p.o.) was administered to animals and one hour later, animals received an intraperitoneal injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to reference group. Data show mean ± S.E.M. of sleep duration of 6-8 mice in each group. *P<0.05, **P<0.01 and ***P<0.001 compared with control group.
Hypnotic effect of Melissa officinalis
M. officinalis extract at a dose of 800 mg/kg and diazepam (3 mg/kg, i.p.) significantly (P<0.05) reduced pentobarbital-induced sleep onset (3.5 ± 1.23 min. in test group, 3.5 ± 0.9 min. in diazepam group and 5.9 ± 0.6 min. in control group) (Fig. 7).
Fig. 7.
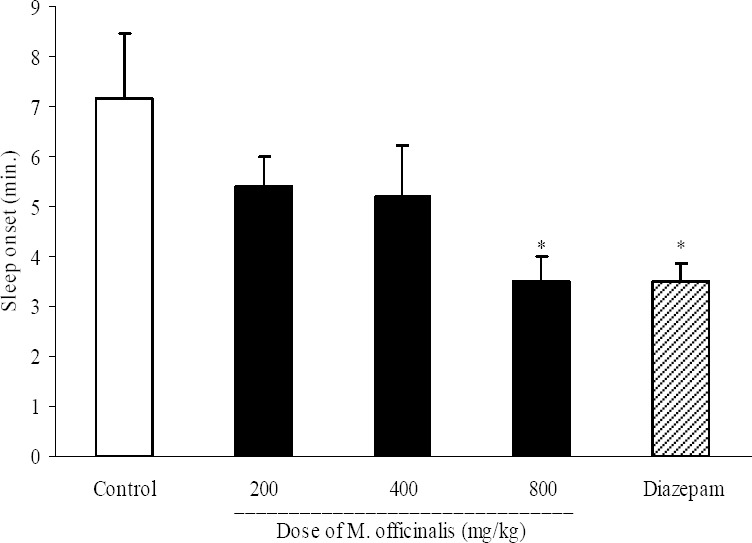
Effect of different doses of Melissa officinalis extract on sleep onset in mice. Melissa Officinalis extract (200, 400 and 800 mg/kg, p.o.) was administered to animals and one hour later, animals received an intraperitoneal injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to reference group. Sleep latency characterized by losing of righting reflex was observed and recorded. Data show mean ± S.E.M. of 6-8 mice in each group. *P<0.05 compared with control group.
Sleep duration results are illustrated in Fig. 8. Doses of 400, 800 mg/kg and also diazepam (3 mg/kg) could change significantly the duration of sleep. The effect of M. officinalis extract on sleep duration was dose dependent and the effect of a dose of 800 mg/kg of the plant extract was nearly equal to that of diazepam (3 mg/kg).
Fig. 8.
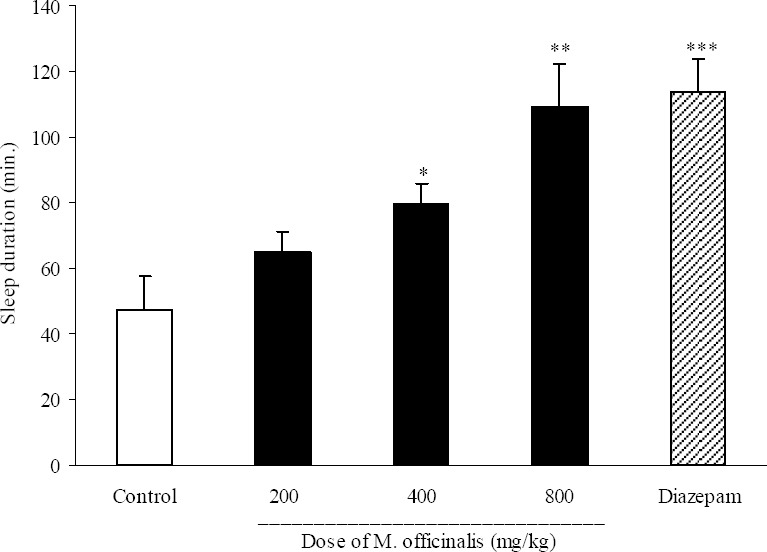
Effect of different doses of Melissa officinalis extract on sleep duration in mice. Melissa officinalis extract (200, 400 and 800 mg/kg, p.o.) was administered to animals and one hour later, animals received an intraperitoneal injection of pentobarbital (50 mg/kg) to induce sleep. Control animals received vehicle (10 ml/kg, p.o.). Diazepam (3 mg/kg, i.p.) was injected to reference group. Data show mean ± S.E.M. of sleep duration of 6-8 mice in each group. *P<0.05, **P<0.01 and ***P<0.001 compared with control group.
Hypnotic effect of combination of M. officinalis and L. angustifolia
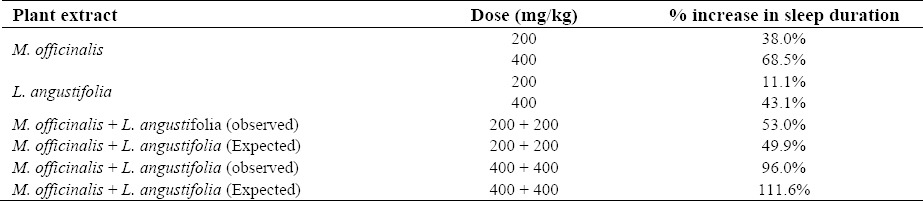
As indicated in Table 1, when combination of M. officinalis and L. angustifolia extracts was administered, the percent increase in sleep duration was close to the mathematical sum of the effects when the extracts were used alone
Table 1.
Hypnotic effect of combinations of L. angustifolia and M. officinalis extracts.
DISCUSSION
The overall objective of the current study was to compare the effect of different doses of four medicinal plants including C. sativum, Z. Jujuba, L. angustifolia and M. officinalis on the duration and onset of pentobarbital-induced sleep in laboratory mice. Traditionally these plants have been used as sleep aid (17) and so far several studies have been conducted to confirm these claims. According to the results obtained from our study, doses of 250, 500 and 1000 mg/kg of C. sativum and Z. jujuba could not produce significant changes in the duration and onset of sleep while L. angustifolia and M. officinalis significantly increased the duration of sleep and led to a decrease in the onset of sleep in tested animals in a shorter duration. Rakhshandeh and coworkers (18) reported sleep prolonging effect of hydroalcoholic, ethyl acetate, and n-butanol fractions prepared from C. sativum aerial parts in mice. In another study conducted by Emamghoreishi and colleagues (11) i.p. administration of aqueous extract of C. sativum seeds showed antianxiety effect in a plus maze model.
In our study C. sativum seed extract administered orally to mice failed to exert sleep-prolonging effect which may be due to the low absorption or high hepatic biotransformation of active constituents via oral route. Z. spinosa has shown hypnotic effect in rats (19) and it is a common ingredient of traditional herbal preparations used to treat insomnia (20). Our findings clearly demonstrated that the extract of this plant even at high doses (e.g. 1000 mg/kg) could not prolong pentobarbital-induced sleep duration and it indicates that extracts of this species are not suitable to be incorporated in sleep aid formulas.
Sedative, antioxidant, antispasmodic, carminative, antibacterial, antiviral and anti-inflammatory activities are amongst the most common therapeutic properties of M. officinalis (21,22). Our results clearly demonstrated that M. officinalis extract at a dose of 800 mg/kg potentiated pentobarbital-induced sleep duration and confirmed a previous study conducted by Souleimani and coworkers. They evaluated the sedative and hypnotic effects of M. officinalis and reported that the plant extract induced the sleep in mice after treatment with an infra-hypnotic dose of pentobarbital and potentiated the sleep induced by a hypnotic dose of pentobarbital (23). Also in agreement with our findings in another study, 600 mg of M. officinalis L. leaf extract per day improved sleep parameters in healthy volunteers (24). Our findings are also consistent with the German Commission E recommendations on the approval of M. officinalis L. extract use for nervous insomnia (25).
Several pharmacological properties including analgesic and anti-inflammatory (26), antibacterial (27), anti-allergic, and anti-asthma (28) have been reported for lavender. It has also a long history of medicinal use for CNS disorders and is believed to possess anticonvulsant, antidepressive, anxiolytic, sedative, and calming properties (29,30). In the present study, lavender shortened pentobarbital sleep induction and also prolonged its hypnotic effect which confirm the sedative and hypnotic effects reported previously (29,30).
Since both Melissa and lavandula showed hypnotic effect, combinations of 200 + 200 mg/kg and 400 + 400 mg/kg of these two plant extracts were also evaluated for the first time. As it is seen in Table 1, combinations produced an additive effect. Physicochemical interaction of active constituents, competition for oral absorption, pharmacodynamic interaction at receptor binding sites and also pharmacokinetic interaction especially at the level of metabolism may explain the lesser effect of a combination of 400 mg/kg of both extracts in comparison with mathematical sum of effects. However further investigation are needed for a definitive elucidation.
CONCLUSION
Results obtained from the present study indicate that L. angustifolia and M. officinalis have considerable hypnotic activity. Combination of these two plant extracts resulted in an increase in sleep time that is very close to mathematical sum of effects and shows a nearly additive effect. It is suggested that a combination of L. angustifolia and M. officinalis may be useful for the treatment of insomnia. Clinical trials are recommended to evaluate the possible superiority of this combination over single application of them.
ACKNOWLEDGEMENTS
This work was financially supported by Barij Essence Pharmaceutical Company (Kashan, Iran).
REFERENCES
- 1.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 2.Zambrano-Sánchez E, Martínez-Cortés JA, Dehesa-Moreno M, del Río-Carlos Y, Poblano A. Correlation between sleep disorder screening and executive dysfunction in children with attention deficit-hyperactivity disorder. Arq Neuropsiquiatr. 2013;71:896–901. doi: 10.1590/0004-282X20130174. [DOI] [PubMed] [Google Scholar]
- 3.Kim WH, Kim BS, Kim SK, Chang SM, Lee DW, Cho MJ, et al. Prevalence of insomnia and associated factors in a community sample of elderly individuals in South Korea. Int Psychogeriatr. 2013;25:1729–1737. doi: 10.1017/S1041610213000677. [DOI] [PubMed] [Google Scholar]
- 4.LeBlanc M, Beaulieu-Bonneau S, Mérette C, Savard J, Ivers H, Morin CM. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res. 2007;63:157–166. doi: 10.1016/j.jpsychores.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Kales A, Allen C, Scharf MB, Kales JD. Hypnotic drugs and their effectiveness. All-night EEG studies of insomniac subjects. Arch Gen Psychiatry. 1970;23:226–232. doi: 10.1001/archpsyc.1970.01750030034006. [DOI] [PubMed] [Google Scholar]
- 6.Reiner PB, Kamondi A. Mechanisms of antihistamine-induced sedation in the human brain: H1 receptor activation reduces backgrounda leakage potassium current. Neuroscience. 1994;59:579–588. doi: 10.1016/0306-4522(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 7.Kales A, Bixler EO, Kales JD, Scharf MB. Comparative effectiveness of nine hypnotic drugs: sleep laboratory studies. J Clin Pharmacol. 1977;17:207–213. doi: 10.1177/009127007701700404. [DOI] [PubMed] [Google Scholar]
- 8.Stewart SH, Westra HA. Benzodiazepine side-effects: from the bench to the clinic. Curr Pharm Des. 2002;8:1–3. doi: 10.2174/1381612023396708. [DOI] [PubMed] [Google Scholar]
- 9.Longo LP, Johnson B. Addiction: Part I. Benzodiazepines-side effects, abuse risk and alternatives. Am Fam Physician. 2000;61:2121–2128. [PubMed] [Google Scholar]
- 10.Emamghoreishi M, Heidari-Hamedani G. Sedative-hypnotic activity of extracts and essential oil of coriander seeds. Iran J Med Sci. 2006;31:22–27. [Google Scholar]
- 11.Emamghoreishi M, Khasaki M, Aazam MF. Coriandrum sativum: evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol. 2005;96:365–370. doi: 10.1016/j.jep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Koetter U, Barrett M, Lacher S, Abdelrahman A, Dolnick D. Interactions of Magnolia and Ziziphus extracts with selected central nervous system receptors. J Ethnopharmacol. 2009;124:421–425. doi: 10.1016/j.jep.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Soltani R, Soheilipour S, Hajhashemi V, Asghari G, Bagheri M, Molavi M. Evaluation of the effect of aromatherapy with lavender essential oil on post-tonsillectomy pain in pediatric patients: a randomized controlled trial. Int J Pediatr Otorhinolaryngol. 2013;77:1579–1581. doi: 10.1016/j.ijporl.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Alnamer R, Alaoui K, Bouidida el H, Benjouad A, Cherrah Y. Sedative and hypnotic activities of the methanolic and aqueous extracts of Lavandul aofficinalis from Morocco. Adv Pharmacol Sci 2012. 2012:270824. doi: 10.1155/2012/270824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibarra A, Feuillere N, Roller M, Lesburgere E, Beracochea D. Effects of chronic administration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice. Phytomedicine. 2010;17:397–403. doi: 10.1016/j.phymed.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Pereira P, Tysca D, Oliveira P, Silva Brum LF, Picada JN, Ardenghi P. Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacol Res. 2005;52:199–203. doi: 10.1016/j.phrs.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Amin Gh. 2nd ed. Tehran: Tchehr Publication; 2011. The most prevalent on Iranian medical herbs; pp. 4–9. [Google Scholar]
- 18.Rakhshandeh H, Sadeghnia HR, Ghorbani A. Sleep-prolonging effect of Coriandrum sativum hydro-alcoholic extract in mice. Nat Prod Res. 2012;26:2095–2098. doi: 10.1080/14786419.2011.613388. [DOI] [PubMed] [Google Scholar]
- 19.Wu SX, Zhang JX, Xu T, Li LF, Zhao SY, Lan MY. Effects of seeds, leaves and fruits of Ziziphus spinosa and jujuboside A on central nervous system function. (703-704).Zhongguo Zhong Yao ZaZhi. 1993;18:685–687. [PubMed] [Google Scholar]
- 20.Wing YK. Herbal treatment of insomnia. HKMJ. 2001;7:392–402. [PubMed] [Google Scholar]
- 21.Koch-Heitzmann I, Schultze W. Melissa officinalis L. an old medicinal plant with new therapeutic action. Dtsc Apothek Ztg. 1984;124:2137–2145. [Google Scholar]
- 22.Lamaison JL, Petitjean-Freytet C, Duband F, Carnat AP. Rosmarinic acid content and antioxidant activity of French lamiaceae. Fitoterapia. 1991;62:166–171. [Google Scholar]
- 23.Soulimani R, Fleurentin J, Mortier F, Misslin R, Derrieu G, Pelt JM. Neurotropic action of the hydroalcoholic extract of Melissa officinalis in the mouse. Planta Med. 1991;57:105–109. doi: 10.1055/s-2006-960042. [DOI] [PubMed] [Google Scholar]
- 24.Wheatley D. Medicinal plants for insomnia: a review of their pharmacology, efficacy and tolerability. J Psychopharmacol. 2005;19:414–421. doi: 10.1177/0269881105053309. [DOI] [PubMed] [Google Scholar]
- 25.Blumenthal M, Goldberg A, Brinckman J. Austin: American Botanical Council; 2000. Expanded commission E monographs. [Google Scholar]
- 26.Hajhashemi V, Ghannadi A, Sharif B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol. 2003;89:67–71. doi: 10.1016/s0378-8741(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 27.Kirmizibekmez H, Demirci B, Yeşilada E, Başer KH, Demirci F. Chemical composition and antimicrobial activity of the essential oils of Lavandula stoechas L. ssp stoechas growing wild in Turkey. Nat Prod Commun. 2009;4:1001–1006. [PubMed] [Google Scholar]
- 28.Ueno-Iio T, Shibakura M, Yokota K, Aoe M, Hyoda T, Shinohata R, et al. Lavender essential oil inhalation suppresses allergic airway inflammation and mucous cell hyperplasia in a murine model of asthma Life Sci. 2014;108:109–115. doi: 10.1016/j.lfs.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Gilani AH, Aziz N, Khan MA, Shaheen F, Jabeen Q, Siddiqui BS, et al. Ethnopharmacological evaluation of the anticonvulsant, sedative and antispasmodic activities of Lavandula stoechas L. J Ethnopharmacol. 2000;71:161–167. doi: 10.1016/s0378-8741(99)00198-1. [DOI] [PubMed] [Google Scholar]
- 30.Cavanagh HMA, Wilkinson JM. Biological activities of lavender essential oil. Phytother Res. 2002;16:301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]


