Abstract
Rosa damascena is a small plant belonging to Rosaceae family which has been used for the treatment of some inflammatory diseases and digestive disorders in the Iranian folk medicine. This study was performed to investigate the effect of R. damascena hydroalcoholic extract (RDHE) and R. damascena volatile oil (RDVO) on ulcerative colitis induced by acetic acid in rats. Different doses of RDHE (250, 500, 1000 mg/kg) and RDVO (100, 200, 400 µl/kg) were given orally (p.o.) and doses of RDHE (125, 250, 500 mg/kg) were administrated intraperitoneally (i.p.) to the male Wistar rats (n=6) 2 h before induction of colitis which continued daily for 4 successive days. Prednisolone (4 mg/kg p.o.) and dexamethasone (1 mg/kg i.p.) were used in the reference groups. Weight/length ratios of wet colon were measured and the tissues were assessed macroscopically, histopathologically, and biochemically via measuring the myeloperoxidase (MPO) activity. Oral RDHE at all doses examined, and the lowest dose of RDVO given p.o. or RDHE administered i.p. reduced all indices of colitis measured in different assays as well as the MPO activity. These results provide encouraging support for the use of hydroalcoholic extract of R. damascena in relieving alimentary inflammatory conditions and reinforce the use of this plant to develop new agents for treating ulcerative colitis.
Keywords: Rosa damascena, Inflammation, Colitis
INTRODUCTION
The inflammatory bowel disease (IBD) refers to a widely chronic gastrointestinal inflammatory condition, which is divided into Ulcerative colitis and Crohn's disease. It's a multifactorial disorder whose etiology is unknown but it's interaction among some risk factors such as immune system, genetics, and bacterial flora may contribute to the disease process (1). Main treatments for IBD are aminosalicylates (mesalamin and sulfasalazine), corticosteroids and immunosuppressive agents (2). However, due to their common adverse effects and lack of evidence on their effectiveness, problems associated with the treatment of IBD have not yet been resolved. Therefore it is imperative to explore new and safe remedies. In this direction, traditional therapies which have been in use for many years could be regarded as potential alternatives (3,4).
Rosa damascena belonging to the Rosaceae family is a small plant with aromatic light pink flowers that grows in spring (5). It is cultivated all over the world including countries such as Syria, Turkey, India, Bulgaria, and Iran (mainly in Kashan) (6,7). This plant is a rich source of flavonoids such as quercetin, kaempferol and their glycoside derivatives (8,9), myrcene, tannins, terpenes and vitamin C (7). Volatile oil obtained from R. damascena cultivated in central region of Iran is mainly comprised of ß-citronellol, nonadecane, geraniol, and docosane (10). R. damascena has traditionally been used as hypnotic (11), cardiotonic (12), cough suppressant (7), anti-inflammatory and anti-ulcer (10), mild laxative (13), and for the treatment of digestive problems (14).
In pharmacological studies, R. damascena has shown both anti-inflammatory action and analgesic effect in animal models (15). Its extract has been shown to have hepatoprotective effects against carbon tetrachloride-induced liver toxicity in rats (16). R. damascena has also been used as mouthwash for the treatment of recurrent aphtous stomatitis in a clinical trial (17). Extract and volatile oil of R. damascena have been reported to be effective as an antispasmodic agent on isolated rat ileum (18,19). Additionally there are numerous studies regarding antioxidant and antibacterial activities of R. damascena flowers (12,20).
Rosa damascena probably has beneficial effect in IBD due to its anti-inflammatory, antioxidant and antispasmodic effects. In the present study, anti-colitis effect of extract and volatile oil of R. damascena was evaluated at various doses administered both orally and intraperitoneally in rats.
METHODS AND MATERIALS
Plant material and preparation of extract and volatile oil
Flowers of the plant were purchased from a local orchard belonging to the Barij Essence Company. The plant was authenticated at the Department of Botany (Faculty of Sciences, Isfahan University, Isfahan, Iran). A voucher specimen of R. damascena (RD-112) was deposited at the Herbarium of Department of Pharmacognosy in the School of Pharmacy and Pharmaceutical Sciences of Isfahan University of Medical Sciences.
Air-dried and finely powdered flowers of the plant (200 g) were macerated with 3000 ml of ethanol (70:30) for 48 h. It was then shaken, filtrated and evaporated in a rotary evaporator under reduced pressure until a semisolid extract was obtained. Afterwards the concentrated extract was freeze-dried to obtain a dry powdered extract according to the British Pharmacopoeia (21,22).
The R. damascena volatile oil (RDVO) was achieved by hydro-distillation of flowers of R. damascena according to the European Pharmacopeia (23) in Barij Essence Co. in Mashhad-Ardehal (Kashan, Iran).
Total phenol assay of the extract
Total phenolic compounds of the R. damascena hydroalcoholic extract (RDHE) of were determined using Folin-Ciocalteu reagent described by Waterhouse and coworkers (24,25). Results are expressed as gallic acid equivalent in mg (GAE)/100 g of the test sample.
Chemicals
Prednisolone and dexamethasone were purchased from Iran Hormone Pharmaceutical Co. (Tehran, Iran). o-dianisidine dihydrochloride and hexadecyltrimethyl-ammonium bromide were procured from Sigma (St. Louis, USA). All the organic solvents and acetic acid were obtained from Merck Company (Darmschtdat, Germany).
Animals
Male Wister rats weighting 230 ± 30 g were used in this study. Animals were housed in groups of six each in wire-bottomed cages under uniform and controlled conditions of temperatures (20-22 °C), humidity and light/dark cycles. They had free access to food and water ad libitum. All animal experiments were approved by the Ethics Committee of Isfahan University of Medical Science and performed in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals (26).
Animal grouping
Fifteen groups of animals were randomly assigned to sham, control, test, and reference groups as following:
Sham groups, treated with vehicle 5 ml/kg per oral (p.o.) and 2 ml/kg intraperitoneal (i.p.) without induction of colitis.
Control groups, treated with vehicle 5 ml/kg p.o. and 2 ml/kg i.p. before induction of colitis.
Volatile oil groups, treated with RDVO p.o. at doses of 100, 200, and 400 µl/kg.
Extract groups, treated with RDHE p.o. at doses of 250, 500, 1000 mg/kg, and i.p. at doses of 125, 250, 500 mg/kg.
Reference groups, treated with prednisolone (4 mg/kg) p.o. and with dexamethasone (1 mg/kg) i.p.
All doses were administrated 2 h before the induction of colitis and continued daily for 4 successive days.
Experimental procedure
Test samples containing suspension of reference drugs and plant extract or emulsion of volatile oil were made freshly using 0.2% tween 40 in distilled water as vehicle, for oral and intraperitoneal administration.
Acute colitis was induced by 2 ml acetic acid (4%) using Mascolo and colleagues method (27). Rats were fasted for 36 h with free access to tap water and were lightly anesthetized with diethylether. Then a rubber plastic catheter with 2 mm in diameter was inserted into the colon, 8 cm proximal to the anus. In sham and control groups, normal saline (2 ml) was instilled. To prevent anal leakage of acid, rats were held in a head-down position for 1 min.
Evaluation of colon macroscopic damage
Rats were euthanized by overdosing of diethylether at 5th day of the experiment; the abdomen was opened and the colon, 8 cm in length and 2 cm proximal to the anus, was excised and incised longitudinally and washed with normal saline. Then the wet colon was weighed and weight/length ratio was determined for each sample.
The tissue was fixed on a transparent sheet and a photo was taken by Sony® camera, Japan. Ulcer area was measured by Fiji Image Processor, China (28). Macroscopic mucosal damage was evaluated using a validated grading scale according to Morris and coworkers (29). Scores were: 0; no ulcer, 1; mucosal erythema only, 2; mild mucosal edema, slight bleeding or slight erosion, 3; moderate edema, bleeding ulcers or erosions, 4; severe ulceration, erosions, edema and tissue necrosis and/or perforation. Ulcer index was detrmined by summing the ulcer score and the ulcer area (cm2) for each colon.
Evaluation of colon histological damage
For further determinations, the samples of tissue were cut into two equal parts along its length, a part was immediately stored at -80 °C for biochemical analysis (myeloperoxidase (MPO) assessments) and the other part was fixed in 10% formalin for pathological evaluation.
Fixed colon tissue was dehydrated, cleared, paraffin embedded, blocked, sectioned in 4-µm thick sections, and stained with haemotoxyline and eosine (H & E). Inflammation severity and extent and crypt damage were specified on H & E stained and coded sections using a validated scoring system described by Cooper and coworkers (30) and Dieleman and colleagues (31). Total colitis index was calculated as the sum of 3 following sub-scores (inflammation severity, inflammation extent and crypt damage). Pathological assessment and scoring was done by using a Zeiss® microscope equipped with a Sony® color video camera for digital imaging.
Evaluation of colonic myeloperoxidase activity
MPO activity was measured according to the modified method of Bradley and coworkers (32). In brief, 0.1 mg of tissue was weighed and homogenized in 1 ml solution containing 0.5% w/v hexadecyltrimethyl-ammonium bromide, dissolved in 50 µM potassium phosphate buffer (pH=6) in an ice bath for 4 × 45 s at 1 min intervals. Then subjected to freezing and thawing for 3 times and sonicated and centrifuged for 15 min at 15000 rpm. To measure the MPO activity, 0.1 ml of the supernatant was added to 2.9 ml of 50 µM phosphate buffer (pH 6) containing 0.167 g o-dianisidine dihydrochloride and 0.005% hydrogen peroxide. The absorbance of the mixture was measured at 450 nm using UV-Vis spectrophotometer (PerkinElmer Co., Germany). The MPO activity was expressed in units (U) per gram of the wet tissue.
Statistical analysis
Data analysis was accomplished by SPSS (version 21) statistical software. Results were expressed as the mean ± standard error of the mean (SEM). The data of weight changes were analyzed using paired student's t-test while other parametric data were analyzed by one-way analysis of variance (ANOVA) with Scheffe as post hoc test. Mann-Whitney U test was used for analysis of non-parametric data. The minimal level of significance was identified at P<0.05.
RESULTS
Pharmacognosy
The hydroalcoholic extract after freeze drying yielded 33% (w/w) dried extract. The total phenol compounds determined by Folin-Ciocalteu reagent showed 15.7 ± 0.2 g of GAE/100 g of the plant.
Macroscopic assessment
Macroscopic observation in control group showed maximum ulcer severity, ulcer area and weight/length ratio, which are indicative of highest level of damage produced by acetic acid compared to sham (normal) group that showed no change (Table 1). Data from the group treated with prednisolone p.o. and dexamethasone i.p. as positive controls showed significant healing (P<0.001) in all macroscopic assessments (Table 1). Pretreatment with RDVO 100 and 200 µl/kg p.o. were effective to reduce all macroscopic parameters including ulcer area, severity and index, and weight/length ratio (at least P<0.05), but at the dose of 400 µl/kg was not effective (P>0.05).
Table 1.
Effects of R. damascena Hydroalcoholic extract (RDHE) and R. damascena volatile oil (RDVO) on macroscopic parameters of colitis induced by acetic acid in rats.
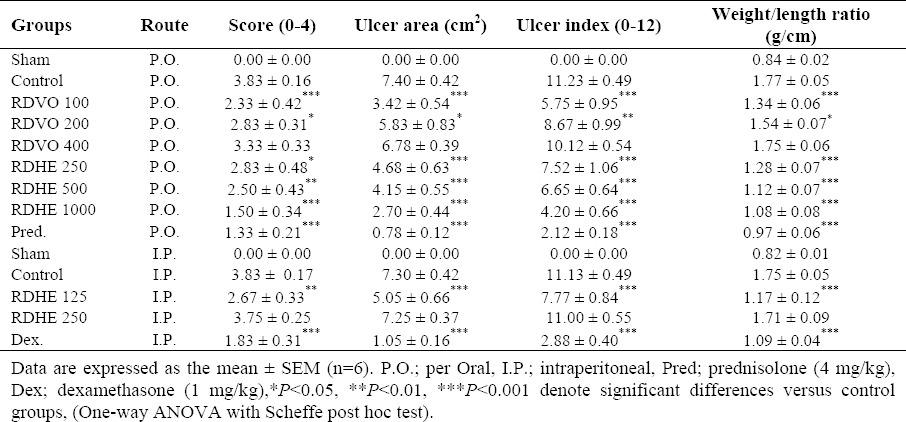
In the groups treated with extract p.o., on the other hand, all the treatments with increasing doses of RDHE were effective to reduce weight/length ratio and ulcer index in colon sample compared to control group (at least P<0.05), and the best results were achieved with RDHE at1000 mg/kg.
Also pretreatment with three doses of RDHE i.p. caused an increase in all macroscopic parameters, leading to death in RDHE 500 mg/kg, i.p. group, with the exception of 125 mg/kg, i.p. which had beneficial effects on colitis parameters (P<0.01) (Table 1).
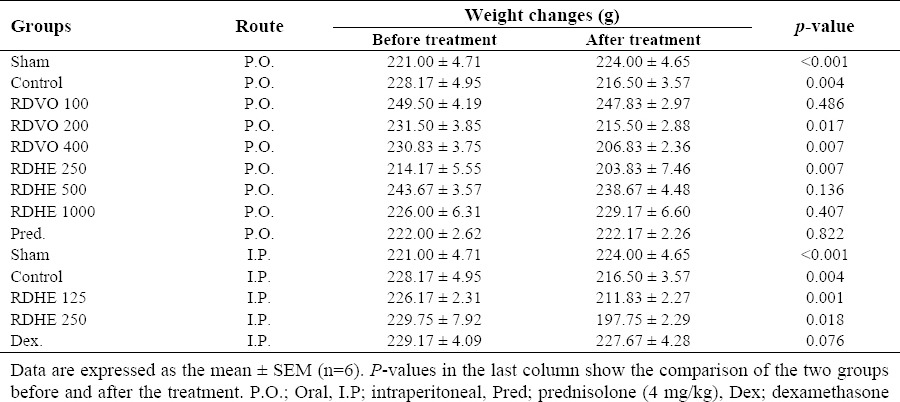
Weight variation
Changes in mean weights before and after the treatment in groups showed that increasing the dose of volatile oil decreased the mean body weight that is not significant with RDVO 100 µl/kg (P>0.05), but at doses of 200 and 400 µl/kg, the differences are significant (P<0.05).
In contrast, groups that are treated with RDHE, reduction in mean body weight is considerably diminished with increasing doses of RDHE giving orally. Then at doses of 1000 and 500 mg/kg p.o. the differences are not significant (P>0.05).
Similar to macroscopic findings, by increasing dose of RDHE administered i.p., weight loss increased leading to death of the animals (data not shown). Body weight reductions were significant (P<0.05) in groups treated i.p. in a dose- related manner (Table 2).
Table 2.
Effects of R. damascena hydroalcoholic extract (RDHE) and R. damascena volatile oil (RDVO) on mean animal weight before and after treatment in rats.
Histological assessments
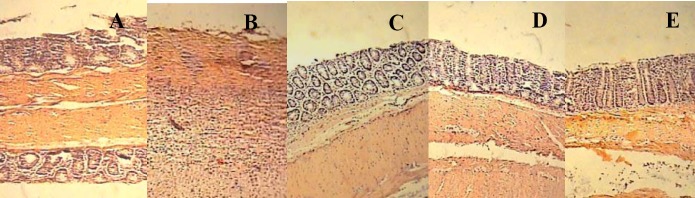
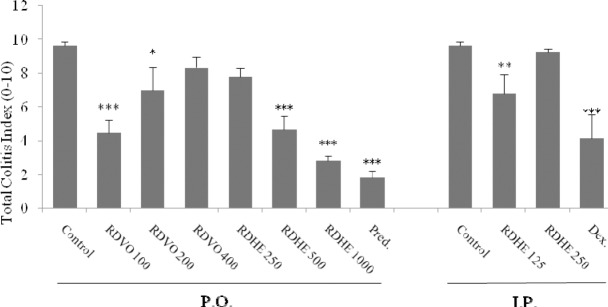
According to Figs. 1 and 2 no histological damage was observed in sham group. In control groups, microscopic assessments revealed severe inflammation and infiltration of white blood cells in mucus and sub-mucosal layers. Pretreatment with prednisolone p.o. and dexamethasone i.p. reduced total colitis index (inflammation severity, inflammation extent, and crypt damage) in injurious colons (P<0.05). As shown in Figs. 1 and 2, the best results are obtained with the smallest does of RDVO and parenteral RDHE and the highest dose of RDHE p.o., considering all histological parameters of colon inflammation and edema (P<0.001).
Fig. 1.
Microscopic presentation of acetic acid-induced colitis in rats. H & E staining with low power. A; Sham, normal colon treated with vehicle, 5 ml/kg; mucus layer and crypts are normal and there is no leucocyte infiltration. B; Control colitis treated with vehicle, 5 ml/kg; mucosal and submucusal inflammation as well as crypt damage and leucocyte infiltration are evident, C; R. damascena volatile oil (RDVO) treated colitis 100 μl/kg, D; R. damascena hydroalcoholic extract (RDHE) treated colitis, 1000 mg/kg; E; Prednisolone treated colitis, 4 mg/kg.
Fig. 2.
Effects of R. damascena hydroalcoholic extract (RDHE) and R. damascena volatile oil (RDVO) on total colitis index induced by acetic acid. The results are expresses as the mean ± SEM, (n=6), P.O.; Oral, I.P.; intraperitoneal, Pred; Prednisolone (4 mg/kg), Dex; dexamethasone (1 mg/kg), *P<0.05, **P<0.01, ***P<0.001 denote significant differences versus control groups, (One-way ANOVA with Scheffe post hoc test).
Biochemical assessment
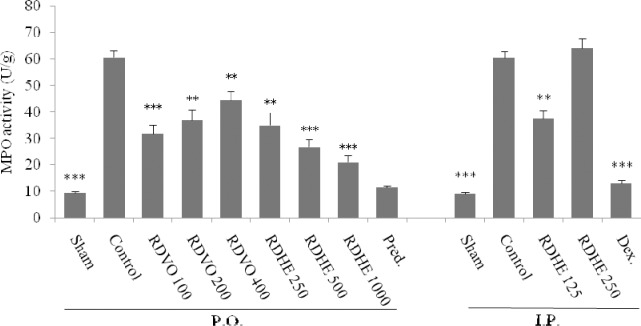
Colitis caused by acetic acid is invariably associated with an increase in tissue MPO activity. As it is shown in Fig. 3, the greatest decline in MPO activity was obtained at lowest doses of RDVO and RDHE i.p. while the highest dose of the RDHE after oral administration had the most activity.
Fig. 3.
Effect of R. damascena hydroalcoholic extract (RDHE) and R. damascena volatile oil (RDVO) on myeloperoxidase activity (MPO, U/g wet tissue) of colon tissue 4 days after acetic acid instillation in rats. The results are expressed as the mean ± SEM, (n=6). P.O.=Oral, I.P.; intraperitoneal, Pred; prednisolone (4 mg/kg), Dex; dexamethasone (1 mg/kg). *P<0.05, **P<0.01, ***P<0.001 denote significant difference versus control groups, (Oneway ANOVA with Scheffe post hoc test).
DISCUSSION
Based on the recent studies, it seems that animal model of IBD has played an important role in delineating disease mechanisms and in appraisal and establishment of new therapeutic agents (33). Acetic acid induced colitis is an easy, available, and cost-effective model with many resemblances to human IBD in terms of pathogenesis, histopathological features and inflammatory mediator profile, rendering it as a beneficial tool for the screening of drugs with anti-colitic activity (34).
The effects of several herbal medicines have been studied on acetic acid-induced colitis such as Cydonia oblonga (22), Rosmarinus officinalis (25) and Prunus armeniaca (35). In the present study, the effects of RDHE and RDVO via two routes of administration on rat colitis were investigated. According to the macroscopic and histopathological assessments, control groups showed the maximum levels of inflammation, tissue necrosis, and infiltration of the immune cells. Reference treated groups demonstrated a significant reduction in all macroscopic and microscopic outputs.
The results showed significant healing for both orally administered RDHE and RDVO, although the effect of RDHE was better and more reliable compared to the effect of RDVO. With increasing doses of hydroalcoholic extract administered p.o., all macroscopic and histopathological parameters were improved dose dependently, while the opposite observed with volatile oil groups. Indeed, by increasing the dose of intraperitoneal administration of the extract no signs of healing were achieved and led to the death of some animals at the dose of 500 mg/kg.
It is assumed that orally treated groups with RDHE demonstrated better outcomes rather than those treated intraperitoneally, which can be attributed to some noxious ingredients that may exist in the extract which could not be absorbed in the GI tract after oral administration but in parenteral route they are readily available in animal body and could exert their harmful interfering effects.
A literature survey revealed that hypnotic, anti-seizure, and laxative effects of R. damascene extract has been investigated following intraperitoneal injection (36,37,38). However, in these studies, 500 to1000 mg/kg of the extract were given as single doses and the animals did not follow up for more than one day. It is interesting that i.p. administration of R. damascena extract caused diarrhea in one study and we know that diarrhea could be harmful in IBD and this might explain our results after parenteral administration (38).
Flowers of R. damascena are rich sources of flavonoids, polyphenols and vitamin C (8,9), and it has been reported that flavonoids and polyphenols have anti-inflammatory effects, especially in gastrointestinal tract (39). Anti-inflammatory properties of flavonoids have been conducted in many studies too (40,41,42). Moreover antioxidant activity of flavonoids in R. damascena flowers (12), and flavonoids in general (43,44) have been proven. Flavonoids also have anti-ulcer action. Quercetin, one of the main flavonoids of R. damascena (12), inhibited gastric damage produced by acidified ethanol in rats (45). As a result, these compounds may have a significant role in anti-colitis effects of RDHE.
According to GC-Mass analysis of RDVO (from Barij Essence), the major components were citronellol and geraniol (19). It has been reported that citronellol and geraniol are responsible for anti-inflammatory and antispasmodic effects of R. damascena volatile oil (19,46). Additionally, anti-inflammatory and immunomodulatory properties of geraniol (47,48) and anti-inflammatory effects of citronellol (49) have been separately investigated. In this study, only the lowest doses of RDVO could alleviate the colitis indices. In the study conducted by Hajhashemi and colleagues (15) investigating the analgesic and anti-inflammatory effects of RDVO, no anti-inflammatory effect was observed with the same doses (100-400 µl/kg) of the volatile oil and the smallest test dose was able to exert analgesic property. Therefore, there are a probable interaction between some active ingredients in volatile oil that oppose with therapeutic actions of citronellol and geraniol. This result suggests that volatile oil should be administrated with dose adjustment.
CONCLUSION
In conclusion, our results clearly demonstrate that oral administration of RDHE has a potent therapeutic activity as an alternative and/or complementary medicine in the management of IBD. More detailed studies are recommended to clarify the underlying mechanisms of anti-inflammatory effect of this plant and to identify the active ingredients which are responsible for its beneficial pharmacological properties.
ACKNOWLEDGMENTS
We are grateful to Dr. Sadraei for the gift of R. damascena volatile oil. The content of this paper is extracted from the Pharm.D thesis NO. 393319 submitted by Gh. Latifi which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Sellin JH, Pasricha PJ. Brunton LL, Lazo JS, Parker KL. Goodman & Gilman's the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill companies; 2006. Pharmacotherapy of inflammatory bowel diseases; pp. 1009–1011. [Google Scholar]
- 2.McQuaid KR. Katzung BG. Basic and clinical pharmacology. 10th ed. New York: McGraw Hill Company; 2007. Drugs used in the treatment of gastrointestinal disease; pp. 1029–1035. [Google Scholar]
- 3.Jagtap AG, Shirke SS, Phadke AS. Effect of polyherbal formulation on experimental models of inflammatory bowel diseases. J Ethnopharmacol. 2004;90:195–204. doi: 10.1016/j.jep.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 4.Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asia Pac J Clin Nutr. 2006;15:143–152. [PubMed] [Google Scholar]
- 5.Mozaffarian V. Tehran: Farhang Mo’aser Publications; 1995. A dictionary of Iranian Plant Names; p. 462. [Google Scholar]
- 6.Groom N. Fulham, London: Blackie Academic Professional; 1997. The perfume handbook; p. 292. [Google Scholar]
- 7.Libster M. Delmar: Thomson Learing Alby; 2002. Delmars integrative herb guide for nurses; pp. 360–370. [Google Scholar]
- 8.Mahmood N, Piacenet S, Pizza C, Bruke A, Khan A, Hay A. The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem Biophys Res Commun. 1996;229:73–79. doi: 10.1006/bbrc.1996.1759. [DOI] [PubMed] [Google Scholar]
- 9.Schiber A, Mihalev K, Berardini N, Mollov P, Carle R. Flavonol glycosides from distilled petals of Rosa damascena Mill. Z Naturforsch C. 2005;60:379–384. doi: 10.1515/znc-2005-5-602. [DOI] [PubMed] [Google Scholar]
- 10.Loghmani-Khozani H, SabziFini O, Safari J. Essential oil composition of Rosa damascena Mill. Cultivated in central Iran. Scientia Iranica. 2007;14:316–319. [Google Scholar]
- 11.Price S, Price L. 2nd ed. London: Churchill Livingston; 1999. Aromatherapy for health professionals; p. 341. [Google Scholar]
- 12.Yassa N, Masoomi F, Rohani Rankouhi SE, Hadjiakhoondi A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan. DARU. 2009;17:175–180. [Google Scholar]
- 13.Zargari A. 5th ed. Tehran: Tehran University Press; 1992. Medicinal plants; pp. 281–284. [Google Scholar]
- 14.Avecina A. Tehran: Soroush; 1990. Law in medicine; pp. 129–131. [Google Scholar]
- 15.Hajhashemi V, Ghannadi A, Hajiloo M. Analgesic and anti-inflammatory effects of Rosa damascena hydroalcoholic extract and its essential oil in animal models. Iran J Pharm Res. 2010;9:163–168. [PMC free article] [PubMed] [Google Scholar]
- 16.Achuthan CR, Babu BH, Padikkala J. Antioxidant and hepatoprotective effects of Rosa damascena. Pharm Biol. 2003;41:357–361. [Google Scholar]
- 17.Hoseinpour H, Peel SA, Rakhshandeh H, Forouzanfar A, Taheri M, Rajabi O, et al. Evaluation of Rosa damascena mouthwash in the treatment of recurrent aphthous stomatitis: a randomized, double-blinded, placebo-controlled clinical trial. Quintessence Int. 2011;42:483–91. [PubMed] [Google Scholar]
- 18.Sadraei H, Asghari G, Emami S. Effect of Rosa damascena Mill. flower extract on rat ileum. Res Pharm Sci. 2013;8:277–284. [PMC free article] [PubMed] [Google Scholar]
- 19.Sadraei H, Asghari G, Emami S. Inhibitor effect of Rosa damascena Mill. flower essential oil, geraniol, citronellol on rat ileum contraction. Res Pharm Sci. 2013;8:17–23. [PMC free article] [PubMed] [Google Scholar]
- 20.Ozkan G, Sagdic O, Baydar NG, Baydar H. Antioxidant and antibacterial activities of Rosa damascena flower extracts. Food Sci Technol Int. 2004;10:277–281. [Google Scholar]
- 21.London: The Stationary Office; 2001. British Pharmacopoeia; pp. 1782–1783. [Google Scholar]
- 22.Minaiyan M, Ghannadi A, Etemad M, Mahzouni P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced ulcerative colitis in rats. Res Pharm Sci. 2012;7:103–110. [PMC free article] [PubMed] [Google Scholar]
- 23.Strasbourg: Council of Europe; 2002. European Pharmacopoeia; pp. 183–184. [Google Scholar]
- 24.Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 25.Minaiyan M, Ghannadi AR, Afsharipour M, Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011;6:13–21. [PMC free article] [PubMed] [Google Scholar]
- 26.Washington DC: The National Academies Press; 2010. Committee for the Update of the guide for the care and use of laboratory animals, national research council. guide for the care and use of laboratory animals. [Google Scholar]
- 27.Mascolo N, Izzo A, Autore G, Maiello F, Dicarlo G, Capsso F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J Pharm ExpTher. 1995;272:469–475. [PubMed] [Google Scholar]
- 28.Motavallian–Naeini A, Minaiyan M, Rabbani M, Mahzuni P. Anti-inflammatory effect of ondansetron through 5-HT3 receptors on TNBS–induced colitis in rat. EXCLI Journal. 2012;11:30–44. [PMC free article] [PubMed] [Google Scholar]
- 29.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuck MR, Wallace JL. Hapten induced mode of inflammation and ulceration in rat colon. Gasteroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 30.Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 31.Dieleman L, Palmen M, Akol H, Bloemena E, Pena A, Meuwissen S. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minaiyan M, Asghari Gh, Taheri D, Saeidi M, Nasr-Esfahani S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna J Phytomed. 2014;4:127–136. [PMC free article] [PubMed] [Google Scholar]
- 33.Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asadi M, Minaiyan M, Ghannadi A, Mahzouni P. Prunus armeniaca L. (Apricot) two fractions ameliorates TNBS-induced ulcerative colitis in rats. Res Pharm Sci. 2014;9:225–231. [PMC free article] [PubMed] [Google Scholar]
- 36.Hosseini M, Ghasemzadeh Rahbardar M, Sadeghnia HR, Rakhshandeh H. Effects of different extracts of Rosa damascena on pentylenetetrazol-induced seizures in mice. Zhong Xi Yi Jie He Xue Bao. 2011;9:1118–1124. doi: 10.3736/jcim20111013. [DOI] [PubMed] [Google Scholar]
- 37.Rakhshandah H, Hosseini M, Dolati K. Hypnotic effect of Rosa damascena in Mice. Iran J Pharmac Res. 2004;3:181–185. [Google Scholar]
- 38.Arezoomandan R, Kazerani HR, Behnam-Rasooli M. The laxative and prokinetic effects of Rosa damascena Mill in rats. Iran J Basic Med Sci. 2011;14:9–16. [Google Scholar]
- 39.González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51:331–362. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- 40.García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 41.Manthey JA. Biological properties of flavonoids pertaining to inflammation. Microcirculation. 2000;7:29–34. [PubMed] [Google Scholar]
- 42.Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–687. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 43.Heim KE, Tagliaferro AR, Bibilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 44.Pier-Giorgio Pietta. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 45.Izzo AA, Di Carlo G, Mascolo N, Capasso F, Autore G. Antiulcer effect of flavonoids. Role of endogenous PAF. Phytother Res. 1994;50:179–181. [Google Scholar]
- 46.Katsukawa M, Nakata R, Koeji S, Hori K, Takahashi S, Inoue H. Citronellol and geraniol, components of rose oil, activate peroxisome proliferator-activated receptor α and ã and suppress cyclooxygenase-2 expression. Biosci Biotechnol Biochem. 2011;75:1010–1012. doi: 10.1271/bbb.110039. [DOI] [PubMed] [Google Scholar]
- 47.Vinothkumar V, Manoharan S, Sindhu G, Nirmal MR, Vetrichelvi V. Geraniol modulates cell proliferation, apoptosis, inflammation, and angiogenesis during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Mol Cell Biochem. 2012;369:17–25. doi: 10.1007/s11010-012-1364-1. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhary SC, Siddiqui MS, Athar M, Alam MS. Geraniol inhibits murine skin tumorigenesis by modulating COX-2 expression, Ras-ERK1/2 signaling pathway and apoptosis. J Appl Toxicol. 2013;33:828–837. doi: 10.1002/jat.2739. [DOI] [PubMed] [Google Scholar]
- 49.Brito RG, Guimaraes AG, Quintans JS, Santos MR, De Sousa DP, Badaue-Passos D, Jr, et al. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. J Nat Med. 2012;66:637–644. doi: 10.1007/s11418-012-0632-4. [DOI] [PubMed] [Google Scholar]