Abstract
Ocimum basilicum belongs to Lamiaceae family and has been used for the treatment of wide range of diseases in traditional medicine in Iranian folk medicine. Due to the progressive need to anti-anxiety medications and because of the similarity between O. basilicum and Salvia officinalis, which has anti-anxiety effects, we decided to investigate the anxiolytic and sedative activity of hydroalcoholic extract and essential oil of O. basilicum in mice by utilizing an elevated plus maze and locomotor activity meter. The chemical composition of the plant essential oil was also determined. The essential oil and hydroalcoholic extract of this plant were administered intraperitoneally to male Syrian mice at various doses (100, 150 and 200 mg/kg of hydroalcoholic extract and 200 mg/kg of essential oil) 30 min before starting the experiment. The amount of hydroalcoholic extract was 18.6% w/w and the essential oil was 0.34% v/w. The major components of the essential oil were methyl chavicol (42.8%), geranial (13.0%), neral (12.2%) and β-caryophyllene (7.2%). HE at 150 and 200 mg/kg and EO at 200 mg/kg significantly increased the time passed in open arms in comparison to control group. This finding was not significant for the dose of 100 mg/kg of the extract. None of the dosages had significant effect on the number of entrance to the open arms. Moreover, both the hydroalcoholic extract and the essential oil decreased the locomotion of mice in comparison to the control group. This study shows the anxiolytic and sedative effect of hydroalcoholic extract and essential oil of O. basilicum. The anti-anxiety and sedative effect of essential oil was higher than the hydroalcoholic extract with the same doses. These effects could be due to the phenol components of O. basilicum.
Keywords: Ocimum basilicum, Anti-anxiety, Sedative, Extract, Essential oil
INTRODUCTION
Anxiety is one of the most common health problems in the world that result in enormous burden on health care system (1) and reduces quality of life (2). By the year 2020, mental disorders are predicted to reach the second place in the ranking of disease burden worldwide (3). The life time prevalence of anxiety varies from 9.2% in Korea to 28.7% in Switzerland (4). In a mental health survey of the adult population in Iran the prevalence of all mental disorders in rural and urban areas was 21.3 and 20.9% respectively. In this range depression and anxiety are more common (5). Anxiety is a mood disorder and patient is waiting for negative events in the future (6). It cause motor and autonomic symptoms such as muscle tension, sweating, elevated heart rate, tremor, etc. (6,7). Patients who suffer from anxiety will experience some other comorbid disease such as depression, drug abuse, asthma, cardiovascular disease (8).
There are some types of treatment for this disorder, including cognitive behavioral therapy, exposure therapy, anxiety management and medications (9). Benzodiazepines are still the main medications which are used for the treatment of anxiety (10). Due to the adverse side effects caused by chemical medications such as muscle relaxation, physical dependence, memory disturbance and interaction with other drugs (11), it would be a progress in the treatment of anxiety to use herbal medications.
In traditional medicine, plants such as Fumeria indica (12), Azadirachta indica (13), Gelsemium sempervirens (14), Piper methysticum, Hypericum perforatum (15), Stachys lavandulifolia (16), Valeriana officinalis (17) and Melissa officinalis (18) have been used for the treatment of anxiety disorder and found effective according to evidences.
Iran has a wide range of medicinal herbs. Ocimum basilicum is one popular edible herb which has also a wide range of use in traditional medicine as a treatment for anxiety, diabetes, cardiovascular disease and headache (19). The main components of essential oil of this plant are geranial, neral, caryophyllene oxide and methyl chavicol (20).
In this study, we investigated the effect of essential oil and hydroalcoholic extract of O. Basilicum on the elevated plus maze (EPM) and loco-motor activity meter (LAM) behavior on mice.
METHODS AND MATERIALS
Plant extraction and fractionation
Arial parts of O. basilicum L. cv. green cultivated in Isfahan province was collected in December 2013. The plant was identified at the Botany Department of the Faculty of Sciences, Isfahan University, and a voucher specimen of the plant has been deposited in the herbarium of the Faculty of Pharmacy, Isfahan University of Medical sciences, Isfahan, Iran (voucher specimen no. 3317).
Extract preparation
Air-dried and powdered aerial parts of O. basilicum (350 g) were extracted at room temperature with 3.9 L ethanol: water (7:3) using maceration method for 24 h. After filtration of the extract by Buchner funnel the solvent was evaporated in a rotary evaporator at 70 °C under reduced pressure to produce concentrated extract which was then freeze-dried to obtain a dry powder. Afterward appropriate amount of the extract was diluted with normal saline (containing 2 drops of Tween 80 per 10 ml) to produce the final concentration.
Isolation of essential oil
The air-dried plant materials were powdered and 350 g of plant materials hydrodistilled in a Clevenger-type apparatus for 3 h according to the method recommended in British Pharmacopoeia (21). The volatile oil was stored in sealed vial at 4 °C until use. The appropriate amount of essential oil of the plant was diluted with normal saline (containing 2 drops of Tween 80 per 10 ml) to produce the final concentration.
Animals
Male Syrian mice (Pasture institute, Iran) weighing 25-35 g were kept in a room with controlled temperature of 22-25 °C and 12 h light/dark cycle. Animals had free access to food and water. One day prior to the experiments mice were brought to the main environment to acclimate. In order to avoid diurnal cycle, all tests were carried out between 9:00 AM to 13:00 PM. Every mice received a single intraperitoneal (i.p.) injection and used just for one experiment. Four experiment groups with at least 6 mice in each group were used for locomotor activity test and six experiment groups with at least 6 mice in each group were used for Elevated plus maze. Animal experiments were approved by the Ethics Committee of Isfahan University of Medical Science and performed in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Diazepam hydrochloride was provided by Caspian Tamin Darou (Iran) as injectable solution in glass ampoule (10 mg/2 ml). The content of the ampoule was emptied and diluted with normal saline containing 0.5% Tween 80 to an appropriate concentration. The plant extract and essential oil were diluted with normal saline and 0.5% Tween 80. All solutions were prepared fresh on the day of experiment and administered i.p. in a volume of 0.1 ml/10 g mice body weight. Control component was made using normal saline containing 0.5% Tween 80.
Elevated plus maze
EPM test was used to measure the anxiolytic activity. The maze has two open (30 × 5× 0.2 cm) and two closed (30 × 5× 15 cm) arms that extend from a central platform (5 × 5 cm) and is 45 cm above the floor. The entire maze is wooden, painted in black and thoroughly cleaned with ethanol and allowed to dry between subjects in order to eliminate any odor cues.
A single i.p. dose of diazepam (0.5 mg/kg), extract at 100, 150, or 200 mg/kg, essential oil at 200 mg/kg, or the control component was administered to a mouse 30 min before the onset of the test. Each mouse was used just once and for one experiment. Experiments were conducted in a sound proof room with only a dim light. At the start of the test the mouse was placed at the center of the maze facing an open arm and during a 5 min observation the number of entries and the time spent in closed and open arms were recorded. Arm entries were defined as entry of all four paws into an arm. For each mouse the percentage of open/closed arm entries is determined as follows:
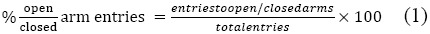
The percentage of time spent in open/closed arm for each animal was determined as follows:

Locomotor activity
In order to monitor spontaneous locomotor activity, a rectangular dark cage with a computerized infrared tracking device which record two parameters of movement (time and count) automatically during a 3 sessions of 5 min observation (total 15 min) were used. The Multiplication of time (s) by number of movement was taken as total locomotor activity count. The unit for the movement count is based on the number of beam breaks and the unit for movement time is the total time of beam breaks by mouse. Each mouse was injected with a single dose of diazepam (0.5 mg/kg), control component, essential oil (150 and 200 mg/kg) or extract (150 mg/kg) 30 min before the onset of test. After each test the cage was cleaned with ethanol in order to avoid any odor cues.
Statistics
Sigma plot (for windows Version 12.3, Build 12.3.0.36, Germany) was used for the analysis of the data. One way analysis of variance (ANOVA) with Duncan's post-hoc test was used and the significant level was assumed as P<0.05.
RESULTS
Pharmacognostic results
In this study the extract and essential oil of O. basilicum were prepared. The amount of extract was 18.6% w/w and the essential oil was 0.34% v/w.
Gas chromatography-mass spectrometry analysis
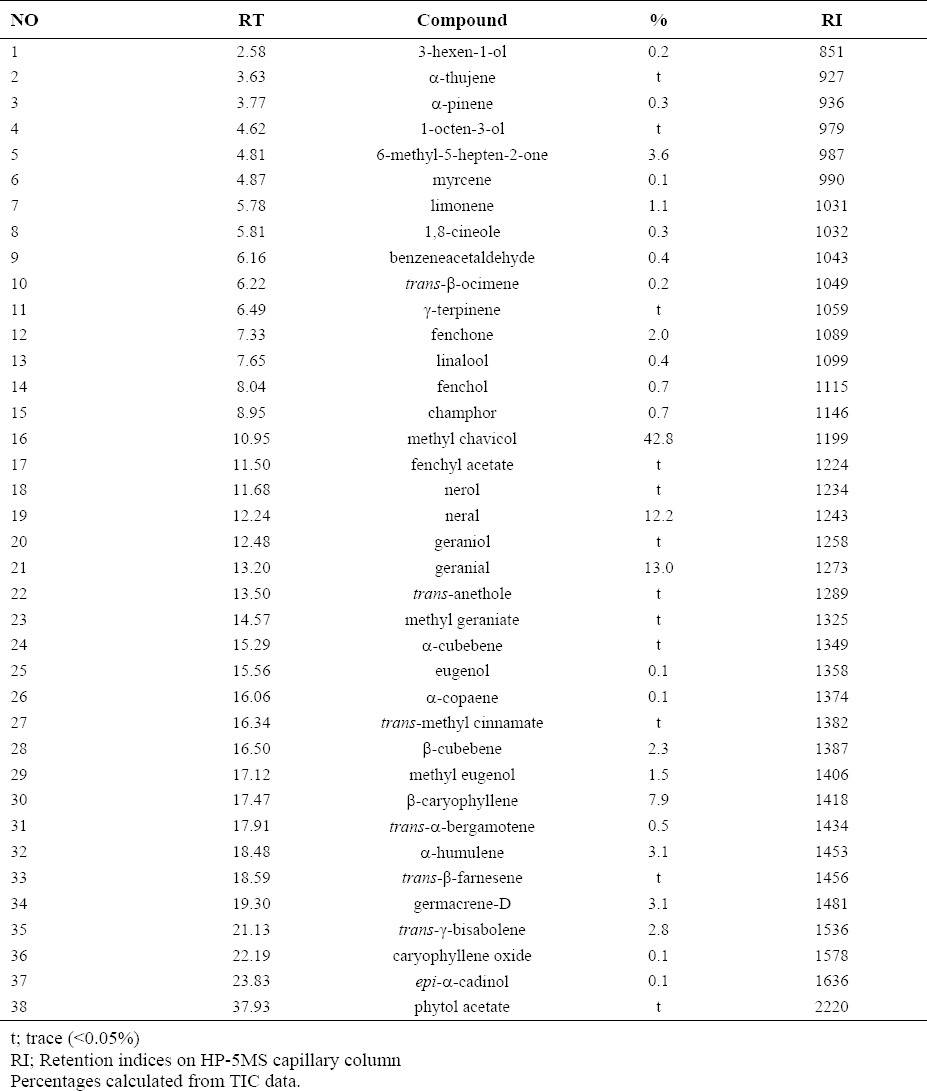
The aerial parts of O. basilicum yielded a 0.34% yellowish essential oil. Table 1 shows identified components of the oil with their percentages according to their elution on HP-5MS column.
Table 1.
Percentage composition of the essential oil of aerial parts of Ocimum basilicum L.
Elevated plus maze
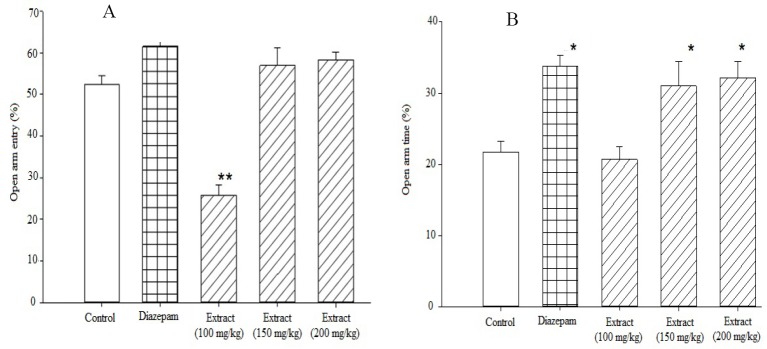
In the elevated plus maze 0.5 mg/kg diazepam significantly increased the percentage of time spent and the number of entries to the open arm (P<0.001 and P<0.05, respectively) (Fig. 1). In order to find the optimum dose of hydroalcoholic extract, three different doses of the extract including 100, 150 and 200 mg/kg were administered. As shown in Fig. 1 the time spent in open arm was significantly increased at 200 and 150 mg/kg (P<0.05). No significant effect was observed at 100 mg/kg dose (P=0.93). None of the three dosage of the injected to animals had significant effect on the number of entries to open arms. Although administration of 200 mg/kg essential oil in comparison with the control solution increased the percentage of the number of open arm entries by 14.2%, but it was not reach to a statistically significant level. The time spent in open arms, however, was significantly increased by 75.8% in comparison to the control group (P<0.001) (Fig. 2).
Fig. 1.
Effects of diazepam, control, and different doses of Ocimum basilicum and hydroalcoholic extract on A; the percentage of entries into the open arms and B; on the percentage of time mice spent in the open arms during a 5 min test. Various doses of the plant's hydroalcoholic extract, diazepam, or control were injected 30 min prior to testing. Data are presented as mean values ± SEM for each group of at least 6 mice. *P<0.05 compared to control group.
Fig. 2.
The effects of diazepam, control and Ocimum basilicam essential oil on A; the percentage of entries into the open arms and B; on the percentage of time mice spent in the open arms during a 5 min test. The plant's essential oil, diazepam, or control was injected 30 min prior to testing. Data are presented as mean values ± SEM for each group of at least 6 mice.*P<0.05 compared to control group. **P<0.001 compared to control group.
Locomotor activity
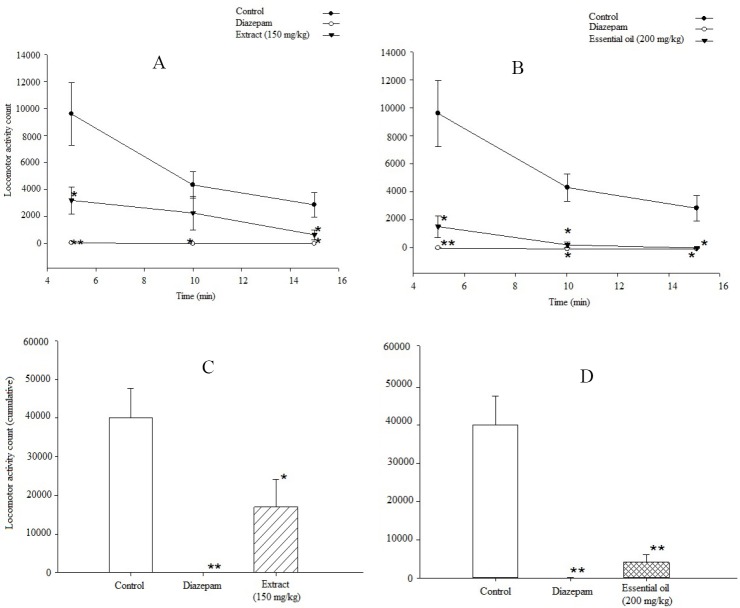
In this experiment, 0.5 mg/kg diazepam decreased the locomotor activity significantly as compared to the control group (P<0.001). As the optimum dose of the extract was found to be 150 mg/kg, in this test this particular dosage was utilized which caused a decline in motor activity of the animal in comparison to control group (P<0.05). The essential oil at dose of 200 mg/kg decreased the motor activity of the animals compared to control group (P<0.001) (Fig. 3).
Fig. 3.
The effects of diazepam, control, and Ocimum basilicum A; hydroalcoholic extract on spontaneous locomotor activity during three 5-min intervals, B; essential oil on spontaneous locomotor activity during three 5-min intervals, C; hydroalcoholic extract on spontaneous locomotor activity during the total 15 min of testing and D; essential oil on spontaneous locomotor activity during the total 15 min of testing. The locomotor activity counts (mean ± SEM) were measured over a 15 min period beginning 30 min after the administration of control, diazepam, plant essential oil or hydroalcoholic extract. Data are presented as mean ± SEM for each group of 6 mice. *P<0.05 compared to control group. **P<0.001 compared to control group.
DISCUSSION
Anxiety is an important health problem which may lead to low quality of the life (2). There are many medical and nonmedical therapies available for anxiety such as antidepressants, benzodiazepines, buspirone and cognitive behavior therapies (9,22). But the adverse effects of these medications may lead to the poor compliance of the patients especially older adults (10). The aim of this study was to investigate the anxiolytic effect of O. basilicum essential oil and hydroalcoholic extract using mice models of anxiety.
According to the results of GC/MS analysis, O. basilicum essential oil contained about 38 compounds and the main components were methyl chavicol (42.8%), geranial (13.0%), neral (12.2%) and ß caryophyllene (7.2%). The difference between this percentages and previous study could be related to the climatic factor that could affect plant growth or plant collecting (20).
In EPM model, based on previous studies, diazepam as an anxiolytic agent increases the time spent and numbers of entries to the open arm (11,23). In our study diazepam increased the time spent in the open arms (P<0.001) as well as the number of entries (P<0.050). As a result diazepam as a benzodiazepine drug increased the percentage of open arm time and entries into the open arms significantly. These observations are consistent with the results of previous studies, which have shown that diazepam like other benzodiazepines exert anxiolytic effects in a variety of anxiolytic screening tests, including EPM (22,23,24,25,26,27), but due to the differences amongst animal species it could be difference in effective doses (28).
Doses of 150 and 200 mg/kg of the HE showed similar results to diazepam. They increased the time significantly (P<0.05), but not the number of entries to the open arm. The result of 100 mg/kg extract was not statistically significant either. As a result the optimum dose of extract was determined to be equal to 150 mg/kg. EO of was another component of O. basilicum which was studied in the current work. In comparison to the negative control solution, essential oil did not increase the number of entries while the time spent in the open arms was no significantly increased.
In this model, both measures including time spent in open arms and the number of entries is important. O. Basilicum was shown to increase the time spent on open arms without affecting the number of entries. The lack of changes in the number of entries to the open arms by O. basilicum could not be due to the sedative effect of O. basilicum, as shown in LAM test, the extract and the essential oil did not affect the LA to the extent observed by diazepam.
Diazepam is positive allosteric modulators of the gamma-aminobutyric acid (GABA) type A (29). Since O. basilicum, unlike diazepam, did not increase open arm entries significantly, it seems that O. basilicum has a different mechanism. In other hand, buspirone is antianxiety drug that commonly used for treating anxiety disorder (29,30). Buspirone functions as a serotonin 5-HT1A receptor partial agonist (31). It is not clear which of the mentioned mechanisms explains the antianxiety effect of O. basilicum; further studies are required to elucidate the exact underlying mechanism.
Another animal model which was used in this study is locomotor activity model. Both the plant hydroalcoholic extract (150 mg/kg) and essential oil (200 mg/kg) significantly decreased the spontaneous locomotor activity of the mice. This reduction in locomotor activity could either be due to the plant's effect on the central nervous system or a direct effect on the periphery. Diazepam decreased spontaneous locomotor activity to a greater extent than plant's extract or essential oil and that difference show that diazepam is more sedative than hydroalcoholic extract and essential oil of O. basilicum. Further studies are needed to determine the exact mechanism for the sedative action of this plant.
Based on previous studies, some of the plant's EO components have anxiolytic and sedative effects such as; 1,8-cineole, linalool, α-pinene, ß-caryophyllene, humulene, citral, myrcene, limonene and methyl chavicol (32,33,34,35), as well as some of the plant's hydroalcoholic components such as malic acid, caffeic acid, kaempferol and Oleanolic acid (36,37,38,39). These studies could verify our result but to find the exact components responsible for the anxiolytic and sedative effects, further experiments and studies are warranted.
CONCLUSION
In conclusion, this study determined the anti-anxiety effects of hydroalcoholic extract and essential oil of O. basilicum and did not cause sedation to the extent observed with diazepam. The anti-anxiety effect of extract at 200 mg/kg was similar to diazepam. The dose of 150 mg/kg extract was determined as the least effective dose. The sedative effect of essential oil was higher than that of the extract. These effects could be due to phenol and terpenoid components of O. basilicum but more tests and studies are required to clarify the exact mechanism and exact effective components.
ACKNOWLEDGMENTS
The content of this paper is extracted from the Pharm.D thesis NO. 393068 submitted by A. Vaezi which was financially supported by the Research Department of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Campos AC, Fogaca MV, Aguiar DC, Guimaraes FC. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr. 2013;35:101–111. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- 2.Sareen J, Jacobi F, Cox BJ, Belik SL, Clara I, Stein MB. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166:2109–2116. doi: 10.1001/archinte.166.19.2109. [DOI] [PubMed] [Google Scholar]
- 3.Brundtland GH. Mental health, A call for action. World Health Organization. 2001 [Google Scholar]
- 4.Mohammadi MR, Davidian H, Noorbala AA, Malekafzali H, Naghavi HR, Pouretemad HR, et al. An epidemiological survey of psychiatric disorders in Iran. Clin Pract Epidemiol Ment Health. 2005;1:16. doi: 10.1186/1745-0179-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noorbala AA, BagheriYazdi SA, Yasamy MT, Mohammad K. Mental health survey of the adult population in Iran. Br J Psychiatry. 2004;184:70–73. doi: 10.1192/bjp.184.1.70. [DOI] [PubMed] [Google Scholar]
- 6.Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE. What is an anxiety disorder? Depress Anxiety. 2009;26:1066–1085. doi: 10.1002/da.20633. [DOI] [PubMed] [Google Scholar]
- 7.Steimer T. Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues Clin Neurosci. 2011;13:495–506. doi: 10.31887/DCNS.2011.13.4/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol. 2011;164:1129–1161. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadock BJ, Sadock VA, Ruiz P. 11th ed. Philadelphia: Williams and Walkins; 2014. Kaplan &Sadock's synopsis of psychiatry behavioral sciences/clinical psychiatry; pp. 387–417. [Google Scholar]
- 10.Lenze EJ, Wetherell JL. A lifespan view of anxiety disorders. Dialogues Clin Neuro Sci. 2011;13:381–399. doi: 10.31887/DCNS.2011.13.4/elenze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Y, Rong C, Wang J, Cui C, Wang L, Feng Z, et al. Mechanism-based anti-anxiety effects of polysaccharides extracted from shudihuang (radix rehmannia epreparata) by two-dimensional electrophoresis analysis in rat hippocampus proteins. J Tradit Chin Med. 2013;33:524–530. doi: 10.1016/s0254-6272(13)60159-4. [DOI] [PubMed] [Google Scholar]
- 12.Singh GK, Chauhan SK, Rai G, Chatterjee SS, Kumar V. Potential antianxiety activity of Fumaria indica: A preclinical study. Pharmacogn Mag. 2013;9:14–22. doi: 10.4103/0973-1296.108129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal AK, Bhattacharya SK, Acharya SB. Anxiolytic activity of Azadirachta indica leaf extract in rats. Indian J Exp Biol. 1994;32:489–491. [PubMed] [Google Scholar]
- 14.Dutt V, Dhar VJ, Sharma A. Antianxiety activity of Gelsemium sempervirens. Pharm Biol. 2010;48:1091–1096. doi: 10.3109/13880200903490521. [DOI] [PubMed] [Google Scholar]
- 15.Saeed SA, Bloch RM, Antonacci DJ. Herbal and dietary supplements for treatment of anxiety disorders. Am Fam Physician. 2007;76:549–556. [PubMed] [Google Scholar]
- 16.Rabbani M, Sajjadi SE, Jalali A. Hydroalcohol extract and fractions of Stachys lavandulifoliaVahl: effects on spontaneous motor activity and elevated plus-maze behaviour. Phytother Res. 2005;19:854–858. doi: 10.1002/ptr.1701. [DOI] [PubMed] [Google Scholar]
- 17.Murphy K, Kubin ZJ, Shepherd JN, Ettinger RH. Valeriana officinalis root extracts have potent anxiolytic effects in laboratory rats. Phytomedicine. 2010;17:674–678. doi: 10.1016/j.phymed.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Taiwo AE, Leite FB, Lucena GM, Barros M, Silveira D, Silva MV, Ferreira VM. Anxiolytic and antidepressant-like effects of Melissa officinalis (lemon balm) extract in rats: Influence of administration and gender. Indian J Pharmacol. 2012;44:189–192. doi: 10.4103/0253-7613.93846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bora KS, Arora S, Shri R. Role of Ocimum basilicum L. in prevention of ischemia and reperfusion-induced cerebral damage, and motor dysfunctions in mice brain. J Ethnopharmacol. 2011;137:1360–1365. doi: 10.1016/j.jep.2011.07.066. [DOI] [PubMed] [Google Scholar]
- 20.Sajjadi SE. Analysis of the essential oil of two cultivated Basil (Ocimum basilicum L.) from Iran. Daru. 2006;14:128–130. [Google Scholar]
- 21.Vol. 2. London: HMSO; 1988. British pharmacopoeia; pp. 137–138. [Google Scholar]
- 22.Gale C, Davidson O. Generalised anxiety disorder. BMJ. 2007;334:579–581. doi: 10.1136/bmj.39133.559282.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabbani M, Sajjadi SE, Sadeghi M. Chemical composition of the essential oil from kelussiaodoratissima Mozaff. and the evaluation of its sedative and anxiolytic effects in mice. Clinics (Sao Paulo) 2011;66:843–848. doi: 10.1590/S1807-59322011000500022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews JS, Stephens DN. Drug discrimination models in anxiety anddepression. Pharmacol Ther. 1990;47:267–280. doi: 10.1016/0163-7258(90)90090-o. [DOI] [PubMed] [Google Scholar]
- 25.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 26.Rabbani M, Sajjadi SE, Mohammadi A. Evaluation of the anxiolytic effect of Nepeta persica Boiss. in mice Evid Based Complement. Alternat Med. 2008;5:181–186. doi: 10.1093/ecam/nem017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabbani M, Sajjadi SE, Zarei HR. Anxiolytic effects of Stachys lavandulifolia Vahl. on the elevated plus-maze model of anxiety in mice. J Ethnopharmacol. 2003;89:271–276. doi: 10.1016/j.jep.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Ohl F, Sillaber I, Binder E, Keek ME, Holsboer F. Differential analysis of basal behavior and diazepam – induced alteration in C57BL/6 and BALB/c mice using the modified hole boaed. J Psychiatry Research. 2001;35:147–154. doi: 10.1016/s0022-3956(01)00017-6. [DOI] [PubMed] [Google Scholar]
- 29.Brunton LL, Lazo JS, Parker KL. 12th ed. New York: McGraw-Hill; 2011. Goodman & Gilman's the pharmacological basis of therapeutics; pp. 453–465. [Google Scholar]
- 30.Schmitt R, Gazalle FK, Lima MS, Cunha A, Souza J, Kapczinski F. The efficacy of antidepressants for generalized anxiety disorder: A systematicreview and meta-analysis. Rev Bras Psiquiatr. 2005;27:18–24. doi: 10.1590/s1516-44462005000100007. [DOI] [PubMed] [Google Scholar]
- 31.Blier P, Bergeron R, de Montigny C. Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response. Neuropsychopharmacology. 1997;16:333–338. doi: 10.1016/S0893-133X(96)00242-4. [DOI] [PubMed] [Google Scholar]
- 32.Edewor-Kuponiyi TI. Plant-derived compounds with potential sedative and anxiolytic activities. Int J Basic Appl Sci. 2013;2:63–78. [Google Scholar]
- 33.do Vale TG, Furtado EC, Santos JG, Jr, Viana GS. Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippiaalba (Mill.) n.e. Brown. Phytomedicine. 2002;9:709–714. doi: 10.1078/094471102321621304. [DOI] [PubMed] [Google Scholar]
- 34.Satou T, Kasuya H, Maeda K, Koike K. Daily inhalation of α-pinene in mice: effects on behavior and organ accumulation. Phytother Res. 2014;28:1284–1287. doi: 10.1002/ptr.5105. [DOI] [PubMed] [Google Scholar]
- 35.Cosentino RM, Norte MC, Lazarini CA. Estragole-induced behavioural changes in rats. Phytother Res. 2004;18:921–924. doi: 10.1002/ptr.1583. [DOI] [PubMed] [Google Scholar]
- 36.Patel M, Antala B, Barua C, Lahkar M. Anxiolytic activity of aqueous extract of Garcinia indica in mice. Int J Green Pharm. 2013;7:332–335. [Google Scholar]
- 37.Takeda H, Tsuji M, Miyamoto J, Masuya J, Iimori M, Matsumiya T. Caffeic acid produces antidepressive- and/or anxiolytic-like effects through indirect modulation of the alpha 1A-adrenoceptor system in mice. Neuroreport. 2003;14:1067–1070. doi: 10.1097/01.wnr.0000073427.02536.b0. [DOI] [PubMed] [Google Scholar]
- 38.Vissiennon C, Nieber K, Kelber O, Butterweck V. Route of administration determines the anxiolytic activity of the flavonolskaempferol, quercetin and myricetin--are they prodrugs? J Nut Biochem. 2012;23:733–740. doi: 10.1016/j.jnutbio.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Fajemiroye JO, Galdino PM, Florentino IF, Da Rocha FF, Ghedini PC, Polepally PR, et al. Plurality of anxiety and depression alteration mechanism by oleanolic acid. J Psychopharmacol. 2014;28:923–934. doi: 10.1177/0269881114536789. [DOI] [PubMed] [Google Scholar]