Abstract
Objective
To test the feasibility of offering rapid, point-of-care human immunodeficiency virus (HIV) testing at community pharmacies and retail clinics.
Design
Pilot program to determine how to implement confidential HIV testing services in community pharmacies and retail clinics.
Setting
21 community pharmacies and retail clinics serving urban and rural patients in the United States, from August 2011 to July 2013.
Participants
106 community pharmacy and retail clinic staff members.
Intervention
A model was developed to implement confidential HIV counseling and testing services using community pharmacy and retail clinic staff as certified testing providers, or through collaborations with organizations that provide HIV testing. Training materials were developed and sites selected that serve patients from urban and rural areas to pilot test the model. Each site established a relationship with its local health department for HIV testing policies, developed referral lists for confirmatory HIV testing/care, secured a CLIA Certificate of Waiver, and advertised the service. Staff were trained to perform a rapid point-of-care HIV test on oral fluid, and provide patients with confidential test results and information on HIV. Patients with a preliminary positive result were referred to a physician or health department for confirmatory testing and, if needed, HIV clinical care.
Main outcome measures
Number of HIV tests completed and amount of time required to conduct testing.
Results
The 21 participating sites administered 1,540 HIV tests, with 1,087 conducted onsite by staff during regular working hours and 453 conducted at 37 different HIV testing events (e.g., local health fairs). The median amount of time required for pretest counseling/consent, waiting for test results, and posttest counseling was 4, 23, and 3 minutes, respectively. A majority of the sites (17) said they planned to continue HIV testing after the project period ended and would seek assistance or support from the local health department, a community-based organization, or an AIDS service organization.
Conclusion
This pilot project established HIV testing in several community pharmacies and retail clinics to be a feasible model for offering rapid, point-of-care HIV testing. It also demonstrated the willingness and ability of staff at community pharmacies and retail clinics to provide confidential HIV testing to patients. Expanding this model to additional sites and evaluating its feasibility and effectiveness may serve unmet needs in urban and rural settings.
Keywords: HIV, point-of-care screening, pharmacist, public health
By the end of 2010, approximately 1.1 million people living in the United States were infected with human immunodeficiency virus (HIV), with approximately 18% of these individuals unaware of their health status.1–4 In recent years, approximately 50,000 new HIV diagnoses have been reported annually.3 To access testing services for HIV, patients must typically visit a health department-supported clinical care site (e.g., sexually transmitted disease [STD] clinic, community health center, or emergency department), physician’s office, or HIV testing site.
Unfortunately, for patients in such areas as the rural Southeast, access to these services may be hindered by both distance and stigma.5 However, 70% of rural consumers live within 15 miles of a pharmacy, and nearly 90% of urban consumers are just 2 miles away from their local pharmacy.6 Additionally, as of 2009, approximately 30% of the U.S. population lived within a 10-minute drive of a retail clinic (typically located within a pharmacy, supermarket, or other retail store).6,7
Going to a pharmacy or retail clinic is considered a socially innocuous act, unlike visiting an HIV testing site. Thus, implementing HIV testing in such settings could potentially overcome the obstacles associated with conventional testing centers.8 Pharmacies and retail clinics offer a vast, largely untapped potential for the delivery of HIV testing in settings that are more accessible and, for some people, less stigmatizing than traditional testing sites.9,10
Published examples of pharmacy-provided HIV testing reveal that some of the sites used third-party partners to conduct the testing,11,12 while others relied on pharmacy staff.13 Pharmacies have been used as HIV testing sites during HIV testing campaigns, such as National HIV Testing Day and, in some cases, as standing sites for third-party HIV testing partners to conduct HIV tests.14,15
We report on a pilot program to determine how to implement confidential HIV testing services in community pharmacies and retail clinics using pharmacy and retail clinic staff as certified testing providers or collaborating with organizations that could provide HIV testing at these sites.
Objective
The objective of this project was to test the feasibility of offering rapid, point-of-care HIV testing at community pharmacies and retail clinics.
Methods
In August 2011, the U.S Centers for Disease Control and Prevention (CDC) awarded a 2-year contract to ASHLIN Management Group, Inc., to develop and implement an HIV testing model that could be adopted by a variety of pharmacies (e.g., chain and independent pharmacies) and retail clinics. An expert panel was formed to provide guidance on development of training for the model and to assist with the identification of sites in the United States that would be willing to sign subcontracts to participate in the project.
Participating sites were required to have or obtain a Clinical Laboratory Improvements Amendments of 1988 (CLIA) Certificate of Waiver, which would allow them to perform laboratory tests that are categorized as “simple laboratory examinations and procedures that have an insignificant risk of an erroneous result.”16 We also developed a curriculum and training module with strategies, materials, and procedures designed to support pharmacists, nurses, and nurse practitioners in the administration of rapid, point-of-care HIV testing at community pharmacies or retail clinics (Table 1). The training sessions, which were delivered either in person or by webinar, provided recommendations and requirements for obtaining patient permission and consent, as well as instructions for staff on how to deliver rapid HIV test results in an accurate, sensitive, and culturally appropriate manner.
Table 1.
Primary training objectives for performing HIV testing in community pharmacies and retail clinics
| Objectives |
|---|
| Understand the current status of the HIV epidemic in the United States and local communities. |
| Summarize the major reasons why routine HIV testing is important. |
| Understand the CDC recommendations for routine testing and describe appropriate HIV testing methods. |
| Identify ways to reach out and support the community for HIV testing. |
| Determine recommendations and requirements for permission, consent, and pre- and posttest counseling in the state in which rapid HIV testing is performed. |
Establish competence in performing, reading, and interpreting a rapid HIV test:
|
| Provide accurate, sensitive, and culturally appropriate information regarding rapid HIV test results. |
| Identify sites for linkage to clinical care and confirmatory testing. |
| Identify and address possible barriers to HIV testing in the setting and/or locale. |
Abbreviations used: HIV, human immunodeficiency virus; CDC, Centers for Disease Control and Prevention.
Each site engaged its local health department for assistance with local norms for HIV point-of-care testing and for help developing a list of sites for confirmatory HIV testing and HIV care services. In one jurisdiction, patients with reactive test results were referred to a patient navigator to facilitate the linkage to confirmatory testing and care. Each site also developed a list of referrals for other issues that might arise during counseling on HIV test results (e.g., evaluation for sexually transmitted infections [STIs]).
Site staff were taught about the variety of point-of-care HIV tests available at the time of the study. We used the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA), the choice of which was made from the available CLIA-waived, rapid, point-of-care HIV tests available in 2011 and is not intended to imply CDC’s endorsement over other products. Staff were trained in how to administer the test on oral fluid by medical professionals hired for this project or by service representatives from the product manufacturer.
Staff were also instructed to provide basic information on HIV risk reduction to those patients with nonreactive HIV test results. Patients with reactive test results (preliminary positive) were informed about the meaning of the test and the need for a confirmatory test, as well as provided a referral for confirmatory testing tailored to local requirements.
We implemented the project in two phases. During Phase 1, seven sites were selected in early 2012 for project participation, six of which received training between March and July 2012. (One of the initial seven sites withdrew before training due to the inability to obtain a CLIA Certificate of Waiver.) After the Phase 1 sites were trained, we refined the training modules and processes. In Phase 2, we selected and trained 16 additional sites from October 2012 through May 2013, with one site withdrawing after training but before any tests were conducted.
The testing service was advertised in a variety of ways, including in-store promotional flyers and pamphlets, local newspaper articles, flyers in customer bags, websites, and social media. For each test, the staff completed a log of the start and stop time of 1) pretest counseling, 2) incubation while waiting for test results (when staff was either not otherwise engaged, was conducting other work in the pharmacy, or seeing another patient in the retail clinic), and 3) posttest counseling. The analysis that follows includes data from the 21 sites that conducted at least one rapid, point-of-care HIV test.
Results
From August 2011 through July 2013, we trained 106 staff at 21 sites (18 community pharmacies, one retail clinic, one nurse-run HIV testing service at an Indian Health Service Clinic, and one multisite venue where faculty from a school of pharmacy partnered with multiple community pharmacies) to conduct HIV tests. Pharmacists at one of these sites had already implemented HIV testing as part of another pilot project,13 while pharmacists at a second site had already implemented both HIV and hepatitis C testing in partnership with the local health department.17 Nineteen of the participating sites used in-store pharmacists or retail clinic staff to conduct the testing; one site partnered with the local health department to do testing every other weekend at the store; and the multisite program relied on pharmacy school faculty to conduct testing.
The six Phase 1 sites began initial testing between May and July 2012. Five of these sites continued to provide testing through July 2013, while the site that partnered with the local health department withdrew from the project after 11 months of participation due to challenges with maintaining staff to support the service. The 15 Phase 2 sites began testing between December 2012 and May 2013, with 14 continuing to provide testing through July 2013 and one withdrawing after 5 months due to the store closing.
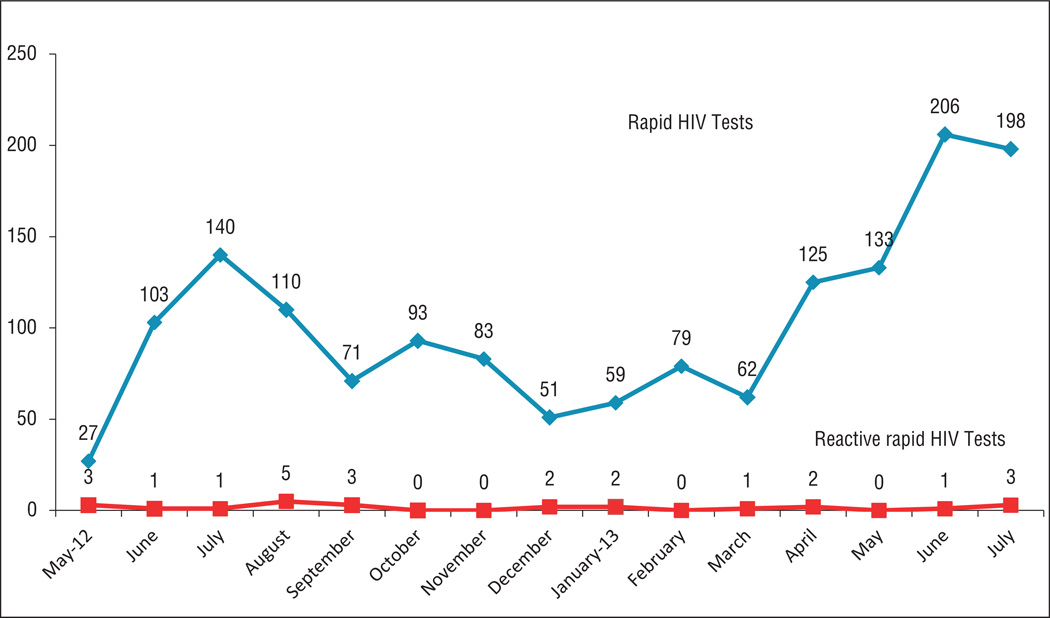
The 21 participating sites conducted 1,540 HIV tests (median: 93; interquartile range [IQR]: 67–129; range: 1–370), with 939 tests completed by the initial six Phase 1 sites and 601 by the 15 Phase 2 sites (Figure 1). On a per-month basis, 11 of the sites conducted 1–5 tests, 5 of the sites conducted 6–10 tests, 4 sites conducted 11–25 tests, and the multisite venue operated by pharmacy school faculty conducted more than 25 tests. The spike in testing in June and July of each year likely corresponds with increased efforts around National HIV Testing Day (June 27).
Figure 1.
Number of HIV tests completed by community pharmacies and retail clinics per month, May 2012 – July 2013
Nine of the sites participated in 37 HIV testing events (e.g., local health fairs, one-time events in a pharmacy), during which 453 tests were conducted. The remaining 1,087 tests were conducted onsite by community pharmacy and retail clinic staff members during regular working hours. There were 1,508 nonreactive test results, 24 (1.6%) reactive test results (preliminary positives), and 8 invalid tests.
A majority of the sites (17) said they planned to continue HIV testing after the project period ended and would seek assistance or support from the local health department, a community-based organization, or an AIDS service organization.
Preliminary positive test results
The 24 reactive test results were identified at 10 of the project sites (range: 1–8 per site). In each instance, the pharmacist or nurse practitioner counseled the patient and provided a referral for confirmatory testing. In 16 of these cases, the outcome of confirmatory testing was unknown to site staff. Of the remaining eight reactive test results, five were determined to be false-positive on confirmatory testing, two involved persons previously diagnosed with HIV, and one was confirmed as a new HIV case.
Staff–patient interaction time
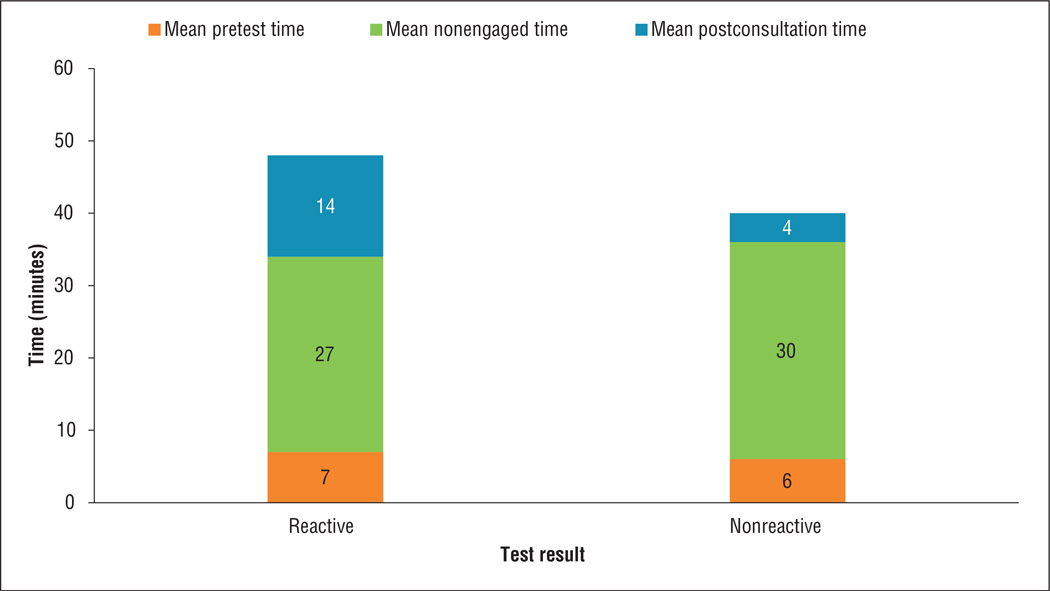
The time required to conduct the rapid point-of-care HIV test was categorized into three components: 1) pretest time, which involved initial patient counseling, obtaining informed consent, and collecting the specimen; 2) time waiting for the test result, during which staff engaged in nontesting-related work; and 3) posttest time, which involved sharing the test result with the patient and providing risk-reduction information for nonreactive test results or making arrangements to obtain a confirmatory test for reactive test results.
Pretest counseling/consent took a median of 4 minutes (IQR: 2–7 minutes), while posttest counseling took a median of 3 minutes (IQR: 2–5 minutes). Posttest counseling took considerably longer for reactive tests compared with nonreactive tests (Figure 2). The time spent waiting for test results (staff nonengaged time) was a median of 23 minutes (IQR: 20–30 minutes). The amount of nonengaged time observed in this study included the 20-minute processing time required for the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test and would likely vary with use of other rapid, point-of-care HIV test kits.
Figure 2.
Mean time to conduct an HIV test in community pharmacies and retail clinics
Discussion
The results of our pilot study suggest that confidential HIV testing services can be administered by existing staff at community pharmacies and retail clinics and can be successfully integrated into a busy work environment with a modest amount of staff–patient interaction time. These novel venues for confidential HIV testing services can supplement conventional HIV testing sites such as health departments, doctors’ offices, and community-based organizations. Additionally, the pharmacists, nurses, and nurse practitioners working at community pharmacies and retail clinics can host or participate in event-based HIV testing activities, extending the model’s reach in a manner not originally anticipated.
Although one participating site was able to successfully partner with its local health department as a third-party provider of onsite testing for a period of time, the site was ultimately unable to continue with this approach as a result of staff and budget constraints. Partnership approaches bring resources to a pharmacy or retail clinic to help conduct HIV testing, but sustainability of the partnership may not be within the pharmacy’s control. Nevertheless, sites that lack the staff to conduct such testing may wish to consider a third-party testing model.
Pharmacies and retail clinics are convenient places to disseminate health information to a broad array of patients seeking medical advice and information who otherwise may have limited access to health care providers.18–20 They may also be points of contact where there are opportunities to reach populations at risk for other STIs or infectious diseases. Therefore, expansion of the testing model studied here to include other point-of-care, CLIA-waived screening tests (e.g., hepatitis C) should be feasible in these types of sites.21
The training provided to study participants focused on educating pharmacy and retail clinic staff about HIV infection and HIV testing, providing results to patients, and having a system for referral for confirmatory HIV testing and linkage to care. Pharmacists, nurses, and nurse practitioners are already skilled in patient counseling and adhere to confidentiality standards at their practice sites; the training offered in this program simply augmented these skills around confidentiality requirements unique to HIV testing and delivery of HIV test results, which has been identified as a need.8,22
The test kit for this project was chosen from among the available options at the time the project started in 2011. By 2014, there were five CLIA-waived, rapid, point-of-care HIV test kits commercially available (with more in the pipeline) from which a pharmacy or retail clinic could choose, as well as a rapid HIV self-test sold for in-home use that could also be administered at a pharmacy or retail clinic.23–25 HIV testing recommendations, local health department preferences for testing, test kit-specific requirements and performance characteristics, and cost should all be considered when deciding which test to use. Additionally, involving the local health department prior to offering confidential HIV testing services is essential to ensuring that procedures for counseling and referral are consistent with local norms or requirements.
An essential component of this project was making arrangements for persons with preliminary positive test results to obtain confirmatory testing; however, we found that pharmacy and retail clinic staff were not always aware of patients’ final testing status, despite reasonable attempts at contacting them. Once a referral is made for confirmatory testing, the responsibility for patient management is turned over to the respective clinician or, in some cases, a patient navigator. Although patients with reactive test results are not obligated to return to the initial testing site, future HIV testing programs in these settings should emphasize methods to assist pharmacists or retail clinic staff in documenting that such patients have followed up with the referred clinician or patient navigator.
Pharmacies and retail clinics could be convenient settings for testing patients at high risk of acquiring HIV for whom repeat (every 3–6 months) HIV testing is recommended. The window of time in which an HIV infection may be detected varies by test, so high-risk persons with repeated potential exposures to HIV may require testing with diagnostic approaches that minimize this window.23,26
Other innovative approaches to the implementation of rapid, point-of-care HIV testing at community pharmacies or retail clinics, which would require further development and evaluation, include use in monitoring persons taking antiretroviral therapy as preexposure prophylaxis against acquisition of HIV or use as a screening test before initiating nonoccupational, post-exposure prophylaxis as part of a collaborative practice model agreement with a primary care provider.
Limitations
The pharmacy and retail clinic partners who agreed to participate in this pilot project may represent early adopters of an innovative service. The model may not be as readily implemented in pharmacies and retail clinics with staffing constraints, inadequate physical infrastructure (e.g., no private testing area), or lack of staff interest. Some sites that were approached to participate in the project declined, but we did not capture their reasons for declining in a systematic fashion.
The number of HIV tests conducted by different pharmacies and retail clinics reflected multiple enabling and hindering factors at each site and should not be considered representative of the maximal capacity to conduct screenings. For example, advertising was noted as a challenge by many sites; while some said advertising was successful in attracting clients to the program, others said it failed to bring in many clients. Many sites also expressed challenges in addressing perceived stigma associated with HIV. We were not able to systematically assess these factors within the scope of this project.
The rate of reactive test results should not be interpreted as reflective of what might be attained if the model is more widely disseminated. For example, while pharmacies and retail clinics in high-prevalence areas may identify more HIV-infected patients, testing sites in some rural areas with low HIV prevalence may have fewer patients with reactive test results yet still serve an important role by providing the service in an area of unmet need for HIV testing.
Pharmacy and retail clinic partners were subcontracted for their participation in this project, and test kits and controls were provided through the project at no cost; the implications of implementing HIV testing services without this support warrant further investigation.
Conclusion
The success of the pilot program reported here demonstrates the feasibility of implementing confidential HIV testing services in community pharmacies and retail clinics at several sites in the United States using pharmacy and retail clinic staff as certified testing providers or collaborating with organizations that could provide HIV testing at these sites. Involvement and support of local health departments was a key feature of this project and can foster meaningful integration with public health promotion activities related to HIV testing at community pharmacies and retail clinics.27,28 Expansion and further evaluation of this model will require securing the support of management of individual sites and training additional pharmacists, nurses, and nurse practitioners interested in offering the service and collecting and analyzing outcome data.
At a Glance.
Synopsis
This pilot project developed and tested a model for implementing confidential human immunodeficiency virus (HIV) counseling and testing services at 21 community pharmacies and retail clinics in the United States. Between August 2011 and July 2013, 106 staff members were trained and conducted 1,540 rapid, point-of-care HIV tests, yielding 1,508 nonreactive results, 24 reactive results (of which only one was confirmed as a new HIV case), and 8 invalid tests. Pretest counseling/consent took a median of 4 minutes, while posttest counseling took a median of 3 minutes. Wait time for test results was a median of 23 minutes, approximately 20 minutes of which included processing time specific to the test kit selected for the pilot project.
Analysis
Access to HIV testing services typically requires that patients visit a health department, physician, or HIV testing site, which in some jurisdictions can require traveling long distances. Pharmacies and retail clinics offer a vast, largely untapped potential for the delivery of HIV testing in settings that are more accessible and, for some people, less stigmatizing than traditional testing sites. The results of this pilot project suggest that confidential HIV testing services can be administered by existing staff at community pharmacies and retail clinics and successfully integrated into a busy work environment with a modest amount of staff– patient interaction time. The success of this model also suggests that community pharmacies and retail clinics may potentially serve as future points of contact for reaching populations at risk for other sexually transmitted infections (STIs) or infectious diseases.
Acknowledgments
Barney’s Pharmacy, Augusta, GA; Bartell Drug Company, Seattle, WA; Causey’s Pharmacy, Natchitoches, LA; Community, A Walgreens Pharmacy, Washington DC; East Pines Pharmacy, Riverdale, MD; Family Medical Services Pharmacy, Bessemer, AL; Family Pharmacy of Neosho, Neosho, MO; Freedom Pharmacy, Orlando, FL; Gateway Apothecary, St. Louis, MO; H & W Drug Store, New Orleans, LA; Indian Health Board, Billings, MT; Long’s Drugs, Columbia, SC; Moose Professional Pharmacy, Concord, NC; Okeechobee Discount Drugs, Okeechobee, FL; Osborn Drugs, Miami, OK; Take Care Clinic, Lithonia, GA; Rite Aid Pharmacy, Atlanta, GA; University of Mississippi School of Pharmacy, Jackson, MS; University Pharmacy, Detroit, MI; Walgreens Pharmacy #03539, Chicago, IL; and Walgreens Pharmacy #10071, Washington, DC.
Funding: Funding provided by CDC contract 200-2009-30908-00004 to ASHLIN Management Group, Inc.
Footnotes
Disclosure: The authors declare no conflicts of interest with or financial interests in any product or service mentioned in this article, including grants, employment, gifts, stock holdings, or honoraria.
Contributor Information
CAPT Paul J. Weidle, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), Atlanta, GA..
CAPT Shirley Lecher, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), Atlanta, GA..
Linda W. Botts, ASH-LIN Management Group, Inc., Greenbelt, MD..
LaDawna Jones, ASH-LIN Management Group, Inc., Greenbelt, MD..
David H. Spach, Division of Allergy, Infectious Diseases, Department of Medicine, University of Washington, Seattle..
Jorge Alvarez, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), Atlanta, GA..
Rhondette Jones, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), Atlanta, GA..
CDR Vasavi Thomas, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), Atlanta, GA..
References
- 1.Centers for Disease Control and Prevention. HIV surveillance report, vol. 23: diagnoses of HIV infection in the United States and dependent areas, 2011. [September 30, 2013]; www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf.
- 2.Centers for Disease Control and Prevention. HIV surveillance supplemental report, 17, no. 3: monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2010, part A. [September 30, 2013]; www.cdc.gov/hiv/pdf/statistics_2010_HIV_Surveillance_Report_vol_17_no_3.pdf.
- 3.Centers for Disease Control and Prevention. HIV surveillance supplemental report, vol. 17, no. 4: estimated HIV incidence in the United States, 2007–2010. [September 30, 2013]; www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf.
- 4.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutton M, Anthony MN, Vila C, et al. HIV testing and HIV/AIDS treatment services in rural counties in 10 southern states: service provider perspectives. J Rural Health. 2010;26:240–247. doi: 10.1111/j.1748-0361.2010.00284.x. [DOI] [PubMed] [Google Scholar]
- 6.Pharmaceutical Care Management Association. Consumer access to pharmacies in the United States. [September 30, 2013];2007 www.pcmanet.org/newsroom/2007/Documents/SKA%20Research%20Consumer%20Access%20to%20Pharmacies%202007%205_1_07.pdf. [Google Scholar]
- 7.Rudavsky R, Pollack CE, Mehrotra A. The geographic distribution, ownership, prices, and scope of practice at retail clinics. Ann Intern Med. 2009;151:315–320. doi: 10.7326/0003-4819-151-5-200909010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryder PT, Meyerson BE, Coy KC, et al. Pharmacists’ perspectives on HIV testing in community pharmacies. J Am Pharm Assoc. 2013:e182–e187. doi: 10.1331/JAPhA.2013.12240. [DOI] [PubMed] [Google Scholar]
- 9.Meyerson B, Barnes P, Emetu R, et al. Institutional and structural barriers to HIV testing: elements for a theoretical framework. AIDS Patient Care STDS. 2014;28:22–27. doi: 10.1089/apc.2013.0238. [DOI] [PubMed] [Google Scholar]
- 10.Dugdale C, Zaller N, Bratberg J, et al. Missed opportunities for HIV screening in pharmacies and retail clinics. J Manag Care Pharm. 2014;20:339–345. doi: 10.18553/jmcp.2014.20.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amesty S, Weidle PJ, Willis L, et al. Support for in-pharmacy HIV testing and prevention services among pharmacists registered in the New York state expanded syringe access program: findings from the PHARM-HIV study. New Orleans: American Pharmacists Association Annual Meeting & Exposition; 2012. Mar, [Google Scholar]
- 12.Calderon Y, Cowan E, Rhee JY, et al. Counselor-based rapid HIV testing in community pharmacies. AIDS Patient Care STDS. 2013;27:467–473. doi: 10.1089/apc.2013.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrobel JM, Scasi KK, Klepser ME, Darin KM. Potential acceptance of community pharmacy based HIV screening: a qualitative evaluation. New Orleans: American Society of Health-System Pharmacists Midyear Clinical Meeting & Exhibition; 2011. Dec, [Google Scholar]
- 14.Greater Than AIDS. [September 30, 2013]; http://greaterthan.org. [Google Scholar]
- 15.Gilead. Gilead’s HIV FOCUS program: HIV on the frontlines of communities in the United States. [September 30, 2013]; www.gilead.com/~/media/Files/pdfs/other/HIV-FOCUS-Program.pdf. [Google Scholar]
- 16.Howerton D, Anderson N, Bosse D, et al. Good laboratory practices for waived testing sites: survey findings from testing sites holding a certificate of waiver under the clinical laboratory improvement amendments of 1988 and recommendations for promoting quality testing. MMWR Recomm Rep. 2005;54:1–25. [PubMed] [Google Scholar]
- 17.OraSure Technologies. Community, A Walgreens Pharmacy in Washington DC becomes first pharmacy in U.S. to launch rapid hepatitis C testing program. [September 30, 2013]; www.testhepc.com/default.aspx?pageid=3037. [Google Scholar]
- 18.Deas C, McCree DH. Pharmacists and HIV/AIDS prevention: review of the literature. J Am Pharm Assoc. 2010;50:411–415. doi: 10.1331/JAPhA.2010.09039. [DOI] [PubMed] [Google Scholar]
- 19.Binkley D, Waller L, Potts L, Bronstein J. Pharmacists as HIV/AIDS information resources: survey of Alabama pharmacists. AIDS Educ Prev. 1995;7:455–466. [PubMed] [Google Scholar]
- 20.Crawford ND, Amesty S, Rivera AV, et al. Randomized, community-based pharmacy intervention to expand services beyond sale of sterile syringes to injection drug users in pharmacies in New York City. Am J Public Health. 2013;103:1579–1582. doi: 10.2105/AJPH.2012.301178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubbins PO, Klepser ME, Dering-Anderson AM, et al. Point-of-care testing for infectious diseases: opportunities, barriers, and considerations in community pharmacy. J Am Pharm Assoc. 2014;54:163–171. doi: 10.1331/JAPhA.2014.13167. [DOI] [PubMed] [Google Scholar]
- 22.Meyerson BE, Ryder PT, von HC, Coy K. We can do more than just sell the test: pharmacist perspectives about over-the-counter rapid HIV tests. AIDS Behav. 2013;17:2109–2113. doi: 10.1007/s10461-013-0427-y. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Advantages and disadvantages of different types of FDA-approved HIV immunoassays used for screening by generation and platform. [September 30, 2013]; www.cdc.gov/hiv/pdf/testing_Advantages&Disadvantages.pdf.
- 24.Centers for Disease Control and Prevention. Rapid HIV tests suitable for use in non-clinical settings (requires CLIA-waiver certificate) [June 25, 2014]; www.cdc.gov/hiv/pdf/testing_nonclinical_cliawaived-tests.pdf.
- 25.Myers JE, El-Sadr WM, Zerbe A, Branson BM. Rapid HIV self-testing: long in coming but opportunities beckon. AIDS. 2013;27:1687–1695. doi: 10.1097/QAD.0b013e32835fd7a0. [DOI] [PubMed] [Google Scholar]
- 26.Patel P, Bennett B, Sullivan T, et al. Rapid HIV screening: missed opportunities for HIV diagnosis and prevention. J Clin Virol. 2012;54:42–47. doi: 10.1016/j.jcv.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyerson BE, Ryder PT, Richey-Smith C. Achieving pharmacy-based public health: a call for public health engagement. Public Health Rep. 2013;128:140–143. doi: 10.1177/003335491312800303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strand MA, Miller DR. Pharmacy and public health: a pathway forward. J Am Pharm Assoc. 2014;54:e220–e224. doi: 10.1331/JAPhA.2014.13145. [DOI] [PubMed] [Google Scholar]