Abstract
There is no current medical therapy for stroke recovery. Principles of physiological plasticity have been identified during recovery in both animal models and human stroke. Stroke produces a loss of physiological brain maps in adjacent peri-infarct cortex and then a remapping of motor and sensory functions in this region. This remapping of function in peri-infarct cortex correlates closely with recovery. Recent studies have shown that the stroke produces abnormal conditions of excitability in neuronal circuits adjacent to the infarct that may be the substrate for this process of brain remapping and recovery. Stroke causes a hypo-excitability in peri-infarct motor cortex that stems from increased tonic γ-aminobutyric acid activity onto neurons. Drugs that reverse this γ-aminobutyric acid signaling promote recovery after stroke. Stroke also increases the sensitivity of glutamate receptor signaling in peri-infarct cortex well after the stroke event, and stimulating α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate glutamate receptors in peri-infarct cortex promotes recovery after stroke. Both blocking tonic γ-aminobutyric acid currents and stimulating α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors promote recovery after stroke when initiated at quite a delay, more than 3 to 5 days after the infarct. These changes in the excitability of neuronal circuits in peri-infarct cortex after stroke may underlie the process of remapping motor and sensory function after stroke and may identify new therapeutic targets to promote stroke recovery.
Stroke is the leading cause of adult disability because of the brain’s limited capacity to repair. Studies of neural repair in stroke have identified several principles of tissue reorganization that might mediate recovery. Stroke induces rapid plasticity in the functional activation of cortical networks. Small cortical strokes in experimental animals cause a loss of responsiveness of surviving cortical areas for several weeks. Sensory inputs to these areas then remap after this time of decreased activity and form new maps or new representations.1 An example is the cortical representation of the forelimb in both rat and mouse stroke models. After stroke, forelimb responses are transferred to the contralateral hemisphere2 and there is decreased activation of the original forelimb cortex near or adjacent to the stroke.2,3 After several weeks, the peri-infarct cortex ipsilateral to stroke again responds to sensory inputs and exhibits remapping of the representation of body regions. In humans, stroke also precipitates a pattern of initially diminished responsiveness in peri-infarct and ipsilateral (to the stroke) brain areas and transfer of language, motor, or sensory activation into contralateral cortex.4 In cases of good recovery, this cortical activation pattern is then transferred back to peri-infarct and ipsilateral networks, but in an altered pattern with new sites of activation compared with those before the stroke.4 These findings indicate that stroke modulates the physiological responsiveness in peri-infarct cortex in a way that is associated with recovery.
These changes in the physiological responsiveness of brain networks occur at the same time as alterations in neuronal excitability. Studies of cellular excitability in the dish, such as measures of neuronal activation in brain slices, indicate altered excitatory inputs after stroke, with prolonged excitatory potentials and enhanced ability to form long-term excitable responses to inputs.5 This last measure, termed long-term potentiation (LTP), is particularly interesting in its modulation after stroke. Long-term potentiation is a measure of the ability of an excitatory synapse to undergo a long-term modification and is the key event underlying learning, memory, and the enhanced excitability that supports neuronal network modification by experience. Cortex normally exhibits LTP in response to afferent input, and cortical LTP is critical to the enhancement of sensory input, memory formation, and learning in the cortex.6 In humans, studies using transcranial magnetic stimulation indicate that there is an enhanced ability to undergo LTP-like phenomena in cortex after stroke. Stimulation parameters using transcranial magnetic stimulation or transcranial direct stimulation that are associated with LTP potentiate short-term recovery of function after stroke and may influence long-term recovery.7 On a clinical level, the effect of magnetic or electrical enhancement of brain excitability after stroke in humans has led to clinical trials for this approach to improve stroke recovery.8 On a basic science level, the data on a role for brain excitability changes in functional recovery in humans and the findings of changes in cortical responsiveness to afferent inputs during recovery suggest that cellular systems that control excitatory signaling in the brain may provide approaches to promote recovery.
BRAIN EXCITABILITY, LEARNING AND MEMORY, AND RECOVERY
Learning and memory processes in the brain involve the modulation of neuronal excitability. Events in memory formation prolong or depress the excitatory responsiveness of specific synapses to incoming signals.6 There are many parallels between the mechanisms of learning and memory and those of stroke recovery. On a neuropsychological level, principles of motor learning also underlie recovery after stroke. Classical learning rules of learned nonuse, mass action, contextual interference, and distributed practice also apply to stroke recovery.9 On a brain imaging level, similar events underlie motor learning and recovery after stroke. For example, in both cases, an attempt at performing the target behavior produces a diffuse and more low-level activation of many different brain areas that is then focused to a more discrete and highly activated network of areas closely associated with the task.10 As noted, on a cellular level, learning and memory processes and recovery after stroke are both identified with changes in network excitability such as LTP. Molecular memory systems that underlie LTP are also induced in peri-infarct cortex after stroke, such as GAP43, SCG10, and MARCKS.11 These similarities between neuropsychological, brain imaging, cellular, and molecular aspects of learning and memory and aspects of stroke recovery suggest that the key elements in memory formation—the regulation and coding of brain excitability—may also play a role in stroke recovery. Several recent studies have directly explored signaling systems that affect neuronal excitability in memory formation for their role in stroke recovery and point the way toward possible new therapies for stroke recovery.
γ-AMINOBUTYRIC ACID SIGNALING IN PERI-INFARCT CORTEX DURING RECOVERY AFTER STROKE
γ-Aminobutyric acid (GABA)–ergic signaling in the brain occurs through 2 main systems (Table).12 Phasic or synaptic GABA signaling is when an interneuron action potential depolarizes the presynaptic bouton, causing release of GABA and an effect on postsynaptic GABA receptors. The GABA type A (GABAA) receptors mediate an inward chloride current and the cell is hyperpolarized in a rapid, transient nature. In synaptic GABA signaling, the transient nature of the response occurs because of the rapid desensitization of the synaptic GABAA receptors and because GABA is taken back out of the synapse by GABA transporters. A second type of GABA signaling is tonic or extrasynaptic GABA signaling. Extra synaptic GABAA receptors respond to ambient GABA levels outside the synapse or to synaptic spillover of GABA. Extrasynaptic GABA receptors desensitize more slowly and exhibit a greater binding affinity to GABA than synaptic GABA receptors. These characteristics mean that extrasynaptic GABA receptors mediate a tonic inhibitor current, or shunt current.12 This current controls the overall membrane potential of the neuron and its propensity to fire. In terms of overall inhibitory current flow in the neuron, the tonic GABA current greatly exceeds the precisely timed and smaller phasic GABA current.12 In simplistic terms, the greater the tonic GABA current is, the more hyperpolarized the cell is and the less likely it is to fire in response to a given excitatory stimulus. The smaller the tonic GABA current is, the more depolarized the cell is and the more likely it is to fire in response to a given excitatory input.
Table.
Brain Excitability Signaling Systems and the Effect of Stroke
| Physiological Process |
Molecular Anatomy |
Neuronal Function |
Response to Stroke in Acute Phase |
Response to Stroke in Chronic Phase |
Role in Functional Recovery |
|---|---|---|---|---|---|
| Tonic GABA signaling | Extrasynaptic inhibition | Slow inhibition, controls normal neuronal excitability | Neuroprotective | Current is activated, hinders recovery | Blocking tonic GABA currents promotes recovery in stroke |
| Phasic GABA signaling | Synaptic inhibition | Fast inhibition within synaptic networks | Neuroprotective | Current is diminished, role in chronic phase not determined | Blocking synaptic inhibition may promote recovery with low risk:benefit ratio |
| AMPA receptor signaling | Synaptic glutamate neurotransmission | Main fast excitatory signaling receptor | Participates in excitotoxic effects | Sensitive to activation and BDNF induction | Agonists promote recovery in stroke |
| NMDA receptor signaling | Activity-dependent glutamate neurotransmission | Slow excitatory signaling, coincidence detector in synaptic activation | Participates in excitotoxic effects | May enhance functional recovery | Agonists promote recovery in some CNS injury models |
| Kainate receptor signaling | Synaptic glutamate neurotransmission, presynaptic glutamate receptor, metabotropic glutamate signaling | Presynaptic inhibition, G protein signaling, fast excitatory signaling | Participates in excitotoxic effects | Not determined | Not determined |
Abbreviations: AMPA, γ-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; BDNF, brain-derived neurotrophic factor; CNS, central nervous system; GABA, γ-aminobutyric acid; NMDA, N-methyl-d-aspartate.
Tonic GABA signaling controls neuronal excitability and is a key target in promoting memory formation. Drugs that block tonic GABA signaling promote neuronal excitability, enhance LTP formation, and lead to long-lasting enhancements in learning paradigms. The LTP in cortex is modulated by GABA inhibition13 in the same circuits that underlie motor learning,14 and motor learning is accompanied in humans by a decrease in cortical GABA.15 Pharmacological approaches to blocking tonic GABA current have been a source of substantial drug development and have focused on the distinct molecular composition of extrasynaptic GABA receptors.12,16 These receptors contain a preponderance of the GABA receptor α5 and δ subunits. Drugs that selectively block α5-containing GABAA receptors will decrease the tonic GABA current. Because this approach acts on tonic GABA signaling and not phasic GABA signaling, there has not been an increase in seizure activity with this approach and several of these drugs have progressed into human trials.17 As noted earlier, peri-infarct cortex exhibits decreased activation to motor and sensory stimulation early after stroke. Because of the prominent role of tonic GABA signaling in setting neuronal excitability, alterations in tonic GABA signaling in stroke may contribute to this diminished cortical responsiveness. However, despite much work on synaptic or phasic GABA signaling in stroke, there have been no studies on tonic GABA signaling in stroke. Tonic GABA signaling has been studied in peri-infarct cortex in experimental stroke in relation to motor cortex activation and behavioral recovery.
Stroke causes an increase in tonic GABA currents for more than 1 month after the stroke. This was studied in a model of experimental stroke in which the infarct is in the portion of the motor cortex that controls the mouse forelimb and the recovering cortex is adjacent to the stroke within other areas of the motor cortex infarct.18 This allows a tight experimental system in which the structure and function of recovering cortex can be specifically studied. In slice physiology studies, neurons in recovering motor cortex are hypoexcitable. There is a substantial 50% increase in the tonic GABA current in peri-infarct motor neurons. This current is not due to altered GABA channel function or to alterations in the neuronal responsiveness to GABA itself. Instead, neurons exhibit an increased tonic GABA current because of diminished GABA uptake: there is more GABA in the recovering peri-infarct cortex after stroke. The GABA uptake occurs through transporters present on neurons and astrocytes.12 It is specifically GABA uptake in astrocytes that is reduced. This is due to a reduced level of the GABA uptake protein GAT-3/4 in reactive astrocytes in peri-infarct cortex.18 In a term, reactive astrocytes have diminished GABA uptake, GABA accumulates, and the GABA stimulates extrasynaptic GABA receptors, which increases the tonic GABA current and lowers the excitability of neighboring neurons. Specific extrasynaptic GABA receptor blockers such as GABAA receptor α5 inverse agonists12,16 reverse this poststroke increase in GABA current. In patch-clamp studies of neurons in a slice, the GABAA receptor α5 inverse agonist L655,708 mediates a remarkable restoration of cellular excitability from the hypoexcitable state in post-stroke pyramidal neurons to that seen in control motor cortical neurons. Thus, on a physiological level, recovering peri-infarct motor cortex is hypoexcitable after stroke, and this hypoexcitability can be specifically reversed by blocking extrasynaptic GABA receptors. What does this mean for behavioral recovery?
Blocking tonic GABA currents also promotes behavioral recovery after stroke.18 Mice administered GABAA receptor α5 inverse agonists or mice with genetic deletions of GABAA receptor α5 or δ subunits exhibit improved recovery after stroke. This recovery is very interesting as it occurs immediately after stroke: mice given a GABAA receptor α5 inverse agonist exhibit the maximal recovery effect within days of initiating the drug. This recovery effect can also be produced with a delay after stroke, when the drug is given beginning 3 days after the infarct. This immediate effect on behavioral recovery differs from the improved recovery seen in other preclinical neural repair therapies that are effective during the recovery phase after stroke, such as growth factor or cell treatments, in which recovery builds over time.19,20 The rapid improvement in recovery may occur because abnormally functioning circuits adjacent to the stroke have a quick normalization in excitability with this approach.
The ability to reverse brain hypo-excitability after stroke and improve recovery in preclinical stroke models has 3 important implications. First, it suggests a novel pharmacological approach to treating stroke recovery. Many efforts at developing clinically relevant tonic GABA antagonists have been published and are ongoing.16 Although developed for other diseases such as Alzheimer disease, these may be applied to stroke. Second, the time window for administering an extrasynaptic GABAA receptor blocker is well after the initial stroke, such as 3 days after the infarct. This opens the clinical window for therapy compared with neuroprotective approaches. Third, the time course of improvement with blocking tonic GABA signaling is such that the effect is maximal and immediate. Thus, tonic GABA antagonists may be applied in conjunction with other approaches that stimulate recovery during a more prolonged period for a multifaceted approach to improve stroke recovery.
There has been much previous work on cortical inhibition after stroke. How do these data on tonic GABA inhibition fit with earlier studies of synaptic inhibition in stroke? The increase in tonic GABA inhibition after stroke occurs because of diminished GABA uptake, so this primary effect (reduced GABA uptake) will not be seen in studies of phasic or synaptic GABA signaling. All previous studies in brain inhibition after stroke have examined phasic or synaptic inhibition. Synaptic inhibition as measured in brain slices by paired-pulse inhibition is decreased, particularly at 7 days after the stroke.21 This was also seen in more recent studies.18 There are inconsistent data on the molecular correlates of this transient decrease in synaptic GABA signaling. It may correspond to a reduced expression of GABAA receptor subunits during this period, although data supporting a reduction in GABAA receptor levels are not consistent across studies.22,23
Human studies with transcranial magnetic stimulation have also looked at synaptic GABA signaling after stroke. Using a paradigm of paired-pulse inhibition in which a magnetic pulse that is insufficient to evoke a movement is delivered just before a suprathreshold pulse (short-interval cortical inhibition), motor cortex ipsilateral to stroke has a reduced paired-pulse inhibition in the early stages after stroke24 that may normalize.25 The animal and human data indicate a possible contrast in the response to GABA systems after stroke: phasic GABA signaling is reduced in the first weeks after stroke, while tonic GABA signaling is potentiated. The behavioral and electrophysiological studies in mice suggest that the overall effect in terms of motor cortex circuitry is one of diminished neuronal excitability, which when reversed leads to recovery.
GLUTAMATE SIGNALING IN PERI-INFARCT CORTEX DURING RECOVERY FROM STROKE
Glutamate signals through α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate (AMPA), N-methyl-d-aspartate, and kainate receptors (Table). Glutamate signaling through AMPA receptors is the major excitatory signaling system in the adult brain. The AMPA receptor signaling has a key role in LTP and memory formation, with alterations in receptor trafficking, membrane insertion, and posttranslational modification in AMPA receptors all contributing to memory formation.26 The role of initial glutamate toxic effects in stroke cell death has been amply documented and has led to the effort for neuroprotection at glutamate blockade. However, glutamate signaling plays an opposite role in recovery after stroke. After human stroke, increases in baseline glutamatergic excitability in peri-infarct tissue parallel recovery, and the level of LTP-like cortical excitability is correlated with recovery. Direct current stimulation of peri-infarct cortex using a protocol that boosts local neuronal excitability improves use of the affected limb in patients with stroke.27 These human stimulation studies and the parallels between memory formation and stroke recovery suggest that glutamate and possibly AMPA receptor signaling may play a role in the synaptic plasticity that underlies recovery.
Positive modulation of AMPA receptor signaling promotes motor recovery after stroke. This was shown in a multistage approach using pharmacological gain and loss of function.28 Drugs that stimulate AMPA receptor activity when glutamate is bound are positive allosteric modulators of the AMPA receptor and have been termed AMPA kines. AMPA kines promote AMPA receptor channel open time and amplitude, enhance LTP, and promote learning and memory in animal models.29 AMPA kine administration also promotes recovery after stroke. Beginning 5 days after stroke, systemic administration of an AMPA kine gradually improves limb motor control in a dose-dependent way.28 This improvement is significant at 1 month after stroke and to a further degree at later periods. This is an important finding as it indicates that treatment with this drug is working at quite a delay after stroke—5 days after the infarct. There is no effect on infarct size with this delayed treatment, indicating an effect on recovering tissue. On the other hand, blocking AMPA receptor signaling during this same period impairs recovery of limb motor control. Thus, AMPA receptor signaling has a delayed and causal effect on motor recovery after stroke.
The potentiation of AMPA receptor signaling may improve motor recovery because it is enhancing overall excitatory glutamatergic communication between neurons or because enhanced AMPA receptor signaling mediates a downstream action such as brain-derived neurotrophic factor (BDNF) release. In support of the former possibility, enhancing N-methyl-d-aspartate signaling at a delay after traumatic brain injury improves recovery.30
However, 2 pieces of evidence support a BDNF action for the recovery effects seen in enhancing AMPA receptor signaling. First, distinct AMPA kines were evaluated for their effect on motor recovery after stroke. Low-impact AMPA kines promote AMPA receptor currents but do not induce BDNF release; high-impact AMPA kines also promote BDNF release.28 Only high-impact AMPA kines significantly improve motor recovery. High-impact AMPA kines induce BDNF signaling during the period in which they enhance recovery. This induction occurs in a widespread area of peri-infarct cortex. Interestingly, although the AMPA kine was given systemically in these studies, it only enhanced BDNF signaling in peri-infarct cortex and not in other brain regions. This suggests that the recovering peri-infarct circuitry may be primed for activity-dependent BDNF release. Blocking BDNF, also only in peri-infarct cortex, blocked the recovery-promoting effect of high-impact AMPA kines. Thus, enhancing AMPA receptor activity mediates improved recovery through its downstream effect of BDNF induction within peri-infarct cortex. Interestingly, blocking BDNF locally in peri-infarct cortex also impaired normal recovery after stroke. Thus, normal brain recovery, at least in this animal model of stroke, occurs through peri-infarct BDNF action.
Brain-derived neurotrophic factor exerts powerful effects on neuronal plasticity, synapse development, and dendritic and axonal sprouting. Selective release of BDNF promotes local and long-distance circuit formation during brain development. Release of BDNF is important in the initiation phases of LTP.31 In stroke, systemic administration of BDNF promotes recovery after stroke.32 Brain infusion of a BDNF antisense oligonucleotide impairs recovery.33 Although these BDNF studies and the AMPA kine study noted previously clearly position this growth factor as a key player in recovery after stroke, the actual mechanisms and translation of these findings may be complex. For example, although BDNF is clearly linked to neuronal plasticity, in stroke it also has an effect through angiogenesis.34 The downstream actions of AMPA kines, N-methyl-d-aspartate receptor stimulation, and BDNF also occur at least in part through the mammalian target of rapamycin system. Neuronal mammalian target of rapamycin signaling promotes a growth state in adult neurons and dramatically enhances axonal sprouting in central nervous system injury models.35 Mammalian target of rapamycin is a prominent target for neural repair in spinal cord injury.36 In studies in stroke, AMPA kine-induced BDNF signaling was not associated with axonal sprouting in peri-infarct cortex.28 However, these data indicate that enhancing excitatory signaling after stroke may have many potential downstream actions in several cellular systems.
YIN AND YANG OF EXCITABILITY IN STROKE
Brain excitability passes through 2 opposite and contradictory phases interlinked through GABA and glutamate signaling during the first weeks after stroke. Acute ischemia triggers neuronal depolarization, glutamate release, calcium entry, further glutamate release and depolarization, and excitotoxic cell death. Inhibition of GABA by hyperpolarizing the cell in part counteracts this excitotoxic cascade. In the acute phase, brain excitability levels are elevated and deleterious. In this phase, blocking glutamate signaling or enhancing GABA signaling promotes neuroprotection, at least in animal models. In the chronic phase, the exact opposite appears to be true. After cell death has transpired, the brain begins to reorganize and repair in the chronic phase of stroke. Tonic GABA currents are enhanced and synaptic glutamate signaling is sensitized to promote BDNF release. Blocking tonic GABA signaling and enhancing glutamate signaling promote recovery (Figure 1). There is a yin and yang to brain excitability (Figure 2): complementary opposites interact within a unified whole of stroke progression. A key element to translating this concept to human neural repair therapies is to determine the inflection point for acute to chronic roles, from yin to yang, in brain excitability effects. As noted, in the mouse this occurs at 3 days after stroke.
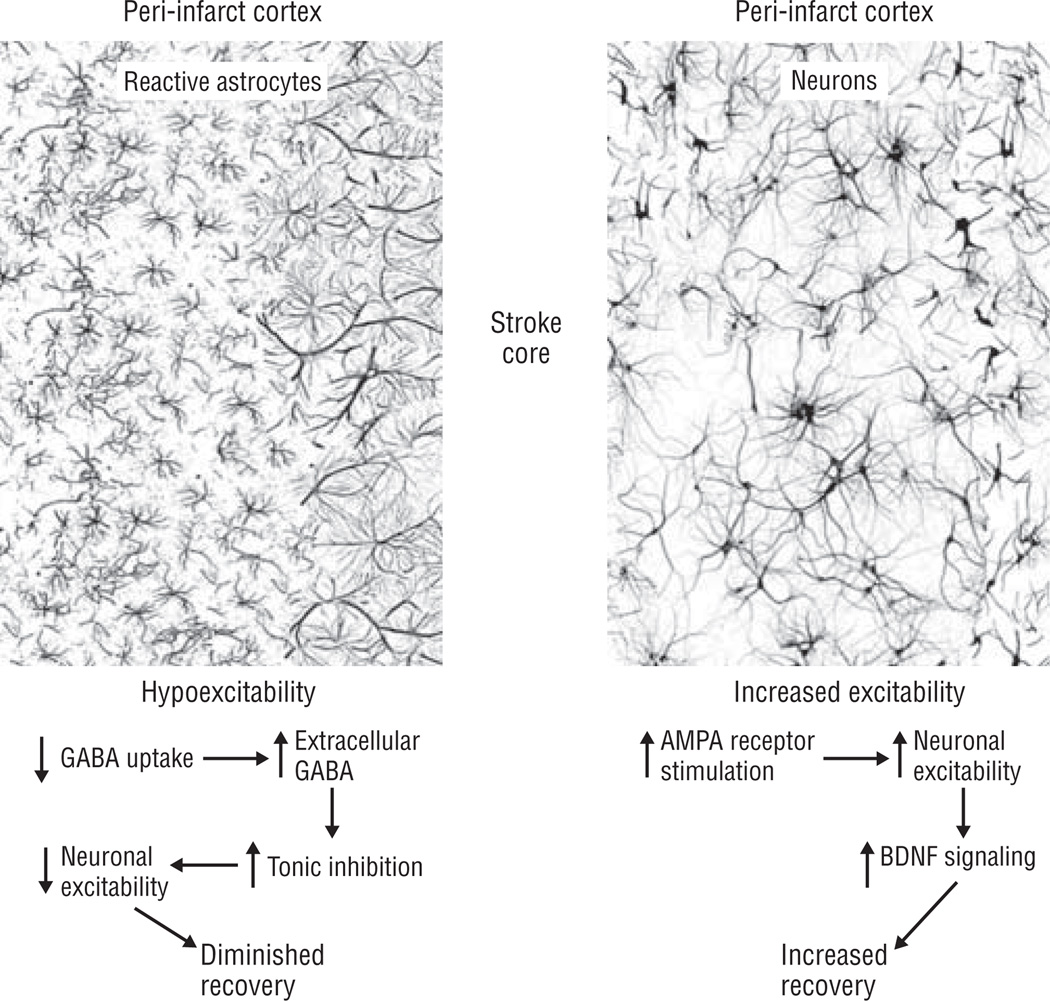
Figure 1.
Peri-infarct cortex exhibits both hypoexcitability and increased excitability responses after stroke. Reactive astrocytes contribute to increased tonic γ-aminobutyric acid (GABA) inhibition by decreasing GABA uptake. Neurons in peri-infarct cortex have an enhanced response to α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor stimulation through brain-derived neurotrophic factor (BDNF) signaling. Pharmacological manipulation of both hypoexcitability and increased excitability promotes behavioral recovery after stroke.
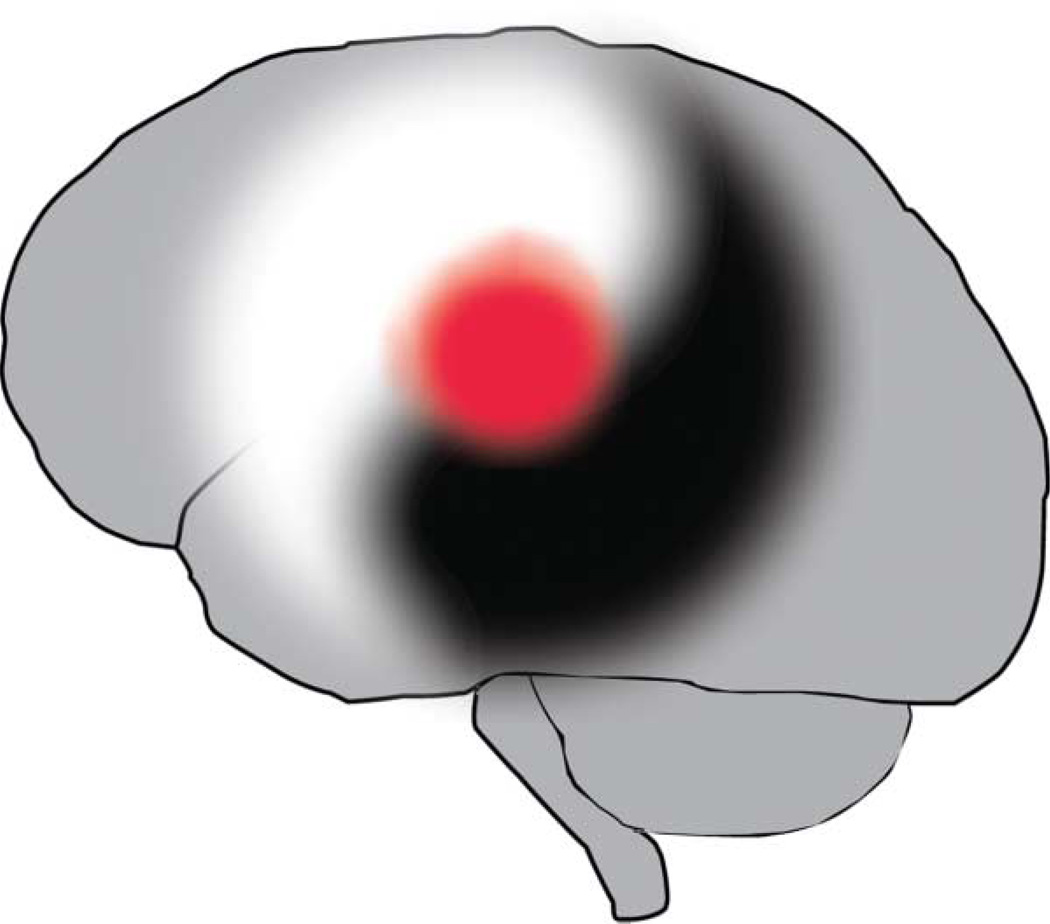
Figure 2.
Yin and yang of brain excitability in stroke. Stroke triggers early increases in excitability that are deleterious. In later phases of recovery, the precise signaling systems in brain excitability that were deleterious now become beneficial. Brain excitability after stroke involves 2 contrary actions of a similar set of signaling systems within an interconnected whole: a yin and yang of stroke progression.
DODGING THE KISS OF DEATH
This idea that some aspect of learning and cellular excitability is the neuronal basis for recovery has led to ad hoc attempts to treat brain-injured patients with any available drug that might also stimulate learning, memory, or attention, including selective serotonin reuptake inhibitors, dopamine agonists, methylphenidate, modafinil, and amphetamine. Because these drugs were developed to treat conditions other than neurorehabilitation from brain injury and because they act with less specificity on many neurotransmitter systems in the brain, their role in promoting neural recovery after brain injury has in general not withstood rigorous clinical trials.37 Recent studies in tonic GABA and AMPA receptor signaling highlight the promising potential for delayed therapies that target neuronal processes that are very specific for stroke recovery: altered neuronal excitability in peri-infarct tissue (Figure 1).
Moving these findings to the clinic is hampered by the sense of despair in the pharmaceutical industry for stroke therapies. This is of course owing to the failures in neuroprotection. It has been said that there is no easier way to clear a meeting room in the pharmaceutical/biotechnology industry than to speak the word “stroke.” However, stroke neural repair provides a highly attractive treatment target. This is because neural repair therapies differ from neuroprotective therapies in at least 3 important ways. Neural repair therapies are given at a delay, well after patients with stroke are in the hospital. Neural repair therapies are given after the phases of cell death, when patients are stable. Also, neural repair therapies target neural systems in which industry research and development have generated promising agents such as tonic GABA signaling and AMPA receptor modulation. If neuroprotection is the immediate kiss of death for a stroke therapy, perhaps a neural repair therapy might offer a more lasting warm embrace.
Acknowledgments
Funding/Support: This work was supported by the Dr Miriam and Sheldon G. Adelson Medical Research Foundation and the Larry L. Hillblom Foundation.
Role of the Sponsors: The sponsors had no role in the design, conduct, or analysis of the study.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 2.Dijkhuizen RM, Ren J, Mandeville JB, et al. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci U S A. 2001;98(22):12766–12771. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29(6):1719–1734. doi: 10.1523/JNEUROSCI.4249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer SC. Repairing the human brain after stroke, I: mechanisms of spontaneous recovery. Ann Neurol. 2008;63(3):272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 5.Hagemann G, Redecker C, Neumann-Haefelin T, Freund HJ, Witte OW. Increased long-term potentiation in the surround of experimentally induced focal cortical infarction. Ann Neurol. 1998;44(2):255–258. doi: 10.1002/ana.410440217. [DOI] [PubMed] [Google Scholar]
- 6.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 7.Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 2009;40(5):1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Wagner CW, Frayne C, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. 2011;42(4):1035–1040. doi: 10.1161/STROKEAHA.110.602128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 10.Ward NS. The neural substrates of motor recovery after focal damage to the central nervous system. Arch Phys Med Rehabil. 2006;87(12 suppl 2):S30–S35. doi: 10.1016/j.apmr.2006.08.334. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193(2):291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56(5):763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75(5):1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- 14.Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1(3):230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 15.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95(3):1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 16.Gabriella G, Giovanna C. γ-Aminobutyric acid type A (GABA(A)) receptor subtype inverse agonists as therapeutic agents in cognition. Methods Enzymol. 2010;485:197–211. doi: 10.1016/B978-0-12-381296-4.00011-7. [DOI] [PubMed] [Google Scholar]
- 17.Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR. Blockade of alcohol’s amnestic activity in humans by an alpha5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology. 2007;53(7):810–820. doi: 10.1016/j.neuropharm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Clarkson AN, Huang BS, MacIsaac S, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468(7321):305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitmeir R, Kilic E, Kilic U, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011;134(pt 1):84–99. doi: 10.1093/brain/awq344. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 21.Neumann-Haefelin T, Hagemann G, Witte OW. Cellular correlates of neuronal hyperexcitability in the vicinity of photochemically induced cortical infarcts in rats in vitro. Neurosci Lett. 1995;193(2):101–104. doi: 10.1016/0304-3940(95)11677-o. [DOI] [PubMed] [Google Scholar]
- 22.Qü M, Buchkremer-Ratzmann I, Schiene K, Schroeter M, Witte OW, Zilles K. Bihemispheric reduction of GABAA receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Res. 1998;813(2):374–380. doi: 10.1016/s0006-8993(98)01063-4. [DOI] [PubMed] [Google Scholar]
- 23.Jolkkonen J, Gallagher NP, Zilles K, Sivenius J. Behavioral deficits and recovery following transient focal cerebral ischemia in rats: glutamatergic and GABAergic receptor densities. Behav Brain Res. 2003;138(2):187–200. doi: 10.1016/s0166-4328(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 24.Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the post-acute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke. 2003;34(11):2653–2658. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- 25.Wittenberg GF, Bastings EP, Fowlkes AM, Morgan TM, Good DC, Pons TP. Dynamic course of intracortical TMS paired-pulse responses during recovery of motor function after stroke. Neurorehabil Neural Repair. 2007;21(6):568–573. doi: 10.1177/1545968307302438. [DOI] [PubMed] [Google Scholar]
- 26.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8(2):101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 27.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5(8):708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 28.Clarkson AN, Overman JJ, Zhong S, Muelle R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31(10):3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8(5):583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 30.Adeleye A, Shohami E, Nachman D, et al. D-cycloserine improves functional outcome after traumatic brain injury with wide therapeutic window. Eur J Pharmacol. 2010;629(1–3):25–30. doi: 10.1016/j.ejphar.2009.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schäbitz WR, Steigleder T, Cooper-Kuhn CM, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38(7):2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 33.Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40(4):1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 34.Qin L, Kim E, Ratan R, Lee FS, Cho S. Genetic variant of BDNF (Val66Met) polymorphism attenuates stroke-induced angiogenic responses by enhancing anti-angiogenic mediator CD36 expression. J Neurosci. 2011;31(2):775–783. doi: 10.1523/JNEUROSCI.4547-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29(27):8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu K, Lu Y, Lee JK, et al. PTEN deletion enhances the regenerative ability of adult cortico-spinal neurons. Nat Neurosci. 2010;13(9):1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berends HI, Nijlant JM, Movig KL, Van Putten MJ, Jannink MJ, Ijzerman MJ. The clinical use of drugs influencing neurotransmitters in the brain to promote motor recovery after stroke: a Cochrane systematic review. Eur J Phys Rehabil Med. 2009;45(4):621–630. [PubMed] [Google Scholar]