Abstract
Status asthmaticus (SA) is a severe, refractory form of asthma that can result in rapid respiratory deterioration and death. Treatment of SA with inhaled anesthetics is a potentially life-saving therapy, but remarkably few data are available about its mechanism of action or optimal administration. In this paper, we will review the clinical use of inhaled anesthetics for treatment of SA, the potential mechanisms by which they dilate constricted airways, and the side effects associated with their administration. We will also introduce the concept of ‘targeted’ delivery of these agents to the conducting airways, a process which may maximize their therapeutic effects while minimizing associated systemic side effects. Such a delivery regimen has the potential to define a rapidly translatable treatment paradigm for this life-threatening disorder.
Introduction
Status asthmaticus (SA) is a severe, refractory exacerbation of asthma that can result in rapid respiratory deterioration and death. Inhaled volatile anesthetics, such as halothane, isoflurane, sevoflurane, and desflurane, are known to be potent bronchodilators, and have been used for several decades as potentially life-saving therapy for the treatment of SA [1-6]. However, remarkably few data are available about its mechanism of action or optimal administration. In this paper, we will review the clinical use of inhaled anesthetics for treatment of SA, the potential mechanisms by which they dilate constricted airways, and the side effects associated with their administration. We will also introduce the concept of ‘targeted’ delivery of these agents to the anatomic deadspace, which is the selective delivery to the conducting airways in order to maximize their therapeutic effects while minimizing their associated systemic side effects. We hypothesize that in vivo inhaled anesthetics exert their bronchodilatory action primarily through direct, diffusion-mediated mechanisms, and that ‘targeted’, selective delivery to the conducting airways will maximize therapeutic effect while minimizing systemic side effects. We will then illustrate how anesthetic delivery to a branching airway network may be further refined and optimized using computational modeling. Finally, we will present promising experimental results using a prototype anesthetic delivery system in a canine model of severe bronchoconstriction.
Asthma And Status Asthmaticus
Asthma is a chronic disease characterized by a hyperresponsiveness of the airway smooth muscle (ASM). Exacerbations may be provoked by a variety of stimuli such as allergens, cold air, and exercise, resulting in airflow obstruction and increased resistance. This leads to reduced ventilation relative to perfusion [7,8]. The disease is distinguished by hypertrophy and inflammation of ASM and bronchial mucosa, which may cause exacerbations of sustained bronchospasm [9]. Airway plugging may also be present due to increased mucus production. For the patient with asthma, these pathophysiologic features result in increased energy expenditure to maintain adequate gas exchange.
Asthma produces heterogeneous constriction of the airway tree, especially in smaller airways [10]. This corresponds to increases in airway and parenchymal tissue resistance, as well as lung elastance [11]. Studies utilizing positron emission tomography (PET) and magnetic resonance imaging with hyperpolarized gases have also demonstrated the presence of so-called ‘ventilation defects’, suggesting that regions of hypoventilation may be due to partial closure of large bronchi [12-14]. By assuming that terminal airways are bi-stable (i.e., existing as either fully opened or nearly closed [15]), computational modeling studies have demonstrated that small airway constriction may lead directly to partial closure of large bronchi [14,16]. This ultimately results in a positive feedback mechanism, by which ventilation defects are clustered within specific regions. Interdependent effects, in which tethered airways expand with surrounding parenchyma, maintain positive transmural pressure across the airway wall and keep airways open. However, this can create a paradoxical feedback mechanism promoting airway closure in large clusters [17]. This phenomenon was recently demonstrated by Venegas et al. [14] using an imaged-based modeling approach in which uniform smooth muscle activation with a small imposed heterogeneity resulted in catastrophic closure of large volumes of lung, with airway closure potentially occurring at the level of the small bronchioles.
Asthma is particularly prevalent in children, and has exhibited a steady increase over the past few decades [9,18]. Acute exacerbations range from mild to severe, with the latter being responsible for nearly half a million admissions to the pediatric intensive care unit (PICU) each year [19-21]. Treatments for severe acute exacerbations may include supplemental oxygen to relieve hypoxia, short-acting β-agonists and / or anticholinergics to alleviate airflow obstruction, and systemic corticosteroids to decrease inflammation [22]. Status asthmaticus (SA) refers to a potentially lethal exacerbation of asthma characterized by severe bronchoconstriction refractory to such standard therapies. Failure to improve symptoms may result in fulminant respiratory failure and death. Treatments for SA may include intravenous administration of β-agonists, magnesium sulfate, epinephrine, or corticosteroids [22]. Endotracheal intubation and supportive mechanical ventilation may be necessary, as well as inhaled helium-oxygen (heliox) gas mixtures. Extracorporeal membrane oxygenation (ECMO) has also been used in the most severe cases [9,23-25].
Bronchodilation With Volatile Anesthetics
As potent bronchodilators, inhaled volatile anesthetics are important components in the armamentarium of the intensivist who manages therapy of the critically-ill asthmatic patient. Treatment with inhaled anesthetics generally results in improvement within 12 hours, although such agents have also been used for periods of several days in refractory cases [26-30]. The exact mechanism of action by which these agents relax airway smooth muscle is not completely understood [31], and their routine clinical use is hampered by predictable hemodynamic and sedative side effects [25]. These include myocardial depression, arrhythmias, intrapulmonary shunting, and cerebral vasodilation [32], all of which may limit the dose that can be administered or necessitate additional pharmacologic treatment. Rarely volatile anesthetics may be a trigger for malignant hyperthermia, and they have varying, agent-specific potential for renal and hepatic toxicity [4,26,33-35]. Withdrawal effects have been noted following prolonged administration [26], and the possibility of long-term neurotoxicity particularly in children has recently been raised [36]. As sedative-hypnotics that promote muscle relaxation and respiratory depression, their use in patients without a secured airway imposes risk. In addition, administration requires either the use of an anesthesia machine with limited ventilator capabilities, or the jury-rigging of an administration system to an ICU ventilator [4], neither of which is ideal. Moreover, the lack of any optimized devices for delivering inhalation anesthetics to ICU patients has resulted in a paucity of dose-response data. Several fundamental questions exist regarding their mechanism of action and optimal administration [1].
Volatile anesthetics cause relaxation of airway smooth muscle in vitro through various mechanisms that reduce intracellular free calcium. These include inhibition of protein kinase C, calcium release from sarcoplasmic reticulum, and voltage-dependent calcium channels [37-39]. In vivo, such mechanisms require that the anesthetic diffuse from lumen through the airway wall to reach the smooth muscle layer [40,41], as shown in Figure 1. This process is mediated by the relative partition coefficients for these agents, with a larger ratio between airway smooth muscle and gas indicating a greater likelihood of diffusion from airway lumen to ASM [41]. Bronchial re-circulation also facilitates drug delivery to poorly-ventilated regions, as well as clearing bronchoconstricting substances [42-44] For example, ß-agonists are effective whether they are administered intravenously or by aerosol [45].
Figure 1.
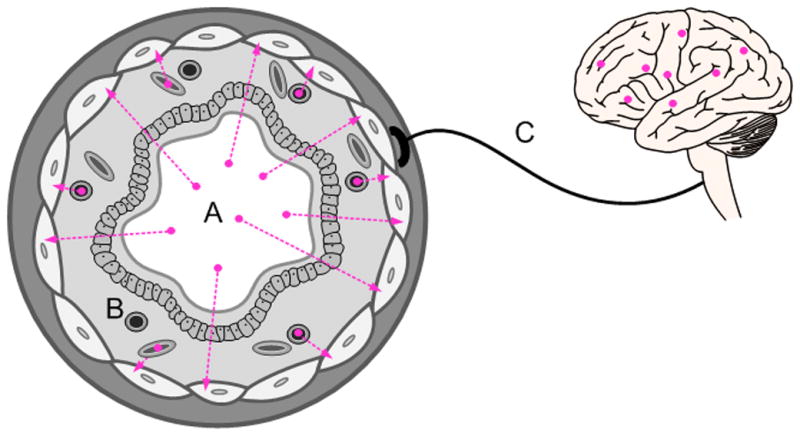
Possible mechanisms by which inhaled anesthetics dilate constricted airways: A) direct action on airway smooth muscle by diffusion from the airway lumen across the airway wall; B) delivery through the bronchial or pulmonary circulations, resulting in a systemic distribution effect; and C) central neurologic, neuroendocrine, or reflex actions.
In addition to direct relaxation effects on ASM, neurally-mediated effects may also be an important component of anesthetic-induced bronchodilation. For example, in vivo airway tone is modulated by a balance between parasympathetic constriction, sympathetic dilation, and non-adrenergic non-cholinergic neural input [46-49]. Previous studies indicate that inhaled anesthetics reduce vagal tone and reflexes [50,51], as well as alter circulating catecholamines and ß receptor sensitivity [52]. Clinically-relevant concentrations of inhaled anesthetics can depress bronchoconstriction produced by either nebulized acetylcholine or vagal nerve stimulation [50].
To summarize, the possible mechanisms by which inhaled anesthetics dilate constricted airways are: 1) direct action on airway smooth muscle by diffusion from the airway lumen across the airway wall; 2) systemic distribution via delivery through the bronchial or pulmonary circulations; and 3) central neurologic, neuroendocrine, or reflex actions. However if inhaled anesthetics work most effectively by direct absorption from airway lumen through the airway walls, then timed-delivery to target the conducting airways during inspiration may have potential to maximize bronchodilation while minimizing systemic effects. Moreover if airways are heterogeneously constricted and are recruited in cascades determined by interdependent effects, then the delivery of bronchodilators to constricted regions may be further optimized by their appropriately timed-delivery to constricted regions, at different points of the inspired tidal volume. This raises the obvious question as to whether sufficient amounts of agent taken from airway lumen to smooth muscle will yield an adequate bronchodilator effect, or whether systemic absorption is necessary for a full therapeutic effect via delivery to under-ventilated regions.
Targeted Anesthetic Delivery
The process of gas transport in the lung occurs via a complex, branching airway network that exponentially increases in cross-sectional area in order to supply a massive exchanging surface. Across this surface, oxygen passively diffuses into the blood, while carbon dioxide is removed. At the end of inspiration, the volume of gas in the conducting airways does not contribute to ventilation, and thus is referred to as the anatomic dead space. Delivery of volatile agents continuously throughout inspiration, as they are administered during surgical procedures, results in drug uptake in the alveoli as well as the conducting airways (Figure 2-A). By contrast, ‘targeted’ delivery of inhaled anesthetics to the dead space offers a scheme for minimizing absorption of the drug into the pulmonary circulation. This approach requires that the concentrations of different gas species vary at different time points of the inspiratory cycle. The concept of selective delivery of an anesthetic to the anatomic deadspace is schematized in Figure 2-B.
Figure 2.
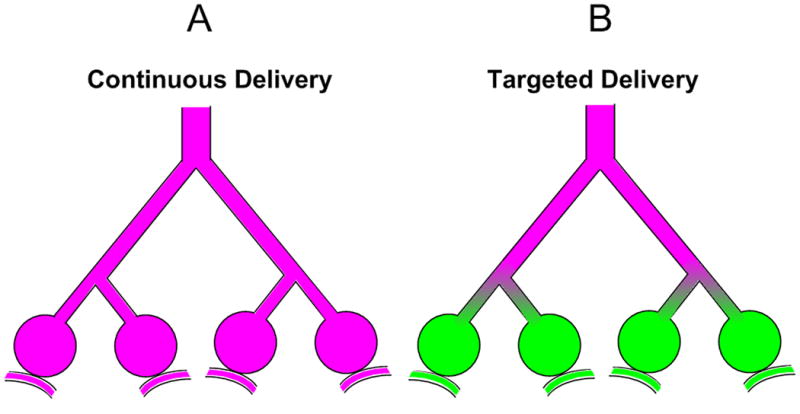
Illustrations of (A) Continuous and (B) Targeted delivery methods for inhaled anesthetics (pink fill). Note that for Targeted delivery, systemic absorption of the agent into the pulmonary circulation is assumed to be minimal.
Such a concept is familiar to anesthesiologists using traditional “Mapleson” style semi-open breathing circuits [53]. Similar passive systems have been employed to control PaCO2 in exercise testing [54]. The feasibility of such a delivery regime has also been demonstrated using inhaled CO2 to modulate ventilation-to-perfusion matching in anesthetized canines [55]. Brogan et al. [55] added CO2 to the conducting airways exclusively via manual injection late in the inspiratory cycle, and demonstrated that such a technique decreases ventilation-to-perfusion heterogeneity, with minimal changes in arterial pH. Active systems in which aerosols [56] or nitric oxide [57] are injected at a specific point in the breathing cycle have also been successfully used to improve delivery to specific lung regions, or to maintain drug concentration during changing airway flows.
Targeted anesthetic delivery also provides a means to distinguish between systemic vs. direct bronchodilator effects of an agent, while characterizing the extent to which inhaled anesthetics diffuse across the airway wall. If volatile anesthetics exert their bronchodilator effects primarily through direct local action on ASM, then selective delivery to the anatomic dead space may be an alternative therapy by which an appropriate balance between bronchodilation and undesirable systemic side effects may be achieved. Moreover if severe bronchoconstriction resolves in patchy serial cascades or avalanches that spread with the assistance of mechanical regional interdependence effects [14,58], then targeted anesthetic delivery has implications for optimal drug administration. Specifically, airway constriction in a symmetric airway tree will lead to asymmetric redistribution of flow, with tidal volume preferentially distributed to less constricted regions [14,59]. Under such conditions, lung recruitment may occur in cascades of large clusters [58]. The delivery of bronchodilators to already well-ventilated regions has the potential to exacerbate ventilation heterogeneity, by reducing the interdependent expansion of constricted areas [14]. However the effects heterogeneous airway constriction on inhaled drug deposition during targeting anesthetic delivery can be explored using computational modeling, as detailed below.
Examples Of Taregted Anesthetic Delivery
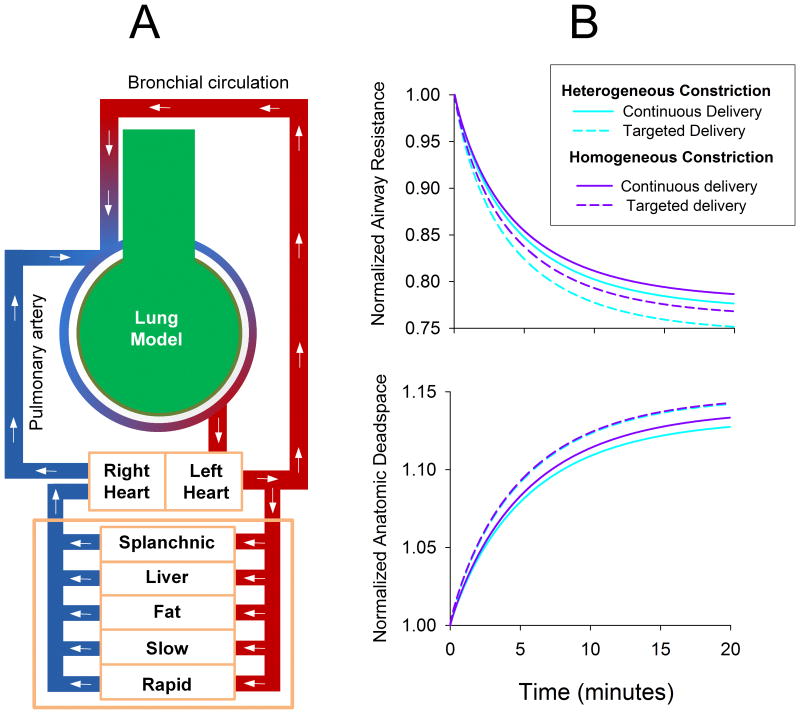
In a previous computational modeling study, Amin et al. [60] demonstrated how an inhaled agonist may be distributed throughout a heterogeneously constricted airway tree, and the implications of such convective transport on ASM activation. Under similar conditions, we compared continuous and targeted delivery of isoflurane during simulated mechanical ventilation of a computational model of the canine lung. The model consisted of a 10 generation airway tree, terminating in 1024 homogeneous parenchymal elastic units and gas volumes. Oxygenated blood was delivered to the airway segments and other tissues using the compartmental model presented by Vinegar et al. [61]. Deoxygenated bronchial blood flow emptied into the pulmonary circulation which, after leaving the lungs, perfused various terminal tissue compartments (Figure 3-A). Homogenous constriction was then simulated by equivalently reducing all airway diameters by 30%, whereas heterogeneous constriction was imposed by stochastically reducing diameters by 25 to 35%. After constriction was established, the anesthetic was deposited directly onto the airway luminal surface, which elicited dose-dependent ASM relaxation. Perfusion via bronchial circulation allowed the anesthetic to reach poorly-ventilated regions. Blood flow throughout the rest of the tissues was simulated via a closed-compartment model to predict anesthetic concentrations in various organs over time [61]. Airway flow, particle transport and deposition, and receptor binding kinetics were all simulated using the previous model of Amin et al. [60]. Figure 3-B shows example tracings of lung resistance and anatomic dead space versus time for these various conditions during mechanical ventilation. As these simulations demonstrate, targeted delivery achieves a greater degree of bronchodilation compared to continuous delivery for equal within-breath doses of isoflurane. This is due to the fact that during targeted delivery, more drug deposits directly onto the airway surface, as opposed to bypassing this diffusive route and being absorbed directly into the pulmonary circulation. Targeted delivery of anesthetic to this model also predicts slightly greater increase in anatomic deadspace compared to continuous delivery, although the absolute difference was minimal.
Figure 3.
(A) Computational model used to simulate the administration of volatile anesthetic during mechanical ventilation of a canine lung. Lung model consisted of a 10 generation airway tree of cylindrical tubes, terminating in homogeneous tissues elastances and gas volumes. Canine-specific lengths and diameters [65] were assigned and normalized to achieve an anatomic dead space of 150 mL. Airways were constricted either 1) homogeneously by reducing all diameters by 30%, or 2) heterogeneously by stochastically reducing all diameters by 25 to 35%. Following bronchoconstriction, inhaled anesthetic was deposited directly onto airway luminal surfaces to elicit dose-dependent ASM relaxation. Bronchial blood flow also allowed the anesthetic to reach poorly-ventilated regions, prior to emptying into the pulmonary circulation, which perfused the terminal compartments uniformly. Systemic blood flow was simulated via a 5-compartment model to predict local anesthetic concentrations in various organs over time [61]. Simulated ventilation was performed using a tidal volume of 350 mL, with constant inspiratory flow and passive exhalation at 20 min-1 for 20 minutes. Inspired air contained equal doses of isoflurane either: 1) continuously throughout inspiration; or 2) only during the last portion of inspiration with a volume equal to the anatomic dead space. Flow calculations, particle transport and deposition, and receptor binding kinetics were all simulated using the model of Amin, et al. [60]. (B) Normalized lung resistance and anatomic deadspace for homogeneous and heterogeneous model conditions for administration of isoflurane in continuous or targeted delivery modes.
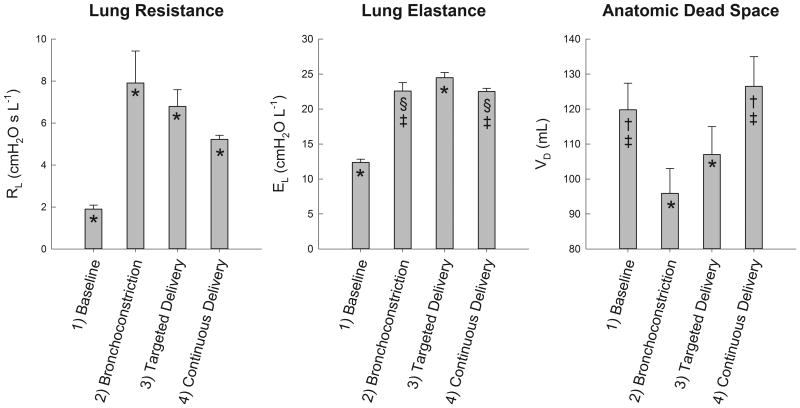
Experimentally, targeted delivery of a volatile anesthetic may be achieved using a delivery system similar to that shown in Figure 4. The system consists of a computer-actuated ventilator circuit designed to divert a portion of inspired volume either through: 1) an anesthetic drawover vaporizer [62], or 2) a bypass limb. For targeted delivery of the anesthetic, only the last portion of the inspired volume is diverted through the vaporizer, with a volume equivalent to the anatomic deadspace. For a continuous delivery, the entire inspired volume of gas is diverted across the anesthetic vaporizer. Using this system, we measured lung resistance (RL), elastance (EL), and anatomic deadspace (VD) during targeted and continuous delivery of isoflurane in a healthy, intubated dog during pharmacologically-induced bronchoconstriction1. The RL and EL were obtained using multiple linear regression on sampled airway flow and transpulmonary pressure waveforms during mechanical ventilation [63], while VD was estimated using the technique of volume capnography [64]. Figure 5 demonstrates that both RL and EL increase compared to baseline conditions with bronchoconstriction, and VD decreases. Following targeted and continuous delivery of isoflurane, both RL and EL were reduced compared to the constricted state, while VD increased. These data demonstrate that both anesthetic delivery regimens achieve bronchodilation of constricted airways, although continuous delivery appeared to achieve greater dilation compared to targeted delivery. This may be due to systemic circulation of drug and/or neurally-mediated effects. Nonetheless significant bronchochodilation was achieved with targeted delivery, as indicted by decreases in RL and increases in VD compared to the bronchoconstricted state. This suggests that one important mechanism by which inhaled volatile anesthetics work is via direct diffusion from the airway lumen to ASM. Additional studies will be required to determine whether such targeted delivery may be further refined for a more optimal delivery pattern; for example, by adjusting the delivered volume of anesthetic in realtime according to breath-by-breath estimation of VD.
Figure 4.
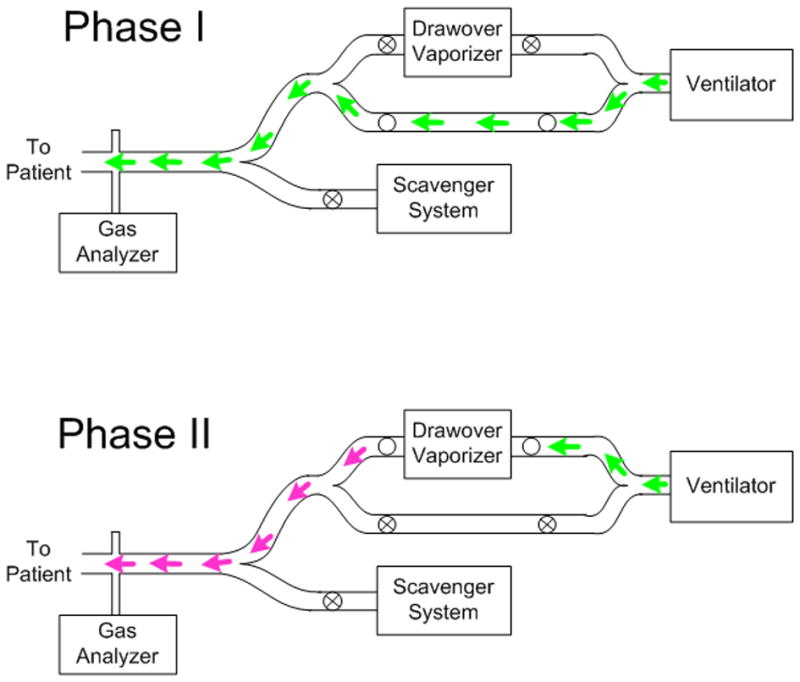
Targeted delivery of anesthesia is achieved by controlling the serial composition of the inspired gas. In Phase I, fresh gas (green) travels directly to the patient through the lower bypass sub-limb. In Phase II, gas is diverted across a drawover vaporizer (pink) to deliver the volatile anesthetic agent at the end of inspiration.
Figure 5.
Lung resistance (RL), elastance (EL), and anatomic deadspace (VD) for a healthy, intubated 25 kg dog during mechanical ventilation. Data are expressed as means ± S.D. of 15-20 breaths, obtained under: 1) baseline conditions; 2) following 10 minutes of intravenous methacholine infusion (200 μg min-1) to cause bronchoconstriction; 3) 10 minutes of targeted isoflurane delivery; and 4) 10 minutes of continuous isoflurane delivery. Statistical significance was assessed via one-way analysis of variance (ANOVA), with post hoc comparisons obtained using the Tukey HSD criterion. Significance level was P ≤ 0.05. *Significantly different compared to all other conditions. §Significantly different compared to baseline condition. †Significantly different compared to bronchoconstriction condition. ‡Significantly different compared to targeted delivery condition.
Conclusions
We anticipate that targeted delivery of inhaled volatile anesthetics to constricted airways can be optimized for patients with severe bronchoconstriction. If clinically significant bronchodilation can be achieved by direct absorption of the anesthetic through the airway walls, then timed-delivery later in inspiration may have the additional advantage of mitigating systemic effects. If airways are heterogeneously constricted and are recruited in cascades determined by interdependent effects, then the delivery of bronchodilators may be further optimized by targeted delivery of the agent to parallel lung regions with different degrees of bronchoconstriction. Additional challenges to selective delivery of anesthetics to conducting airways will include gas mixing at the interface of alveolar gas, as well as parallel heterogeneity of ventilation distribution which may result in short time constant regions receiving agent intended for conducting airways. Fundamental questions remain as to whether there can be sufficient direct uptake of agent through airway walls to attain an effect, or whether systemic absorption is necessary for a full therapeutic effect. Nonetheless, such an optimized delivery regime for inhaled anesthetics would provide a breakthrough treatment paradigm for status asthmaticus, and may result in more widespread clinical use of these agents.
Acknowledgments
Work supported in part by National Institutes of Health grant UM1 HL108724. We are grateful to Impact Instrumentation, Inc. and General Anesthetic Services, Inc. for equipment loans, as well as the Department of Anesthesia, Critical Care, and Pain Medicine at Beth Israel Deaconess Medical Center for additional support of this work. The authors acknowledge Dr. John H. Arnold of Boston Children's Hospital and Dr. Béla Suki of Boston University for their thoughtful insights and criticism of the technique of targeted anesthetic delivery.
Footnotes
Subject was a healthy, intubated 25 kg canine, with general anesthesia maintained by continuous intravenous infusion of midazolam (0.25 mg kg-1 hr-1) and fentanyl (2-5 μg kg-1 hr-1). Neuromuscular blockade was obtained using intermittent 1-2 mg boluses of vecuronium. Ventilation was maintained using volume-cycled ventilation (Impact Instrumentation, Inc., Model 754 Eagle™). Airway flow was measured with a pneumotachograph, and transpulmonary pressure was obtained as the difference between tracheal pressure and esophageal pressure. Bronchoconstriction was induced with a continuous intravenous infusion of methacholine (200 μg min-1) Additional experimental details can be found in Kaczka et al. [63]. Experiment was conducted in the Animal Research Facility at Beth Israel Deaconess Medical Center. The protocol (#048-2012) was approved by the Institutional Animal Care and Use Committee to ensure humane treatment during the course of the study.
Conflict Of Interest: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyer NE, Schotz S. Relief of severe intractable bronchial asthma with cyclopropane anesthesia: Report of a case. Journal of Allergy. 1939;10:239–240. [Google Scholar]
- 2.Schultz TE. Sevoflurane administration in status asthmaticus: a case report. AANA J. 2005;73(1):35–36. [PubMed] [Google Scholar]
- 3.Shankar V, et al. Isoflurane therapy for severe refractory status asthmaticus in children. Intensive care medicine. 2006;32(6):927–933. doi: 10.1007/s00134-006-0163-0. [DOI] [PubMed] [Google Scholar]
- 4.Tobias JD. Therapeutic applications and uses of inhalational anesthesia in the pediatric intensive care unit. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2008;9(2):169–179. doi: 10.1097/PCC.0b013e31816688ef. [DOI] [PubMed] [Google Scholar]
- 5.Turner DA, Arnold JH. Improving our approach to sedation in the pediatric intensive care unit: Is it time to inhale? Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2008;9(2):233–234. doi: 10.1097/PCC.0b013e318166d135. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler DS, et al. Isoflurane therapy for status asthmaticus in children: A case series and protocol. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2000;1(1):55–59. doi: 10.1097/00130478-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Lutchen KR, Gillis H. Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. Journal of Applied Physiology. 1997;83(4):1192–1201. doi: 10.1152/jappl.1997.83.4.1192. [DOI] [PubMed] [Google Scholar]
- 8.Lutchen KR, et al. Airway constriction pattern is a central component of asthma severity: the role of deep inspirations. Am J Respir Crit Care Med. 2001;164(2):207–215. doi: 10.1164/ajrccm.164.2.2008119. [DOI] [PubMed] [Google Scholar]
- 9.Nievas IF, Anand KJ. Severe acute asthma exacerbation in children: a stepwise approach for escalating therapy in a pediatric intensive care unit. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG. 2013;18(2):88–104. doi: 10.5863/1551-6776-18.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaczka DW, et al. Emergent behavior of regional heterogeneity in the lung and its effects on respiratory impedance. Journal of Applied Physiology. 2011;110(5):1473–1481. doi: 10.1152/japplphysiol.01287.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaczka DW, et al. Airway and lung tissue mechanics in asthma. Effects of albuterol. Am J Respir Crit Care Med. 1999;159(1):169–178. doi: 10.1164/ajrccm.159.1.9709109. [DOI] [PubMed] [Google Scholar]
- 12.Tzeng YS, et al. Investigation of hyperpolarized 3He magnetic resonance imaging utility in examining human airway diameter behavior in asthma through comparison with high-resolution computed tomography. Academic radiology. 2008;15(6):799–808. doi: 10.1016/j.acra.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzeng YS, et al. The difference in ventilation heterogeneity between asthmatic and healthy subjects quantified using hyperpolarized 3He MRI. J Appl Physiol (1985) 2009;106(3):813–822. doi: 10.1152/japplphysiol.01133.2007. [DOI] [PubMed] [Google Scholar]
- 14.Venegas JG, et al. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature. 2005;434(7034):777–782. doi: 10.1038/nature03490. [DOI] [PubMed] [Google Scholar]
- 15.Anafi RC, Wilson TA. Airway stability and heterogeneity in the constricted lung. J Appl Physiol. 2001;91(3):1185–1192. doi: 10.1152/jappl.2001.91.3.1185. [DOI] [PubMed] [Google Scholar]
- 16.Winkler T, Venegas JG. Complex airway behavior and paradoxical responses to bronchoprovocation. Journal of Applied Physiology. 2007;103:655–663. doi: 10.1152/japplphysiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 17.Venegas JG, et al. The distribution of ventilation during bronchoconstriction is patchy and bimodal: a PET imaging study. Respiratory physiology & neurobiology. 2005;148(1-2):57–64. doi: 10.1016/j.resp.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Newth CJ, et al. Fatal and near-fatal asthma in children: the critical care perspective. Journal of Pediatrics. 2012;161(2):214–221. doi: 10.1016/j.jpeds.2012.02.041. e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birken CS, et al. Asthma severity scores for preschoolers displayed weaknesses in reliability, validity, and responsiveness. Journal of clinical epidemiology. 2004;57(11):1177–1181. doi: 10.1016/j.jclinepi.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Kelly CS, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2000;84(5):509–516. doi: 10.1016/S1081-1206(10)62514-8. [DOI] [PubMed] [Google Scholar]
- 21.Mannino DM, et al. Surveillance for asthma--United States, 1980-1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 22.NHLBI. Expert Panel Report 3: Guidlelines for the Diagnosis and Management of Asthma 2007 [Google Scholar]
- 23.Hebbar KB, et al. Experience with use of extracorporeal life support for severe refractory status asthmaticus in children. Crit Care. 2009;13(2):R29. doi: 10.1186/cc7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irazuzta J, et al. Feasibility of short-term infusion of magnesium sulfate in pediatric patients with status asthmaticus. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG. 2012;17(2):150–154. doi: 10.5863/1551-6776-17.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobias JD, Garrett JS. Therapeutic options for severe, refractory status asthmaticus: inhalational anaesthetic agents, extracorporeal membrane oxygenation and helium/oxygen ventilation. Paediatr Anaesth. 1997;7(1):47–57. doi: 10.1046/j.1460-9592.1997.d01-33.x. [DOI] [PubMed] [Google Scholar]
- 26.Arnold JH, et al. Prolonged administration of isoflurane to pediatric patients during mechanical ventilation. Anesth Analg. 1993;76(3):520–526. doi: 10.1213/00000539-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Best A, et al. Prolonged use of isoflurane in asthma. Can J Anaesth. 1994;41(5 Pt 1):452–453. doi: 10.1007/BF03009879. [DOI] [PubMed] [Google Scholar]
- 28.Bierman MI, et al. Prolonged isoflurane anesthesia in status asthmaticus. Crit Care Med. 1986;14(9):832–833. doi: 10.1097/00003246-198609000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Miyagi T, et al. Prolonged isoflurane anesthesia in a case of catastrophic asthma. Acta Paediatr Jpn. 1997;39(3):375–378. doi: 10.1111/j.1442-200x.1997.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 30.Mori N, et al. Prolonged sevoflurane inhalation was not nephrotoxic in two patients with refractory status asthmaticus. Anesth Analg. 1996;83(1):189–191. doi: 10.1097/00000539-199607000-00035. [DOI] [PubMed] [Google Scholar]
- 31.Yamakage M. Effects of anaesthetic agents on airway smooth muscles. British Journal of Anaesthesia. 2002;88(5):624–627. doi: 10.1093/bja/88.5.624. [DOI] [PubMed] [Google Scholar]
- 32.O'Rourke PP, Crone RK. Halothane in status asthmaticus. Crit Care Med. 1982;10(5):341–343. doi: 10.1097/00003246-198205000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Mazze RI, et al. Inorganic fluoride nephrotoxicity: prolonged enflurane and halothane anesthesia in volunteers. Anesthesiology. 1977;46(4):265–271. [PubMed] [Google Scholar]
- 34.Spencer EM, et al. Plasma inorganic fluoride concentrations during and after prolonged (greater than 24 h) isoflurane sedation: effect on renal function. Anesth Analg. 1991;73(6):731–737. doi: 10.1213/00000539-199112000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Truog RD, Rice SA. Inorganic fluoride and prolonged isoflurane anesthesia in the intensive care unit. Anesth Analg. 1989;69(6):843–845. [PubMed] [Google Scholar]
- 36.Perouansky M, Hemmings HC., Jr Neurotoxicity of general anesthetics: cause for concern? Anesthesiology. 2009;111(6):1365–1371. doi: 10.1097/ALN.0b013e3181bf1d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamakage M, Namiki A. Cellular mechanisms of airway smooth muscle relaxant effects of anesthetic agents. J Anesth. 2003;17(4):251–258. doi: 10.1007/s00540-003-0194-4. [DOI] [PubMed] [Google Scholar]
- 38.Yamakage M, et al. Volatile anesthetics inhibit voltage-dependent Ca2+ channels in porcine tracheal smooth muscle cells. Am J Physiol. 1995;268:L187–L191. doi: 10.1152/ajplung.1995.268.2.L187. [DOI] [PubMed] [Google Scholar]
- 39.Pabelick CM, et al. Effect of halothane on intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol. 1999;276:L81–L89. doi: 10.1152/ajplung.1999.276.1.L81. [DOI] [PubMed] [Google Scholar]
- 40.Schimmel C, et al. Soluble gas exchange in the pulmonary airways of sheep. Journal of Applied Physiology. 2004;97:1702–1708. doi: 10.1152/japplphysiol.01272.2003. [DOI] [PubMed] [Google Scholar]
- 41.Swenson ER, et al. Conducting airway gas exchange: diffusion-related differences in inert gas elimination. J Appl Physiol (1985) 1992;72(4):1581–1588. doi: 10.1152/jappl.1992.72.4.1581. [DOI] [PubMed] [Google Scholar]
- 42.Kelly L, et al. Bronchial blood flow affects recovery from constriction in dog lung periphery. J Appl Physiol. 1986;60(6):1954–1959. doi: 10.1152/jappl.1986.60.6.1954. [DOI] [PubMed] [Google Scholar]
- 43.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butler J. The bronchial circulation. News in Physiological Sciences. 1991;6:21–25. [Google Scholar]
- 45.Pierce RJ, et al. Comparison of intravenous and inhaled terbutaline in the treatment of asthma. Chest. 1981;79(5):506–511. doi: 10.1378/chest.79.5.506. [DOI] [PubMed] [Google Scholar]
- 46.Werner HA. Status asthmaticus in children: a review. Chest. 2001;119(6):1913–1929. doi: 10.1378/chest.119.6.1913. [DOI] [PubMed] [Google Scholar]
- 47.Wiklund CU, et al. Relaxation by sevoflurane, desflurane and halothane in the isolated guinea-pig trachea via inhibition of cholinergic neurotransmission. British Journal of Anaesthesia. 1999;83(3):422–429. doi: 10.1093/bja/83.3.422. [DOI] [PubMed] [Google Scholar]
- 48.Wiklund CU, et al. Interactions of volatile anesthetics with cholinergic, tachykinin, and leukotriene mechanisms in isolated Guinea pig bronchial smooth muscle. Anesth Analg. 2002;95:1650–1655. doi: 10.1097/00000539-200212000-00032. [DOI] [PubMed] [Google Scholar]
- 49.Lindeman KS, et al. Interaction between halothane and the nonadrenergic, noncholinergic inhibitory system in porcine trachealis muscle. Anesthesiology. 1994;81:641–648. doi: 10.1097/00000542-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 50.Warner DO, et al. Direct and neurally mediated effects of halothane on pulmonary resistance in vivo. Anesthesiology. 1990;72(6):1057–1063. doi: 10.1097/00000542-199006000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Brown RH, et al. Comparison of low concentrations of halothane and isoflurane as bronchodilators. Anesthesiology. 1993;78(6):1097–1101. doi: 10.1097/00000542-199306000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Klide AM, Aviado DM. Mechanism for the reduction in pulmonary resistance induced by halothane. The Journal of pharmacology and experimental therapeutics. 1967;158(1):28–35. [PubMed] [Google Scholar]
- 53.Lovich MA, et al. A mass balance model for the Mapleson D anaesthesia breathing system. Can J Anaesth. 1993;40(6):554–567. doi: 10.1007/BF03009741. [DOI] [PubMed] [Google Scholar]
- 54.Sommer LZ, et al. A simple breathing circuit minimizing changes in alveolar ventilation during hyperpnoea. The European respiratory journal. 1998;12(3):698–701. doi: 10.1183/09031936.98.12030698. [DOI] [PubMed] [Google Scholar]
- 55.Brogan TV, et al. Carbon dioxide added late in inspiration reduces ventilation-perfusion heterogeneity without causing respiratory acidosis. Journal of Applied Physiology. 2004;96(5):1894–1898. doi: 10.1152/japplphysiol.00160.2003. [DOI] [PubMed] [Google Scholar]
- 56.Farr SJ, et al. Aerosol deposition in the human lung following administration from a microprocessor controlled pressurised metered dose inhaler. Thorax. 1995;50(6):639–644. doi: 10.1136/thx.50.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mourgeon E, et al. Distribution of inhaled nitric oxide during sequential and continuous administration into the inspiratory limb of the ventilator. Intensive care medicine. 1997;23(8):849–858. doi: 10.1007/s001340050421. [DOI] [PubMed] [Google Scholar]
- 58.Suki B, et al. Avalanches and power-law behaviour in lung inflation. Nature. 1994;368(6472):615–618. doi: 10.1038/368615a0. [DOI] [PubMed] [Google Scholar]
- 59.Amini R, Kaczka DW. Impact of ventilation frequency and parenchymal stiffness on flow and pressure distribution in a canine lung model. Ann Biomed Eng. 2013;41(12):2699–2711. doi: 10.1007/s10439-013-0866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amin SD, et al. Modeling the dynamics of airway constriction: effects of agonist transport and binding. Journal of Applied Physiology. 2010;109(2):553–563. doi: 10.1152/japplphysiol.01111.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vinegar A. PBPK modeling of canine inhalation exposures to halogenated hydrocarbons. Toxilogical Sciences. 2001;2001(1):20–27. doi: 10.1093/toxsci/60.1.20. [DOI] [PubMed] [Google Scholar]
- 62.Lunn DV, Young PC. The Ohmeda Universal PAC drawover apparatus. A technical and clinical evaluation. Anaesthesia. 1995;50:870–874. doi: 10.1111/j.1365-2044.1995.tb05854.x. [DOI] [PubMed] [Google Scholar]
- 63.Kaczka DW, et al. Assessment of time-domain analyses for estimation of low-frequency respiratory mechanical properties and impedance spectra. Ann Biomed Eng. 1995;23:135–151. doi: 10.1007/BF02368321. [DOI] [PubMed] [Google Scholar]
- 64.Blanch L, et al. Volumetric capnography in the mechanically ventilated patient. Minerva Anestesiologica. 2006;72(6):577–585. [PubMed] [Google Scholar]
- 65.Majumdar A, et al. Relating airway diameter distributions to regular branching asymmetry in the lung. Physical review letters. 2005;95(16):168101. doi: 10.1103/PhysRevLett.95.168101. [DOI] [PubMed] [Google Scholar]