Abstract
Objective
To explore and facilitate the multifaceted process of drug development and regulatory approval in ovarian cancer.
Methods
The Society of Gynecologic Oncology (SGO) recently sought and received input from multiple stakeholders including the National Cancer Institute's (NCI) Clinical Therapy Evaluation Program (CTEP), the Food and Drug Administration (FDA), pharmaceutical industry, and patient advocates. This whitepaper is the work product and opinion solely of the SGO.
Results
This document summarizes the SGO's interpretation of these meetings and the current regulatory environment where there has been a paucity of recent approvals in the United States. It provides guidance in clinical trial design with the express purpose of encouraging novel drug development in ovarian cancer. Points of emphasis include: ovarian cancer heterogeneity (histologic subtypes and molecular genetic alterations), clinical trial design elements, surrogate as well as composite endpoints, and the four principles of clinical drug development (unmet medical need, discovery, safety, and efficacy).
Conclusions
There has been an evolution in the acceptance of surrogate endpoints depending upon the clinical setting in ovarian cancer. While overall survival (OS) remains the most objective clinical trial endpoint, there is now realization that demanding OS as the primary endpoint has many obstacles. Ovarian cancer is a heterogeneous disease that is now divided by histologic subtypes. Future registration strategies will need to address disease heterogeneity. The exploration of currently acceptable clinical trial endpoints and alternative regulatory strategies will hopefully stimulate interest in novel drug development for patients with ovarian cancer.
Keywords: Ovarian cancer, Clinical trial endpoints, Progression free survival, Overall survival, Patient reported outcomes
Introduction
The Society of Gynecologic Oncology (SGO) recognizes the evolving challenges in cancer drug development. These challenges, particularly in ovarian cancer, have adversely influenced the portfolio expansion of approved agents. The perception that overall survival (OS) is the only acceptable clinical trial endpoint has challenged the interpretation of several recent trials and has deterred drug development in ovarian cancer. As ovarian, fallopian tube and peritoneal cancers, collectively known as epithelial ovarian cancer, are characterized by a long initial post progression survivorship, the unbalanced and frequent use of active treatment, including frequent crossover treatment, as well as the length and cost of clinical trials may make OS an imprecise and impractical endpoint [1].
To address this problem, the SGO first sought to better understand the key issues responsible for this dynamic paradigm whereby no new ovarian cancer drug approvals have occurred in the United States since 2006. Thus a task force was formed to examine the issues. The role of clinical trial endpoints was seen as one of the contributory factors, and an SGO white paper was published by the task force to provide insight into pivotal regulatory issues, the patients' perspective, the unique features of ovarian cancer, and the potential role of surrogate clinical trial endpoints in clinical trials designed for new drug approvals [1] (Table 1).
Table 1.
Endpoints and study settings. In addition to statistically significant difference, other means of benefit would need to be demonstrated such as significant difference in time off therapy or at least an OS trend. Opportunities to develop metrics of clinical benefit that integrate response elements with context to better define treatment effect. Modified from Herzog TJ, Armstrong DK, Brady MF, Coleman RL, Einstein MH, Monk BJ, Mannel RS, Thigpen JT, Umpierre SA, Villella JA, and Alvarez RD. Gynecol Oncol. 2014 Jan; 132 (1):8–17.doi: 10.1016/j.ygyno.2013.11.008. Reproduced with permission of Elsevier.
| Frontline | Platinum-sensitive | Platinum-resistant | |
|---|---|---|---|
| OS | Approve | Approve | Approve |
| PFS (statistically significant) + other (QoL/PRO) | Approve | Approve | Consider |
| PFS (statistically significant) with clinically meaning MOE | Consider | Consider | Consider |
| Response Rate/CBR Overall- high grade serous | No | No | Consider |
| Response rate/CBR selected histologies (eg. clear cell, mucinous, and low grade serous) | Consider | Consider | Consider |
MOE = magnitude of effect; QOL = quality of life; PRO = patient reported outcomes; CBR = clinical benefit rate.
One of the most significant developments influencing drug development and therefore drug regulatory approval in oncology is the rapid growth and discovery in cancer biology. The molecular and/or genetic etiologies of many cancers are now known, and the molecular-genetic characteristics of others are well established. Innovation to develop targeted agents to leverage these molecular–genetic aberrations has advanced rapidly. The discovery of actionable mutations has outpaced our ability to clinically validate many of these intriguing targets.
In many solid tumors, including ovarian cancer, these developments have prompted the division of relatively homogenous populations into smaller and even more homogenous subgroups. For instance, most epithelial ovarian cancers were initially considered biologically similar. However, it has become apparent that certain histologic subtypes are more clinically diverse than previously thought based upon origin and response to chemotherapeutics [2]. More recently, it has been noted that even more heterogeneity exists, even within the same histology, and gene signatures that demonstrate both prognostic and predictive roles for therapy and survivorship are emerging [3]. Further complicating our understanding of this process is the role of varying host responses within the tumor microenvironment and the critical role of poorly understood immunologic variables. Together, these rapidly changing forces will have significant implications on the design of future clinical trials in ovarian cancer.
Recognizing the multifaceted process of drug development and regulatory approval both inside the United States as well as abroad, the SGO recently sought and received input from multiple stakeholders including the National Cancer Institute's (NCI) Clinical Therapy Evaluation Program (CTEP), the Food and Drug Administration (FDA), pharmaceutical industry, and patient advocates. This document summarizes the SGO's interpretation of the current regulatory environment and provides guidance in clinical trial design with the express purpose of encouraging novel drug development in ovarian cancer. This document is the work product and opinion solely of the SGO. Official endorsement or approval from any governmental, industry, or advocacy groups has not been sought and/or independently provided.
Methods
Subsequent to the publication of the SGO White Paper on Clinical Trial Endpoints in Ovarian Cancer, an SGO task force was assembled and a meeting with the FDA was convened in March of 2014. During this meeting, information about the SGO professional organization and its mission, as well as the unique features of ovarian cancer was discussed. Several key agenda items, such as factors associated with extending median survival in advanced ovarian cancer and the continued poor long-term outcomes associated with advanced stage ovarian cancer were reviewed and discussed. In addition, the task force asked for input and response to the SGO White Paper emphasizing clinical trial endpoints (OS, PFS, response rate, CA125 levels, quality of life and patient reported outcomes). Deliberations of this meeting were discussed by the SGO task force at the 2014 SGO Annual Meeting on Women's Cancers, and the outline and content for the current manuscript were formulated. The final document was reviewed by the SGO's Publication Committee and its Executive Board prior to submission and represents the opinions of the SGO task force after careful consideration and input from a number of stakeholders in ovarian cancer drug development.
Statistical considerations in clinical trial design
Clinical drug development focuses on four principles: unmet medical need, discovery, safety and efficacy. In traditional development strategies, these are interrogated generally in sequence with increasingly more restricted sample populations. The central tenet guiding the ongoing dialog with the FDA and industry partners has been to identify and establish a definition of “meaningful clinical benefit” linked to a particular therapy and a cohort of ovarian cancer patients. This issue is difficult to globally define because, while the magnitude of effect is relatively equipoise (as quantified by Hazard Ratios), the context impacts the size of this effect. For instance, a hazard ratio of 0.67 may represent a median survival outcome delta between two regimens ranging from 3 to 12 months, depending on the sample size and whether PFS or OS is the focus of the analysis. However, this conundrum does not mean to imply that there are no standards against which to establish a meaningful precedent of effect.
With efficacy usually being evaluated in phase III randomized, placebo-controlled trials, involving unselected large numbers of patients with multiple stratifications to account for post-randomization effects that may affect the trial's endpoints, preserving power to evaluate both OS and PFS endpoints generally lead to large sample sizes. This results in over-powering for the PFS endpoint (risking a clinically unimpressive significant effect) and wasting valuable resources and time. In addition, given that survival endpoints are dependent on events, clinical context can paradoxically increase the chance of making a type II statistical error. This is particularly vulnerable when there is long post progression survival, as is seen in most ovarian cancer patients.
One strategy discussed with the FDA provided a development path that may be a reasonable option for some agents. FDA representatives postulated that agents or strategies providing clinical benefit in resistant/refractory populations, where the unmet medical need is high, could be represented by objective measures (e.g. RECIST response) and be filed for accelerated approval. Customarily, this would be followed by a larger randomized clinical trial that met regulatory standards and was supported by a meaningful statistical result (e.g. PFS). By way of discovery, the Gynecologic Oncology Group (GOG, now a part of NRG Oncology) has evaluated multiple agents in single arm, open label phase II trials in women with recurrent/refractory ovarian cancer under similar eligibility criteria. The statistical design was represented by a two-stage accrual strategy, where a limited number of patients would be exposed to ineffective therapy (response rate of 10%), yet sufficient power would accompany a point estimate of effective therapy (response rate of 25%) [4]. This algorithm limited the sample size to approximately 50 patients and served well as guidance to direct future investigation. For regulatory purposes, the SGO task force would propose a similar design to estimate a true response rate of at least 30% and a sample size of approximately 100–120 patients. This would ensure adequate power (>90%) to exclude a true response rate below 25% yet provide adequate sample size to estimate safety ahead of the regulatory phase III trial. It was agreed that in recurrent resistant/refractory patients, this degree of efficacy is clinically meaningful and would represent an important advance in therapy. It was appreciated that these meaningful estimates may vary by disease setting, but in principle would be consistent and should be conducted with oversight to limit ineffective and/or unsafe drug exposure. Moreover, the duration of response and the number of complete responders (CRs) should also be considered in this framework as well as the final tenet of safety (toxicity). One strategy to increase the numbers of CRs and thereby increasing the confidence in deriving clinical benefit from therapy is to limit enrollment to predictive biomarker positive patients (discussed below) who are most likely to respond to a targeted agent.
Patient reported outcomes
Patient reported outcomes (PROs) are attractive because they may provide a better global assessment of clinical effectiveness in balance with treatment toxicity. Surveys of ovarian cancer patients specifically indicate that these women are initially very willing to pursue increased toxicity for a higher chance of cure. But as these women develop recurrent disease where cure is not possible, minimizing toxicity and achieving stable disease become much more important aspects of clinical benefit [1]. Ameliorating pain, reducing the need for paracentesis and improving intestinal function are very important aspects of quality of life for ovarian cancer patients [5].
It is common in studies that evaluate PROs in patients with ovarian cancer for there to be a considerable amount of missing data and to have an imbalance of measurements between study arms [6]. Unless patients are blinded, there is also the risk of considerable bias in how PROs are reported [7]. In addition, toxicity will always need to be evaluated separately from PROs to establish the safety of any new drug.
Another confounding aspect of the natural history of ovarian cancer is that many women are asymptomatic at the time of a tumor-marker or radiologic recurrence. It is difficult to show improvement in PROs among treated cohorts of asymptomatic participants. Even in the AURELIA trial that evaluated PROs in women with platinum resistant recurrent ovarian cancer, only 65% suffered sufficient symptoms at baseline for subsequent differences to be detectable [6,7]. Other patients may have symptoms related to recent cytoreductive surgery. Accurately attributing symptoms and toxicity to disease, surgical intervention or treatment in many instances can be complex and may be interrelated. Most patients in the front line setting will not have any symptoms from cancer once the acute side effects of surgery have resolved.
Composite end points
Since standard clinical trial endpoints may not accurately portray the benefits and risks of therapy, assessments that consider multiple tumor- and patient-centric endpoints (survival, response rate, toxicity, and PRO), may help health care providers, cancer patients, and decision-makers to better understand the total clinical benefit of therapeutic interventions. One method to optimize clinical trial endpoints in the modern era of cancer therapeutics is to integrate multiple endpoints into a single metric — a composite endpoint. A composite endpoint allows for simultaneous change in multiple outcomes and enhances our ability to capture therapeutic benefit including the patient experience and objective outcomes, and may enhance statistical power by decreasing the number of comparisons. Current endpoints don't adequately define the clinical effects of treatment, especially when post-progression survival is longer than 12 months [8]. While there may be a role for newer composite endpoints to establish drug efficacy, very few of these measures have undergone evaluation to establish the reliability and validity needed for regulatory approval.
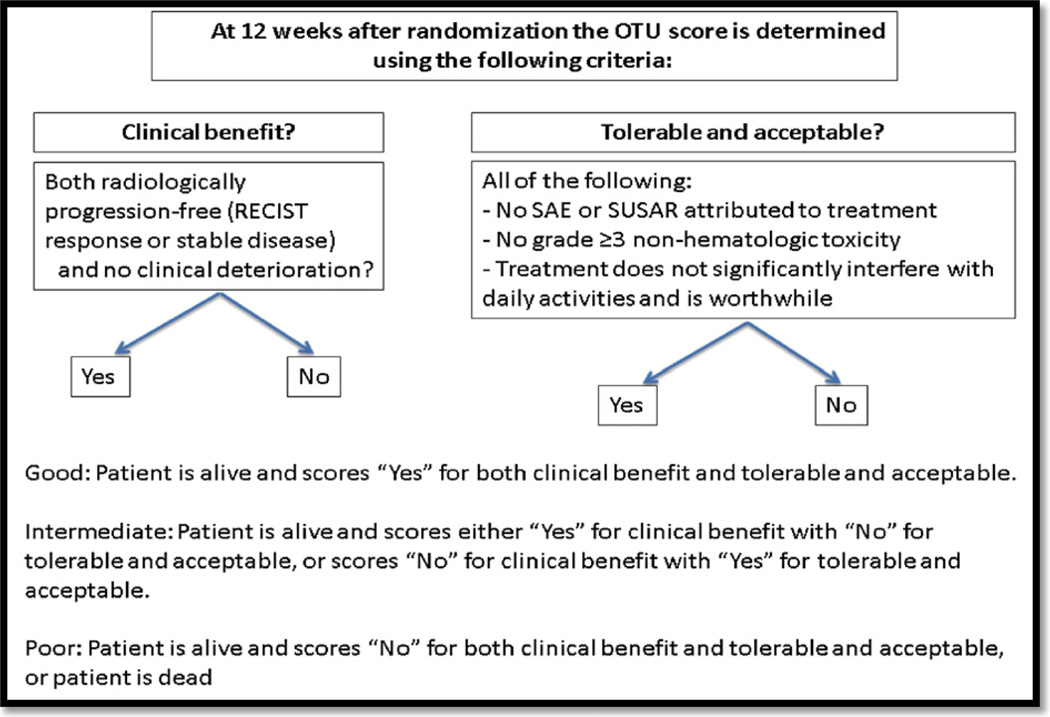
The Clinical Benefit Response composite endpoint, not to be confused with clinical benefit rate — complete + partial responses + stable disease, was first utilized by Burris and colleagues in the landmark pancreatic cancer study [9]. Clinical Benefit Response is a composite of objective and subjective elements — pain (measured as analgesic consumption and a patient-reported pain intensity scale), clinician-assessed performance status, and weight. Subsequently several trials have evaluated patient-centered composite and a variety of different novel composite endpoints have been studied including: 1) a modified Burris' Clinical Benefit Response endpoint with the inclusion of alleviation of tumor-related symptoms [10]; and 2) time until definitive deterioration [11,12]; 3) net clinical benefit [13]; and 4) overall treatment utility (OTU) (Fig. 1).
Fig. 1.
Overall treatment utility (OTU) schematic. Adapted from Seymour et al. [14]. OTU is a novel composite measure to determine if therapy was meaningful from both the patient's and the clinician's viewpoint. OTU was scored at 12 weeks and was designated as poor, intermediate, or good. Poor OTU indicated either disease progression or clinical deterioration and either a major negative treatment effect attributed to therapy, or an episode of grade ≥ 3 non-hematologic toxicity or patient perspective that treatment significantly interfered with normal daily activities and has not been worthwhile. Intermediate OTU signified either RECIST response or SD with no clinical deterioration in addition to a significant negative treatment effect or lack of patient acceptability, or disease progression or clinical deterioration combined with no negative treatment effect, or patient acceptability. Good OTU was defined as RECIST response or SD associated with no major negative treatment effects and positive patient acceptability.
Numerous studies demonstrated improvement of traditional endpoints as well as patient-centric composite endpoints [10–12, 14–16]. Table 2 details the relative advantages and disadvantages of composite endpoints.
Table 2.
Advantages and disadvantages of composite endpoints modified from Herzog TJ, Armstrong DK, Brady MF, Coleman RL, Einstein MH, Monk BJ, Mannel RS, Thigpen JT, Umpierre SA, Villella JA, Alvarez RD. Gynecol Oncol. 2014 Jan;132 (1):8–17.doi: 10.1016/j.ygyno.2013.11.008. Reproduced with permission of Elsevier.
| Advantages | Disadvantages |
|---|---|
| Unmet need | Complicated |
| Does not require OS | May be disease specific |
| Comprehensive overview treatment effect | Subjective weighting |
| Theoretically more reflective of total experience | Will need years to develop and assure accuracy |
| Compilation of variables | Lacks validation in ovarian cancer |
| Enhance statistical power via decreasing multiple comparisons | Dilution of composite score if not all components are effected equally by treatment |
Clinical trial endpoints in the era of modern ovarian cancer therapeutics need to be reassessed to guarantee that the endpoints assessed reflect the realities of differing outcomes and meaning of those outcomes to women afflicted. The development of patient-centric composite endpoints in ovarian cancer clinical trials may achieve this objective. Research is ongoing to determine the appropriate component weighting for an ovarian cancer-specific composite endpoint. In the interim, the SGO has developed a disease specific clinical outcomes registry that incorporates patient reported symptom data. Such a large and complete data set could serve in further testing and validating composite clinical trial endpoints that may be useful in future clinical trials.
Clinical trial evolution
A recent publication entitled “The Gynecologic Oncology Group: Report of 35 Years of Excellence in Clinical Research” summarizes how impactful the GOG's cooperative group trials have been in defining the standard of care for managing gynecologic oncology patients [17]. It also reveals that over the past 40 years there has been a continuing evolution of what questions are being asked and how trials are designed to provide the best answer. Beginning in the 1970s the focus was on using stage of disease and organ of origin as the basis for trial eligibility. Many of our foundational trials were surgical staging trials designed to better understand the natural disease progression of uterine, ovarian, and cervical cancer [18,19].
As our knowledge matured, therapeutic trials were designed that studied large groups of patients based on stage of disease or the presence of recurrence. Landmark trials on the role of chemoradiation in advanced stage cervical cancer and the role of intraperitoneal therapy in ovarian cancer led to NCI Clinical Alerts [20,21]. Other trials established the role of chemotherapy in advanced endometrial cancer as well as the role of anti-angiogenesis agents in cervical and ovarian cancer [22–24]. These large trials included multiple histologies in the eligibility criteria. Post hoc analysis has raised the question of the importance of histology in response to therapy and over the past 20 years rigorous debates have centered on the question of all-inclusive versus selective histologic eligibility criteria in trial design. For example, more recent ovarian cancer trials have separated mucinous, clear cell, and low-grade serous ovarian cancer from those patients with high-grade serous cancers. But the fundamental question remains whether histology is the best discriminator for determining the potential response therapy.
More recent information has confirmed that in spite of a homogenous histologic appearance, there can be a wide discrepancy in response to standardized therapy in many gynecologic malignancies. For example, ovarian TCGA analysis shows a great deal of variability in the genetic mutations seen in high-grade serous ovarian cancers [25]. Subsets of high-grade serous ovarian cancers which have genetic mutations in the BRCA 1 or BRCA 2 genes show tremendous improvement in PFS to PARP inhibitors with hazard ratios thought impossible to achieve just a few years ago [26]. Similar results have been noted in other cancer types. The NCI and FDA are looking to capitalize on the emerging technologies of genomics and proteomics to shift cancer research and care away from strict adherence to site of origin, stage, and histology with more emphasis placed on molecularly determined biomarker driven trials. The NCI defines integral biomarkers as genetic, molecular, or imaging signatures used in the trial design to select treatment and considers use of these biomarkers as the cornerstone for evaluating novel therapies in the future. Designing a trial evaluating the effectiveness of PARP inhibitors in BRCA1 and 2 germ line mutation ovarian cancer patients would be an example of an integral biomarker. The NCI is also interested in developing new biomarker candidates by funding translational research looking at exploratory integrated biomarkers in large phase II and phase III trials. The NCI has adopted the term precision medicine to capture this new philosophy. Future trials will have eligibility confined to a smaller more homogenous populations based on biomarkers with endpoints anticipated to be much more robust in terms of response and hazard ratios for progression free survival.
Conclusions
There has been an evolution in the acceptance of surrogate endpoints depending upon the clinical setting in ovarian cancer.
While OS remains the most objective clinical trial endpoint, there is now realization that demanding OS as the primary endpoint has many obstacles including size, length, expense, and clinical relevance of a registration trial.
Ovarian cancer is a heterogeneous disease that is now divided by histologic subtypes and moving to even more differentiation based on molecular genetic alterations.
Future registration strategies will need to address disease heterogeneity by designing trials that enroll smaller more homogenous populations that will then require utilization of surrogate endpoints.
Composite endpoints should be explored for feasibility and validated in future clinical trials in order to examine novel ways to evaluate treatment on patient outcomes.
At this time, registration-quality therapeutic trials need to demonstrate a predefined clinical benefit with no decrement in OS as well as provide sufficient data to clearly define safety.
HIGHLIGHTS.
Clinical trials demonstrating a benefit in progression-free survival frequently fail to preserve that effect in overall survival.
Ovarian cancer is a heterogeneous disease defined by histologic subtypes and activated biologic pathway aberrations, which are impacting drug development.
Alternative clinical trial endpoints should be explored in regulatory strategies this should be its own section as in the instructions.
Acknowledgments
Special thanks to the SGO staff especially Jessica Oldham and to Patrick Timmins, MD for their assistance in the preparation of this manuscript.
Footnotes
Conflict of interest statement
None of the authors has declared a conflict of interest with the material presented in this manuscript.
References
- 1.Herzog TJ, et al. Ovarian cancer clinical trial endpoints: Society of Gynecologic Oncology white paper. Gynecol Oncol. 2014;132(1):8–17. doi: 10.1016/j.ygyno.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galic V, Coleman RL, Herzog TJ. Unmet needs in ovarian cancer: dividing histologic subtypes to exploit novel targets and pathways. Curr Cancer Drug Targets. 2013 Jul;13(6):698–707. doi: 10.2174/15680096113139990002. [DOI] [PubMed] [Google Scholar]
- 3.Barlin JN, Jelinic P, Olvera N, Bogomolniy F, Bisogna M, Dao F, et al. Validated gene targets associated with curatively treated advanced serous ovarian carcinoma. Gynecol Oncol. 2013 Mar;128(3):512–517. doi: 10.1016/j.ygyno.2012.11.018. http://dx.doi.org/10.1016/j.ygyno.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–2312. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Pujade-Lauraine E. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. JCO. 2014 doi: 10.1200/JCO.2013.51.4489. http://dx.doi.org/10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 6.Stolcker JM. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. JCO. 2014 May 1;:1309–1316. doi: 10.1200/JCO.2013.51.4240. http://dx.doi.org/10.1200/JCO.2013.51.4240. published online on March 31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JF. Emerging role for bevacizumab in combination with chemotherapy for patients with platinum-resistant ovarian cancer. 2014 Mar 17; doi: 10.1200/JCO.2013.54.7299. http://dx.doi.org/10.1200/JCO.2013.54.7299 [published online ahead of print at www.jco.org on]. [DOI] [PubMed] [Google Scholar]
- 8.Amir E, Seruga B, Kwong R, Tannock IF, Ocana A. Poor correlation between progression-free and overall survival in modern clinical trials: are composite endpoints the answer? Eur J Cancer. 2012;48:385–388. doi: 10.1016/j.ejca.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–2069. doi: 10.1200/JCO.2003.11.126. [DOI] [PubMed] [Google Scholar]
- 11.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 12.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouche O, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong TS, Wefel JS, Wang M, Gilbert MR, Won M, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31:4076–4084. doi: 10.1200/JCO.2013.49.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Bellmunt J, Theodore C, Demkov T, Komyakov B, Sengelov L, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 17.DiSaia P, et al. The gynecologic oncology group: report of 35 years of excellence in clinical research. 2006 [Google Scholar]
- 18.Morrow CP, Bundy B, Kurman R, Creasman WT, Heller P, Homesley H, et al. Relationship between surgical-pathological risk factors and outcome in clinical stages I and II carcinoma of the endometrium (a Gynecologic Oncology Group study) Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 19.Lagasse LD, Creasman WT, Shingleton HM, Ford JH, Blessing JA. Results and complications of operative staging in cervical cancer: experience of the gynecologic oncology group. Gynecol Oncol. 1980;9(1):90–98. doi: 10.1016/0090-8258(80)90013-x. [DOI] [PubMed] [Google Scholar]
- 20.NCI Clinical Announcement on Intraperitoneal Therapy for Ovarian Cancer. http://www.cancer.gov/clinicaltrials/developments/IPchemo-digest.
- 21.NCI Clinical Announcement on Chemo-radiation for Cervical Cancer. http://www.cancer.gov/newscenter/newsfromnci/1999/cervicalcancer.
- 22.Randall M, Filiaci V, Muss H, Spirtos N, Mannel R, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24(1):36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 23.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer, a GOG study. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 24.Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, et al. Incorporation of bevacizumab significantly improves overall survival in advanced cervical cancer: a phase III randomized trial of the Gynecologic Oncology Group. N Engl J Med. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spellman, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011 Jun 30; doi: 10.1038/nature10166. http://dx.doi.org/10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledermann, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. [April 12, 2012];N Engl J Med. 2012 366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]