Abstract
The rapid development of high-volume horizontal hydraulic fracturing for mining natural gas from shale has posed potential impacts on human health and biodiversity. The produced flow back waters after hydraulic stimulation is known to carry high levels of saline and total dissolved solids. To understand the toxicity and potential carcinogenic effects of these waste waters, flow back water from five Marcellus hydraulic fracturing oil and gas wells were analyzed. The physicochemical nature of these samples was analyzed by inductively coupled plasma mass spectrometry and scanning electron microscopy / energy dispersive X-ray spectroscopy. A cytotoxicity study using colony formation as the endpoint was carried out to define the LC50 values of test samples using human bronchial epithelial cells (BEAS-2B). The BEAS-2B cell transformation assay was employed to assess the carcinogenic potential of the samples. Barium and strontium were among the most abundant metals in these samples and the same metals were found elevated in BEAS-2B cells after long-term treatment. BEAS-2B cells treated for 6 weeks with flow back waters produced colony formation in soft agar that was concentration dependant. In addition, flow back water-transformed BEAS-2B cells show a better migration capability when compared to control cells. This study provides information needed to assess the potential health impact of post-hydraulic fracturing flow back waters from Marcellus Shale natural gas mining.
Keywords: Marcellus Shale, Strontium, Barium, Cytotoxicity, Mutagenesis, Epigenetics
INTRODUCTION
Natural gas is believed to possibly be a bridge to transitioning from coal dependence. Currently natural gas fuels nearly 40 percent of the U.S. electricity generation, and the Marcellus shale formation in the Appalachian Basin is on the forefront of gas-shale drilling for natural gas production in the United States (Pritz, 2010). Mining natural gas is not new, but the volume has soared in recent years because the new technique of high-volume horizontal hydraulic fracturing (HVHHF). The concern surrounding the environmental, public health and social impacts of this method has increased accordingly. HVHHF is an advanced technology that injects water, sand, and other ingredients at very high pressure vertically into a well about 6,000 to 10,000 feet deep (Penningroth et al., 2013). The high pressure creates small fractures in the rock that extend out as far as 1,000 feet away from the well. The pressure is reduced after the fractures are created, which allows water from the well to return to the surface, also known as flow back water (Veil, 2010). The flow back water contains complex proprietary chemical mixtures, but also naturally occurring toxins such as metals, volatile organics, and radioactive compounds that are destabilized during gas extraction (Warner et al., 2012). On average, about 5.5 million gallons of water is used on average to hydraulically fracture each shale gas well, and 30% to 70% of the volume returns as flow back water (Veil, 2010). Currently discharge options of flow back water are: inject underground through an onsite or offsite disposal well; discharge to a nearby surface water body; transport to a municipal wastewater treatment plant or publicly owned treatment works; transport to a commercial industrial wastewater treatment facility; and/or reuse for a future hydraulic fracturing job either with or without some remediation (Pritz, 2010). Some commercial wastewater disposal facilities accept flow back and discharge the water after treatment under their own national pollutant discharge elimination system permits (Veil, 2010).
Metal pollution is a serious problem as they are taken up readily in the digestive tract and exhibit harmful effects on many tissues (Alomary et al., 2013; Rasmussen et al., 2013). Barium and strontium are abundant in the Marcellus shale formation, and are easily dissolved and transported in waste water after gas drilling activity (Pritz, 2010), which could potentially pose a threat to drinking water (EPA).
In contrast to the increased support of drilling and exploration by U.S. government agencies and rising concerns of impact on human and animal health within close proximity of the drilling sites (Bamberger and Oswald, 2012), knowledge of the health risks associated with the gas drilling waste water is sparse. The question posed here is whether flow back water specific to the Marcellus Shale malignantly transforms cells, and if it does, what’s the mechanism underlying tumorigenic potential of produced flow back water.
It has been challenging yet critical to choose a proper human cellular model to address this question. Immortalized human bronchial epithelial cells (BEAS-2B) have been widely used as a malignant cell transformation model to estimate the carcinogenesis capability of various environmental toxicants (Liao et al., 2007; Chang et al., 2010; Son et al., 2012; Yang et al., 2013). It has wild-type and functional p53 gene expression due to the loss of SV40 in passages after immortalization process, which provides a low spontaneous anchorage free growth, a quality of good cellular model for malignant cell transformation analysis(Lehman et al., 1993).
In this study we employed BEAS-2B as well established models (Lee et al., 1993; Chen et al., 2006; Sun et al., 2011; Passantino et al., 2013) to investigate the malignant cell transformation of Marcellus shale gas drilling flow back water.
MATERIAL AND METHODS
Cell culture and exposure
BEAS-2B (ATCC, Manassas, VA) cells were cultured as previously described (Sun et al., 2011; Passantino et al., 2013) at 37°C in a humid 5% CO2 atmosphere. BEAS-2B cells were seeded at 3×105 into 25 cm2 polystyrene tissue culture flasks. The cells were treated with filtered (0.22μm filter) produced flow back water collected from Bradford County, PA (a generous gift from Dr. Carl Kirby and Dr. Judy Zelikoff), and diluted with the appropriate medium to 0.13%, 0.25%, 0.5%, 1%, 2%, 4% or 8% (v/v). Control cells received distilled water or filtered water from a pristine lake located in Sterling Forest (SF), NY, diluted with the designated medium to 4 %(v/v). The cells were cultured for various time intervals as indicated. Every 3 to 4 day, the cells were trypsinized, counted, and re-seeded into fresh 25 cm2 flasks at a density of 3×105 viable cells per flask, and provided fresh media with the appropriate concentration of the flow back water sample.
Scanning electron microscopy (SEM)/energy dispersive X-ray spectroscopy (EDX)
The water samples were briefly sonicated in bath sonication for 1 min to make sure the solution was uniform. Both high density and low density samples were prepared. High density water samples were prepared with one drop of 100 μl of each sample dried on carbon tape in a class 100 clean room. For low density samples, 100 μl of each sample were spin coated onto a carbon tape surface pre-mounted to a SEM sample holder at 200rpm before being air dried overnight in a class 100 clean room. Field Emission Scanning Electron Microscopy (FEI, The Netherlands) and Energy Dispersive Spectrometry (model Genesis 60S, by EDAX Company, USA) were performed to identify any particles in the sample and the chemical components of those particulates were analyzed by EDX.
Inductively coupled plasma mass spectrometry (ICP-MS)
A volume of 0.1mL of each sample including flow back water and their filtrates were ionized in tubes with 1 mL HNO3 (70%) at 140°C for 5h. Concentrations of the heavy metals were determined by an ICP-MS (Perkin Elmer, Warsaw, Poland). BEAS-2B cells after 5w of treatment with flow back water at 0.5% (v/v) were trypsinized and counted to determine the total cell number. The cell pellets with same amount of cells were then mixed with 3 ml of HNO3 (70%) and incubated at 80°C for 48h, followed by cooling for 1h to room temperature. After cooling, 3 ml of hydrogen peroxide (30%) was added to each tube, followed by incubation of the solution at 80°C for 3h. After suitable dilution of the digested materials with ultrapure water, levels of elements in the samples were determined by ICP-MS.
Colony formation and soft agar assay
Following treatment, BEAS-2B cells were trypsinized and counted using a hemocytometer to determine viability. Colony formation and soft agar assay were then conducted, cells exhibited anchorage free growth were collected for wound healing assay according to previously published procedure (Passantino et al., 2013), for detailed information, please see supplemental materials.
Cell migration assays
Matrigel (BD Biosciences, Bedford, MA) was reconstituted on the top surfaces of Transwell membranes at 100 μg protein/ cm2 of surface area. Transformed BEAS-2B cells (5×104 in 100 μl) were added to the upper chamber in serum free medium supplemented with 0.5 μM plasminogen. The bottom chamber contained DMEM supplemented with 10% FBS. The cells were allowed to invade for 24 hours at 37°C, at which time the Matrigel and cells that were associated with the top surfaces of the membranes were removed with cotton swabs. Cells that penetrated through the Matrigel to the underside surfaces of the membranes were fixed and stained with 0.1% Crystal Violet. Cells on the lower surface of the filter were enumerated using an ocular micrometer. Five fields were counted. Each experiment was performed twice with triplicate samples.
Wound healing assay: Cells (2 X 105) from each clone extracted from soft agar were plated into 35 mm culture dishes with a grid etched into the bottom. The cells were cultured in 1X DMEM complete media until 100% confluent (4d). The media were then replaced with 1X PBS, and a single scratch was made across the monolayer using a 1 mL pipette tip held perpendicular to the plate bottom. The plate was washed twice with 1X PBS to remove any floating cells released during the scratching process. 1X DMEM was added back into the plates to nourish the cells and photographs were taken at 6h and 36h after the scratch using a Leica SP5 microscope (Leica Microsystems, Buffalo Grove, IL). The exact same field of view was captured at each time point with location guidance from the culture plate grid.
Mouse studies
All mouse experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and all efforts were made to minimize suffering. Animals were maintained under protocols approved by the New York University School of Medicine Animal Care and Use Committee (IACUC). Female athymic nude mice (Nu/J) aged 6–8 weeks were injected subcutaneously with 1×107 various transformed BEAS-2B cells and control cells in the left and right flank to generate solid human tumor xenografts. All mice were housed in specific pathogen free (SPF) environments and observed for tumor growth twice a week. Euthanasia was performed through carbon dioxide overexposure per the American Veterinary Medical Association (AVMA) guidelines.
RNA isolation and RNA-Sequencing (RNA-Seq)
Total RNA was isolated from control or treated cells in biological replicates (duplicates for control and triplicates for well water treated samples) and polyA+ RNA was purified using poly-T oligo-attached magnetic beads in accordance to Illumina’s protocol for sequencing of mRNA (RNA-Seq). Following purification, the mRNA was fragmented into small pieces using divalent cations under elevated temperature. Then the cleaved RNA fragments were copied into first strand cDNA using reverse transcription and random primers, and followed by second strand cDNA synthesis using DNA Polymerase I and RNaseH. These cDNA fragments were then end repaired, and a single ′A′ base was added to allow blunt-end ligation of adapters. These products were then purified and enriched with PCR to create the final cDNA library suitable for high throughput DNA sequencing on the Illumina Cluster Station and Genome Analyzer.
For RNA sequencing, each mRNA sample was uploaded onto one lane of flow cell and sequenced in 36-nucleotide single-end run by Illumina Genome Analyzer II (GAII). For each sample, about 20 million raw reads were generated. Over 90% raw reads were able to be mapped to human genome (GRCh37.71/hg19) by using Bowtie aligner (0.12.9) with v2 and m1 parameters. Mapped reads were subsequently subjected to PCR duplicates removal and reads assignment back to gene model was done by feature counts package. To obtain significant differential expressed genes, the surface water and agar clone experiments were treated as single factor multiple group design while well water experiment was treated as two factors (time and dose) multiple levels design. The TMM normalization method in edgeR package (3.4.2) was applied for data normalization cross sample groups before applying general linear model for statistical estimate and analysis for either single factor or two factor designed experiments. FDR adjustment was used for multiple hypothesis test to obtain adjusted p value. A proper FDR cutoff was set to select significant differential expressed genes for subsequent clustering or functional analysis. The results were then validated using quantitative real-time PCR.
RESULTS
High Barium and Strontium levels in flow back water samples
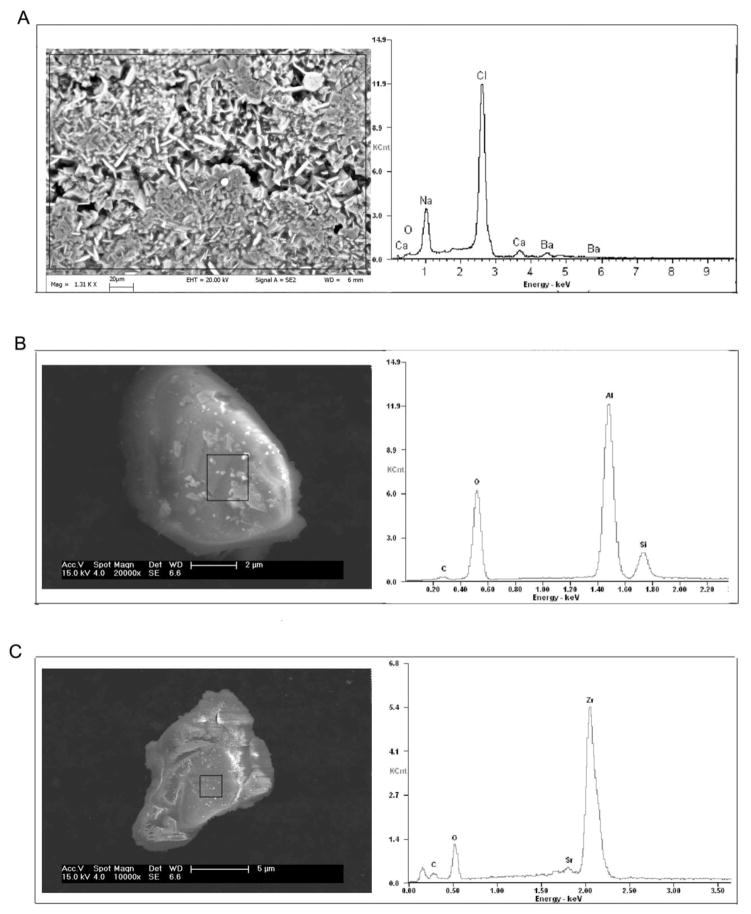
Elemental analysis from ICP-MS indicated high amounts of strontium (Sr, ranged from 1339 mg/L to 3728 mg/L), and barium (Ba, ranged from 3237 mg/L to 4989 mg/L) beside high sodium (Na, ranged from 28,600 mg/L to 46,100 mg/L) and calcium (Ca, ranged from 6,010 mg/L to15,500 mg/L) levels in these samples (Table S1), which are consistent with the results from the measurements conducted when the water samples were originally collected (Pritz, 2010). Only small amounts of hexacosane and octacosane were detected from three of the five samples (Table S1). To remove any large organics or other confounding biotic before treating the cells, the water samples were filtered through 0.22μm polyethersulfone membrane filters and the filtrate was again measured via ICP-MS. As shown in Table 1, Sr and Ba levels remained highly elevated in the filtrates, although substantially reduced from that observed prior to filtration; a phenomenon that was also observed with the other constituents of the samples as well. To characterize the morphological features as well as size parameters of the particles in these samples, SEM/EDX was used. Figure 1 shows the representative SEM morphology of the particles in flow back water from well 1 (Figure 1A shows whole water with high density sample preparation, Figure 1B and 1C show filtered water with low density preparation). Amorphous silicon aluminum oxide (SiAlOx) nano/micro-particles ranging from 70–285nm and amorphous zirconium oxide (ZrOx) particles ranging from 40–140nm, were detected in filtered flow back samples using SEM/EDX analysis, indicating small particle aggregates pass through the 0.22um pore of the filters. The EDX spectrum on individual particles in Figure 1B and 1C confirmed the SiAlOx and ZrOx chemistry of the particles but without stoichiometric balancing, the exact oxidation state is not known. Radiation emission detected was not higher than normal background levels in our samples when measured by beta detectors or gamma scintillation counters (data not shown).
Table 1.
ICP-MS analysis of filtered Marcellus Shale flow back
| Concentration (mg/L) | Well 1 | Well 2 | Well 3 | Well 4 | Well 5 | SF |
|---|---|---|---|---|---|---|
| Al | 0.301 | 0.044 | 1.268 | 1.102 | 1.057 | N.D. |
| B | 0.315 | 0.252 | 0.408 | 0.388 | 0.370 | N.D. |
| Ba | 452.4 | 474.6 | 596.5 | 606.8 | 701.6 | N.D. |
| Bi | 0.110 | 0.164 | 0.180 | 0.158 | 0.059 | N.D. |
| Ca | 470.7 | 974.9 | 707.8 | 608.9 | 502 | 1,537 |
| Co | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Fe | 3.506 | 1.343 | 4.491 | N.D. | N.D. | N.D. |
| K | 23.43 | 237.4 | 180.8 | 203.4 | 345.1 | 0.248 |
| Mg | 52.89 | 91.70 | 109.1 | 115.1 | 105.0 | 41.20 |
| Mn | 0.341 | 0.357 | 2.58 | 0.43 | 0.34 | N.D. |
| Na | 61.48 | 60 | 62.2 | 57.94 | 90 | 91.62 |
| S | 8.74 | 70.69 | 9.884 | 9.18 | 14.5 | 0.781 |
| Si | 0.675 | 0.931 | 1.762 | 0.713 | 0.759 | N.D. |
| Sr | 183.3 | 281.2 | 361.4 | 353.4 | 339.7 | 1.01 |
| Ti | 0.079 | 0.067 | 0.045 | 0.067 | 0.074 | 0.008 |
| V | 0.206 | 0.267 | 0.441 | 0.512 | 0.457 | 0.006 |
| W | 1.926 | 3.419 | 9.343 | N.D. | N.D. | 3.28 |
| Zn | 0.062 | 0.049 | 0.054 | 0.003 | 0.030 | 59.02 |
| Zr | 0.066 | 0.073 | 0.033 | 0.008 | N.D. | N.D. |
N.D.: Not detected
SF: pristine lake water from Sterling Forest
Figure 1.
SEM/EDX analysis of Marcellus flow back water. Representative SEM images reveal the morphology of crystals and particles in flow back samples. The rectangles indicate the regions that were analyzed by the EDX for the composition of the particulates. Samples were prepared at high (A) and low densities (B, C). At low density conditions, individual particles can be isolated and EDX can be performed on each particle.
Flow back water samples transformed BEAS-2B cells in vitro
BEAS-2B cells were employed as a non-organ-specific assay system to test the transformation activity of the flow back samples. Due to the comparability of components of five flow back samples (shown in Table S1 and 1), filtered samples from well 3 (representative flow back water after multiple use) and well 1 (representative flow back water after single use) were chosen to treat BEAS-2B cells.
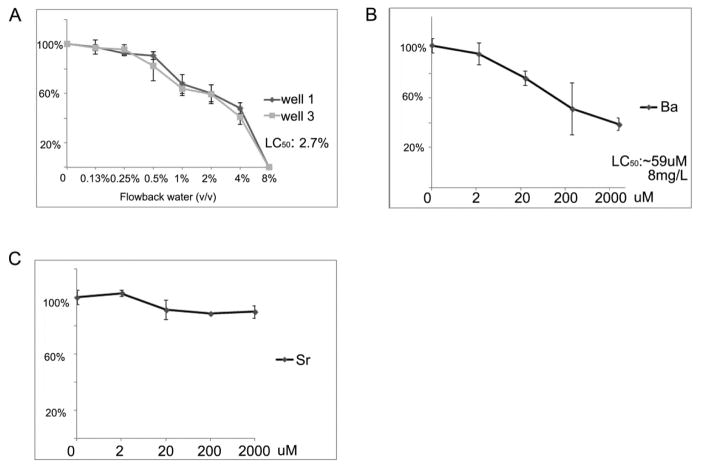
Up to 8% (v/v) dosages were used to assay cytotoxicity. The colony formation assay was carried out to determine an appropriate dose of flow back water samples for treatment of the BEAS-2B cells. This assay was performed with three biological replicates, and the total number of colonies in each replicate fell within two standard deviations of each other. The dose response curve (Figure 2A) demonstrated that cell survival decreased in a dose-dependent manner. The LC50 following treatment for 7d was calculated to be ~2.7% (v/v). Based on these data, dosages of 0.13%, 0.25%, 0.5%, 1%, 2%, and 4% were subsequently selected to determine cytotoxicity from long-term water treatment. After 10 days, the BEAS-2B cells treated with 4% flow back failed to survive, while cells treated with the lower doses all survived and were subsequently exposed to flow back water for total 6 weeks. Soluble barium chloride (Ba II) and strontium chloride (Sr II) were used to determine individual cytotoxicity of each metal. The LC50 of Ba (II) following treatment for 7d was calculated to be ~8mg/L (Figure 2B), this concentration is parallel to the concentrations of Ba when the two studied flow back samples were diluted to 2.7% (12.21mg/L for well 1 and 16.11mg/L for well 3), indicating Ba plays a major role in cytotoxicity of Marcellus Shale flow back water. Sr (II) showed no significant cytotoxicity even at the 2mM concentration in the culture medium (Figure 2C), which indicated that the cytotoxicity of Ba was not driven by an elevation in osmolality (2mM SrCl2 generates same amount of osmolality as 2mM BaCl2).
Figure 2.
Cytotoxicity of Marcellus flow back, soluble barium and strontium. The colony formation curve was generated by culturing replated BEAS-2B cells for 16 days after exposed to various concentrations of flow back (A), soluble barium (B) and strontium (C)for 3 weeks. (B) and then the number of surviving colonies was determined.
A subset of treated BEAS-2B cells (at 5 week) was collected and the uptake of metals by the cells was then examined by ICP-MS, the metals that increased significantly in flow back water treated cells are shown in Table 2. As expected, Ba and Sr levels increased significantly in cells after long-term treatments when compared to levels in control treated cells (Table 2).
Table 2.
ICP-MS analysis of BEAS-2B cells from long-term treatment by Marcellus Shale flow back at 0.5% (v/v)
| Concentration (μg/million cells) | Ctrl | Well 1 | Well 3 | SF |
|---|---|---|---|---|
| Ba | 0.0017 | 0.1418 | 0.1378 | N.D. |
| Sr | 0.003 | 0.3941 | 0.4724 | 0.044 |
N.D.: Not detected
SF: cells exposed to filtered pristine lake water from Sterling Forest
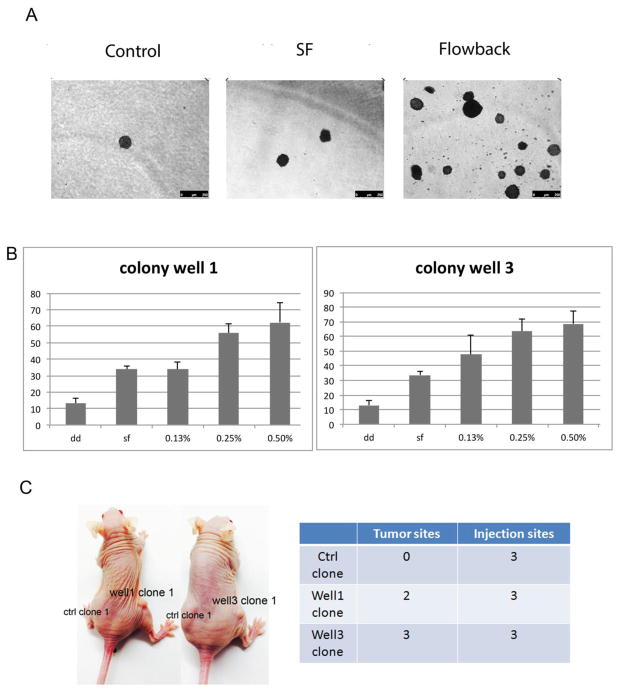
BEAS-2B cells treated with flow back water for 6 weeks were tested for anchorage independent growth using the soft agar colony-forming assay (Figure 3A and B). The soft agar assay was performed using three biological replicates, and the total number of colonies in each replicate fell within two standard deviations of each other. A dose-dependent increase in colony number in soft agar was observed, with the greatest number of colonies arising from cells treated with 0.5% flow back water recovered from wells 1 and 3 (Figure 3B). A small number of control cells spontaneously formed colony like clusters in agar; however the numbers were significantly fewer and sizes were considerably smaller (Figure 3B).
Figure 3.
Anchorage free growth of BEAS-2B cells exposed to Marcellus flow back. BEAS-2B cells were exposed to various concentrations of flow back for 6 weeks, and assessed for anchorage free growth using a soft agar assay. 3w later, cell colonies were stained with INT/BCIP and photographed. Panel A shows representative plates in soft agar assay, control: distilled water, SF: filtered pristine lake water from Sterling Forest. Numbers of colonies formed by BEAS-2B cells exposed to flow back well 1 and flow back well 3 (B) were counted and presented as the mean ± SD (n = 3), dd: distilled water, SF: filtered pristine lake water from Sterling Forest. Panel C shows representative image and the actual number of tumor formation in nude mice after subcutaneously injection of control clones (ctrl clone 1) and flow back water well 1-transformed cells (Well1 clone 1) and flow back water well 3-transformed cells (Well3 clone 1).
For further transformation studies, the 0.5% (v/v) dose was selected as this dose generated the greatest number of flow back water transformed clones without being cytotoxic. Colonies were then isolated from soft agar, trypsinized and expanded in monolayer culture. Five out of six mice injected with cells transformed from well water treatments developed tumors, while the mice injected with control clones did not form tumors after 6 months (Figure 3C), indicating the produced flow back water is capable of neoplastic transformation in vitro.
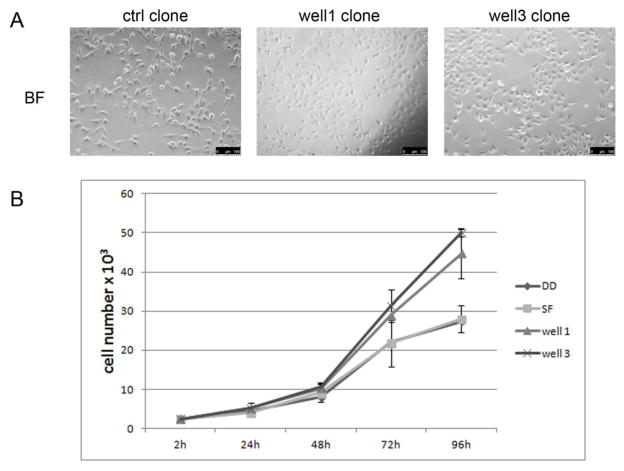
After expansion in monolayer culture, clones generated from flow back water treatments exhibited a distinct cell morphology compared to those derived from control clones. As shown in Figure 4A, control clone cells were flat, diamond-shaped, and similar to their parental BEAS-2B cells, while clones generated from cells treated with flow back water from wells 1 or 3 were rounder, forming a more compact cobblestone-like monolayer. In addition, these clones exhibited a slightly faster growth rate as compared to control cells after 72 hour cell culture (Figure 4B).
Figure 4.
Analysis of transformed cells derived from soft agar. (A) representative image of normal BEAS-2B cells derived from spontaneously derived colonies of untreated cells (ctrl clone), flow back well 1 transformed cells (well 1 clone) or flow back well 3 transformed cells (well 3 clone) grown in low density. (B) control and flow back transformed cells were seeded at 2500 cells/well in 24-well plates. Cells were trypsinized and counted at the indicated time point. Results were represented as mean ± SD (n=3), DD: distilled water, SF: filtered pristine lake water from Sterling Forest.
Five out of six mice injected with cells transformed from well water treatments developed tumors as early as 3 months after the injection. One mouse carrying xenograft tumor developed from well1-flow-back-water-transformed-BEAS-2B was sacrificed 5 months after the injection when the tumor diameter reached 1cm, others were kept in SPF facility until 6 months after the injection; the tumor diameters ranged from 0.2cm to 0.6cm. The mice injected with control clones did not form tumors after 6 months (Figure 3C); indicating the produced flow back water is capable of neoplastic transformation in vitro.
Enhanced migration of flow back water transformed BEAS-2B
After expansion in monolayer culture, clones generated from flow back water treatments exhibited a distinct cell morphology compared to those derived from control clones. As shown in Figure 4A, control clone cells were flat, diamond-shaped, and similar to their parental BEAS-2B cells, while clones generated from cells treated with flow back water from wells 1 or 3 were rounder, forming a more compact cobblestone-like monolayer. In addition, these clones exhibited a slightly faster growth rate as compared to control cells after 72h cell culture (Figure 4B).
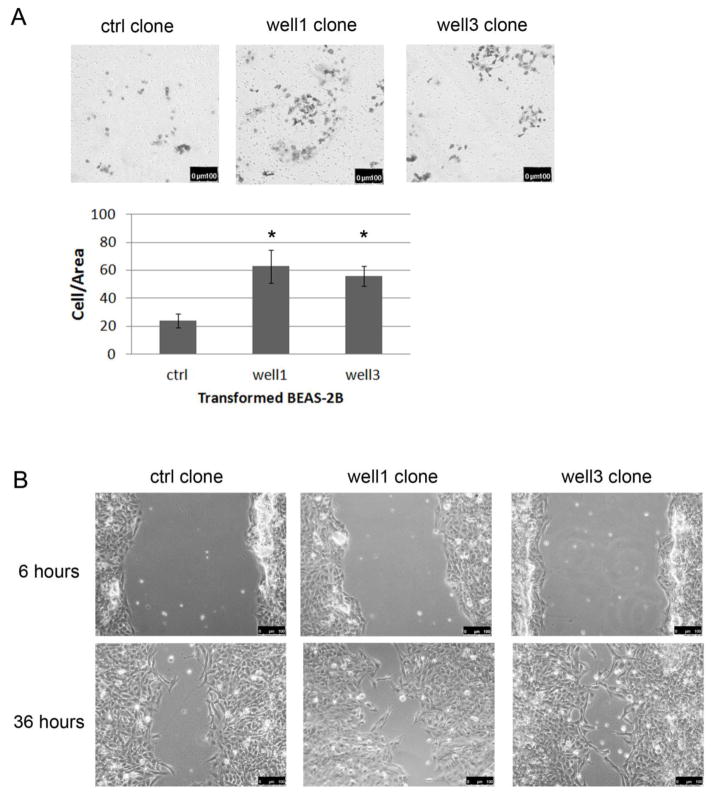
Surviving clones from both treatment groups were then assayed for cell migration. Matrigel assay showed that BEAS-2B cells transformed by flow back well water samples presented an enhanced migration capacity than control cells (Figure 5A). In line with the results from Matrigel assay, all of the clones generated from flow back water treatments were able to heal the wound by 36h post-scratch while control clones failed to heal the wound in the same amount of time (Figure. 5B).
Figure 5.
Enhanced cell migration of transformed BEAS-2B. (A) Representation (top) and quantification (bottom) of matrigel invasion assay showing the in vitro migration of transformed BEAS-2B cells. *p<0.05 (B) flow back transformed clones healed the wound faster than the control clones. All images were captured at 100 X magnification, and the same field of view was captured at each time point with guidance from the grid on the cell culture plates.
Altered transcription profile in flow back water transformed BEAS-2B
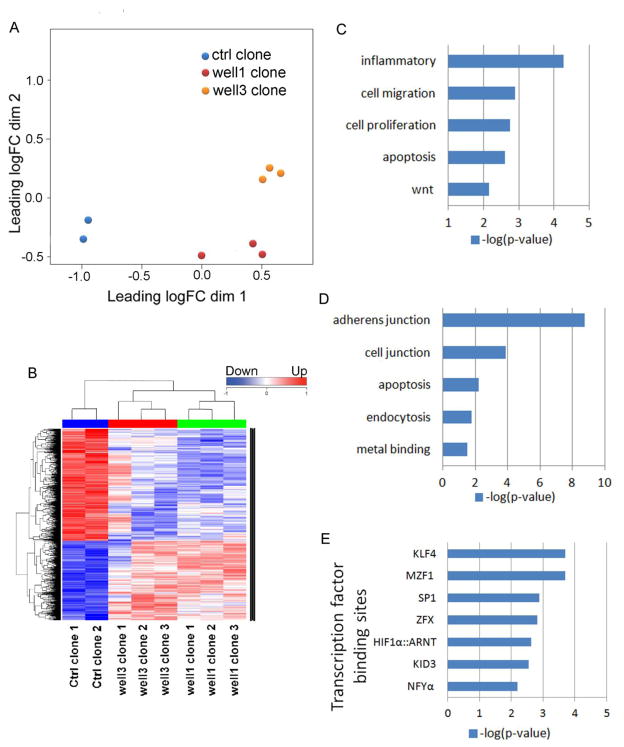
We analyzed transcription profiles of transformed cells by sequencing the RNA libraries prepared from clones generated from flow back water treatments and control clones (Figure 6). Multidimensional scaling and hierarchical clustering dendrogram revealed that transformed clones cluster according to their treatment group. The transcription profiles were then analyzed by DAVID Bioinformatics Resources 6.7(National Institute of Allergy and Infectious Diseases, NIH). The differentially expressed genes have been listed in Table S3 (well1 clones Vs ctrl clones) and Table S4 (well3 clones Vs ctrl clones). For DAVID analysis, differentially expressed genes (Table S5) that are common in both well1 and well3 clones were used. The genes with up-regulated expression in transformed clones were over-presented by inflammation, cell migration, cell proliferation and wnt signaling pathway (Figure 6C), while down-regulated genes were over-presented by adherens junction, apoptosis, endocytosis (Figure 6D), which are consistent with the phenotypes we observed in transformed clones (Figure 4 and 5).
Figure 6.
RNA-Seq analysis of transformed BEAS-2B cells. (A) Hierarchical clustering dendrogram shows 1298 genes resulting from a multiple hypothesis test, FDR<0.05, for all 8 clones (6 flow back water-transformed clones, 2 control clones). Genes that were increased in expression compared to their control clones are shown in red while under-expressed genes are depicted in blue. (B) A Multidimensional scaling plot shows that clones derived from individual flow back water wells treated cells generally cluster together.(C and D) GO analyses showing the major canonical pathways associated with the genes (C)upregulated and (D)downregulated in flow back well water sample transformed BEAS-2B cells. (E) Identification of the overrepresented transcription factor binding motifs at the promoters of genes upregulated in flow back well water sample transformed BEAS-2B cells.
The transcription factor binding motifs overrepresented at the differentially expressed genes’ promoter sequences (−250 and +50 base pairs around the transcription start site) were identified using Opossum 3.0 with JASPAR CORE transcription factor binding profiles. The conservation cutoff were set at 0.6 and only those transcription binding sites with Z-score >=10 and Fisher score >=7 were shown in Figure 6E (transcription binding sites associated with up-regulated genes) and Table S6 (transcription binding sites associated with down-regulated genes). Interestingly, the over-represented conserved transcription binding sites in up-regulated genes (Figure 6E) are of transcription factors that are known to associate with cancer (SP1, HIF-1α, MZF1) and stem cell self-renewal (KLF4, NFYα).
DISCUSSION
The objective of this study was to investigate the potential cytotoxicity and transforming activity of Marcellus shale well flow back water to mammalian cells. To the best of our knowledge, this is a first report of this nature. Human and animal exposure to flow back water occurred through leakage or improper fencing of impoundments, and/or via alleged compromise of a liner in an impoundment to drain fluid, direct discharging of the flow back water to the creeks and nearby land (Bamberger and Oswald, 2012).
Ba and Sr appeared to be metals with high concentrations in these aged flow back samples that were found elevated in cells after long-term treatment. There are reports showing arsenic and selenium are constituents of gas containing rock bed (Haluszczak et al, 2013; Jackson, 2012), however, these elements were below the detection limit of ICP-MS in these specific five flow back water samples. Based on this study and previous report(Jackson,2012), the high Ba and Sr levels are not likely due to fracking procedure but rather the nature of Marcellus Shale. Sr and Ba are two alkaline elements that mimic calcium in the body of living organisms, therefore high concentration of Ca will reduce the absorption of these two metals (Comar et al, 1957). In these flow back water samples, we found high concentration of Ca, however, after long-term treatment, the absorption of Ba and Sr were still detectable by ICP-MS.
Although the World Health Organization (WHO) 4th edition of health-based guidelines sets 700 μg/L as a guideline for Ba levels in drinking water, a lower level (i.e., 343.3–686.6 μg/L) of Ba alone promotes transforming activity of several cell lines (Thang et al., 2011). It’s notable that the concentrations of Ba in the flow back water we used were at 2262 μg/L (well 1) and 2982.5 μg/L (well 3). In Iran, Sr concentrations in drinking water, soil, and grain samples are much higher (3437.3 μg/L) in areas with a high esophageal cancer incidence rate (i.e. Gonbad–Dashlibroon and Marave Tappeh regions) compared to that in areas where the esophageal cancer incidence is lower (Keshavarzi et al., 2012a; Keshavarzi et al., 2012b), suggesting adverse impacts of Sr to human health. Notably, the concentrations of Ba and Sr in our whole flow back water samples are at least a thousand-fold higher than concentrations in the drinking water from these areas, making direct discharge of flow back to surface water body a pressing water and soil pollution problem, which in turn could affect the health of animals and humans in close proximity. Furthermore, we believe the major components in the aged water samples that we tested in this study are also present in conventional gas drilling flow back waters. Our work suggested the importance of proper waste water regulation and treatment before its discharge to surface water, and it is applicable to both conventional and unconventional gas drilling.
In response to concern over flow back and produced water discharges, the Pennsylvania Department of Environmental Protection (PADEP, Pennsylvania is the state where the waste water in this study was originated from) proposed a new strategy that would add effluent standards for oil and gas wastewaters of 500 mg/L for total dissolved solids, 250 mg/L for sulfates, 250 mg/L for chlorides, and 10 mg/L for total Ba and total Sr (Veil,2010). Moreover, the Pennsylvania Environmental Quality Board approved the new discharge requirements as revisions to the Pennsylvania regulations (Veil, 2010). As the Marcellus Shale development grows in popularity, operators seek to bring more truckloads of salty flow back and produced water to the treatment plants. In this case, it is most likely that the increased input of total dissolved solids will result in increased levels of total dissolved solids in the discharge. Close monitoring and restrictions on the discharge should be conducted to limit the impact on animal and human health. National pollutant discharge elimination system that requires that the volume of wastewater from oil and gas sources may not exceed 1% of the average daily flow has been adopted by many of the water treatment works (Veil, 2010); whether this regulation is sufficient to prevent the environmental impact on the eco system warrants further investigation.
The flow back water used in this study while transformative may not be truly representative as it was aged prior to the physical-chemical characterization necessary for in this set of experiments; and thus neither significant amount of radioactivity or organic compounds were present. While the time of laboratory storage accounted for changes in the chemistry of the samples, it could be noted that some waste water generated from hydraulic fracturing has been stored in disposal pits for years and may therefore be more representative of the long term hazards posed by improper disposal or containment. Results from this study suggest that even aged flow back water could pose substantial health threats to exposed humans. The absence of volatile organic compounds aided in the identification of toxicity due mostly from Ba, Sr, or other metals in the flow back waters. Due to limited resource, we were not able to measure Radium (Ra226 and/or Ra228 which have been reported in some of the other Marcellus Shale flow back water samples (Pritz, 2010). Ra226, an alpha emitter, has a half-life of about 1600 years with accompanying gamma radiation; and Ra228, a beta emitter, has a half-life of 5.76 years.). We attempted to determine whether there was any gamma radiation emitted from the water using a gamma counter but we did not detect any radiation. The measurements of metals in the filtrates and cells exposed to the filtrates helped us to assess the presence of toxic metals and their relative concentrations.
The cells transformed by flow back water formed tumors in athymic nude mice and exhibit higher migration ability in transwell assay and wound healing assay. These phenomena have been further supported by gene expression analysis. Cell migration pathways are up-regulated and adherent junction pathways are down-regulated in flow back water transformed cells. This is a common phenomenon for malignant cell transformation which we have reported earlier nickel, arsenic or vanadium transformed BEAS-2B cells (Clancy et al, 2012). However, the transcription profile alteration induced by flow back water which contains various kinds of elements still has its distinguish signature. The genes with increased expression level in flow back water transformed cells are over-represented by inflammation, while the down-regulated genes are over-represented by endocytosis; indicating the cells went through an inflammation reaction and a reduction of endocytosis to cope with elevated metal levels in their micro-environment upon their encounter with metal enriched flow back water. However, based on the data we gathered, we are not able to tell whether the malignant transformation activity were solely from Ba, Sr or other metals that have been detected in the flow back waters at lower levels.
It is worth noting that binding sites of a few transcription factor that are associated with stem cell self-renewal (KLF4, NFYα) and cancer (SP1, HIF-1α, MZF1) are found over-represented in genes with up-regulated expression in flow back water transformed cells. MZF1 (myeloid zinc finger 1) is also up-regulated at its gene expression level. The overexpression of MZF1 was reported to inhibit apoptosis and induce migration, invasion, tumor formation, and metastasis in vivo in cultured colorectal and cervical cancer cells (Mudduluru et al, 2010). Our expression analysis against transformed cells provided a potential molecular mechanism of how flow back water transforms human cells at gene expression level. In addition, it has been previously shown that there is a finger print of gene expression in transformed clones that is characteristic for each metal that was used to induce the anchorage independent growth (Clancy et al, 2012). This seems to be also true for the flow back water induced transformation and this finding argues that Ba and perhaps Sr were the major carcinogenic species in the flow back water.
CONCLUSIONS
Our work has provided the first line of evidence that Marcellus Shale flow back water induces malignant cell transformation in vitro. The BEAS-2B cells exposed to flow back water up to six weeks appeared to be transformed and exhibiting altered morphology as compared to parental cells. The present work also provided Ba and Sr as hydraulic fracturing-related target pollutants in addition to the more classically-studied fracking contaminants (i.e., radioisotopes and methane) for further investigation. Research to determine whether fracking-associated pollutants can migrate to private or public drinking wells, to identify early warning indicators of exposure and effect, and to identify suitable remediation approaches are urgently needed. Descriptive and analytical epidemiological studies along with animal model studies will help to better understand the health impact associated with unconventional shale gas production.
Supplementary Material
HIGHTLIGHTS.
This is the first report of potential cytotoxicity and transforming activity of Marcellus shale gas mining well flow back water to mammalian cells.
Barium and Strontium were elevated in flow back water exposed cells.
Flow back water malignantly transformed cells and formed tumor in athymic nude mice.
Flow back water transformed cells exhibited altered transcriptome with up-regulated cell migration pathway and down-regulated adherent junction pathway.
Acknowledgments
We thank Dr. Carl S Kirby from Bucknell University and Dr. Judith T Zelikoff from New York University for generously providing flow back water; laboratory colleagues and EHSCC inter-Center Work Group for their valuable discussions and suggestions. This work was supported by a supplement to NIEHS Center grant #ES 000260 awarded to Costa and by grant R01ES023174, R01ES022935 and the Fundamental Research Funds for the Central Universities #30920130111020 awarded to Wu.
Footnotes
Competing Financial Interests: The authors have no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alomary A, Al-Momani IF, Obeidat SM, Massadeh AM. Levels of lead, cadmium, copper, iron, and zinc in deciduous teeth of children living in Irbid, Jordan by ICP-OES: some factors affecting their concentrations. Environmental monitoring and assessment. 2013;185:3283–3295. doi: 10.1007/s10661-012-2790-y. [DOI] [PubMed] [Google Scholar]
- Bamberger M, Oswald RE. Impacts of gas drilling on human and animal health. New solutions : a journal of environmental and occupational health policy : NS. 2012;22:51–77. doi: 10.2190/NS.22.1.e. [DOI] [PubMed] [Google Scholar]
- Chang Q, Pan J, Wang X, Zhang Z, Chen F, Shi X. Reduced reactive oxygen species-generating capacity contributes to the enhanced cell growth of arsenic-transformed epithelial cells. Cancer research. 2010;70:5127–5135. doi: 10.1158/0008-5472.CAN-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ke Q, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Molecular and cellular biology. 2006;26:3728–3737. doi: 10.1128/MCB.26.10.3728-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy H, Sun H, Passantino L, Kluz T, Munoz A, Zavadilb J, Costa M. Gene expression changes in human lung cells exposed to arsenic, chromium, nickel or vanadium indicate the first steps in cancer. Metallomics. 2012;4:784–793. doi: 10.1039/c2mt20074k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comar C, Russell R, Wasserman R. Strontium-calcium movement from soil to man. Science. 1957;126:485–492. doi: 10.1126/science.126.3272.485. [DOI] [PubMed] [Google Scholar]
- EPA, United States Environmental Protection Agency. Drinking Water Contaminants. Retrieved from http://water.epa.gov/drink/contaminants.
- Haluszczak L, Rose A, Kump L. Geochemical evaluation of flowback brine from Marcellus gas wells in Pennsylvania, USA. Applied Geochemistry. 2013;28:55–61. [Google Scholar]
- Jackson R. Hydraulic fracturing, water resources and human health. Paper presented at the Institute of Medicine the Health Impact Assessment of New Energy Sources: Shale Gas Extraction; Washington, DC. 2012. [Google Scholar]
- Kato M, Kumasaka MY, Ohnuma S, Furuta A, Kato Y, Shekhar HU, Kojima M, Koike Y, Dinh Thang N, Ohgami N, Ly TB, Jia X, Yetti H, Naito H, Ichihara G, Yajima I. Comparison of Barium and Arsenic Concentrations in Well Drinking Water and in Human Body Samples and a Novel Remediation System for These Elements in Well Drinking Water. PloS one. 2013;8:e66681. doi: 10.1371/journal.pone.0066681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi B, Moore F, Najmeddin A, Rahmani F. The role of selenium and selected trace elements in the etiology of esophageal cancer in high risk Golestan province of Iran. The Science of the total environment. 2012a;433:89–97. doi: 10.1016/j.scitotenv.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Keshavarzi B, Moore F, Najmeddin A, Rahmani F, Malekzadeh A. Quality of drinking water and high incidence rate of esophageal cancer in Golestan province of Iran: a probable link. Environmental geochemistry and health. 2012b;34:15–26. doi: 10.1007/s10653-011-9377-3. [DOI] [PubMed] [Google Scholar]
- Lee YW, Pons C, Tummolo DM, Klein CB, Rossman TG, Christie NT. Mutagenicity of soluble and insoluble nickel compounds at the gpt locus in G12 Chinese hamster cells. Environmental and molecular mutagenesis. 1993;21:365–371. doi: 10.1002/em.2850210408. [DOI] [PubMed] [Google Scholar]
- Lehman TA, Modali R, Boukamp P, Stanek J, Bennett WP, Welsh JA, Metcalf RA, Stampfer MR, Fusenig N, Rogan EM, et al. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- Liao WT, Lin P, Cheng TS, Yu HS, Chang LW. Arsenic promotes centrosome abnormalities and cell colony formation in p53 compromised human lung cells. Toxicology and applied pharmacology. 2007;225:162–170. doi: 10.1016/j.taap.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Mudduluru G, Vajkoczy P, Allgayer H. Myeloid zinc finger 1 induces migration, invasion, and in vivo metastasis through Axl gene expression in solid cancer. Molecular cancer research : MCR. 2010;8:159–169. doi: 10.1158/1541-7786.MCR-09-0326. [DOI] [PubMed] [Google Scholar]
- Passantino L, Munoz AB, Costa M. Sodium metavanadate exhibits carcinogenic tendencies in vitro in immortalized human bronchial epithelial cells. Metallomics : integrated biometal science. 2013;5:1357–1367. doi: 10.1039/c3mt00149k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penningroth SM, Yarrow MM, Figueroa AX, Bowen RJ, Delgado S. Community-based risk assessment of water contamination from high-volume horizontal hydraulic fracturing. New solutions : a journal of environmental and occupational health policy : NS. 2013;23:137–166. doi: 10.2190/NS.23.1.i. [DOI] [PubMed] [Google Scholar]
- Pritz ME. Honor’s Theses. 2010. Geochemical Modeling and Analysis of the Frac Water Used in the Hydraulic Fracturing of the Marcellus Formation, Pennsylvania. Paper 69. [Google Scholar]
- Rasmussen PE, Levesque C, Chenier M, Gardner HD, Jones-Otazo H, Petrovic S. Canadian House Dust Study: population-based concentrations, loads and loading rates of arsenic, cadmium, chromium, copper, nickel, lead, and zinc inside urban homes. The Science of the total environment. 2013;443:520–529. doi: 10.1016/j.scitotenv.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Son YO, Wang L, Poyil P, Budhraja A, Hitron JA, Zhang Z, Lee JC, Shi X. Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3beta/beta-catenin signaling. Toxicology and applied pharmacology. 2012;264:153–160. doi: 10.1016/j.taap.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Clancy HA, Kluz T, Zavadil J, Costa M. Comparison of gene expression profiles in chromate transformed BEAS-2B cells. PloS one. 2011;6:e17982. doi: 10.1371/journal.pone.0017982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thang ND, Yajima I, Kumasaka MY, Ohnuma S, Yanagishita T, Hayashi R, Shekhar HU, Watanabe D, Kato M. Barium promotes anchorage-independent growth and invasion of human HaCaT keratinocytes via activation of c-SRC kinase. PloS one. 2011;6:e25636. doi: 10.1371/journal.pone.0025636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner NR, Jackson RB, Darrah TH, Osborn SG, Down A, Zhao K, White A, Vengosh A. Geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11961–11966. doi: 10.1073/pnas.1121181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YX, Li XL, Wang L, Han SY, Zhang YR, Pratheeshkumar P, Wang X, Lu J, Yin YQ, Sun LJ, Budhraja A, Hitron AJ, Ding SZ. Anti-apoptotic proteins and catalase-dependent apoptosis resistance in nickel chloride-transformed human lung epithelial cells. International journal of oncology. 2013;43:936–946. doi: 10.3892/ijo.2013.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.