Abstract
Background
Testicular cancer (TC) is one of the most curable cancers. Given survival rates of close to 100% with appropriate therapy, ensuring proper treatment is essential. We reviewed and summarized the literature on the association of socioeconomic position (SEP) along the cancer control spectrum from risk factors to survivorship.
Methods
We searched PubMed from 1966 to 2011 using the following terms: testicular cancer, testicular neoplasm, poverty, and socioeconomic factors, retrieving 119 papers. After excluding papers for the non-English (10) language and non-relevance (46), we reviewed 63 papers. We abstracted information on socioeconomic position (SEP), including occupation, education, income, and combinations of the 3. Five areas were examined: risk factors, diagnosis, treatment, survival, and survivorship.
Results
Most studies examined area-based measures, not individual measures of SEP. The majority of studies found an increased risk of developing TC with high SEP though recent papers have indicated increased risk in low-income populations. Regarding diagnosis, recent papers have indicated that lower levels of education and SEP are risk factors for later-stage TC diagnosis and hence higher TC mortality. For treatment, 1 study that examined the use of radiation therapy (RT) in stage I seminoma reported that living in a county with lower educational attainment led to lower use of RT. For survival (mortality), several studies found that men living in lower SEP geographic areas experience lower survival and higher mortality.
Conclusion
The strongest evidence for SEP impact on testicular germ cell tumor (TGCT) was found for the risk of developing cancer as well as survival. The association of SEP with TGCT risk appears to have changed over the last decade. Given the highly curable nature of TGCT, more research is needed to understand how SEP impacts diagnosis and treatment for TGCT and to design interventions to address disparities in TGCT outcomes and SEP. Published by Elsevier Inc.
Keywords: Testicular cancer, Socioeconomic status, Socioeconomic position, Occupation, Income, Education
Introduction
Testicular germ cell tumors (TGCT), broadly classified as seminomas and nonseminomas, are the most common cancer in men between 20 and 39 years old and represent the leading cause of cancer-related morbidity and mortality in this age group [1]. TGCTs represent one-third of cancers among boys and young men between 15 and 29 years [2]. In 2007, more than 7,700 men were diagnosed and more 350 men died of TC in United States [3]. Incidence varies by country with the highest incidence rates in European countries [4]. With the introduction of platinum-based therapy in the 1970s, TGCT represents one of the most curable cancers with approximately 96% of men surviving 5 years or more today vs. 83% in the 1970s [5].
TGCT staging is based upon extent of disease at diagnosis, which includes lymph node status and whether distant metastases are present. After orchiectomy, careful work-up is done to determine clinical stage, histology of the tumor, and the need for treatment after surgery. Men with advanced disease are categorized into risk categories (good, intermediate, and poor) that represent site of metastases, histology, and the level of tumor markers (β HCG and & α feto-protein) produced by the cancer [6]. Advanced seminomas are classified as good and intermediate risk (i.e., good/intermediate survival) and nonseminoma are classified as good, intermediate, and poor. Winter and Albers recently provided an excellent summary of treatment options in Nature Reviews: Endocrinology [7]. Treatment is based on stage at diagnosis as well as risk classification. Treatment interventions includes surgery, radiation therapy, chemotherapy, and close observation for men with stage I seminoma and nonseminoma. All men with advanced stage TGCT regardless of histology should receive chemotherapy. The extent of treatment is determined by risk classification [7]. For men with nonseminoma, those who do not respond to chemotherapy, salvage therapies such as stem cell transplant need to be considered.
Relationship of socioeconomic position and cancer outcomes
For this review, we use the term “socioeconomic position” (SEP) as an encompassing term, inclusive of socioeconomic status, which indicates both resource-based and prestige-based indicators as defined by Krieger et al. and Galobardes et al. [8–10] According to Krieger et al., SEP indicators include “income, wealth, and educational credentials… as well as access to and consumption of goods, services, and knowledge, as linked to their occupational prestige, income, and education level” [10]. SEP, especially the lack of social prestige or resources, is associated with cancer outcomes for many types of cancer [11]. Many studies have examined race as a proxy for SEP in the United States [12–14]. As we were concerned about direct measures of SEP and previous papers have reported on racial differences in TGCT [2,15–17], this study does not include papers that focused only on racial disparities in TGCT risk, diagnosis, treatment, and survival. We concentrate our review on the association of SEP with risk factors for developing TGCT, as well as SEP differences in diagnosis, treatment, survival, and survivorship in TGCT patients.
Literature review
We searched PubMed from 1966 to 2011 using the following terms: TC, testicular neoplasm, poverty, socioeconomic position, and socioeconomic factors, retrieving 119 papers. We initially excluded papers that were not written in English (10) and papers that were not relevant (46). We fully reviewed 63 papers and found that 41 papers [18–59] were relevant. Papers were further excluded for the following reasons: there was no SEP information reported in the paper (10), they were letters or commentaries (3), or they were older reviews (9). The reference lists of the remaining 41 papers were reviewed and 5 [60–64] additional papers were identified. We abstracted information on SEP, including occupation, education, income, and combinations of the 3. Five areas were examined: risk factors, diagnosis, treatment, survival, and survivorship.
SEP and risk for developing testicular cancer
Cryptorchidism, familial and genetic factors, height, and early life/prenatal exposures have been suggested as risk factors for TC [65,66]. However, there is limited evidence of socioeconomic differences in these risk factors that may explain differences in incidence and survival from TC. While 1 study in the United States reported paternal SEP to be associated with a higher risk of cryptorchidism, a study in Nordic countries did not show SEP to be associated with cryptorchidism [67,68]. Familial and genetic factors play a role in the development of some TCs. However, only a limited number of specific susceptibility genes for TC have been identified [69,70]. Exposures early in life, including the prenatal period, have also been hypothesized to contribute to increasing TC risk [65,71]. However, literature examining the effect of these factors on population differences in TC incidence is limited.
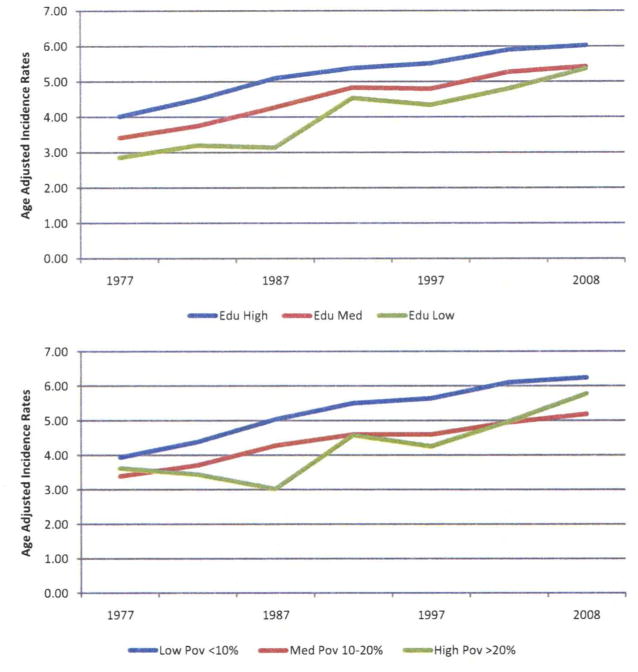
Numerous studies report a higher risk of TC in men of high social class compared with men of lower social class [22,25,53,54,56,72,73]. However, more recent reports suggest that the association has decreased over time [55] and that the risk appears to be reversed with a higher risk of TC in men with lower SEP [52,55]. We also found that the change SEP differences have narrowed using testicular cancer incidence data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program (Fig. 1). During 1973–2008, the age-adjusted TC incidence rate for persons living in high poverty areas was lower than the rate for those in low poverty areas, but increased at a faster rate. In 2008, the cancer incidence rate for persons living in high poverty areas (5.8/100,000) is approaching the rate for persons living in low poverty areas (6.2/100,000) in 2008.
Fig. 1.
Trends in testicular cancer incidence by county socioeconomic measures*, Surveillance, Epidemiology, and End Results (SEER) (9 registries), data, 1975–2008. Rates were calculated as 5-year moving averages and were age-adjusted to the 2000 U.S. standard population. Data were released in April 2011 and based on the November 2010 submission (http://www.seer.cancer.gov/resources/). *Educational attainment: Low is defined as ≥25% of men without a high school diploma. Medium is defined as 15%–24.9% of men without a high school diploma. High is defined as <15% of men without a high school education. Poverty: High is defined as ≥20% of individuals below the poverty level. Medium is defined as 10%–20% of individuals below the poverty level. Low is defined as <10% of individuals below the poverty level. (Color version of figure is available online).
Contrary to reports suggesting associations between TC incidence and SEP, others have found no evidence that SEP is associated with the risk for TC. Prener et al. found no association between socioeconomic class and risk of TC [51]. Similarly, the United Kingdom Cancer Study Group found no association between social class and TC risk [50]. Marsa et al. found no association between education, disposable income, and TC risk [49]. Parental SEP has a variable association with developing TC. Children of women with high SEP have been shown to have a higher risk of nonseminomas [54]. Paternal SEP has not been associated with an increased risk of TC [48,59].
While the literature does not consistently report an association between specific occupations and TC, numerous studies have examined this topic [21,47,74–76]. Occupation is defined in many ways, making comparisons difficult. Men with manual occupations and lower education have been found to have a higher incidence of TC [22,46]. Various studies have shown an increased risk of TC with numerous occupations, including metal workers, agricultural workers, and equipment technicians [21,42–45,77]. One study that examined chemical exposures reported an increased risk with fertilizers, phenols, and fumes [58]. Contrary to these studies, others have found no association between occupational exposure and TC risk [24,41]. Taken together, SEP in general, and occupation and education of parents, and at the individual level have shown variable associations with developing TC.
SEP and stage at diagnosis
Few studies have examined SEP and stage at diagnosis among TC patients, and it is conceivable that patients with lower SEP and limited access to care would have delays in diagnosis [40,60]. Late stage diagnosis of TGCT, particularly nonseminomatous TC, has been shown to be a risk factor for increased TGCT-specific mortality [40,60]. We were able to find 2 studies that specifically address the association of stage at diagnosis of TC and SEP. Dieckmann et al. found that lower educational levels were related to delays in diagnosis for nonseminomatous TGCT in 180 German men [60]. In contrast, Toklu and colleagues reported on 140 Turkish men seen in their clinic from 1994–1995 and found that stage of diagnosis did not statistically differ by income level or between college graduates versus non-college graduates [40]. Recent SEER data (Table 1) showed that distant stage TC diagnoses are higher for persons living in areas of high-poverty >20% county poverty) and low educational attainment (>25% of men have not graduated from high school).
Table 1.
Testicular germ cell tumor stage at diagnosis (2000–2008) and 5-year relative survival rates (2000–2003) by socioeconomic position, surveillance, epidemiology, and end results (SEER) (17 registries) data
| County-level poverty*
|
County-level education attainment**
|
|||||||
|---|---|---|---|---|---|---|---|---|
| High | Medium | Low | P value | Low | Medium | High | P value | |
| SEER summary stage at diagnosis | <0.01 | <0.01 | ||||||
| Localized | 68.0% | 69.8% | 73.0% | 68.0% | 71.2% | 73.4% | ||
| Regional | 17.3% | 17.9% | 17.5% | 18.2% | 17.6% | 17.5% | ||
| Distant | 14.7% | 12.2% | 9.5% | 13.8% | 11.2% | 9.1% | ||
| 5-year relative survival by Age | <0.01 | <0.01 | ||||||
| Age < 40 | 93.5% | 95.0% | 96.9% | 93.9% | 95.5% | 97.6% | ||
| Age ≥ 40 | 87.6% | 94.1% | 96.9% | 91.9% | 94.9% | 97.6% | ||
Data were released in April 2011 and based on the November 2010 submission (http://www.seer.cancer.gov/resources/).
Poverty: High is defined as ≥20% individuals below the poverty level. Medium is defined as 10%–20% individuals below the poverty level. Low is defined as <10% individuals below the poverty level.
Education attainment: Low is defined as ≥25% of men without a high school diploma. Medium is defined as 15%–24.9% of men without a high school diploma. High is defined as <15% of men without a high school diploma.
SEP and treatment
Treatment for TGCT is multimodal with surgery, radiation therapy, and chemotherapy [7]. All of these treatments have varying side effect profiles, including infertility, chronic medical problems such as cardiovascular disease, neurotoxicity, nephrotoxicity, pulmonary toxicity from treatments, and second cancers and psychological problems [78]. Given the excellent survival among TGCT patients, treatment strategies continued to be explored that will reduce the toxicity of treatment while preserving long-term survival [79]. We did not find any studies of long-term side effects of TC treatment and SEP.
We found only 1 paper examining the association between treatment and county level SEP. This paper focused on adjuvant radiation therapy (ART) for stage I seminoma and county-level SEP [39]. Hoffman et al. reported that men living in counties with higher educational levels were more likely to receive ART after surgery. These findings are difficult to interpret as the treatment recommendations for stage I seminoma have evolved to the point where close follow-up after surgery is an acceptable alternative to ART [7]. We found no studies of TGCT treatment and individual-level SEP.
SEP and survival
Although cisplatin was available starting in 1977, Fossa et al. note that it was not widely used until the mid-1980s [38]. Seven studies were reviewed on survival and SEP [18,37,38,49,62–64]. There were 2 distinct time survival periods based upon the availability of effective chemotherapy treatment with cisplatin. Davies analyzed a cohort of men diagnosed before the effective chemotherapy in 1975 and reported higher mortality in higher SEP populations [20]. Six studies examined SEP and survival in TC patients in the cisplatinera (after 1977) [18,37,49,62–64]. Sun et al. reviewed 22,553 TGCT cases in SEER databases from 1998–2006 and used a composite, area-based SEP score based upon median family income, percentage of individuals living below the poverty line, and percentage of individuals without a high school diploma. “Low” and “High” SEP categories were created using median score as the cut point. In multivariate analysis, individuals living in areas with “Low” SEP scores (vs. “High” SEP) had significantly higher cancer-specific mortality and overall mortality HR = 1.41 (P = 0.002) and 1.28 (P < 0.001), respectively [64]. Davies et al. also reported higher mortality for men with professional jobs [20]. Hussain et al. reported on 1,094 cases of TC in Swedish men diagnosed from 1990–2006. Men with 12–13 years of education had significantly lower HR of TGCT-specific mortality versus those with <9 years HR = 0.07 (95% CI 0.01–0.55). Yet this did not hold true for those with more than 13 years of education HR = 0.30 (95% CI 0.06–1.47) [62]. Nur and colleagues studied 18,605 men in England and Wales from 1986 to 1999 and found decreased survival in the most economically-deprived TGCT group compared with the most affluent group [37]. Power et al. reported similar results [18]. Conversely, Marsa et al. studied 1,770 Danish men from 1994–2003 and found no difference in 5-year survival rate with regard to disposable income, education, or employment status [49]. Similarly, Mackillop et al. did not find any significant TGCT survival differences by income level. [63]Overall, studies published to date have found that TGCT mortality and survival has markedly improved with cisplatin-based chemotherapy but a gap remains between survival in high versus low-SEP populations. Current SEER data confirm these findings (Table 1). Men with TC diagnosed in counties with high poverty and low educational attainment have lower survival compared with their counterparts. These effects were especially strong for men over 40 years similar to reports from Fossa et al. [38].
SEP and survivorship
Studies have shown that TC survivors experience a variety of negative psychosocial outcomes following diagnosis and treatment of TGCT [57,80–82]. The issue of quality of life is particularly important among TC survivors as they are often diagnosed at a younger age. Over 80% of boys and men with TGCT are between 14 and 44 [2]. This makes infertility an issue for TC survivors [78,83], but we found no studies that described the association of infertility and SEP. Distress about loss of fertility as a psychosocial outcome is addressed by 1 of the articles in our review. For this review, we identified 8 articles that examined how psychosocial outcomes are influenced by SEP in TC survivors [29–36]. The relationship between psychological outcomes and SEP was not consistent in TC survivors. While Skaali et al. [33] and Tuinman et al. [29] did not find that SEP was associated with TC-related distress and mental health, respectively, in survivors, others have reported an association between lower SEP and poor psychological outcomes. Fleer et al. investigated cancer-related stress symptoms in The Netherlands [36]. The study showed that survivors with less education and without paid employment were more likely to experience cancer-related distress and higher levels of avoidance coping. Rutskij et al. also found that lower levels of SEP measured as less than 12 years of schooling was associated with an avoidance coping style in a univariate model. Multivariate analysis using these data showed a small, significant association between not being in paid work and approach coping; there was no significant association between education and coping strategy [34]. Using data from the same study of Norwegian survivors, Skaali et al. found that unemployment, men with 12 or fewer years of education, and economic problems were significantly associated with fear of recurrence [32].
Rieker et al. reported that men with less than a college education, lower income, and lower occupational status experienced higher rates of distress about loss of fertility. Lower educational level and occupational status were also associated with higher sexual performance distress [35]. In a study of cancer survivors of many cancer types, Taskila et al. reported that male cancer survivors with less than a college degree and lower occupational status had a greater need for support in the workplace and from occupational health personnel [31]. The same authors found that male cancer survivors with a university degree were 10 times less likely to report impairment of ability to work than male cancer survivors who had the lowest level of education [30].
Conclusion
Testicular cancer represents a modern medical triumph with more than 95% of men living 10 years or more [78]. This has been made possible through advances in diagnosis and treatment. We found evidence that socioeconomic position is associated with TC risk, diagnosis, treatment, survival, and psychological outcomes among men who are cured. Given the younger ages of adolescents and men affected by TC, long-term physical and psychological outcomes must be addressed as well. As with other cancers, there is evidence that TC outcomes are worse in older persons [78] and minorities, especially African Americans [64]. Access to care and receipt of state of the art treatment should be given to ensure equitable outcomes.
We have identified SEP gaps along the cancer spectrum from risk of developing TC to psychological outcomes. Why has there been a shift in the occurrence of TC in lower SEP populations? Over time, researchers have noted that the incidence gap between higher and lower SEP groups has narrowed, which is illustrated by SEER data in this paper. How do these changes relate to the etiology of TC? At the point of diagnosis, men of lower SEP are diagnosed at a later stage of cancer and have a lower survival. What role does increased awareness among providers and patients play in closing these gaps? Very little data exist about treatment differences by SEP and outcomes. More research is needed elucidate the determinants associated with treatment and whether these differ by SEP. We noted an almost 10% difference in 5 year survival for men over 40 years diagnosed with TC living in counties with >20% poverty rates compared with those living in counties with <10% poverty.
Men of lower SEP may have fewer resources at their disposal to deal with the diagnosis, treatment, and long-term physical effects of their treatment. Though cured, men will need to be enrolled in a surveillance program to prevent new problems and diagnose complications early. For example, men surviving TC have an observed to expected ratio of heart attack of 7.1 (95%CI: 1.9–18.3) compared with controls [78]. Surveillance programs are being put into place and more research is needed to provide the evidence-base to provide the best care [78].
Opportunities for research in TC spans the spectrum from elucidating risk to designing evidence-based survivor follow-up models. Future research should both verify the findings presented in previous studies and provide an evidence basis for interventions thought to be helpful in addressing the disparities noted in this review.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Richardson LC, Wingo PA, Zack MM, et al. Health-related quality of life in cancer survivors between ages 20 and 64 years—population-based estimates from the behavioral risk factor surveillance system. Cancer. 2008;112:1380–9. doi: 10.1002/cncr.23291. [DOI] [PubMed] [Google Scholar]
- 2.Townsend JS, Richardson LC, German RR. Incidence of testicular cancer in the United States, 1999–2004. Am J Mens Health. 2010;4:353–60. doi: 10.1177/1557988309356101. [DOI] [PubMed] [Google Scholar]
- 3.United States Cancer Statistics Working Group. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Vol. 2011 Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. [Google Scholar]
- 4.Chia VM, Quraishi SM, Devesa SS, et al. International Trends in the Incidence of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev. 2010;19:1151–9. doi: 10.1158/1055-9965.EPI-10-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institutes of Health, National Cancer Institute Cancer of the Testis. NCI. Bethesda, MD: National Institutes of Health; 2007. Trends in SEER Incidence and U.S. Mortality Using the Joinpoint Regression Program. [Google Scholar]
- 6.Mead GM, Stenning SP, Cook P, et al. International germ cell consensus classification: A prognostic factor-erased staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 7.Winter C, Albers P. Testicular germ cell tumors: Pathogenesis, diagnosis, and treatment. Nat Rev Endocrinol. 2011;7:43–53. doi: 10.1038/nrendo.2010.196. [DOI] [PubMed] [Google Scholar]
- 8.Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 1) J Epidemiol Community Health. 2005;60:7–12. doi: 10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 2) J Epidemiol Community Health. 2006;60:95–101. doi: 10.1136/jech.2004.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: Concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 11.Singh GK, Miller BA, Hankey BF, et al. National Cancer Institute. Bethesda, MD: NIH; 2003. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. vol. NCI Cancer Surveillance Monograph Series, Number 4. Publication No. 03–5417. [Google Scholar]
- 12.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research—one size does not fit all. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 13.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: What the patterns tell us. Am J Public Health. 2010;100:S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99:1013–23. [PMC free article] [PubMed] [Google Scholar]
- 15.Biggs ML, Schwartz SM. Differences in testis cancer survival by race and ethnicity: A population-based study, 1973–1999 (United States) Cancer Causes Control. 2004;15:437–44. doi: 10.1023/B:CACO.0000036443.95995.40. [DOI] [PubMed] [Google Scholar]
- 16.Gajendran VK, Nguyen M, Ellison LM. Testicular cancer patterns in African-American men. Urology. 2005;66:602–5. doi: 10.1016/j.urology.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland FD, Hunt WC, Key CR. Trends in the survival of American Indian, Hispanic, and non-Hispanic white cancer patients in New Mexico and Arizona, 1969–1994. Cancer. 1998;82:1769–83. doi: 10.1002/(sici)1097-0142(19980501)82:9<1784::aid-cncr26>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Power DA, Brown RSD, Brock CS, et al. Trends in testicular carcinoma in England and Wales, 1971–1999. BJU Int. 2001;87:361–5. doi: 10.1046/j.1464-410x.2001.00078.x. [DOI] [PubMed] [Google Scholar]
- 19.Morrison AS. Some social and medical characteristics of Army men with testicular cancer. Am J Epidemiol. 1976;104:511–6. doi: 10.1093/oxfordjournals.aje.a112323. [DOI] [PubMed] [Google Scholar]
- 20.Davies JM. Testicular cancer in England and Wales—some epidemiological aspects. Lancet. 1981;1:928–32. doi: 10.1016/s0140-6736(81)91625-1. [DOI] [PubMed] [Google Scholar]
- 21.Mills PK, Newell GR, Johnson DE. Testicular cancer associated with employment in agriculture and oil and natural gas extraction. Lancet. 1984;1:207–10. doi: 10.1016/s0140-6736(84)92125-1. [DOI] [PubMed] [Google Scholar]
- 22.Nethersell ABW, Sikora K. Testicular cancer and social class in East Anglia. Br J Cancer. 1984;50:537–40. doi: 10.1038/bjc.1984.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decarli A, La Vecchia C. Environmental factors and cancer mortality in Italy: Correlational exercise. Oncology. 1986;43:116–26. doi: 10.1159/000226347. [DOI] [PubMed] [Google Scholar]
- 24.Sewell CM, Castle SP, Hull HF. Testicular cancer and employment in agriculture, and oil and natural gas extraction: Testicular Cancer and employmnet in agriculture and oil and natural gas extraction. Lancet. 1984;1:553. doi: 10.1016/s0140-6736(86)90902-5. [DOI] [PubMed] [Google Scholar]
- 25.Rimpela AH, Pukkala EI. Cancers of affluence positive social class gradient and rising incidence trend in some cancer forms. Soc Sci Med. 1987;24:601–6. doi: 10.1016/0277-9536(87)90064-5. [DOI] [PubMed] [Google Scholar]
- 26.Garland FC, Gorham ED, Garland CF, et al. Testicular cancer in US Navy personnel. Am J Epidemiol. 1988;127:411–14. doi: 10.1093/oxfordjournals.aje.a114815. [DOI] [PubMed] [Google Scholar]
- 27.Mller H, Skakkebaek NE. Risks of testicular cancer and cryptorchidism in relation to socioeconomic status and related factors: Case-control studies in Denmark. Int J Cancer. 1996;66:287–93. doi: 10.1002/(SICI)1097-0215(19960503)66:3<287::AID-IJC2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Mickisch GH. Prognostic parameters for the management of advanced testis tumors. Cur Opin Urol. 2000;10:465–71. doi: 10.1097/00042307-200009000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Tuinman MA, Hoekstra HJ, Fleer J, et al. Self-esteem, social support, and mental health in survivors of testicular cancer: A comparison based on relationship status. Urol Oncol. 2006;24:279–86. doi: 10.1016/j.urolonc.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Taskila T, Martikainen R, Hietanen P, et al. Comparative study of work ability between cancer survivors and their referents. Eur J Cancer. 2007;43:914–20. doi: 10.1016/j.ejca.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Taskila T, Lindbohm ML, Martikainen R, et al. Cancer survivors’ received and needed social support from their work place and the occupational health services. Support Care Cancer. 2006;14:427–35. doi: 10.1007/s00520-005-0005-6. [DOI] [PubMed] [Google Scholar]
- 32.Skaali T, Fossa SD, Bremnes R, et al. Fear of recurrence in long-term testicular cancer survivors. Psycho-oncology. 2009;18:580–8. doi: 10.1002/pon.1437. [DOI] [PubMed] [Google Scholar]
- 33.Skaali T, Fossa SD, Andersson S, et al. Is psychological distress in men recently diagnosed with testicular cancer associated with their neuropsychological test performance? Psychooncology. 2011;20:369–77. doi: 10.1002/pon.1737. [DOI] [PubMed] [Google Scholar]
- 34.Rutskij R, Gaarden T, Bremnes R, et al. A study of coping in long-term testicular cancer survivors. Psychol Health Med. 2010;15:146–58. doi: 10.1080/13548501003623955. [DOI] [PubMed] [Google Scholar]
- 35.Rieker PP, Fitzgerald EM, Kalish LA, et al. Psychosocial factors, curative therapies, and behavioral outcomes—a comparison of testis cancer survivors and a control group of healthy men. Cancer. 1989;64:2399–407. doi: 10.1002/1097-0142(19891201)64:11<2399::aid-cncr2820641134>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Fleer J, Sleijfer D, Hoekstra H, et al. Objective and subjective predictors of cancer-related stress symptoms in testicular cancer survivors. Patient Educ Coun. 2006;64:142–50. doi: 10.1016/j.pec.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Nur U, Rachet B, Mitry E, et al. Survival from testicular cancer in England and Wales up to 2001. B JC. 2008;99:S80–2. doi: 10.1038/sj.bjc.6604597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fossa SD, Cvancarova M, Chen LL, et al. Adverse Prognostic Factors for testicular cancer-specific survival: A population-based study of 27,948 patients. J Clin Oncol. 2011;29:963–70. doi: 10.1200/JCO.2010.32.3204. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman KE, Chen MH, Punglia RS, et al. Influence of year of diagnosis, patient age, and sociodemographic status on recommending adjuvant radiation treatment for stage I testicular seminoma. J Clin Oncol. 2008;26:3937–42. doi: 10.1200/JCO.2008.16.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toklu C, Ozen H, Sahin A, et al. Factors involved in diagnostic delay of testicular cancer. Int Urol Neph. 1999;31:383–8. doi: 10.1023/a:1007134421608. [DOI] [PubMed] [Google Scholar]
- 41.Walschaerts M, Muller A, Auger J, et al. Environmental, occupational and familial risks for testicular cancer: A hospital-based case-control study. Intl J Androl. 2007;30:222–9. doi: 10.1111/j.1365-2605.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 42.McDuffie HH, Quail J, Ghosh S, et al. Host factors, occupation, and testicular cancer in Saskatchewan, Canada: 1979–2002. J Agric Saf Health. 2007;13:247–58. doi: 10.13031/2013.23350. [DOI] [PubMed] [Google Scholar]
- 43.Kelleher C, Newell J, MacDonagh-White C, et al. Incidence and occupational pattern of leukemias, lymphomas, and testicular tumors in Western Ireland over an 11-year period. J Epidemiol Community Health. 1998;52:651–6. doi: 10.1136/jech.52.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollan M, Gustavsson P, Cano MI. Incidence of testicular cancer and occupation among Swedish men gainfully employed in 1970. Ann Epidemiol. 2001;11:554–62. doi: 10.1016/s1047-2797(01)00234-4. [DOI] [PubMed] [Google Scholar]
- 45.Guo J, Pukkala E, Kyyronen P, et al. testicular cancer, occupation, and exposure to chemical agents among Finnish men in 1971–1995. Cancer Causes Control. 2005;16:97–103. doi: 10.1007/s10552-004-2236-0. [DOI] [PubMed] [Google Scholar]
- 46.Dusek L, Abrahamova J, Lakomy R, et al. Multivariate analysis of risk factors for testicular cancer: A hospital-based case-control study in the Czech Republic. Neoplasma. 2008;55:356–68. [PubMed] [Google Scholar]
- 47.Van den Eeden SK, Weiss NS, Strader CH, et al. Occupation and the occurrence of testicular cancer. Am J Ind Med. 1991;19:327–37. doi: 10.1002/ajim.4700190307. [DOI] [PubMed] [Google Scholar]
- 48.Richiardi L, Akre O, Lambe M, et al. Birth order, sibship size, and risk for germ-cell testicular cancer. Epidemiology. 2004;15:323–9. doi: 10.1097/01.ede.0000120043.45185.7e. [DOI] [PubMed] [Google Scholar]
- 49.Marsa K, Johnsen NF, Bidstrup PE, et al. Social inequality and incidence of and survival from male genital cancer in a population-based study in Denmark, 1994 –2003. Eur J Cancer. 2008;44:2018–29. doi: 10.1016/j.ejca.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Social, behavioral and medical factors in the etiology of testicular cancer: Results from the UK study. UK Testicular Cancer Study Group. Br J Cancer. 1994;70:513–20. doi: 10.1038/bjc.1994.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prener A, Engholm G, Hsieh CC, et al. Birth order and risk of testicular cancer. Cancer Causes Control. 1992;3:265–72. doi: 10.1007/BF00124260. [DOI] [PubMed] [Google Scholar]
- 52.Sarfati D, Shaw C, Blakely T, et al. Ethnic and socioeconomic trends in testicular cancer incidence in New Zealand. Int J Cancer. 2011;128:1683–91. doi: 10.1002/ijc.25486. [DOI] [PubMed] [Google Scholar]
- 53.Swerdlow AJ, Douglas AJ, Huttly SR, et al. Cancer of the testis, socioeconomic status, and occupation. Br J Ind Med. 1991;48:670–4. doi: 10.1136/oem.48.10.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akre O, Ekbom A, Hsieh CC, et al. Testicular nonseminoma and seminoma in relation to perinatal characteristics. J Natl Cancer Inst. 1996;88:883–9. doi: 10.1093/jnci/88.13.883. [DOI] [PubMed] [Google Scholar]
- 55.Pukkula E, Weiderpass E. Socioeconomic differences in incidence rates of cancers of the male genital organs in Finland, 1971–1995. Int J Cancer. 2002;102:643–8. doi: 10.1002/ijc.10749. [DOI] [PubMed] [Google Scholar]
- 56.Harding M, Hole D, Gillis C. The epidemiology of non-seminomatous germ cell tumors in the west of Scotland 1975–1989. Br J Cancer. 1995;72:1559–62. doi: 10.1038/bjc.1995.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bloom JR, Fobair P, Gritz E, et al. Psychosocial outcomes of cancer a comparative analysis of Hodgkin’s disease and testicular cancer. J Clin Oncol. 1993;11:979–88. doi: 10.1200/JCO.1993.11.5.979. [DOI] [PubMed] [Google Scholar]
- 58.Haughey BP, Graham S, Brasure J, et al. The epidemiology of testicular cancer in upstate New York. Am J Epidemiol. 1989;130:25–36. doi: 10.1093/oxfordjournals.aje.a115319. [DOI] [PubMed] [Google Scholar]
- 59.Swerdlow AJ, Huttly SR, Smith PG. Prenatal and familial associations of testicular cancer. Br J Cancer. 1987;55:571–7. doi: 10.1038/bjc.1987.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dieckmann KP, Becker T, Bauer HW. Testicular tumors presentation and role of diagnostic delay. Urol Int. 1987;42:241–7. doi: 10.1159/000281948. [DOI] [PubMed] [Google Scholar]
- 61.Moul JW. Timely diagnosis of testicular cancer. Urol Clin North Am. 2001;34:109–17. doi: 10.1016/j.ucl.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Hussain SK, Lenner P, Sundquist J, et al. Influence of education level on cancer survival in Sweden. Ann Oncol. 2008;19:156–62. doi: 10.1093/annonc/mdm413. [DOI] [PubMed] [Google Scholar]
- 63.Mackillop WJ, Zhang Salomons J, Groome PA, et al. Socioeconomic status and cancer survival in Ontario. J Clin Oncol. 1997;15:1680–9. doi: 10.1200/JCO.1997.15.4.1680. [DOI] [PubMed] [Google Scholar]
- 64.Sun M, Abdollah F, Liberman D, et al. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: a survival analysis. Cancer. 2011;117:4277–85. doi: 10.1002/cncr.25969. [DOI] [PubMed] [Google Scholar]
- 65.Garner MJ, Turner MC, Ghadirian P, et al. Epidemiology of testicular cancer: An overview. Int J Cancer. 2005;116:331–9. doi: 10.1002/ijc.21032. [DOI] [PubMed] [Google Scholar]
- 66.Litwin MS, Saigal CS, editors. Urologic Diseases in America. Bethesda, MD: National Institutes of Health; 2007. [Google Scholar]
- 67.McGlynn KA, Graubard BI, Klebanoff MA, et al. Risk factors for cryptorchism among populations at differing risks of testicular cancer. Int J Epidemiol. 2006;35:787–95. doi: 10.1093/ije/dyl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Möller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among 5-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. J Clin Oncol. 2001;19:3173–81. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 69.Lutke-Holzik MF, Rapley EA, Hoekstra HJ, et al. Genetic predisposition to testicular germ-cell tumors. Lancet Oncol. 2004;5:363–71. doi: 10.1016/S1470-2045(04)01493-7. [DOI] [PubMed] [Google Scholar]
- 70.Horvath A, Korde L, Greene MH, et al. Functional phosphodiesterase 11A mutations may modify the risk of familial and bilateral testicular germ cell tumors. Cancer Res. 2009;69:5301–6. doi: 10.1158/0008-5472.CAN-09-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Myrup C, Westergaard T, Schnack T, et al. Testicular cancer risk in first- and second-generation immigrants to Denmark. J Natl Cancer Inst. 2008;100:41–7. doi: 10.1093/jnci/djm276. [DOI] [PubMed] [Google Scholar]
- 72.Harding M, Cole D, Gillis C. The epidemiology of non-seminomatous germ cell tumors in the west of Scotland 1975–1989. Br J Cancer. 1995;72:1559–62. doi: 10.1038/bjc.1995.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrison AS. Some social and medical characteristics of Army men with testicular cancer. Am J Epid. 1976;104:511–16. doi: 10.1093/oxfordjournals.aje.a112323. [DOI] [PubMed] [Google Scholar]
- 74.Dubrow R, Wegman DH. Setting priorities for occupational cancer research and control synthesis of the results of occupational disease surveillance studies. J Natl Cancer Inst. 1983;71:1123–42. [PubMed] [Google Scholar]
- 75.Marshall EG, Melius JM, London MA, et al. Investigation of a testicular cancer cluster using a case-control approach. Int J Epidemiol. 1990;19:269–73. doi: 10.1093/ije/19.2.269. [DOI] [PubMed] [Google Scholar]
- 76.McDowall M, Balarajan R. Testicular cancer and employment in agriculture. Lancet. 1984;323:510–11. doi: 10.1016/s0140-6736(84)92875-7. [DOI] [PubMed] [Google Scholar]
- 77.Garland FC, Gorham ED, Garland CF, et al. Testicular cancer in US Navy personnel. Am J Epid. 1988;127:411–4. doi: 10.1093/oxfordjournals.aje.a114815. [DOI] [PubMed] [Google Scholar]
- 78.Travis L, Beard C, Allan J, et al. Testicular cancer survivorship: Research strategies and recommendations. J Natl Cancer Inst. 2010;102:1114–30. doi: 10.1093/jnci/djq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brydy M, Foss S, Klepp O, et al. Paternity and testicular function among testicular cancer survivors treated with 2 to 4 cycles of cisplatin-based chemotherapy. Eur Urol. 2010;58:134–40. doi: 10.1016/j.eururo.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 80.Arai Y, Kawakita M, Hida S, et al. Psychosocial aspects in long-term survivors of testicular cancer. J Urol. 1996;155:574–8. [PubMed] [Google Scholar]
- 81.Fossa SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Oncol. 2003;21:1249–54. doi: 10.1200/JCO.2003.08.163. [DOI] [PubMed] [Google Scholar]
- 82.Gotay CC, Muraoka MY. Quality of life in long-term survivors of adult-onset cancers. J Natl Cancer Inst. 1998;90:656–67. doi: 10.1093/jnci/90.9.656. [DOI] [PubMed] [Google Scholar]
- 83.Matos E, Škrbinc B, Zakotnik B. Fertility in patients treated for testicular cancer. J Cancer Surviv. 2010;4:274–8. doi: 10.1007/s11764-010-0135-9. [DOI] [PubMed] [Google Scholar]