Abstract
Years of searching and researching have finally yielded a few leads in the quest to identify molecules required for mechanosensory transduction in the mammalian inner ear. Studies of human and mouse genetics have raised the profile of several molecules that are critical for the function sensory hair cells. Follow up studies have begun to define the molecular function and biochemical interactions of several key proteins. These studies have exposed a sensory transduction apparatus that is more complex than originally envisioned and have reinvigorated the search for additional molecular components required for normal inner ear function.
Introduction
Sensory hair cells of the inner ear are highly specialized for the detection of the mechanical forces of sound and head movements. Their crowning achievement is an array of modified microvilli, known as stereocilia (Figure 1). Each hair cell includes 50–100 tightly clustered stereocilia that are polarized to detect nanometer-scale movement along a physiologically-defined axis of sensitivity. The axis of physiological sensitivity corresponds to an axis of morphological polarity as the stereocilia are arranged in a stair case pattern. Deflection of the bundle of stereocilia, collectively known as the hair bundle, toward the tallest stereocilia initiates an excitatory response, while deflection toward the shortest is inhibitory. The hair bundle is unresponsive to orthogonal deflections. Excitatory hair bundle deflections generate inward cation currents that tend to depolarize the hair cell and initiate graded receptor potentials which are transmitted at the hair cell – afferent synapse to neurons of the 8th cranial nerve. The molecules that mediate sensory transduction in inner ear hair cells have been the focus of intensive research over the past few years. Recent progress toward identification of hair cell transduction molecules will be reviewed herein.
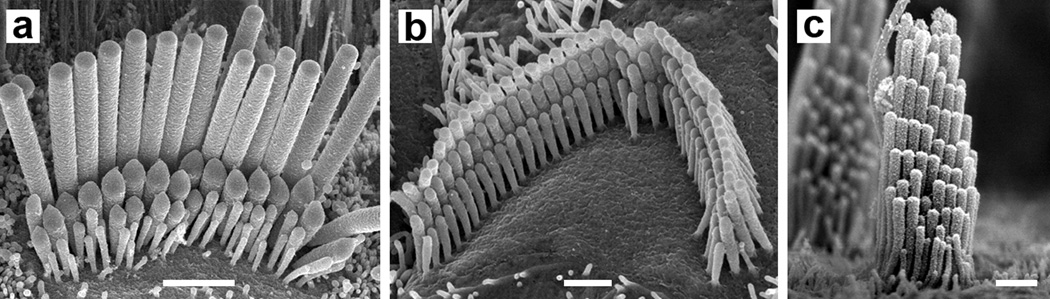
Figure 1.
Scanning electron micrographs of hair bundles from the rodent inner ear. (a) Hair bundle from a P15 rat inner hair cell. Scale bar = 2 µm. Reprinted from Beurg et al. (2006). (b) Hair bundle from a mouse hair outer cell. Scale bar = 2 µm. Reprinted with permission from David Furness. (c) Hair bundle from a mouse vestibular hair cell. Scale bar = 2 µm. Reprinted from Holt et al. (2002).
Models of sensory transduction
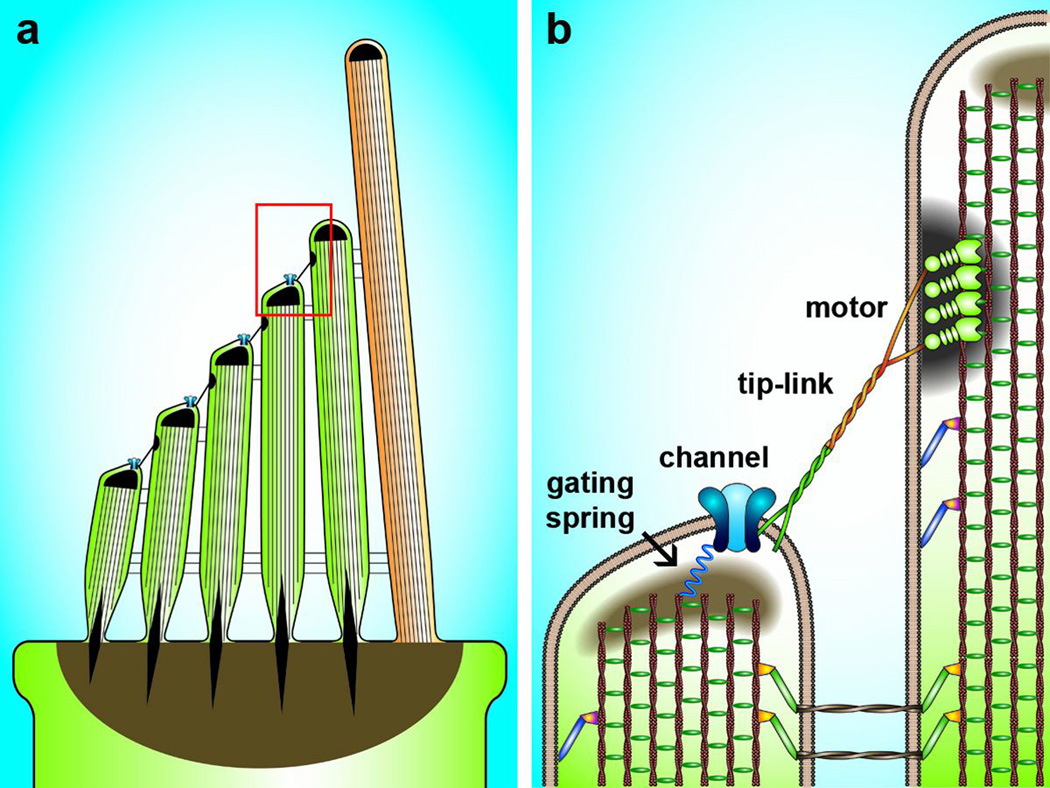
Since the first biophysical characterization of hair cell transduction, ~35 years ago (Corey and Hudspeth, 1979), inner ear biologists have sought to identify the molecular and genetic basis of this fundamental mechanosensory process. The biophysical properties of hair cell transduction lead to the generation of a simple model for how the process might occur (Corey and Hudspeth 1983). The model has been depicted in schematized cartoons that have been published multiple times since their first appearance in the 1980s and includes several basic elements: a gating spring, an extracellular tip-link, a motor that provides feedback, and at its core, a mechanosensitive ion channel (Figure 2). The model has been tweaked along the way (Howard and Hudspeth, 1988; Assad and Corey, 1992; Shepherd and Corey, 1994; Kachar et al., 2000; Beurg et al., 2009), but has remained largely intact. Based on this simple model investigators have focused on molecular identification of each of these biophysically defined entities. Unfortunately, identification of mechanosensory molecules in hair cells has proven challenging for a number of reasons: 1) There are only a few thousand hair cells per cochlea in the mouse inner ear, as opposed to a few million photoreceptors in each retina. 2) Hair cells do not replicate, so their numbers cannot be easily expand in vitro. 3) Each hair cell contains very few sensory transduction complexes: 50–100/cell as opposed to a few million/photoreceptor cell. These limitations have precluded classical biochemical approaches for identification of hair cell transduction molecules. However, several intriguing molecules have emerged recently based on genetic evidence. In a number of cases, genetic information from both human and mouse studies have converged to raise the profile of a few key elements, some of which may be components of elusive mechanosensory transduction complex. Yet, it is now becoming increasingly clear that the hair cell transduction apparatus is more complex than the simple biophysical models have indicated. Indeed, there may multiple molecular components that are required to form the fully functional mechanosensory complex. It also seems plausible that each biophysically-defined component may be comprised of multiple molecular components. For example, the biophysically-defined gating spring may be composed of the collective elasticity of several molecules in series within a molecular chain rather than a single protein as is often depicted in cartoons.
Figure 2.
Schematic diagrams of the mechanosensory hair bundle of the mammalian inner ear. (a) Hair bundles are comprised of 50–100 modified microvilli, known as stereocilia (pictured in green). They are arranged in a stair case pattern of increasing height. At the tall edge is a single true cilium, known as the kinocilium which is present in vestibular hair bundles and auditory bundles during development. Stereocilia are held together in a compact bundle by extracelluar linkages know as ankle links and horizontal top connectors. The bundle is anchored to the cuticular plate at the apical surface of the hair cell cell-body. Deflection of the hair bundle toward the kinocilium is excitatory. The region within the red box is enlarged at the right to show greater detail. (b) Stereocilia have cores of actin filaments crosslinked with Espin and Fimbrin. Several unconventional myosins, shuttle cargo up and down the stereocilia, function to maintain bundle lingages and hold the cell membrane in place around the actin core. Four key components of the biophysically-defined model for hair cell sensory transduction are shown here: the gating spring, the mechanosensitive channel, the tip-link and the adaptation motor. Modified from Géléoc and Holt (2014). The molecular composition of the hair cell sensory transduction apparatus is under investigation.
PCDH15 and CDH23 form tip-links
One success story thus far is the identification of tip-link molecules. There is now compelling evidence showing that tip-links are composed of a heterophilic interaction between cadherin-23 at the upper end of the tip-link and protocadherin-15 at the lower end. This model is now widely accepted by scientists in the field and is based on studies of human and mouse genetics (Ahmed et al., 2001; Alagramam et al., 2001; Di Palma et al., 2001), immunolocalization (Siemens et al., 2004; Söllner et al., 2004; Ahmed et al., 2006; Kazmierczak et al., 2007), physiological properties (Lelli et al., 2010; Alagramam et al., 2011; Geng et al., 2013) and molecular structures (Sotomayor et al., 2010; 2012), all of which support the PCDH15/CDH23 hypothesis. A contentious issue that remains is precisely which alternative splice forms of CDH23 and PCDH15 contribute to formation of tip-links (Ahmed et al., 2006; Webb et al., 2011), but recent evidence favors the CD2 splice form of PCDH15 (Pepermans et al., 2014).
The elusive mechanosensory transduction channel
Other molecular components of the hair cell transduction apparatus are poorly defined. At the core of the complex is a mechanosensory ion channel, for which the molecular composition remains to be clarified. Current strong candidates for the pore-forming subunits of the channel are proteins known as Transmembrane channel-like (TMC) 1 and 2 (Kawashima et al., 2011). Mutations in TMC1 cause deafness in humans and similar mutations in the murine homolog, Tmc1 cause deafness in mice (Kurima et al., 2002). Absence of TMC1 and TMC2 renders hair cells unresponsive to sensory stimulation, but reintroduction of the coding sequence for either gene restores sensory transduction (Kawashima et al., 2011). The biophysical properties of sensory transduction differ in hair cells that express wild-type TMC1 or TMC2 (Pan et al., 2013; Kim and Fettiplace 2013). The strongest evidence implicating a direct role for TMC1 in hair cell transduction is derived from mice with a point mutation in TMC1, known as Beethoven (Bth). The Bth mutation causes a methionine to lysine substitution at position 412 in mouse (Vreugde et al., 2002) and an identical mutation has been identified in the orthologous position in human TMC1 (Zhao et al., 2014). In mouse, the Bth mutation causes a decrease in single-channel conductance and calcium-selectivity and an increase in calcium-dependent block (Pan et al., 2013), all of which are core properties of an ion channel pore. Recently, two groups have shown compelling evidence for a biochemical interaction between TMC1, TMC2 and the intracellular domain of the tip-link protein PCDH15 (Maeda et al., 2014; Beurg et al., 2015). These observations are consistent with the hypothesis that TMC1 and TMC2 are pore-forming subunits of the transduction channel, but they do not provide irrefutable proof. Certainly TMC1 and TMC2 expression affects ion selectivity (Pan et al., 2013; Kim et al., 2013) and single-channel properties (Pan et al., 2013; Beurg et al., 2014), thus there is agreement that they are components of the channel complex but whether they function as subunits that form the channel pore or provide some other essential function is not yet clear. Several alternate possibilities (Figure 3) have been proposed. 1) The location of the Bth mutation (p.M412K) is in a putative extracellular loop between transmembrane domains 2 and 3. Therefore, it has been suggested that this loop may be part of vestibule that serves to funnel cations into the ion channel pore (Pan et al., 2013; Holt et al., 2014). Indeed, the mutation substitutes a neutral amino acid for a positively charged one, which may act to repel cations. 2) TMC1/2 may be essential components of a protein complex that helps to stabilize pore-forming subunits composed of other proteins. In this scenario the Bth point mutation must be a critical residue for stabilizing pore-forming subunits. 3) They may be linker proteins required to convey mechanical tension to the channel. Work aimed at distinguishing these possibilities is ongoing. For all of these scenarios, TMC1/2 must be either part of the pore or in close enough proximity to the pore to affect permeation properties and therefore must be components of the mechanosensory channel complex. It is also plausible that TMCs perform several functions: pore, vestibule and linker.
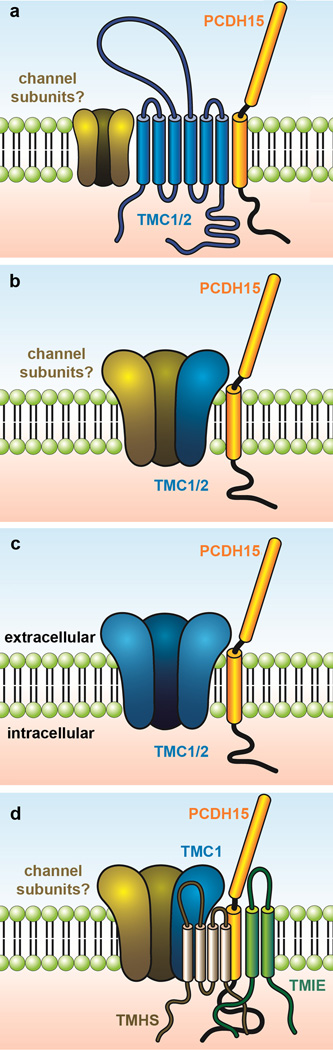
Figure 3.
Three possible configurations of TMC1 and TMC2 within the sensory transduction channel complex. Modified from Kawashima et al. (2014). (a) TMC1/TMC2 may be linker proteins that bind PCDH15 and convey tension to pore-forming subunits of the transduction channel. In this configuration, extracellular domains of TMC1/TMC2 may be within close enough proximity to the mouth of the pore to modify calcium-selectivity and single-channel conductance. (b) TMC1/TMC2 may be essential components of the channel linking directly with PCDH15. In this case, TMC1/TMC2 are part of an ion channel complex that includes other, as yet unknown, ion channel subunits. (c) In this scenario, TMC1/TMC2 are pore-forming subunits of the transduction channel and are coupled directly PCDH15. Modified from Kawashima et al. (2014). (d) A model based on the most recent biochemical data which suggest interactions between PCDH15 and TMC1 (Maeda et al., 2014), PCDH15 and TMIE (Zhao et al., 2014), PCDH15 and TMHS and shows that TMHS is necessary for TMC1 localization at the tips of sterocilia (Beurg et al., 2015).
Anomalous mechanotransduction
An alternate proposal that TMCs may be accessory or beta subunits (Kim et al., 2013) is inconsistent with ion channel convention (Hille, 2001). Accessory or beta subunits in other channel complexes can modify channel properties but those subunits are, by definition, non-essential, meaning the channel can function and respond to the appropriate stimulus even in their absence. In hair cells, sensory transduction does not function at all in the absence of TMC1 and TMC2 (Kawashima et al., 2011; Pan et al., 2013; Kim et al., 2013), ruling out the notion that they are merely accessory subunits.
The suggestion that TMCs may be accessory subunits arose from recent observations of a second form of mechanosensitivity that is present in wild-type hair cells and in hair cells that lack sensory transduction (Kim et al., 2013). Upon strong stimulation with a fluid jet directed at the apical surface of hair cells, several groups have described a form of “anomalous” mechanotransduction (Alagramam et al., 2011; Kim et al., 2013; Marcotti et al., 2014). The anomalous currents are most easily isolated and studied in cells that lack conventional bundle-dependent sensory transduction as the latter is more sensitive and tends to obscure the less sensitive, smaller and more variable anomalous currents (Marcotti et al., 2014). The anomalous currents have a biophysical and pharmacological properties that differ from conventional sensory transduction in a number of respects (Marcotti et al., 2014).
Whether anomalous mechanotransduction currents have any relation to conventional sensory transduction currents in hair cells is not yet clear (Barr-Gillespie and Nicolson, 2013). They may be related developmentally or under pathological conditions or they may be distinct, carried by distinct classes of ion channel proteins. The notion that a single cell type may express multiple classes of ion channels is not without precedent. For example, sensory neurons of the dorsal root ganglion express multiple types of mechanosensitive currents (Hao and Delmas, 2010). Hair cells, like many excitable cells, express multiple forms of potassium channels (Kros, 2007). Thus, it seems plausible that hair cells may also express multiple forms of mechanosensitive ion channels. Interestingly, the anomalous mechanotransduction currents are transient and fade during the second postnatal week (Kim et al., 2013), suggesting they may have a developmental role. While the function of the anomalous mechanosensitive response remains a mystery, it is also possible that it may be without function, a consequence of loosely regulated gene expression or an artifact of excessive mechanical stimulation.
The adaptation molecules
Sensory adaptation or the decay of the response in the presence of a constant stimulus occurs in hair cells on different time scales (Fettiplace and Ricci, 2003). There is a slow component that occurs over tens of milliseconds and is most prominent in vestibular hair cells and a fast component that occurs within a millisecond or two and is more prominent in auditory hair cells, though there is some evidence the two decay rates may coexist in a single cell (Eatock, 2000; Holt and Corey 2000). The rate of current decay for both fast and slow adaptation varies as a function of external calcium. Although numerous studies have examined the calcium dependence of adaptation (Eatock et al., 1987; Assad and Corey, 1992; Holt et al., 1997; Ricci and Fettiplace, 1997; Kennedy et al., 2003; Farris et al., 2006), one recent report suggested adaptation may be calcium independent (Peng et al., 2013). However, a follow up report has tipped the scales back in favor of traditional calcium-dependent adaptation (Corns et al., 2014).
Slow adaptation in vestibular hair cells seems to depend on the activity of myosin molecules. Myosins at the upper end of the tip-link may serve to regulate tension within the transduction complex in a calcium-dependent manor (Assad and Corey, 1992). Myo1c has been localized to the site of vestibular hair cell adaption (Garcia et al., 1998) where it may interact with CDH23 (Phillips et al., 2006). Chemical-genetic inhibition of Myo1c slows adaptation in vestibular hair cells (Holt et al., 2002; Stauffer et al., 2005), yet whether Myo1c or other myosins contribute to adaptation in auditory hair cells has not been determined. However, since sensory transduction channels appear to be localized at the lower end of tip-links (Beurg et al., 2009), calcium entry through transduction channels may be insufficient to regulate adaptation motors at the upper end, consistent with a less prominent role for slow adaptation in auditory hair cells.
The molecules that mediate fast adaptation are unknown, though based on the time course of fast adaptation, they must be in close proximity to transduction channels (Wu et al., 1999). Fast adaptation may result from calcium binding directly to the channel or to other nearby proteins that modulate channel open probability. Interestingly, Pan et al. (2013) found that adaptation was slower and to a lesser extent in auditory inner hair cells that expressed TMC2 than in those that expressed TMC1, suggesting that TMC1 and TMC2 may contribute to adaptation as well. Another candidate for the fast adaptation calcium sensor is CIB2 (Riazuddin et al., 2012). CIB2 is a calcium-binding protein localized to the tips of cochlear hair cell stereocilia that causes deafness when mutated.
Other components required for mechanotransduction
Other possible components of the sensory transduction apparatus, including TMHS (also known as LHFPL5) and TMIE, have been proposed, but their role is poorly defined (Xiong et al., 2012; Zhao et al., 2014). TMIE (Transmembrane Inner Ear) protein has been linked to deafness in mice and humans (Mitchem et al. 2002; Naz et al., 2002) and several studies have shown that TMIE is localized to hair bundles (Su et al., 2008; Shin et al., 2010) and required for sensory transduction (Shen et al., 2008; Shin et al., 2008; Gleason et al., 2009; Park et al., 2013). Recently, Zhao et al. (2014) showed that TMIE interacts with PCDH15 in HEK293 cells but were unable to identify the molecular function for TMIE in hair cells. Interestingly, Zhao et al. (2014) demonstrated the presence of anomalous mechano-transduction currents in TMIE-deficient hair cells, thus, it could be argued that TMIE is an accessory protein and is not essential for mechanotransduction. However, using conventional nomenclature (Hille, 2001), TMIE cannot be considered an accessory protein for sensory transduction as conventional hair cell transduction was completely absent in TMIE-deficient cells. Unlike TMC1, TMIE mutations have not been identified that alter the biophysical properties of sensory transduction channels. Thus, TMIE seems to be an essential component of the transduction apparatus but may be more remotely involved, perhaps as a linker required to convey mechanical tension to mechanosensory channels.
TMHS has also been localized to hair bundles and causes deafness in mice and humans when mutated (Longo-Guess et al., 2005; Kalay et al., 2006; Shabbir et al., 2006). Xiong et al. (2012) suggested TMHS may be a component of the transduction apparatus in hair cells based on biochemical interactions with PCDH15. However, since TMHS expression is transient during early development (Shabbir et al., 2006) and hair cells deficient in TMHS are dysmorphic (Xiong et al., 2012), it is not clear whether TMHS plays a direct role in mechanotransduction or a developmental role during hair bundle morphogenesis. Recent work suggested that TMHS expression is required for proper targeting of TMC1 to the tips of hair cell stereocilia and may help stabilize an interaction between TMC1 and the tip-link protein PCDH15 (Beurg et al., 2015). In the absence of TMHS expression, TMC1 localization at the tips of stereocilia is lost, the number of tip-links is reduced by ~66% and the whole-cell currents are reduced by 70–80%. TMHS deletion does not disrupt TMC2 localization, suggesting that another unknown protein may be responsible for targeting TMC2. Since TMHS is required for proper targeting of TMC1, deletion of both TMHS and TMC2 resulted in complete loss of residual transduction currents, consistent with prior observations that proper expression of TMC1 or TMC2 are required for sensory transduction in hair cells (Kawashima et al., 2011). A model that includes TMHS and TMIE is shown in Figure 3D.
Conclusions
The molecular complexity of the hair cell transduction apparatus and the heterogeneity in hair cells of different organs and species is becoming increasingly apparent. How the various components of this complex fit together to form one of nature’s most exquisite mechanosensory devices is not yet clear, but these question surely will keep investigators busy for some time to come. Recent progress in the field has already fuelled expanded interest and research into the molecules and mechanisms of hair cell sensory transduction. We anticipate that identification of the molecular composition of the sensory transduction apparatus will facilitate a better understanding of basic biology of inner ear function and dysfunction and will help translate these discoveries into treatment strategies designed to restore hearing and balance function in deaf and dizzy patients.
Highlights.
The molecular mechanisms of sensory transduction in hair cells are more complex than depicted in cartoons based on biophysical models.
Tip-links are composed of cadherin-23 and protocadherin-15.
TMC1 and TMC2 function as components of sensory transduction channels in hair cells.
The molecules, mechanisms and functions of anomalous mechanotransduction are unknown.
Myosins contribute to slow adaptation in vestibular hair cells. The molecular mechanisms of fast adaptation are unknown.
Acknowledgements
We thank Alice Galvin for assistance with manuscript preparation. The authors are supported by an NIH/NIDCD grant (RO1-DC013521).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing financial interests (BP and JRH).
References and recommended reading
- 1.Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, et al. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. doi: 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Riazuddin S, Wilcox ER. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alagramam KN, Goodyear RJ, Geng R, Furness DN, van Aken AF, Marcotti W, Kros CJ, Richardson GP. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS One. 2011;6:e19183. doi: 10.1371/journal.pone.0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet. 2001;27:99–102. doi: 10.1038/83837. [DOI] [PubMed] [Google Scholar]
- 5. Assad JA, Corey DP. An active motor model for adaptation by vertebrate hair cells. J Neurosci. 1992;12:3291–3309. doi: 10.1523/JNEUROSCI.12-09-03291.1992. This study presented a quantitative description of slow adaptation in bullfrog saccular hair cells. The authors provided a theoretical framework for slow adaptation, known as the motor model.
- 6.Barr-Gillespie PG, Nicolson T. Who needs tip links? Backwards transduction by hair cells. J Gen Physiol. 2013;142:481–486. doi: 10.1085/jgp.201311111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beurg M, Evans MG, Hackney CM, Fettiplace R. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci. 2006;26:10992–11000. doi: 10.1523/JNEUROSCI.2188-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beurg M, Fettiplace R, Nam JH, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci. 2009;12:553–558. doi: 10.1038/nn.2295. This study used high-speed imaging of calcium-sensitive dyes to localize calcium entry in rat auditory hair cells. The images showed calcium entry at the lower end of tip-links and little evidence of calcium entry at the upper end and concluded that transduction channels are located at the lower end.
- 9. Beurg M, Xiong W, Zhao B, Müller U, Fettiplace R. Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc Natl Acad Sci USA. 2015;112:1589–1594. doi: 10.1073/pnas.1420906112. This study demonstrated that TMHS expression is required for proper targeting of TMC1 but not TMC2 to the tips of hair cell stereocilia.
- 10.Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281:675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- 11. Corey DP, Hudspeth AJ. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983;3:962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. This is a hallmark paper that showed rapid kinetics of sensory transduction in bullfrog vestibular hair cells. The speed of the activation kinetics were too fast for second messanger cascades which had been identified for sensory transduction in photoreceptors. Thus, the authors proposed direct mechanical gating of ion channel to account for the submillisecond activation in hair cell mechanotransduction.
- 12. Corns LF, Johnson SL, Kros CJ, Marcotti W. Calcium entry into stereocilia drives adaptation of the mechanoelectrical transducer current of mammalian cochlear hair cells. Proc Natl Acad Sci U S A. 2014;111:14918–14923. doi: 10.1073/pnas.1409920111. This paper is the most recent showing that sensory adaptation is calcium-dependent. The authors present compelling evidence from mouse auditory hair cells using a fluid-jet stimulator.
- 13.Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet. 2001;27:103–107. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- 14.Eatock RA. Adaptation in hair cells. Annu Rev Neurosci. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- 15.Eatock RA, Corey DP, Hudspeth AJ. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J Neurosci. 1987;7:2821–2836. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farris HE, Wells GB, Ricci AJ. Steady-state adaptation of mechanotransduction modulates the resting potential of auditory hair cells, providing an assay for endolymph [Ca2+] J Neurosci. 2006;26:12526–12536. doi: 10.1523/JNEUROSCI.3569-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fettiplace R, Ricci AJ. Adaptation in auditory hair cells. Curr Opin Neurobiol. 2003;13:446–451. doi: 10.1016/s0959-4388(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 18.Garcia JA, Yee AG, Gillespie PG, Corey DP. Localization of myosin-Ibeta near both ends of tip links in frog saccular hair cells. J Neurosci. 1998;18:8637–8647. doi: 10.1523/JNEUROSCI.18-21-08637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Géléoc GS, Holt JR. Sound strategies for hearing restoration. Science. 2014;344:1241062. doi: 10.1126/science.1241062. This review article discusses recent progress in gene therapy, stem cell therapy and pharmocological approaches being development to treat hearing loss.
- 20.Geng R, Sotomayor M, Kinder KJ, Gopal SR, Gerka-Stuyt J, Chen DH, Hardisty-Hughes RE, Ball G, Parker A, Gaudet R, et al. Noddy, a mouse harboring a missense mutation in protocadherin-15, reveals the impact of disrupting a critical interaction site between tip-link cadherins in inner ear hair cells. J Neurosci. 2013;33:4395–4404. doi: 10.1523/JNEUROSCI.4514-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gleason MR, Nagiel A, Jamet S, Vologodskaia M, Lopez-Schier H, Hudspeth AJ. The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc Natl Acad Sci U S A. 2009;106:21347–21352. doi: 10.1073/pnas.0911632106. The authors show lovely functional data demonstrating that Tmie is required for sensory transduction in zebrafish hair cells. Mutations in Tmie caused defects in hair bundle morphology.
- 22.Hao J, Delmas P. Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. J Neurosci. 2010;30:13384–13395. doi: 10.1523/JNEUROSCI.2926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt JR, Corey DP. Two mechanisms for transducer adaptation in vertebrate hair cells. Proc Natl Acad Sci U S A. 2000;97:11730–11735. doi: 10.1073/pnas.97.22.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt JR, Corey DP, Eatock RA. Mechanoelectrical transduction and adaptation in hair cells of the mouse utricle, a low-frequency vestibular organ. J Neurosci. 1997;17:8739–8748. doi: 10.1523/JNEUROSCI.17-22-08739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, Gillespie PG. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. For this study the authors generated mice that carried a point mutation in Myo1c which sensitized the molecule to an otherwise non-toxic ADP analog. Inhibition of the mutant Myo1c with the ADP analog decreased the rate and extent of slow adaptation in vestibular hair cells.
- 26.Holt JR, Pan B, Koussa MA, Asai Y. TMC function in hair cell transduction. Hear Res. 2014;311:17–24. doi: 10.1016/j.heares.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howard J, Hudspeth AJ. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron. 1988;1:189–199. doi: 10.1016/0896-6273(88)90139-0. This classic paper established a direct link between the mechanical motion of bullfrog hair bundles and gating of transduction channels. It is the strongest evidence supporting direct mechanical activation sensory transduction channels in hair cells.
- 28. Kachar B, Parakkal M, Kurc M, Zhao Y, Gillespie PG. High-resolution structure of hair-cell tip links. Proc Natl Acad Sci U S A. 2000;97:13336–13341. doi: 10.1073/pnas.97.24.13336. This manuscript presented lovely SEM images showing tip-link structure. Based on these images the authors proposed that tip-links are not coiled springs but behave more as rigid chains that convey tension from the side of one sterocilium to tip of an adjacent lower stereocilium.
- 29.Kalay E, Li Y, Uzumcu A, Uyguner O, Collin RW, Caylan R, Ulubil-Emiroglu M, Kersten FF, Hafiz G, van Wijk E, et al. Mutations in the lipoma HMGIC fusion partner-like 5 (LHFPL5) gene cause autosomal recessive nonsyndromic hearing loss. Hum Mutat. 2006;27:633–639. doi: 10.1002/humu.20368. [DOI] [PubMed] [Google Scholar]
- 30. Kawashima Y, Geleoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, et al. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest. 2011;121:4796–4809. doi: 10.1172/JCI60405. This was a landmark paper showing the TMC1 or TMC2 are required for sensory transduction in auditory and vestibular hair cells. Double knockout mice that lacked both TMC1 and TMC2 lacked sensory transduction and suffered deafness and balance dysfunction.
- 31.Kawashima Y, Kurima K, Pan B, Griffith AJ, Holt JR. Transmembrane channel-like (TMC) genes are required for auditory and vestibular mechanosensation. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1582-3. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. This manuscript used high-resolution immunolocalization to establish that tip-links are composed of CDH23 at their upper end and PCDH15 at the lower end. In vitro experiments also showed that the molecules interacted at their N-termini to form protein chains of sufficent length to span the ~150 nm required for tip-links.
- 33.Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci. 2003;6:832–836. doi: 10.1038/nn1089. [DOI] [PubMed] [Google Scholar]
- 34.Kim KX, Beurg M, Hackney CM, Furness DN, Mahendrasingam S, Fettiplace R. The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. J Gen Physiol. 2013;142:493–505. doi: 10.1085/jgp.201311068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KX, Fettiplace R. Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. J Gen Physiol. 2013;141:141–148. doi: 10.1085/jgp.201210913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kros CJ. How to build an inner hair cell. challenges for regeneration. Hear Res. 2007;227:3–10. doi: 10.1016/j.heares.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 37. Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 2002;30:277–284. doi: 10.1038/ng842. This was the landmark study that identified TMC1 as a gene that required for auditory function in humans and mice. The authors identified TMC1/Tmc1 mutations that caused deafness in humans and mice.
- 38. Lelli A, Kazmierczak P, Kawashima Y, Muller U, Holt JR. Development and regeneration of sensory transduction in auditory hair cells requires functional interaction between cadherin-23 and protocadherin-15. J Neurosci. 2010;30:11259–11269. doi: 10.1523/JNEUROSCI.1949-10.2010. The study presented the first electrophysiological evidence of a direct protein interaction between CDH23 and PCDH15 in hair cells, establishing these molecules as functionally required for tip-links and sensory transduction in mouse auditory hair cells.
- 39.Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, Johnson KR. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci U S A. 2005;102:7894–7899. doi: 10.1073/pnas.0500760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, Zhao H, Clemens-Grisham R, Barr-Gillespie PG, Nicolson T. Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc Natl Acad Sci U S A. 2014;111:12907–12912. doi: 10.1073/pnas.1402152111. This important study demonstrated a biochemical interaction between PCDH15 and either TMC1 or TMC2 in zebrafish and mice.
- 41. Marcotti W, Corns LF, Desmonds T, Kirkwood NK, Richardson GP, Kros CJ. Transduction without tip links in cochlear hair cells is mediated by ion channels with permeation properties distinct from those of the mechano-electrical transducer channel. J Neurosci. 2014;34:5505–5514. doi: 10.1523/JNEUROSCI.4086-13.2014. This study provided a charaterization of anomalous mechanotransduction in outer hair cells of wild type mice and in mice that carry mutations in Myo7a and CDH23.
- 42.Mitchem KL, Hibbard E, Beyer LA, Bosom K, Dootz GA, Dolan DF, Johnson KR, Raphael Y, Kohrman DC. Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Hum Mol Genet. 2002;11:1887–1898. doi: 10.1093/hmg/11.16.1887. [DOI] [PubMed] [Google Scholar]
- 43.Naz S, Giguere CM, Kohrman DC, Mitchem KL, Riazuddin S, Morell RJ, Ramesh A, Srisailpathy S, Deshmukh D, Riazuddin S, et al. Mutations in a novel gene, TMIE, are associated with hearing loss linked to the DFNB6 locus. Am J Hum Genet. 2002;71:632–636. doi: 10.1086/342193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pan B, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron. 2013;79:504–515. doi: 10.1016/j.neuron.2013.06.019. This manuscript presented evidence that TMC1 and TMC2 function as components of sensory transduction channels in auditory inner hair cells and vestibular hair cells. The evidence is based on data from Tmc1/Tmc2-deficient hair cells and those that carried the Beethoven mutation in Tmc1.
- 45.Park S, Lee JH, Cho HJ, Lee KY, Kim MO, Yun BW, Ryoo Z. tmie Is required for gentamicin uptake by the hair cells of mice. Comp Med. 2013;63:136–142. [PMC free article] [PubMed] [Google Scholar]
- 46. Peng AW, Effertz T, Ricci AJ. Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron. 2013;80:960–972. doi: 10.1016/j.neuron.2013.08.025. These authors suggest that sensory adaptation does not involve calcium entry. The conclusion is controversial and based on the presence of transduction currents that decay at depolarized potentials, which the authors argue should reduce calcium entry and adaptation.
- 47.Pepermans E, Michel V, Goodyear R, Bonnet C, Abdi S, Dupont T, Gherbi S, Holder M, Makrelouf M, Hardelin JP, et al. The CD2 isoform of protocadherin-15 is an essential component of the tip-link complex in mature auditory hair cells. EMBO Mol Med. 2014;6:984–992. doi: 10.15252/emmm.201403976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips KR, Tong S, Goodyear R, Richardson GP, Cyr JL. Stereociliary myosin-1c receptors are sensitive to calcium chelation and absent from cadherin 23 mutant mice. J Neurosci. 2006;26:10777–10788. doi: 10.1523/JNEUROSCI.1847-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricci AJ, Fettiplace R. The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol. 1997;501(Pt 1):111–124. doi: 10.1111/j.1469-7793.1997.111bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shabbir MI, Ahmed ZM, Khan SY, Riazuddin S, Waryah AM, Khan SN, Camps RD, Ghosh M, Kabra M, Belyantseva IA, et al. Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet. 2006;43:634–640. doi: 10.1136/jmg.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen YC, Jeyabalan AK, Wu KL, Hunker KL, Kohrman DC, Thompson DL, Liu D, Barald KF. The transmembrane inner ear (tmie) gene contributes to vestibular and lateral line development and function in the zebrafish (Danio rerio) Dev Dyn. 2008;237:941–952. doi: 10.1002/dvdy.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shepherd GM, Corey DP. The extent of adaptation in bullfrog saccular hair cells. J Neurosci. 1994;14:6217–6229. doi: 10.1523/JNEUROSCI.14-10-06217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin MJ, Lee JH, Yu DH, Kim BS, Kim HJ, Kim SH, Kim MO, Park C, Hyun BH, Lee S, et al. Ectopic expression of tmie transgene induces various recovery levels of behavior and hearing ability in the circling mouse. Biochem Biophys Res Commun. 2008;374:17–21. doi: 10.1016/j.bbrc.2008.06.064. [DOI] [PubMed] [Google Scholar]
- 54.Shin MJ, Lee JH, Yu DH, Kim HJ, Bae KB, Yuh HS, Kim MO, Hyun BH, Lee S, Park R, et al. Spatiotemporal expression of tmie in the inner ear of rats during postnatal development. Comp Med. 2010;60:288–294. [PMC free article] [PubMed] [Google Scholar]
- 55.Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Muller U. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- 56.Sollner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, Muller U, Nicolson T. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- 57.Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structural determinants of cadherin-23 function in hearing and deafness. Neuron. 2010;66:85–100. doi: 10.1016/j.neuron.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature. 2012;492:128–132. doi: 10.1038/nature11590. This crystal structure analysis showed the distal tips of CDH23 and PCDH15 at the point where they interact. The structure revealed an overlaping interaction rather than a tip-to-tip interaction and demonstrated how calcium serves to stablize the complex.
- 59.Stauffer EA, Scarborough JD, Hirono M, Miller ED, Shah K, Mercer JA, Holt JR, Gillespie PG. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron. 2005;47:541–553. doi: 10.1016/j.neuron.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su MC, Yang JJ, Chou MY, Hsin CH, Su CC, Li SY. Expression and localization of Tmie in adult rat cochlea. Histochem Cell Biol. 2008;130:119–126. doi: 10.1007/s00418-008-0385-z. [DOI] [PubMed] [Google Scholar]
- 61. Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, Kurima K, Wilcox ER, Friedman TB, Griffith AJ, Balling R, et al. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet. 2002;30:257–258. doi: 10.1038/ng848. This manuscript was published at that same time as reference #36 above and identifed the dominant Bth point mutation Tmc1. Dominant mutations in human TMC1 cause DFNA36.
- 62.Webb SW, Grillet N, Andrade LR, Xiong W, Swarthout L, Della Santina CC, Kachar B, Muller U. Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Development. 2011;138:1607–1617. doi: 10.1242/dev.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu YC, Ricci AJ, Fettiplace R. Two components of transducer adaptation in auditory hair cells. J Neurophysiol. 1999;82:2171–2181. doi: 10.1152/jn.1999.82.5.2171. [DOI] [PubMed] [Google Scholar]
- 64.Xiong W, Grillet N, Elledge HM, Wagner TF, Zhao B, Johnson KR, Kazmierczak P, Muller U. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell. 2012;151:1283–1295. doi: 10.1016/j.cell.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao B, Wu Z, Grillet N, Yan L, Xiong W, Harkins-Perry S, Muller U. TMIE Is an Essential Component of the Mechanotransduction Machinery of Cochlear Hair Cells. Neuron. 2014;84:954–967. doi: 10.1016/j.neuron.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y, Wang D, Zong L, Zhao F, Guan L, Zhang P, Shi W, Lan L, Wang H, Li Q, et al. A novel DFNA36 mutation in TMC1 orthologous to the Beethoven (Bth) mouse associated with autosomal dominant hearing loss in a Chinese family. PLoS One. 2014;9:e97064. doi: 10.1371/journal.pone.0097064. [DOI] [PMC free article] [PubMed] [Google Scholar]