Abstract
Importance
Epidemiologic evidence suggests that physical activity benefits cognition, but results from randomized trials are limited and mixed.
Objective
To determine whether a 24-month physical activity program results in better cognitive function and/or lower risk of mild cognitive impairment (MCI) or dementia compared to a health education program.
Design, Setting, and Participants
The Lifestyle Interventions and Independence for Elders (LIFE) study was a multicenter, randomized clinical trial that enrolled 1635 community-living participants at 8 centers in the U.S. from February 2010 until December 2011. Participants were sedentary adults aged 70–89 years at risk for mobility disability, but able to walk 400m.
Intervention
Participants were randomized to a structured, moderate-intensity physical activity program (n=818) that included walking, resistance training, and flexibility exercises or to a health education program (n=817) of educational workshops and upper extremity stretching.
Outcomes and Measures
Pre-specified secondary outcomes of the LIFE study included cognitive function measured by the Digit Symbol Coding task (0–133 scale, higher=better) and Hopkins Verbal Learning Test-Revised (12-word list recall) assessed in 1,476 (90.3%) participants. Tertiary outcomes included global and executive cognitive function and incident MCI or dementia at 24 months. Pre-specified subgroups analyses were performed based on age, sex, baseline physical performance, and baseline Modified Mini-Mental State Examination score.
Results
At 24 months, DSC and HVLT-R scores (adjusted for clinic site, gender, and baseline values) were not different between groups. Mean DSC scores were 46.26 points for physical activity vs. 46.28 for health education; mean difference −0.014 points, 95% CI −0.80 to 0.77, p= 0.97. Mean HVLT-R delayed recall scores were 7.22 for physical activity vs. 7.25 for health education; mean difference −0.03 words, 95% CI −0.29 to 0.24, p= 0.84.
No differences for any other cognitive or composite measures were observed. Participants randomized to physical activity who were ≥80 years (n=307) and those with poorer baseline physical performance (n=328) had better changes in executive function composite scores vs. those randomized to health education (interaction p=0.01, respectively). Incident MCI or dementia occurred in 98 (13.2%) participants randomized to physical activity and 91 (12.1%) participants randomized to health education (OR 1.08, 95% CI 0.80 to 1.46).
Conclusions and Relevance
Among sedentary older adults, a 24-month moderate intensity physical activity program, compared to a health education program, did not result in improvements in global or domain-specific cognitive function.
Introduction
Epidemiologic evidence suggests that regular physical activity is associated with lower rates of cognitive decline. Exercise is associated with improved cerebral blood flow and neuronal connectivity,1 maintenance or improvement in brain volume,2;3 and favorable changes in brain-derived neurotrophic factor and neurogenesis.4;5 In transgenic Alzheimer’s mouse models, exercise reduces beta-amyloid deposition.6
Randomized clinical trials (RCT) assessing the effect of physical activity on cognitive function are equivocal,7–9 perhaps due to small sample sizes, short intervention periods, and differences in cohorts and protocols, particularly physical activity intensity.7 Two recent small RCTs of physical activity found no benefit of a structured physical activity program vs. no intervention or cognitive training in non-demented older adults with cognitive complaints or at risk of cognitive decline.10;11 However, a 6-month RCT of a home-based physical activity program vs. usual care in participants with memory complaints or mild cognitive impairment found a modest cognitive benefit.12 The LIFE Pilot study showed a correlation between changes in physical and cognitive performance in a 12 month exercise trial.13
Here we report the pre-specified secondary cognitive outcomes of the LIFE Study, the largest and longest RCT to assess the effect of a standardized physical activity intervention on cognitive function and cognitive impairment in sedentary older adults at risk for mobility disability.14 We hypothesized that compared to health education, physical activity for 24 months would result in better cognitive function and lower risk of incident all-cause mild cognitive impairment (MCI) or dementia.
Methods
Trial design and participants
The LIFE study was a multicenter, single-blinded, randomized clinical trial of a physical activity intervention versus a health education control conducted at 8 field centers across the U.S. Participants were from rural and urban communities. (See acknowledgment section for the field centers.) Details of the LIFE Study design and results have been published.14;15 The study included sedentary men and women aged 70–89 years who were at high risk for mobility disability based on objectively assessed lower extremity functional limitations defined as a Short Physical Performance Battery (SPPB)16 score ≤9 (out of 12), but who could walk 400 m in 15 minutes at baseline without assistance. Eligible participants had no diagnosis of dementia or significant cognitive impairment on the Modified Mini-Mental State Examination17 (3MSE) based on education- and race-specific norms. Participants with <9 years of education were excluded if the screening 3MSE score was <70 for African Americans and Spanish Speakers or <76 for English-speaking non-African Americans. Participants with ≥9 years of education were excluded if their 3MSE score was <76 for African Americans and <80 for Spanish speakers and English-speaking non-African Americans. Race and ethnicity were self-reported and collected as required by the National Institutes of Health.
Recruitment was predominantly by mass mailing to age-eligible residents. Additional strategies included newspaper, radio and television advertisements, and presentations at health fairs, senior centers, medical clinics, and churches.
The LIFE study was approved by the institutional review boards at all participating sites and monitored by a data safety monitoring board appointed by the National Institute on Aging. Written informed consent was obtained from all participants.
Interventions
Participants were randomly assigned, using a secure web-based data management system (permuted block algorithm with random block lengths), with equal probability to either a physical activity intervention or a successful aging health education program, stratifying by field center and sex. The physical activity intervention focused on walking, strength, flexibility, and balance training. Participants were expected to attended two center-based visits per week and perform home-based activity 3–4 times per week. The physical activity sessions progressed towards a goal of 30 min of walking at moderate intensity, 10 min of primarily lower extremity strength training with ankle weights, 10 min of balance training, and large muscle group flexibility exercises.
The health education group attended weekly health education workshops during the first 26 weeks of the intervention and at least monthly sessions thereafter. Sessions lasted 60–90 minutes and consisted of interactive and didactic presentations, facilitator demonstrations, guest speakers, or field trips. Sessions included approximately 10 minutes of group discussion and interaction and 5- to 10-minutes of upper extremity stretching and flexibility exercises. Example topics included travel safety, age appropriate preventive services, legal/financial issues, and nutrition. The intervention committee ensured that health education activities were consistent across sites and unlikely to increase physical activity.
Measurements
Outcome assessments were conducted in person by masked staff every six months. Home, telephone, and proxy assessments were attempted if participants could not attend clinic visits. Information on demographics, medical and hospitalization history, medication inventory, quality of well-being, and functional limitation was based on self-report.18 Usual physical activity was assessed by self-report using the CHAMPS questionnaire to measure total weekly minutes in walking and strength training19 and objectively using an Actigraph accelerometer to measure total minutes of at least moderate activity (>760 counts/minute) over seven days14.
Cognitive assessment
A previously described neuropsychological battery of tests was administered by trained and certified examiners at baseline and 24 months post-randomization.20 Three computerized tasks were administered at baseline and either 18 or 30 months depending on when the participant was enrolled.20
Neuropsychological battery
Cognitive tests at baseline included the Modified Mini-Mental State Examination (3MSE),17 a 100-point test of global cognitive function; the Wechsler Adult Intelligence Scale-III Digit Symbol Coding (DSC),21 a test of psychomotor speed, attention, and working memory; the Hopkins Verbal Learning Test-Revised (HVLT-R),22 a 12-item word list learning and recall task; and a modified version of the Rey-Osterrieth Complex Figure to assess visuospatial function (copy) and figural memory (immediate recall). At 24 months these measures were repeated along with the Boston Naming Test, a measure of language;23 the Trail Making Test24 parts A (measuring attention, concentration, and psychomotor speed) and B (executive function); and Category Fluency for animals, a measure of executive function. In all tests except Trail Making, higher scores indicate better performance.
Computerized battery
Using a laptop computer, participants were administered 3 tasks that were chosen for added sensitivity in assessing speed of processing and executive function: the n-back (1-back and 2-back) task,25 the Eriksen Flanker task,26 and a task switching paradigm.27
Mild Cognitive Impairment (MCI) and Dementia outcome determinations
At baseline and 24 months post-randomization, all participants were assigned one of the following cognitive classifications: No Cognitive Impairment, MCI, or Dementia. Participants who scored ≤88 on the 3MSE were sent for central adjudication by a panel of eight clinical experts in the diagnosis of late life cognitive impairment, blinded to treatment assignment.20 Each case was assigned to 2 independent adjudicators; disagreements were resolved by the full panel. Adjudicators reviewed data from the neuropsychological battery, medical history, medications, discharge diagnoses for hospitalizations during the trial, Center for Epidemiology Studies-Depression (CESD) scores,28 self-reported disability, and informant-reported functional status (Functional Assessment Questionnaire, FAQ).29 The FAQ is a 10-item interviewer-administered questionnaire assessing degree of dependence in cognitively challenging activities of daily living such as preparing balanced meals, traveling outside the neighborhood, and managing finances. The FAQ was administered to the participant’s proxy for all participants with a 3MSE score ≤88 at baseline and 24 months. MCI and dementia were adjudicated based on the 2011 National Institute on Aging/Alzheimer’s Association criteria.30;31
Statistical analyses
The LIFE protocol specified DSC (total score) and HVLT-R (mean of the immediate and delayed recall subscales) as the two primary cognitive outcomes for assessing cognitive decline, each tested according to the intention to treat principle with analysis of covariance using 24-month data and covariate adjustment for field center, gender, and the baseline value. Additional pre-specified cognitive outcomes were based on scores from the computerized battery. Raw scores from this battery were first winsorized to limit the influence of extreme values: this was done by replacing scores less than the 1st percentile of the cohort wide distribution with the value of the 1st percentile and replacing scores greater than the 99th percentile with the 99th percentile value. Z-scores were formed for each cognitive test score by dividing their difference from the baseline mean by the baseline standard deviation. Composite scores for the HVLT-R (immediate and delayed recall scores), n-back (1- and 2-back scores), task switching (no switch and switch reaction times), and flanker tasks (congruent and incongruent reaction times) were formed by averaging the z-scores for their two individual components. The global cognitive function score was the average of scores from these composites and the z-transformed DSC, renormalized to have mean 0 and standard deviation 1 at baseline. The executive function composite score was the renormalized average of scores from the n-back, task switching, and Flanker tasks. In creating these composite scores, averages were taken of all available data, i.e., missing data if participants did not complete the full battery were ignored. Supporting analyses were conducted using multiple imputation in which missing measures and exam scores were imputed to create five databases that were analyzed in parallel.32
Subgroup comparisons using interaction terms were pre-specified for gender and baseline SPPB (<8 versus ≥8), 3MSE (<90 versus ≥90), and age (70–79 years versus ≥80 years). Associations between changes in cognitive function and changes in objective and subjective physical activity were assessed using linear regression and tests of interactions.
Progression in cognitve impairment (i.e., from baseline normal cognitive function to either MCI or dementia or from baseline MCI to dementia), was a tertiary outcome. Logistic regression was used to compare progression rates between intervention groups. Participants with prevalent MCI (n=141) at baseline were not included in the incidence of MCI, but were included in the incident dementia outcome if they progressed to dementia at 24 months. Seven participants were adjudicated to have dementia at the baseline visit (in spite of otherwise meeting LIFE entry criteria). These participants were excluded from the incident dementia outcome analysis.
Analyses were conducted using SAS 9.4; two-sided inferences with p<0.05 were considered statistically significant. The targeted sample size (N=1600) was expected to provide 87% power to detect mean differences between groups of 0.15 SD for cognitive tests. This was projected to correspond to mean differences of 1.8 units for DSC scores and 0.8 units for HVLT immediate memory scores.
Results
From February 2010 to December 2011, 1,635 participants were randomized (818 to physical activity and 817 to health education) (Figure 1). Analyses are limited to the 1476 (90.3%) participants with cognitive data during follow-up. Compared to participants without follow-up cognitive data, included participants had faster gait speeds (p<0.001). 24-month retention rates were 89.8% in the physical activity and 90.7% in the health education group (p=0.56).
Figure 1.
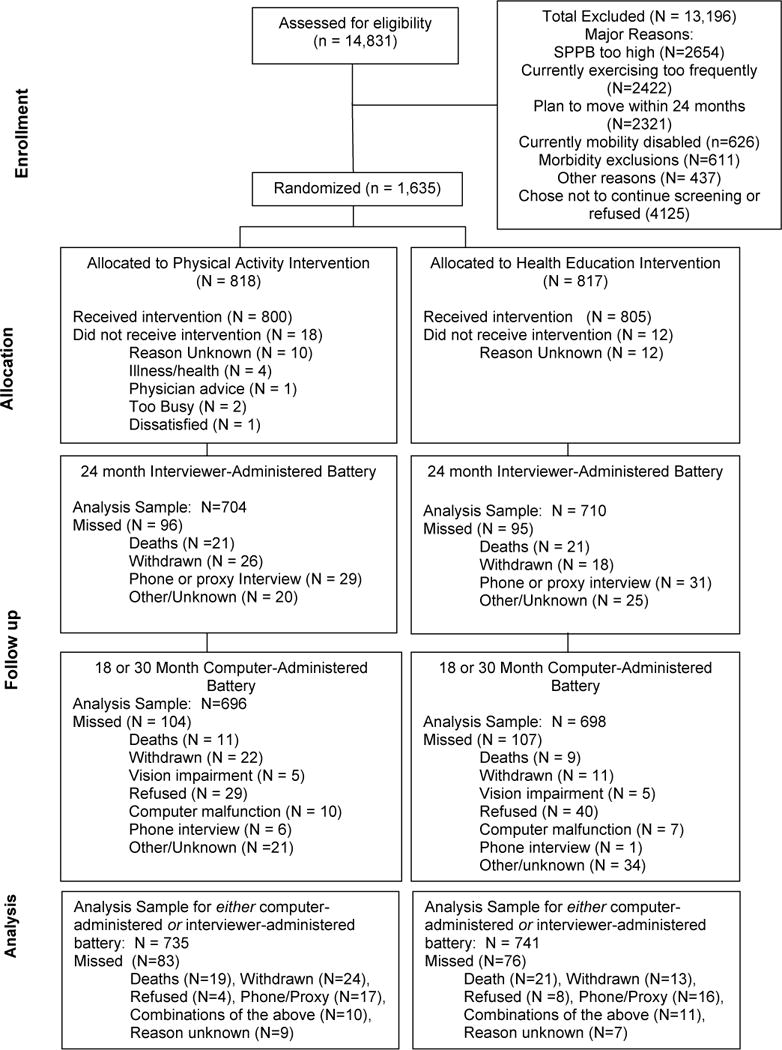
Consort Diagram
Table 1 shows characteristics of participants. Overall, mean (SD) age was 78.9 (5.2) years, 68% were women, and 67% had a college education. The mean 3MSE score was 91.7 (5.4) (range 71 to 100). There were more African-Americans in the physical activity than the health education group.
Table 1.
Baseline characteristics: The Lifestyle Interventions and Independence of Elders (LIFE) Study.
| Physical Activity N=735 | Health Education N=741 | |
|---|---|---|
| Age | ||
| 70–79 years | 428 (58.2%) | 413 (55.7%) |
| 80–89 years | 307 (41.8%) | 328 (44.3%) |
|
| ||
| Women | 496 (67.5%) | 503 (67.9%) |
|
| ||
| Education, Miss=4 | ||
| High School or less | 249 (33.9%) | 237 (32.1%) |
| College or more | 485 (66.1%) | 501 (67.9%) |
|
| ||
| Race, Miss=4 | ||
| African-American | 148 (20.2%) | 112 (15.2%) |
| Non-Hispanic White | 542 (73.9%) | 580 (78.5%) |
| Other | 43 (5.9%) | 47 (6.4%) |
|
| ||
| SPPB score | ||
| <8 | 309 (42.0%) | 341 (46.0%) |
| 8–9 | 426 (58.0%) | 400 (54.0%) |
|
| ||
| 400 m walking speed (m/sec) | 0.83 (0.16) | 0.82 (0.16) |
|
| ||
| Body mass index (kg/m2) | 30.2 (5.8) | 30.2 (6.1) |
|
| ||
| Minutes/week walking and strength training | 75.1 (125.6) | 86.7 (134.4) |
|
| ||
| History of hypertension | 552 (75.1%) | 554 (74.8%) |
|
| ||
| Diabetes status | ||
| None | 366 (49.8%) | 375 (50.6%) |
| Impaired fasting glucose | 173 (23.5%) | 154 (20.8%) |
| Diabetes | 196 (26.7%) | 212 (28.6%) |
|
| ||
| History of cardiovascular disease | 210 (28.6%) | 225 (30.4%) |
|
| ||
| History of stroke | 53 (7.2%) | 48 (6.5%) |
|
| ||
| ApoE-e4 allele | ||
| 0 | 525 (64.2%) | 529 (64.8%) |
| 1 | 146 (17.8%) | 153 (18.7%) |
| 2 | 10 (1.2%) | 9 (1.1%) |
| Missing | 137 (16.8%) | 126 (15.4%) |
|
| ||
| 3MSE (0–100) | 91.61 (5.54) | 91.71 (5.28) |
| % with score <90 | 230 (31.3%) | 236 (31.8%) |
|
| ||
| DSC score (0–133) | 45.99 (13.04) | 47.01 (12.72) |
|
| ||
| HVLT-R, number correct | ||
| Sum of 3 Immediate recall trials (0–36) | 23.44 (5.12) | 23.18 (5.44) |
| Delayed recall (0–12) | 7.79 (2.73) | 7.70 (2.92) |
|
| ||
| N-back, % correct (0–100) | ||
| 1-back | 81.58 (17.85) | 82.11 (16.30) |
| 2-back | 51.04 (19.84) | 50.68 (21.47) |
|
| ||
| Task Switching, reaction time (seconds) | ||
| No switch | 1.46 (0.73) | 1.41 (0.69) |
| Switch | 2.44 (1.04) | 2.35 (1.01) |
|
| ||
| Flanker, reaction time (seconds) | ||
| Congruent | 0.65 (0.19) | 0.65 (0.20) |
| Incongruent | 0.72 (0.22) | 0.73 (0.24) |
Data are N (%) or means (standard deviations); SPPB = Short Physical Performance Battery; 3MSE= Modified Mini-Mental State Examination (higher scores indicate better performance); DSC = Digit Symbol Coding (higher scores indicate better performance); HVLT-R = Hopkins Verbal Learning Test-Revised (higher scores indicate better performance); for task switching and flanker reaction times, larger values indicate slower (worse) performance.
Intervention adherence
Based on accelerometry data, and compared to the health education group, the physical activity group maintained moderate/vigorous physical activity between baseline and 24-month follow-up (mean −2.1 minutes/week (95% CI −9.7 to 13.9) vs −40.4 minutes/week (95% CI −29.4 to −51.4), p<0.001). Based on CHAMPS questionnaire data, and compared to the health education group, the physical activity group had greater increase in self-reported physical activity from baseline to 24 months (mean +130.4 min/week (95% CI 116.7 to 144.1) vs +30.5 min/week (95% CI 18.9 to 42.1), p<0.001). The median attendance at physical activity sessions was 71%, excluding medical leave.
Cognitive function results
At baseline, interviewer-administered cognitive assessments were collected on all participants. Computer-based assessments were collected on 85.5% (2-back) to 96.2% (Flanker) of participants. There were no differences between groups on any cognitive tests at baseline.
Table 2 presents the raw scores and z-transformed cognitive outcomes, adjusting for clinic site, gender, and baseline values. Z-scores are interpreted as the change from baseline in standard deviations. The adjusted mean raw DSC scores (range 1–133) at follow up were not different between groups (46.26 points (95% CI 45.71 to 46.82) in the physical activity group and 46.28 (95% CI 45.73 to 46.83) in the health education; mean difference −0.01 points, 95% CI −0.80 to 0.77, p= 0.97). Similarly, adjusted mean HVLT-R delayed recall scores (range 0–12) were not different between groups (7.22 words (95% CI 7.03 to 7.41) for physical activity vs. 7.25 (95% CI 7.06 to 7.44) for health education; mean difference −0.03 words, 95% CI −0.29 to 0.24, p= 0.84). There were no between-group differences in the executive function composite z-score (p=0.59) or mean global composite score (p=0.40). Additional adjustment for race/ethnicity and education did not change the results.
Table 2.
Mean (95% Confidence Interval) adjusted raw and z-transformed follow-up cognitive function scores by intervention assignment: The LIFE Study
| Physical Activity Mean [95% CI] N=735 | Health Education Mean [95% CI] N=741 | Mean Difference [95% CI] | p-value | |
|---|---|---|---|---|
|
| ||||
| DSC | ||||
| Raw | 46.26 [45.75 to 46.82] |
46.28 [45.72 to 46.83] |
−0.01 [−0.80 to 0.77] |
0.97 |
| z-score | −0.003 [−0.046 to 0.040] |
−0.002 [−0.045 to 0.041] |
−0.001 [−0.063 to 0.060] |
|
|
| ||||
| HVLT-R | ||||
|
| ||||
| Immediate | ||||
| Raw | 22.83 [22.52 to 23.14] |
22.97 [22.67 to 23.28] |
−0.14 [−0.58 to 0.29] |
0.52 |
| z-score | −0.073 [−0.132 to −0.014] |
−0.046 [−0.105 to 0.013] |
−0.027 [−0.110 to 0.055] |
|
|
| ||||
| Delayed | ||||
| Raw | 7.22 [7.03 to 7.41] |
7.25 [7.06 to 7.44] |
−0.03 [−0.29 to 0.24] |
0.84 |
| z-score | −0.167 [−0.234 to −0.100] |
−0.157 [−0.224 to −0.090] |
−0.010 [−0.103 to 0.084] |
|
|
| ||||
| HVLT-R Composite z-score | −0.130 [−0.187 to −0.073] |
−0.106 [−0.163 to −0.049] |
−0.024 [−0.105 to 0.057] |
0.56 |
|
| ||||
| Executive Function N-back | ||||
| 1-back, % correct | 83.7 [82.5 to 84.9] |
82.9 [81.8 to 84.1] |
0.7 [−0.9 to 2.4] |
0.39 |
| 2-back, % correct | 53.2 [51.6 to 54.8] |
51.9 [50.4 to 53.5] |
1.3 [−0.9 to 3.5] |
0.26 |
| Task Switching reaction time | ||||
| no switch, seconds | 1.47 [1.42 to 1.51] |
1.46 [1.42 to 1.51] |
0.01 [−0.06 to 0.07] |
0.86 |
| switch, seconds | 2.43 [2.37 to 2.49] |
2.39 [2.33 to 2.45] |
0.04 [−0.05 to 0.13] |
0.37 |
| Flanker reaction time | ||||
| Congruent, seconds | 0.65 [0.64 to 0.67] |
0.67 [0.66 to 0.68] |
−0.02 [−0.03 to −0.01] |
0.04 |
| Incongruent, seconds | 0.73 [0.72 to 0.74] |
0.75 [0.73 to 0.76] |
−0.02 [−0.04 to 0.00] |
0.07 |
| Composite z-score | −0.003 [−0.060 to 0.054] |
−0.025 [−0.080 to 0.030] |
0.022 [−0.057 to 0.101] |
0.59 |
|
| ||||
| Overall Composite z-score | −0.052 [−0.099 to −0.005] |
−0.081 −0.128 to −0.034] |
0.029 [−0.038 to 0.095] |
0.40 |
DSC = Digit Symbol Coding; HVLT-R = Hopkins Verbal Learning Test-Revised
Covariate adjustment for sex, clinic site, and baseline value
Composite scores are ordered so that positive values reflect better performance on tasks
Executive function composite: n-back, task switching, and flanker tasks
Overall composite includes: DSC, HVLT-R immediate and delayed recall, n-back, task switching and flanker tasks
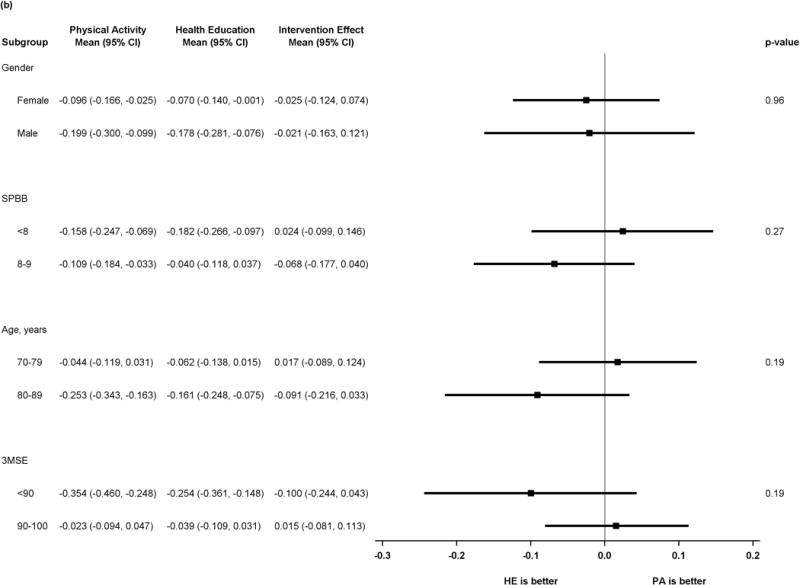
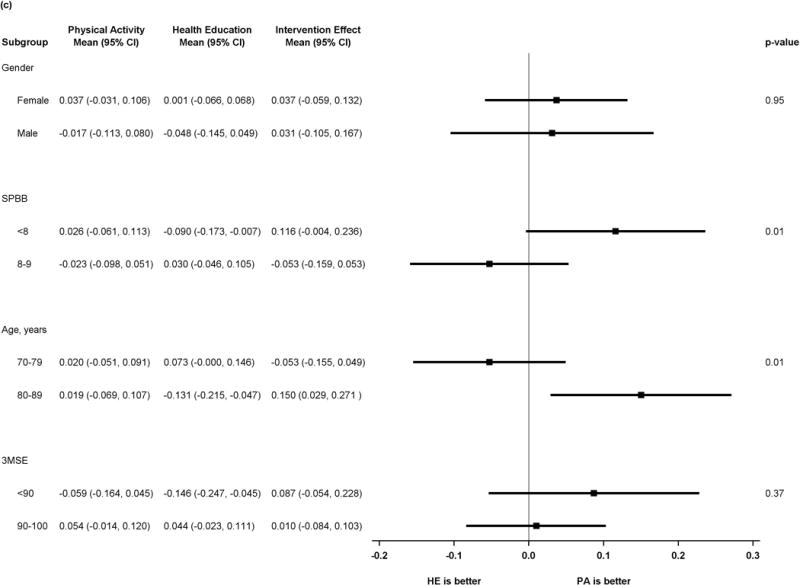
Figure 2 shows results of pre-specified subgroup comparisons. Intervention effects did not vary by gender or baseline 3MSE. For the executive function composite, however, there was heterogeneity in intervention effects, suggesting benefit in executive function associated with the physical activity intervention (p=0.01 for tests of interaction) for participants with baseline SPPB <8 or age ≥80. Details of the scores for all subgroups and outcomes can be found in the supplemental table.
Figure 2.
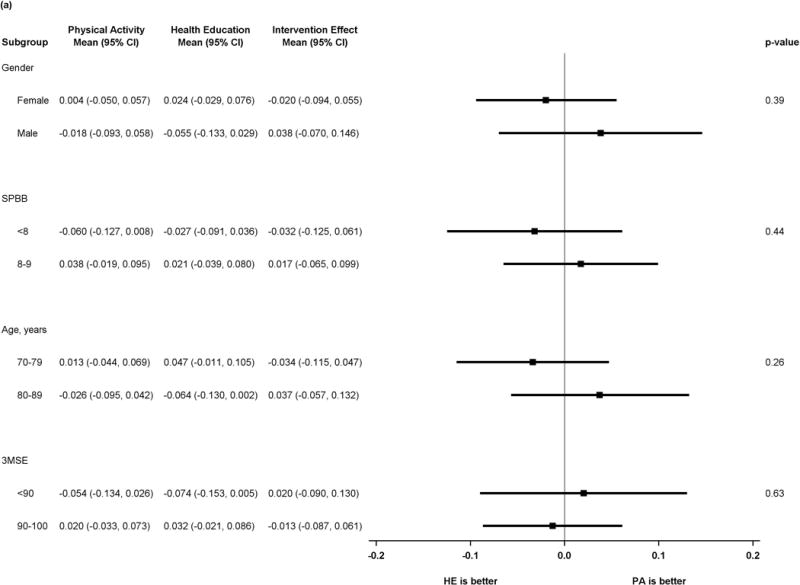
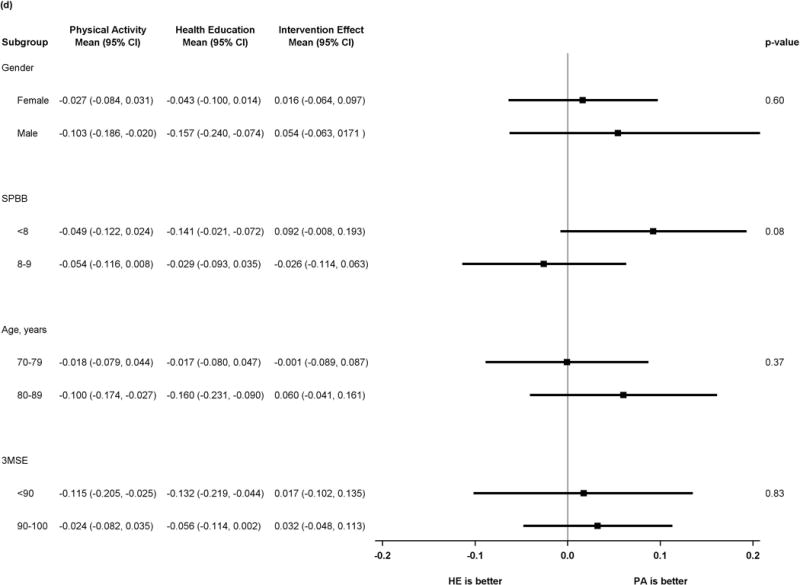
a) Forest plot of intervention effects on Z-transformed Digit Symbol Coding
b) Forest plot of intervention effects on z-transformed Hopkins Verbal Learning Test
c) Forest plot of intervention effects on z-transformed executive function composite
d) Forest plot of intervention effects on z-transformed cognitive function composite
P values represent test of interaction in all plots
Relationships with changes in physical activity
24-month changes in the four cognitive function measures were not correlated with changes in moderate physical activity as measured by accelerometry (p>0.30) among the 697 participants with 24-month data. 24-month changes in weekly walking and strength training from the CHAMPS were modestly associated with global cognitive function (r=0.07; p=0.006) and executive function (r=0.06; p=0.04). These relationships were not different between the two intervention groups (interaction tests p>0.70). Results were unchanged when using 12 month change in physical activity.
Incident MCI or Dementia
There was no difference between groups in the incidence of MCI, dementia, or both combined; 13.2% of participants randomized to physical activity developed MCI or dementia by 24 months compared to 12.1% randomized to health education (unadjusted OR [95% CI] 1.08 [0.80 to 1.46]; p=0.61) (Table 3). There were no between group differences within MCI subtypes: incident amnestic MCI was 5.5% for physical activity and 5.7% for health education (p= 0.85); non-amnestic MCI was 4.6% and 3.2% (p=0.16), respectively.
Table 3.
Incident mild cognitive impairment (MCI) or dementia at 24 months by intervention assignment: The LIFE Study
| Physical Activity | Health Education | OR [95% CI]a | p-value | |
|---|---|---|---|---|
| MCIb | 70/686 (10.2%) | 62/682 (9.1%) | 1.14 [0.79 to 1.62] | 0.48 |
|
| ||||
| Dementiac | 28/743 (3.8%) | 29/747 (3.9%) | 0.96 [0.57 to1.63] | 0.88 |
|
| ||||
| MCI or Dementia | 98/743 (13.2%) | 91/747 (12.1%) | 1.08 [0.80 to 1.46] | 0.61 |
Odds ratio from unadjusted logistic regression;
Of those free of MCI or dementia at baseline;
Of those free of dementia at baseline; Denominators are slightly larger than in table 1 because some participants were adjudicated, but did not get cognitive testing at 24 months (deaths, for example).
Discussion
The LIFE Study’s structured, 24-month moderate-intensity physical activity intervention did not result in better global or domain-specific cognition compared with a health education program in older, sedentary adults. There was also no difference between groups in the incidence of MCI or dementia, though this was an exploratory outcome with limited statistical power. However, participants randomized to the physical activity group who were ≥80 years old and those with lower baseline physical functioning levels experienced benefits in executive functioning compared to the health education group. Cognitive function remained stable over 2 years for all participants. We cannot rule out that both interventions were successful at maintaining cognitive function.
Despite epidemiologic evidence supporting benefits of exercise and physical activity on cognition, the results of the LIFE Study are consistent with some other randomized trials.7 In the MAX trial,10 a structured aerobic physical activity intervention was not superior to a stretching exercise control or mental activity control in sedentary older adults. The Look AHEAD trial found no benefit of diet plus physical activity on cognitive function over 8 years.33 A large trial of a multifactorial intervention including diet, physical activity, cognitive training, social activity, and management of metabolic and vascular risk factors showed a small, statistically significant benefit on global and executive cognitive function at 2 years.34 However, it is difficult to compare this trial with the LIFE Study because the population was 10 years younger, physically active at baseline, and had a multi-factorial intervention.
Possible explanations for the lack of cognitive benefit of the LIFE physical activity intervention include: a) the dose of physical activity may have been insufficient to produce changes in the cognitive measures despite its effect on physical function;14 b) improvements in cognitive function in some shorter clinical trials, including the LIFE pilot,13 may dissipate by 24 months and thus may have been missed in LIFE, especially if adherence to the physical activity intervention wanes over time;14 c) the LIFE population was not specifically selected for cognitive vulnerability, though poor physical function, especially gait speed, has been shown to be a risk for cognitive decline;35;36 d) LIFE participants were well educated, with over 2/3 having gone to college, and high cognitive reserve may have protected against cognitive decline over 2 years;39 and e) the health education intervention may have benefited cognition.10;37 The LIFE health education group consisted of interactive seminars providing both cognitive and social stimulation. Both cognitive and social stimulation have been shown to preserve cognition in older adults.10;37
The dose-response relationship between physical activity and cognition is not well-understood.7;38 The LIFE physical activity intervention was designed to provide moderate-intensity aerobic walking activity and was consistent with American College of Sports Medicine recommendations. However, we recruited a population with limited physical ability. It is possible that impaired lower extremity functioning and the high prevalence of comorbidities limited participants’ ability to exercise at sustained levels sufficient to improve cognition. Nonetheless, the LIFE physical activity group had significantly greater physical activity levels than controls, and a more intensive, sustained intervention that could be translatable at the population level would be difficult to achieve.
Despite the lack of overall benefit, our pre-specified subgroup analyses of participants ≥80 years and those with lower baseline physical performance demonstrated that the physical activity group had better performance on executive function tasks than those in the health education group at 24 months. This finding is important because executive function is the most sensitive cognitive domain to exercise interventions40 and preserving executive function is required for independence in instrumental activities of daily living. Future physical activity interventions in particularly vulnerable older adult groups (e.g., those 80 years and older and those with especially diminished physical functioning levels) may be warranted.
To our knowledge, the LIFE Study is the largest, longest randomized clinical trial of a physical activity intervention in sedentary older adults at increased risk for mobility disability. Other strengths include high retention rates, without differential loss to follow-up in the 2 groups; comprehensive standardized, well-validated cognitive assessments; and blinded adjudication of MCI and dementia. However, there are several limitations. First, while cognitive function and incident MCI/dementia were a priori outcomes for the LIFE Study, the LIFE Study was not specifically powered for these outcomes and may have been too short to affect incident events. Second, the intensity of the physical activity intervention was moderate by design. While sufficient to increase physical activity and reduce incident mobility disability,14 it may have been insufficient to produce cognitive effects. Third, the components of the health education intervention, including the cognitive and social components, may have improved or prevented cognitive decline. Fourth, we did not measure changes in mechanistic surrogate outcomes, such as brain volumes or cerebrospinal fluid amyloid beta levels.
Conclusions
Among sedentary older adults, a 24-month moderate intensity physical activity program, compared to a health education program, did not result in improvements in global or domain-specific cognitive function.
Supplementary Material
Acknowledgments
Mark Espeland had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Valerie K. Wilson, MD (Adjunct Instructor, Wake Forest School of Medicine, Winston Salem, NC and Staff physician, Veterans Affairs, Johnson City, TN) contributed to the work reported in this manuscript as an adjudicator of cognitive outcomes. She received compensation for her effort.
Funding:
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes on Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
This research is also supported by the following Claude D. Pepper Older Americans Independence Centers: Boston (1P30AG031679), Florida (1 P30 AG028740), Pittsburgh (P30 AG024827), Wake Forest (1 P30 AG21332) and Yale (P30AG021342).
Dr. Ried’s contribution is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
Role of the Sponsors: The NIH sponsor was avoting member (1 of 12 votes) of the LIFE Steering Committee, which approved the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Appendix: Research Investigators for the LIFE Study
Northwestern University, Chicago, IL
Mary M. McDermott, MD – Field Center Principal Investigator
Bonnie Spring, PhD – Field Center Co-Principal Investigator
Joshua Hauser, MD
Martha Gulati, MD
Sanjiv Shah, MD
Kathryn Domanchuk
Rex Graff
Kasia Kadela
Alvito Rego
Pennington Biomedical Research Center, LA
Timothy Church, MD, PhD, MPH – Field Center Principal Investigator
Steven Blair, PED (University of South Carolina)
Valerie Myers, PhD
Ron Monce, PA-C
Nathan Britt, NP
Melissa Nauta, BS
Ami Parks, MPA, BS
Ruben Rodarte, MBA, MS, BS
Heidi Millet, BS
Catrine Tudor-Locke, PhD, FACSM
Ben Butitta, BS
Sheletta Donatto, MS, RD, LDN, CDE
National Heart, Lung and Blood Institute, Bethesda, MD
Denise Bonds, MD
National Institute on Aging, Bethesda, MD
Evan C. Hadley, MD
Kushang V. Patel, PhD
Sergei Romashkan, MD, PhD
Office of Behavioral and Social Sciences Research, National Institutes of Health
Robert M. Kaplan, PhD
Stanford University, Palo Alto, CA
Abby C. King, PhD – Field Center Principal Investigator
Cynthia M. Castro, PhD
William L. Haskell, PhD
Randall S. Stafford, MD, PhD
Veronica Yank, MD
Leslie A. Pruitt, PhD
Kathy Berra, MSN, NP-C, FAAN
Carol Bell, NP
Rosita Thiessen
Kate P. Youngman, MA
Selene B. Virgen, BAS
Kristina N. Tarin, MS, CSCS
Heather Klaftenegger
Carolyn A. Prosak, RD
Ines Campero, BA
Dulce M. Garcia
José Soto, BA
Linda Chio
David Hoskins, MS
Tufts University, Boston, MA
Roger Fielding, PhD – Field Center Principal Investigator
Miriam Nelson, PhD
Sara Folta, PhD
Edward Phillips, MD
Christine Liu, MD
Erica Cifarelli, MS
Kieran Reid, MSc, MPH
Paige Lacasse, BS
Dylan Kirn, BS
Evan Pasha, BS
Sonnly Ribourg, BS
Karen Ruais, NP
Won Kim, BS
Gregory Cloutier, BS
University of Florida, Gainesville, FL
- Todd Manini, PhD
Field Center Principal Investigator
Marco Pahor, MD – Field Center Co-Principal Investigator
Stephen Anton, PhD
Thomas Buford, PhD
Susan Nayfield, MD, MSc
Joe Nocera, PhD
Michael Marsiske, PhD
Bhanu Sandesara, MD
Jocelyn Lee, PhD
Deborah Hiatt-Jensen, ARNP, MPH
Mieniecia Black, MS
Kimberly Case, PhD
Katherine Herring, BS
Amber Schwier-Delvisco, BS
Bill Burk
Brian Hoover, BS
Jeffrey Knaggs, BS
Allison Martin, MS, RCEP
Brooks Center for Rehabilitation Studies (Satellite Site)
Chonglun Xie, MD
Holly Morris, RN
Flo Singletary, MS, CCC-SLP
Jackie Causer
Susan Yonce, RN
Christine Del Boccio
Charles Gay, D.C.
Tangerica Peavy Alexander
Administrative Coordinating Center
Marco Pahor, MD – Principal Investigator of the LIFE Study
Stephen Anton, PhD
Thomas Buford, PhD
Christiaan Leeuwenburgh, PhD
Susan Nayfield, MD, MSc
Todd Manini, PhD
Connie Caudle
Lauren Crump, MPH
Latonia Holmes
Jocelyn Lee, PhD
Ronald Lester, PhD, MBA
Ching-ju Lu, MPH
Ryan O’Mara
Electrocardiogram Reading Center
Carl J. Pepine MD, MACC
Mario Ariet, PhD
Eileen Handberg, PhD, ARNP
Daniel Deluca, BS
James Hill, MD, MS, FACC
University of Maryland School of Medicine, Baltimore, MD
Jack M. Guralnik, MD, PhD – Co-Principal Investigator of the LIFE Study
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, MD, MPH – Field Center Principal Investigator
Stephanie A. Studenski, MD, MPH – Field Center Co-Principal Investigator
Bret H. Goodpaster, PhD
Oscar Lopez, MD
Nancy W. Glynn, PhD
Janet T. Bonk, MPH, RN
Jennifer Rush, MPH
Piera Kost, BA
Allison Gerger, BS
Pamela Vincent, CMA
LaTisha Davis, BS
Mark A. Newman, PhD
George Grove, MS
Kathy Williams, RN, BSEd, MHSA
VA San Diego Healthcare System and University of California, San Diego, San Diego, CA
Erik J. Groessl, PhD
Wake Forest University, Winston-Salem, NC
Stephen B. Kritchevsky, PhD – Field Center Principal Investigator
Anthony Marsh, PhD – Field Center Co-Principal Investigator
Tina Brinkley, PhD
Jamehl Demons, MD
Kaycee Sink, MD, MAS
Kimberly Kennedy, BA, CCRC
Rachel Shertzer-Skinner, MA, CCRC
Abbie Wrights, MS
Rose Fries, RN, CCRC
Deborah Barr, MA, RHEd, CHES
Cognition Reading Center
Jeff Williamson, MD, MHS – Center Principal Investigator
Kaycee M Sink, MD, MAS – Center Co-Principal Investigator
Hugh C. Hendrie, MB, ChB, DSc (Indiana University)
Stephen R. Rapp, PhD
Joe Verghese, MBBS (Albert Einstein College of Medicine of Yeshiva University)
Nancy Woolard
Valerie K Wilson, MD
Data Management, Analysis and Quality Control Center (DMAQC)
Michael E. Miller, PhD – DMAQC Principal Investigator
Mark Espeland, PhD – DMAQC Co-Principal Investigator
Walter Ambrosius, PhD
Don Babcock, BSEE, PE
Daniel Beavers, PhD, MS
Robert P. Byington, PhD, MPH, FAHA
Delilah Cook
Curt Furberg, MD, PhD
Candace Goode
Jason Griffin, BS
Lea Harvin, BS
Leora Henkin, MPH, Med
John Hepler, BFA
Fang-Chi Hsu, PhD
Kathy Joyce
Laura Lovato, MS
Wesley Roberson, BSBA
Julia Robertson, BS
Julia Rushing, BSPH, MStat
Scott Rushing, BS
Cynthia L. Stowe, MPM
Michael P. Walkup, MS
Don Hire, BS
Jack Rejeski, PhD
Jeff Katula, PhD, MA
Peter H. Brubaker, PhD
Shannon Mihalko, PhD
Janine M. Jennings, PhD
Kathy Lane, BA
Yale University, New Haven, CT
Thomas M. Gill, MD – Field Center Principal Investigator
Robert S. Axtell, PhD, FACSM – Field Center Co-Principal Investigator (Southern Connecticut State University, Exercise Science Department)
Susan S. Kashaf, MD, (VA Connecticut Healthcare System)
Nathalie de Renekeire, MD
Joanne M. McGloin, MDiv, MS, MBA
Raeleen Mautner, PhD
Sharon M. Huie-White, MPH
Luann Bianco, BA
Janice Zocher
Denise M. Shepard, RN, MBA
Barbara Fennelly, MA, RN
Sean Halpin, MA
Theresa Barnett, MS, APRN
Karen C. Wu, RN
Lynne P. Iannone, MS
Julie A. Bugaj, MS
Christine Bailey, MA
Spirometry Reading Center
Geoffrey Chupp, MD
Gail Flynn, RCP, CRFT
Thomas M. Gill, MD
John Hankinson, PhD (Hankinson Consulting, Inc.)
Carlos A. Vaz Fragoso, MD
Footnotes
Disclosures:
Dr. Lopez is a consultant for Baxter, Lilly, Grifols, and Lundbeck
Trial Registration: ClinicalsTrials.gov identifier NCT01072500.
Contributor Information
Kaycee M. Sink, Email: kmsink@wakehealth.edu.
Mark A. Espeland, Email: mespelan@wakehealth.edu.
Cynthia M. Castro, Email: Cynthia.Castro@stanford.edu.
Timothy Church, Email: tim.church@pbrc.edu.
Ron Cohen, Email: roncohen@ufl.edu.
John A. Dodson, Email: john.dodson@nyumc.org.
Jack Guralnik, Email: jguralnik@epi.umaryland.edu.
Hugh C. Hendrie, Email: hhendri@iupui.edu.
Janine Jennings, Email: jjennings@wfu.edu.
Jeffery Katula, Email: katulaj@wfu.edu.
Oscar L. Lopez, Email: lopezol@upmc.edu.
Mary M. McDermott, Email: mdm608@northwestern.edu.
Marco Pahor, Email: mpahor@ufl.edu.
Kieran F. Reid, Email: Kieran.reid@tufts.edu.
Julia Rushing, Email: jrushing@wakehealth.edu.
Joe Verghese, Email: joe.verghese@einstein.yu.edu.
Stephen Rapp, Email: srapp@wakehealth.edu.
Jeff D. Williamson, Email: jwilliam@wakehealth.edu.
Reference List
- 1.Burdette JH, Laurienti PJ, Espeland MA, et al. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 3.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35(Suppl 2):S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduroz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2013;56:10–15. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15:99–108. doi: 10.31887/DCNS.2013.15.1/kerickson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snowden M, Steinman L, Mochan K, et al. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59:704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- 8.Denkinger MD, Nikolaus T, Denkinger C, Lukas A. Physical activity for the prevention of cognitive decline: current evidence from observational and controlled studies. Z Gerontol Geriatr. 2012;45:11–16. doi: 10.1007/s00391-011-0262-6. [DOI] [PubMed] [Google Scholar]
- 9.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes DE, Santos-Modesitt W, Poelke G, et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173:797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legault C, Jennings JM, Katula JA, et al. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: the Seniors Health and Activity Research Program Pilot (SHARP-P) study, a randomized controlled trial. BMC Geriatr. 2011;11:27. doi: 10.1186/1471-2318-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 13.Williamson JD, Espeland M, Kritchevsky SB, et al. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. J Gerontol A Biol Sci Med Sci. 2009;64:688–694. doi: 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fielding RA, Rejeski WJ, Blair SN, et al. The Lifestyle Interventions and Independence for Elders (LIFE) Study: Design and Methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 18.Rejeski WJ, Fielding RA, Blair SN, et al. The Lifestyle Interventions and Independence for Elders (LIFE) Pilot Study: Design and Methods. Contemp Clin Trials. 2005;26:141–154. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sink KM, Espeland MA, Rushing J, et al. The LIFE Cognition Study: design and baseline characteristics. Clin Interv Aging. 2014;9:1425–1436. doi: 10.2147/CIA.S65381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wechsler D. WAIS-III manual. New York: Psychological Corporation; 1997. [Google Scholar]
- 22.Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised Professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 23.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd. Philadelphia, PA: 1983. [Google Scholar]
- 24.Reitan R. Trail Making Test. Manual for administration and scoring. Tucson, AZ: Neuropsychological Laboratory; 1992. [Google Scholar]
- 25.Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55:352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- 26.Eriksen B, Eriksen CW. Effects of noise letters upon the identificaiton of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- 27.Rogers R, Monsell S. Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen. 1995;124:207–231. [Google Scholar]
- 28.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 30.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan Y. Multiple Imputation Using SAS Software. J Statistical Software. 2011;45:1–25. doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espeland MA, Rapp SR, Bray GA, et al. Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci. 2014;69:1101–1108. doi: 10.1093/gerona/glu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 35.Robertson DA, Savva GM, Coen RF, Kenny RA. Cognitive function in the prefrailty and frailty syndrome. J Am Geriatr Soc. 2014;62:2118–2124. doi: 10.1111/jgs.13111. [DOI] [PubMed] [Google Scholar]
- 36.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 38.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


