Abstract
A study of workers exposed to jet fuel propellant 8 (JP-8) was conducted at U.S. Air Force bases and included the evaluation of three biomarkers of exposure: S-benzylmercapturic acid (BMA), S-phenylmercapturic acid (PMA), and (2-methoxyethoxy)acetic acid (MEAA). Postshift urine specimens were collected from various personnel categorized as high (n = 98), moderate (n = 38) and low (n = 61) JP-8 exposure based on work activities. BMA and PMA urinary levels were determined by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS), and MEAA urinary levels were determined by gas chromatography–mass spectrometry (GC-MS). The numbers of samples determined as positive for the presence of the BMA biomarker (above the test method’s limit of detection [LOD = 0.5 ng/ml]) were 96 (98.0%), 37 (97.4%), and 58 (95.1%) for the high, moderate, and low (control) exposure workgroup categories, respectively. The numbers of samples determined as positive for the presence of the PMA biomarker (LOD = 0.5 ng/ml) were 33 (33.7%), 9 (23.7%), and 12 (19.7%) for the high, moderate, and low exposure categories. The numbers of samples determined as positive for the presence of the MEAA biomarker (LOD = 0.1 μg/ml) were 92 (93.4%), 13 (34.2%), and 2 (3.3%) for the high, moderate, and low exposure categories. Statistical analysis of the mean levels of the analytes demonstrated MEAA to be the most accurate or appropriate biomarker for JP-8 exposure using urinary concentrations either adjusted or not adjusted for creatinine; mean levels of BMA and PMA were not statistically significant between workgroup categories after adjusting for creatinine.
Biomarkers of exposure are important tools for use in exposure assessment and toxicological research. As the term implies, biomarkers of exposure are those related to exposure and the internal levels of some agent or chemical. A well-chosen biomarker of exposure should have several qualities. Primarily, the biomarker should be specific for the exposure of interest; some metabolites are common to multiple parent chemical substances and therefore may not be suitably specific biomarkers. Second, the biomarker needs to be associated with the exposure, and it needs to provide good predictive value to a specific health status. Third, a biomarker needs to have reference values in the population if possible (B’Hymer and Cheever 2010). With these qualities being considered, JP-8, the primary fuel used by the Department of Defense, becomes an interesting challenge with respect to assessing exposure. JP-8 is a kerosene-based complex chemical mixture containing hundreds of aliphatic and aromatic hydrocarbons with various isomer forms plus several additives (Ritchie et al. 2003). The fuel is formulated to meet military performance specifications, and therefore, the overall chemical composition varies from batch to batch with the exception of an anti-icing component (NRC 2003). With the many constituent chemicals present at varying concentrations, the best or most accurate biomarker for JP-8 exposure has not been extensively addressed in the literature, and this was the main objective of the current study. Three potential biomarkers of JP-8 exposure were compared since a previous study had investigated only one of these potential biomarkers (B’Hymer et al. 2012).
The specifications for JP-8 include a maximum olefin content of 5%, a maximum aromatic content of 22%, and a maximum sulfur content of 0.3%. On average, the composition is approximately 33–61% alkanes, 10–45% cycloalkanes, 12–22% aromatics and 0.5–5% olefins (Vere 2003). Toluene and benzene are two important aromatic compounds in JP-8. Biotransformation of these two aromatic compounds has been thoroughly studied, and the applicability of their metabolites as possible biomarkers of exposure in humans has been discussed (Kim et al. 2006; Manini et al. 2004; Qu et al. 2000). Another important component of JP-8 is 2-(2-methoxyethoxy)ethanol; this is added to the fuel as an anti-icing agent and is formulated at a consistent concentration of 0.1%. 2-(2-Methoxyethoxy)ethanol metabolites have been previously discussed with respect to their applicability for possible use as JP-8 biomarkers of exposure (B’Hymer and Cheever 2010). The toxicity of many of the component chemicals in jet fuel has been well established, and reviews on this topic appeared elsewhere (Mattie and Sterner 2011; Ritchie et al. 2003) and thus are not discussed further.
Two of the known common urinary metabolites of toluene include S-benzylmercapturic acid (BMA) and hippuric acid (Marchese et al. 2004). A simplified metabolic scheme for toluene is shown in Figure 1, and a full description of the metabolic pathway can be found elsewhere (Rietveld et al. 1983; Angerer et al. 1998). O-Cresol and hippuric acid are the traditional urinary metabolites used to measure toluene exposure and are the more abundant metabolites of toluene exposure. The American Conference of Governmental Industrial Hygienists (ACGIH) has set biological exposure indices (BEI) levels for these two compounds. The BEI level for o-cresol is 0.5 mg/L, and the BEI level for hippuric acid is 1.6 mg/mg creatinine in urine samples collected at the end of a shift (ACGIH 2011). However, hippuric acid is not specific to toluene exposure. Other studies showed BMA to be the preferred metabolite for use as a biomarker of toluene exposure (Angerer et al. 1998; Inoue et al. 2002), and therefore BMA was selected for the current study.
FIGURE 1.
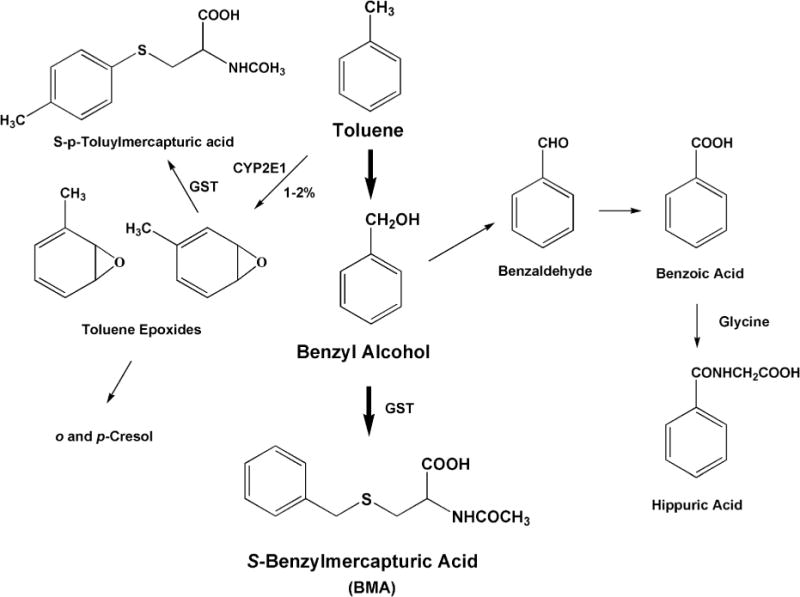
The metabolic formation of S-benzylmercapturic acid (BMA) from toluene, a common component in jet fuel. BMA was one of the metabolites evaluated in this study.
Some of the common urinary metabolites of benzene include S-phenylmercapturic acid (PMA), trans,trans-muconic acid (t,t-MA), hydroquinone, catechol, phenol, and trihydrobenzene (Qu et al. 2000; Sabourin et al. 1988). A simplified metabolic scheme for benzene is shown in Figure 2 and is not described in further detail here. The ACGIH has set recommended BEIs for an end-of-shift urine sample containing PMA at 25 ng/mg creatinine and t,t-MA at 500 ng/mg creatinine (ACGIH 2001). Although phenol and its conjugates are the more abundant biotransformation products for benzene, these compounds are not suitable as specific biomarkers for benzene as they can also be the metabolic products of other common chemicals. As with BMA from its parent toluene, PMA is the preferred metabolite for use as biomarker for benzene exposure (Lovreglio et al. 2010; Melikian et al. 1999) and is the target for the current study.
FIGURE 2.
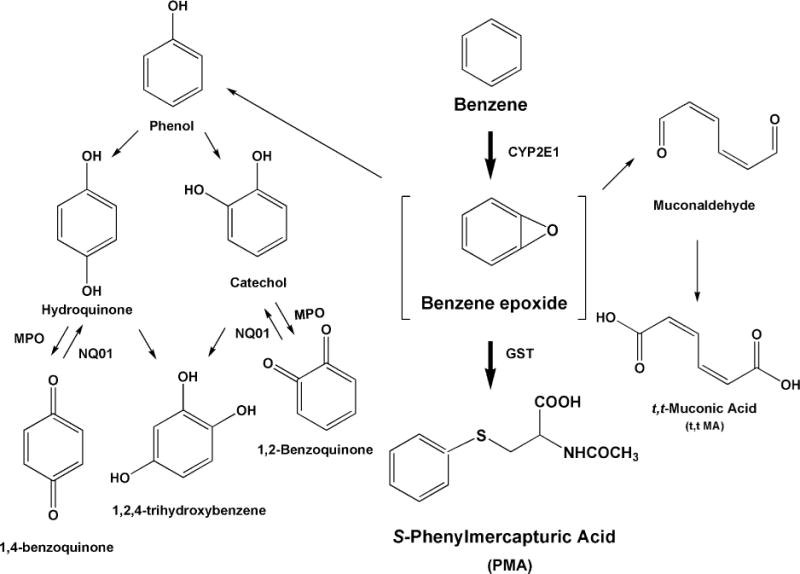
The metabolic formation of S-phenylmercapturic acid (PMA) from benzene, a common component in jet fuel. PMA was one of the metabolites evaluated in this study.
The compound 2-(2-methoxyethoxy) ethanol (also known as diethylene glycol monomethyl ether [DiEGME] and under the trade name of methyl carbitol, CAS 111-77-3) is another component of JP-8. It is unique in being an additive in the formulation of JP-8 for the military and is at a consistent concentration of 0.1% (v/v) for every batch of the fuel as previously described. 2-(2-Methoxyethoxy)ethanol, a glycol ether, has a limited number of industrial uses, including in formulation of inks and some paints. Although benzene and toluene are commonly used in many home products and other fuels, 2-(2-methoxyethoxy)ethanol is encountered much less frequently in the general environment. The corresponding metabolite of this glycol ether is (2-methoxyethoxy)acetic acid (MEAA). Because of the low frequency of use of the parent compound, the MEAA metabolite has not been detected in the general population and should not appear in any significant quantity in a control population. MEAA has been studied for use as a biomarker previously in both humans (B’Hymer et al. 2012) and animals (B’Hymer et al. 2005a; Richards et al. 1993). It has also been demonstrated that MEAA is the urinary metabolite best suited for use as a short-term biomarker for exposure to 2-(2-methoxyethoxy)ethanol (Richards et al. 1993). Animal studies demonstrated a rapid conversion of the parent compound to MEAA (Daniel et al. 1991; Richards et al. 1993). The metabolism of 2-(2-ethoxyethoxy)ethanol is complex, and the mechanism based on animal studies (Cheever et al. 1988; Sumner et al. 1992) is presented in Figure 3. MEAA is the predominate metabolite in animals and the urinary levels of this compound are fairly abundant. It was found that male rats dosed with 14C-labeled parent 2-(2-methoxyethoxy)ethanol excreted 68–70% of the label as the MEAA metabolite by means of ADH conversion (Cheever et al. 1988). The same study showed that only trace levels of 2-(2-methoxyethoxy)ethanol or methoxyethoxyacetaldehyde were detected in the urine. Further, cumulative excretion of 14C-tagged compounds showed rapid excretion (50% of administered dose excreted in 8 h) during rat oral dosing studies (Cheever et al. 1988). Study results from this laboratory also demonstrated that rat urinary MEAA is in the free form, not conjugated. Therefore, MEAA represents a viable candidate for analytical quantitation and was used as basis for comparison of JP-8 exposure biomarkers in the current study.
FIGURE 3.
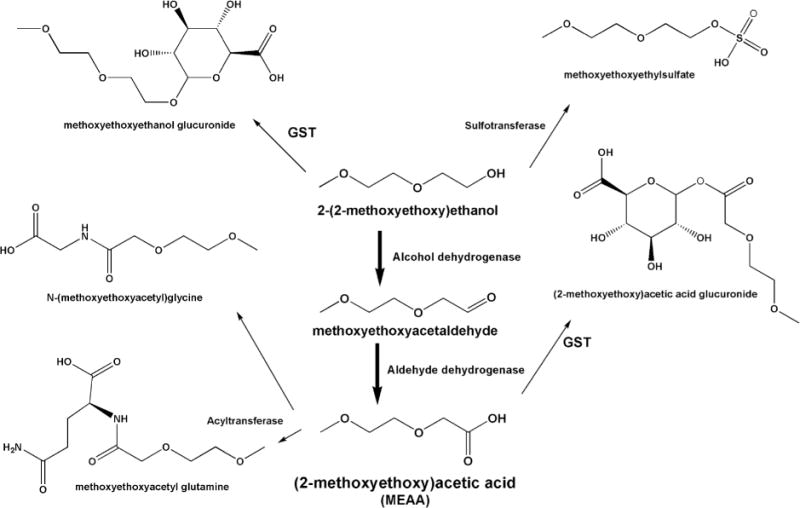
The metabolic formation of (2-methoxyethoxy)acetic acid (MEAA) from the JP-8 anti-icing agent 2-(2-methoxyethoxy)ethanol. MEAA is one of the metabolites evaluated in this study.
MATERIALS AND METHODS
Study Participants
This study was conducted on six USAF bases located within the continental United States. The participants were recruited with jobs rated as high, moderate, or low potential for jet fuel exposure. The high-exposure work-group category consisted of aircraft fuel-system maintenance workers, that is, those whose jobs included maintaining and repairing aircraft fuel tanks (termed fuel cells), fuel lines, and associated structures. Workers were classified as “entrant,” “attendant,” or “runner,” based on the duties performed the day of observation during the study. The “entrant” had the job of actually entering the aircraft fuel tank or fuel cell wearing a supplied-air respirator. The “attendant” assisted the entrant and stayed outside the tank at all times. The “runner” stayed outside the tank and handled moving fuel-soaked foam that had been removed from the tank to a storage location or obtained tools for the “entrant.” Protective equipment was limited to forced air respirators, gloves, and cotton overalls for the “entrant.” The “attendant” was limited to cotton overalls. The moderate-exposure group consisted of personnel who did not perform fuel-tank maintenance but conducted work that involved regular contact with jet fuel, such as fueling aircraft or maintaining fuel storage facilities. The low-exposure group consisted of workers whose jobs covered a wide variety of activities that did not require exposure to the jet fuel on the air force bases, and this group was used as a control group for the purpose of this study.
Collection of Urine Samples
Urine samples were collected from participants in the low (n = 61), moderate (n = 38), and high (n = 98) groups at the end of a 4-h work shift for urinary measurements. The samples were packed with frozen Blue Ice (Newell Rubbermaid, Inc., Atlanta, GA) and shipped to arrive within 24 h at the NIOSH laboratory. After arrival to the laboratory, samples were stored at −80°C until analysis.
Chemicals and Reagents
The BMA (S-benzylmercapturic acid, N-acetyl-S-benzyl-DL-cysteine, CAS number 19542-77-9) reference standard was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). The PMA (S-phenylmercapturic acid, N-acetyl-S-phenyl-DL-cysteine, CAS number 20640-68-0) reference standard was purchased from Tokyo Chemical Industry Co., Ltd. (TCI, Tokyo). The deuterated analogs, S-benzyl-d5-mercapturic acid and S-phenyl-d5-mercapturic acid, were purchased from CDN Isotopes (Quebec, Canada). Standard compounds of (2-methoxyethoxy)acetic acid (MEAA, CAS number 16024-59-9) and the deuterated 2-butoxyacetic acid (d-BAA), used as an internal standard, were synthesized and described previously (Cheever et al. 1988; Brown et al. 2003). All other reagents used were analytical grade and are regularly available for laboratory use.
BMA and PMA HPLC-MS/MS Analysis
Urinary BMA and PMA levels were measured using a method previously validated and described (B’Hymer 2011). Briefly, a 4-ml aliquot of urine was spiked with deuterated analogs of BMA and PMA to act as internal standards. The target analytes were extracted by solid-phase extraction using Bond Elut C18 cartridges (Varian, Inc., Harbor City, CA) containing 500 mg of solid-phase bed. The analytes were recovered by acetone washes and evaporated to dryness. The dry extracts were dissolved in a 5/95/0.1% (v/v/v) solution of acetonitrile/water/acetic acid. A high-performance liquid chromatograph (HPLC) equipped with a tandem mass spectrometric detector (MS/MS) and a Zorbax RX C18 column (Agilent Technologies, Santa Clara, CA) with gradient elution (acetonitrile/water/acetic acid) was used to analyze the samples and prepared standards. The limit of detection (LOD) for this method was 0.5 ng/ml for both BMA and PMA.
MEAA GC-MS Analysis
Urinary MEAA levels were measured using a method previously validated and described in the literature (B’Hymer et al. 2003; 2005b). Briefly, a 4-ml aliquot of urine was acidified with 12 N hydrochloric acid to pH 1–1.5, and the sample was spiked with d-BAA to act as an internal standard. The MEAA and internal standard were extracted by liquid–liquid extraction (LLE) using ethyl acetate, and the extraction solvent was evaporated to a 1-ml volume. Ethanol and concentrated sulfuric acid were used to react with the target analytes to form the corresponding ethyl esters. These products were extracted by LLE using methylene chloride and the methylene chloride solvent was reduced to a 1-ml volume by evaporation. Standard MEAA was used to spike unexposed urine at various levels and treated similarly to create the calibration curves for quantitation. A gas chromatograph (GC) equipped with a mass spectrometric detector and an HP-1 capillary column (Agilent Technologies, Santa Clara, CA) was used to analyze the sample and prepared standard solutions. The LOD for this method was 0.1 μg MEAA/ml urine.
Creatinine Determination
Urine samples were diluted 1:20 to measure creatinine concentrations using a Vitros 250 Chemistry Analyzer (Ortho-Clinical Diagnostics, Rochester, NY), which employs a slide composed of a dry, multilayered analytical element coated on a polyester support (Findlay et al. 1985; Mauck et al. 1986). Creatinine measurements were calibrated with a 3-level set of standards, the highest being 17 mg/dl, which corresponds to a creatinine concentration of 340 mg/dl for urine samples diluted 20-fold. Urine control pools (Bio-Rad Laboratories, Hercules, CA) and instrument Vitros Performance Verifier pools (Ortho-Clinical Diagnostics) were assayed in duplicate at the front, middle, and rear of each analytical run.
Statistical Analysis
Pairwise t-tests for unequal variances (Cochran and Cox 1950) were used to test for differences in mean concentrations between the workgroups. Analyses of the concentrations both not adjusted and adjusted for creatinine were performed. Measurements below the limit of detection (LOD, 0.5 ng/ml for BMA and PMA, 0.1 μg/ml for MEAA) were assigned a value of the limit of detection divided by the square root of 2. Hornung and Reed (1990) recommend this method for data that are lognormal distributed and not highly skewed. All calculations were made using SAS (Version 9.2, SAS Institute, Inc., Cary, NC).
RESULTS AND DISCUSSION
The BMA and PMA results of this study are summarized in Table 1 and are compared to a summary of MEAA data that were collected and reported previously (B’Hymer et al. 2012). The numbers of samples determined as positive for the presence of the BMA biomarker, that is, above the test method’s LOD (0.5 ng/ml), were 96 (98.0%), 37 (97.4%), and 58 (95.1%) for the high, moderates and low exposure work-group categories, respectively. The means of the three workgroups are also presented in Table 1 and graphically in Figure 4A. Toluene is commonly encountered in the general environment from its use in most liquid fuels; therefore, its metabolite would be expected to be present in most of the individuals tested including the low exposure control group. Our modern motor vehicle-based society represents one of the main emission sources for toluene; thus, the study population, as well as the general population, undergoes a lifelong exposure to this chemical. Statistical analysis of the means for the three exposure workgroups was also undertaken (see Table 1). For BMA (ng/ml), the mean for the high group was greater than the mean for the low group, and this difference was statistically significant, while the difference between the means of the high and moderate group was not markedly different. In addition, when the mean urinary BMA levels were adjusted for creatinine (ng/mg creatinine), none of the means from the workgroups had differences of statistical significance. Owing to the high incidence of BMA metabolite being present in all samples (greater than 95%) and the lack of statistical significance detected between the means of the work groups after adjusting for creatinine, it can be safely concluded that BMA is not an ideal research tool for use as a biomarker of JP-8 exposure. It should also be noted that two individuals in the low exposure group had exceedingly high levels of urinary BMA and were dropped from the statistical analysis. Those two outlier values were 144 and 244 ng/ml; these values were confirmed by triplicate analysis. These two individuals had job descriptions obviously excluding them from exposure to JP-8 fuel. It was concluded that their exposure was from another environmental source, and this further weakened BMA for use as an accurate biomarker of JP-8 exposure.
TABLE 1.
Postshift Urinary Metabolite Measurements of U.S. Air Force Personnel
| Exposure group | n | Mean | Standard deviation | Minimuma | Maximum | N > LODb (%) |
|---|---|---|---|---|---|---|
| BMA (ng/ml) | ||||||
| Low | 593c | 8.7 | 7.8 | 0.35 | 32.5 | 58 (95.1) |
| Medium | 38 | 8.9 | 8.1 | 0.35 | 35.5 | 37 (97.4) |
| High | 98 | 11.9e | 7.7 | 0.35 | 33.9 | 96 (98.0) |
| PMA (ng/ml) | ||||||
| Low | 61 | 0.5 | 0.3 | 0.35 | 1.5 | 12 (19.7) |
| Medium | 38 | 0.5 | 0.4 | 0.35 | 2.7 | 9 (23.7) |
| High | 98 | 1.0e,f | 1.6 | 0.35 | 12.5 | 33 (33.7) |
| MEAA (μg/ml)d | ||||||
| Low | 61 | 0.07 | 0.02 | 0.07 | 0.2 | 2 (3.3) |
| Medium | 38 | 0.42e | 0.89 | 0.07 | 4.8 | 13 (34.2) |
| High | 98 | 6.8e,f | 15.2 | 0.07 | 110 | 92 (93.9) |
| BMA (ng/mg creatinine) | ||||||
| Low | 59c | 7.8 | 13.4 | 0.36 | 1.5 | — |
| Medium | 38 | 5.2 | 3.5 | 0.47 | 2.7 | — |
| High | 98 | 4.9 | 4.9 | 1.32 | 16.6 | — |
| PMA (ng/mg creatinine) | ||||||
| Low | 61 | 0.44 | 0.34 | 0.10 | 1.5 | — |
| Medium | 38 | 0.44 | 0.57 | 0.09 | 3.4 | — |
| High | 98 | 0.44 | 0.54 | 0.07 | 3.5 | — |
| MEAA (μg/mg creatinine) | ||||||
| Low | 61 | 0.08 | 0.07 | 0.02 | 0.30 | — |
| Medium | 38 | 0.26e | 0.44 | 0.02 | 2.3 | — |
| High | 98 | 2.9e,f | 6.4 | 0.02 | 45 | — |
| Creatinine (mg/ml) | ||||||
| Low | 61 | 1.50 | 0.81 | 0.23 | 3.59 | — |
| Medium | 38 | 1.76 | 0.91 | 0.10 | 4.04 | — |
| High | 98 | 2.48 | 1.10 | 0.25 | 5.50 | — |
Minimum = minimum concentration; 0.35 ng/ml for BMA and PMA and 0.07 μg/ml for MEAA.
LOD = limit of detection; 0.5 ng/ml for BMA and PMA and 0.1 μg/ml for MEAA. Samples with less than the LOD are coded as 0.35 ng/ml for BMA and PMA and 0.07 μg/ml for MEAA for use in statistical evaluation only. This is the LOD divided by the square root of 2 (see the text for a full explanation).
Two individuals from the low exposure group had BMA levels far exceeding any other measured value: 144 and 244 ng/ml. These were treated as outliers for purposes of determining the mean and standard deviation for this workgroup; thus, n = 59 for the mean and standard deviation (see the text for a full explanation).
MEAA data alone reported previously (B’Hymer et al. 2012).
The difference from the low exposure (control) group was statistically significant, p < .05.
The difference from the medium exposure group was statistically significant, p < .05.
FIGURE 4.
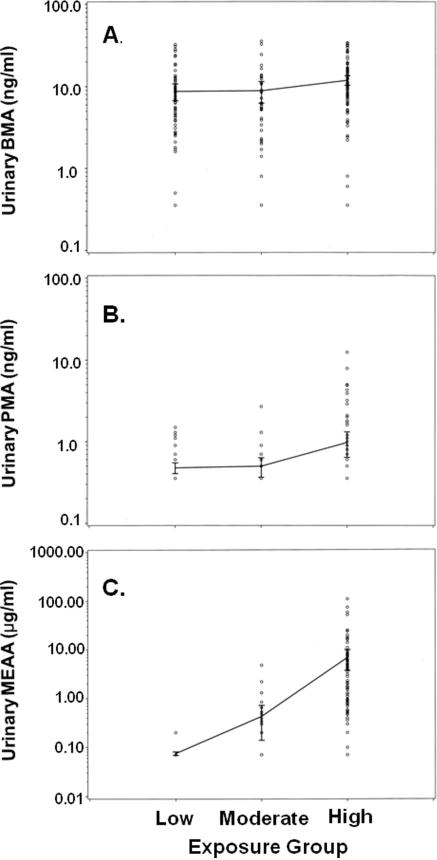
Comparisons of urinary metabolite levels in low, moderate, and high jet-fuel exposure categories. All urinary concentrations are on a log10 scale. (A) BMA is measured in ng/ml. (B) PMA is measured in ng/ml. (C) MEAA is measured in μg/ml.
The numbers of samples determined above the LOD for benzene metabolite PMA (0.5 ng/ml) were 33 (33.7%), 9 (23.7%), and 12 (19.7%) for the high, moderate, and low exposure workgroup categories, respectively. The means of the three workgroups are also presented in Table 1 and graphically in Figure 4B. For PMA (ng/ml), the mean for the high group was greater than the means for the low and the moderate groups, and the differences were statistically significant in each case. However, after PMA was adjusted for creatinine (ng/mg creatinine), none of the workgroup means had statistically significant differences from one another. Benzene represents a fairly small component in the fuel and is quickly lost by expiration. Therefore, low urinary levels can be expected. Detection limits could have been reduced by a methodology utilizing extraction of the PMA metabolite from larger volumes of urine, but this would have led to a more expensive test. PMA can also be confounded by smoking since benzene is a minor product of tobacco combustion. Considering these factors and the evaluation of this study’s results, PMA was determined not to be an ideal tool for use as a biomarker of JP-8 exposure.
The numbers of samples determined above the LOD for MEAA (0.1 μg/ml) were 92 (93.4%), 13 (34.2%), and 2 (3.3%) for the high, moderate, and low (control) exposure categories. The means of the three workgroups are also presented in Table 1 and graphically in Figure 4C. For MEAA (μg/ml), the mean for the high group was greater than the means of both the low and the moderate workgroup categories, and the differences were statistically significant in each case. The mean of the moderate exposure category was greater than the mean of the low category and was also statistically significant. Similar results of statistical significance were determined for the means of the workgroups when adjusting for creatinine. Again for MEAA, the mean (μg/ mg creatinine) of the high category was greater than the means for the low and the moderate workgroup categories, and these differences were statistically significant in each case. The mean for the moderate category was greater than the mean for the low category and was statistically significant. Given the wide differences in the number of samples positive for the presence of MEAA and differences in the mean levels of urinary MEAA between the workgroups, MEAA was clearly demonstrated to be the best metabolite of the three evaluated for use as an accurate biomarker of JP-8 exposure. Also, comparison of the graphed data (see Figure 4) shows less concentration overlap of the three exposure categories for MEAA versus PMA and BMA, as well as the more significant difference in mean concentration values between the three exposure categories.
A discussion about creatinine urine levels is also in order. There were differences in the creatinine levels between the work groups. The mean levels of creatinine are shown in Table 1, and the mean of the high exposure group was greater than other groups with statistically significant differences in each case. This was likely owing to dehydration and the increased physical workloads of participants in the high exposure group. The ACGIH, as well as other authorities, often uses creatinine-adjusted urinary levels for its BEI recommendations for this very reason. Creatinine adjustment is a traditional normalization procedure for many urinary analytes. For MEAA, its concentration in urine and that adjusted for creatinine show statistically similar results for the high, moderate, and low (control) exposure categories. This was not an unexpected result; the use of creatinine to normalize urinary analyte concentrations has been extensively reported to not necessarily improve correlation of dose to exposure for other urinary components (Allessio et al. 1985; Boeniger et al. 1993; Carrieri et al. 2001). Gaines et al. (2010) suggested the use of urine specific gravity for biomarker normalization as an alternative to creatinine. However, for the current JP-8 exposure study, creatinine-adjusted and nonadjusted values were the only normalization schemes investigated.
In summary, MEAA fits the qualities of a well-chosen biomarker of exposure. First, it appears to be relatively specific for the exposure of interest, namely, JP-8. There are few outside sources for its parent compound, unlike PMA and BMA from benzene and toluene, respectively. Second, MEAA is easily detectable at levels to distinguish exposure group categories of workers potentially exposed to JP-8. The high exposure category from this study had significantly higher levels of MEAA. The mean of the high exposure group was 6.8 μg/ml, which was 68-fold greater than the LOD for the test procedure. Statistically, the MEAA means of the high, moderate, and low (control) exposure categories differ significantly for both creatinine-adjusted and nonadjusted concentration levels, unlike BMA and PMA. The gas chromatographic method (B’Hymer et al. 2003; 2005a) used in this study appears to have adequate MEAA sensitivity for its use as a biomarker of exposure. MEAA, therefore, is clearly associated with JP-8 exposure work-groups and has the potential for good predictive value, a desirable characteristic of a well-chosen biomarker of occupational exposure. Finally, the gas chromatographic test method is relatively inexpensive to use; liquid–liquid extraction was used with low-cost solvents and acid-catalyzed esterification, which avoided more expensive chemical derivatizing reagents.
However, there are obvious limitations for the use of MEAA as a biomarker of JP-8 exposure. MEAA represents a metabolite of an additive component of JP-8; it does not represent a metabolite of any of the other components within the fuel itself, and therefore has little clinical value for an individual. Obviously, the toluene and benzene biomarkers, BMA and PMA, would have a greater utility in the assessment of an individual’s exposure to the respective toxic parent compounds. The rate of skin penetration and bioavailability from inhalation would be different between the toxic components in JP-8, so that a single biomarker can only be used as an indirect biomarker for the other toxic components in the jet fuel. The metabolic conversion of 2-(2-methoxyethoxy)ethanol to MEAA is also fairly rapid; the half-life was determined to be approximately 8 h in various animal studies (Cheever et al. 1988; Daniel et al. 1991; Richards et al. 1993). MEAA, therefore, represents a biomarker of acute exposure to JP-8. Finally, MEAA production is based upon aldehyde dehydrogenase and alcohol dehydrogenase conversion metabolism. In this study, over 90% of the participants were ethnic Caucasian or African and had not consumed alcohol within 24 h of the work shift and urine collection. MEAA would not be a useful biomarker of JP-8 exposure in individuals with reduced metabolism, whether through mutations or hereditary factors, since the target urinary biomarker would not be produced.
CONCLUSIONS
This study evaluated and compared BMA, PMA, and MEAA for use as a biomarker of JP-8 exposure. Despite BMA and PMA being described in outside studies as the preferred metabolites for toluene and benzene exposure, urinary MEAA analysis yielded the best statistical results to distinguish between workgroups having high, moderate, and low exposure to the JP-8 fuel. The proportions of positive measurements for MEAA (greater than the test method’s LOD [0.1 μg/ml]) were 93.9%, 34.2%, and 3.3% for the high, moderate, and low exposure categories, respectively. The toluene metabolite BMA was present in most urine samples and was probably due to the widespread existence of toluene in the general environment. Therefore, BMA was not a useful biomarker for JP-8 exposure, but may be used as biomarker for general exposure to toluene. The benzene metabolite PMA likely would not be a useful metabolic biomarker for JP-8 exposure owing to the low concentration benzene in the batches of fuel and the need for more sensitive instrumentation or a greater expense for laboratory testing. MEAA, therefore, represents an ideal research tool for use as a biomarker of JP-8 exposure with a sensitive and relatively low-cost analysis methodology.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH) or the Centers for Disease Control and Prevention (CDC). Mention of company names and/or products does not constitute an endorsement by NIOSH.
The authors thank the Department of Defense and the U.S. Air Force for their cooperation and help with this study. The authors also thank Jeanette Krause, Dennis Lynch, John Lipscomb, David R. Mattie, and Deborah Keil for their help in reviewing and editing this article.
References
- Allessio L, Berlin A, Dell’Orto A, Toffoletto F, Chezzi I. Reliability of urinary cratinine as a parameter used to adjust values of urinary biological indicators. Int Arch Occup Environ Health. 1985;55:99–106. doi: 10.1007/BF00378371. [DOI] [PubMed] [Google Scholar]
- American Conference of Governmental Industrial Hygienists. TLVs and BEIs for chemical substances and physical agents. Cincinnati, OH: ACGIH; 2011. [Google Scholar]
- Angerer J, Schilbach M, Kramer A. S-p-Toluylmercapturic acid in the urine of workers exposed to toluene: A new biomarker of toluene exposure. Arch Toxicol. 1998;72:119–23. doi: 10.1007/s002040050478. [DOI] [PubMed] [Google Scholar]
- B’Hymer C. Validation of an HPLC-MS-MS method for the determination of urinary S-benzylmercapturic acid and S-phenylmercapturic acid. J Chromatogr Sci. 2011;49:547–53. doi: 10.1093/chrsci/49.7.547. [DOI] [PubMed] [Google Scholar]
- B’Hymer C, Cheever KL. Biomarkers and metabolites: HPLC/MS analysis. In: Cazes J, editor. Encyclopedia of chromatography. 3rd. Boca Raton, FL: CRC Press/Taylor & Francis; 2010. pp. 238–46. [Google Scholar]
- B’Hymer C, Cheever KL, Butler MA, Brown KK. Procedure for the quantification of the biomarker (2-methoxyethoxy)acetic acid in human urine. J Chromotogr A. 2003;795:145–50. doi: 10.1016/s1570-0232(03)00552-x. [DOI] [PubMed] [Google Scholar]
- B’Hymer C, Keil DE, Cheever KL. A test procedure for the determination of (2-methoxyethoxy)acetic acid in urine from jet fuel-exposed mice. Toxicol Mech Methods. 2005a;15:367–73. doi: 10.1080/153765291009976. [DOI] [PubMed] [Google Scholar]
- B’Hymer C, Butler MA, Cheever KL. A comparison and evaluation of analysis procedures for the quantification of (2-methoxyethoxy)acetic acid in urine. Anal Bioanal Chem. 2005b;383:201–209. doi: 10.1007/s00216-005-0048-z. [DOI] [PubMed] [Google Scholar]
- B’Hymer C, Mathias P, Krieg E, Cheever KL, Toennis CA, Clark JC, Kesner JS, Gibson RL, Butler MA. (2-Methoxyethoxy)acetic acid: A urinary biomarker of exposure for jet fuel JP-8. Int Arch Occup Environ Health. 2012;85:413–20. doi: 10.1007/s00420-011-0687-7. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am Ind Hyg Assoc J. 1993;54:615–27. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Brown KK, Cheever KL, Butler MA, Shaw P, McLaurin JL. Synthesis, characterization and use of 2-[(2H9)butoxy]acetic acid and 2-(3-methylbutoxy)acetic acid as an internal standard and instrument performance surrogate, respectively, for the gas chromatographic-mass spectrometric determination of 2-butoxyacetic acid, a human metabolite of 2-butroxyethanol. J Chromatogr B. 2003;792:150–59. doi: 10.1016/s1570-0232(03)00256-3. [DOI] [PubMed] [Google Scholar]
- Carrieri M, Tevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: Correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001;74:63–67. doi: 10.1007/s004200000190. [DOI] [PubMed] [Google Scholar]
- Cheever KL, Richards DL, Weigel WW, Dinsmore AM, Daniel FB. Metabolism of bis(2-methoxethyl)ether in the adult male rat—Evaluation of the principle metabolite as a testicular toxicant. Toxicol Appl Pharmacol. 1988;94:150–59. doi: 10.1016/0041-008x(88)90345-6. [DOI] [PubMed] [Google Scholar]
- Cochran WG, Cox GM. Experimental designs. New York: John Wiley & Sons; 1950. [Google Scholar]
- Daniel FB, Cheever KL, Begley KB, Richards DE, Weigel WW. Bis(2-methoxyethyl)ether: Metabolism and embryonic disposition of a developmental toxicant in the pregnant CD-1 mouse. Fundam Appl Toxicol. 1991;16:567–75. doi: 10.1016/0272-0590(91)90096-m. [DOI] [PubMed] [Google Scholar]
- Findlay JB, Wu AL, Knott V, Mauck L, Frickey PH, Norton GE. Development of a Kodak Ektachem® clinical chemistry slide for CK-B activity. Clin Chem. 1985;31:1000. [Google Scholar]
- Gaines LGT, Fent KW, Flack SL, Thomasen JM, Ball LM, Zhou H, Whittaker SG, Nylander-French LA. Effect of creatinine and specific gravity normalization on urinary biomarker 1,6-hexamethylene diamine. J Environ Monit. 2010;12:591–99. doi: 10.1039/b921073c. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Inoue Q, Kanno E, Yusa T, Kalizaki M, Ukai H, Okamoto S, Higashikawa K, Ikeda M. Urinary benzylmercapturic acid as a marker of occupational exposure to toluene. Int Arch Occup Environ. 2002;75:341–47. doi: 10.1007/s00420-002-0322-8. [DOI] [PubMed] [Google Scholar]
- Lovreglio P, Barbieri A, Carrieri M, Sbabatini L, Fracasso ME, Doria D, Drago I, Basso A, D’Errico MN, Bartolucci GB, Vilante FS, Soleo V. Validity of new biomarkers of internal dose for use in biological monitoring of occupational and environmental exposure to low concentrations of benzene and toluene. Int Arch Occup Environ Health. 2010;83:341–56. doi: 10.1007/s00420-009-0469-7. [DOI] [PubMed] [Google Scholar]
- Kim S, Vermeulen R, Waidyanatha S, Johnson BA, Lan Q, Li G, Shen M, Yen S, Rappaport SM. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis. 2006;27:772–81. doi: 10.1093/carcin/bgi297. [DOI] [PubMed] [Google Scholar]
- Manini P, Andreoli R, Niessen WMA. Liquid chromatography-mass spectrometry in occupational toxicology: A novel approach to the study of biotransformation of industrial chemicals. J Chromatogr A. 2004;1058:21–37. [PubMed] [Google Scholar]
- Marchese S, Cruini R, Gentili A, Perret D, Rocca LM. Simultaneous determination of urinary metabolites of benzene, toluene, xylene and styrene using high-performance liquid chromatography/hybrid quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:265–72. doi: 10.1002/rcm.1323. [DOI] [PubMed] [Google Scholar]
- Mattie DR, Sterner TR. Past, present and emerging toxicity issues for jet fuel. Toxicol Appl Pharmacol. 2011;254:127–32. doi: 10.1016/j.taap.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Mauck JC, Mauck L, Novros J, Norton GE, Toffaletti J. Development of a single slide Kodak Ektachem® thin-film assay for serum and urine creatinine. Clin Chem. 1986;32:1197–98. [Google Scholar]
- Melikian AA, O’Connor R, Prahalad AK, Hu P, Heyi L, Kagan M, Thompson S. Determination of the urinary benzene metabolites S-phenylmercapturic acid and trans, trans-muconic acid by liquid chromatography-tandem mass spectrometry. Carcinogenesis. 1999;20:719–26. doi: 10.1093/carcin/20.4.719. [DOI] [PubMed] [Google Scholar]
- National Research Council. Toxicologic assessment of jet-propulsion fuel 8. Washington, DC: National Academies Press; 2003. National Research Council of the National Academies. [PubMed] [Google Scholar]
- Qu QS, Melikian AA, Li GL, Shore R, Chen LC, Cohen B, Yin SN, Kagan MR, Li HY, Meng M, Jin XM, Winnik W, Li YY, Mu RD, Li KQ. Validation of biomarkers in humans exposed to benzene: Urine metabolites. Am J Ind Med. 2000;37:522–31. doi: 10.1002/(sici)1097-0274(200005)37:5<522::aid-ajim8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Richards DE, Begley KB, DeBord DG, Cheever KL, Weigel WW, Tirmenstein MA, Savage RE. Comparative metabolism of bis(2-methoxyethyl)ether in isolated rat hepatocytes and in the intact rat-effects of ethanol on in vitro metabolism. Arch Toxicol. 1993;67:531–37. doi: 10.1007/BF01969265. [DOI] [PubMed] [Google Scholar]
- Rietveld EC, Plate R, Seutter-Berlage F. Mechanism of formation of mercapturic acids from aromatic aldehydes in vivo. Arch Toxicol. 1983;52:199–207. doi: 10.1007/BF00333899. [DOI] [PubMed] [Google Scholar]
- Ritchie GD, Still KR, Rossi J, Bekkedal MYV, Boff AJ, Arfsten DP. Biological and health effects of exposure to kerosene-based jet fuels and performance additives. J Toxicol Environ Health B. 2003;6:357–451. doi: 10.1080/10937400306473. [DOI] [PubMed] [Google Scholar]
- Sabourin PJ, Bechtold WE, Henderson RF. A high pressure liquid chromatographic method for the separation and quantitation of water-soluble radiolabeled benzene metabolites. Anal Biochem. 1988;170:316–27. doi: 10.1016/0003-2697(88)90637-9. [DOI] [PubMed] [Google Scholar]
- Sumner SCJ, Stedman DB, Clark DO, Welsch F, Fennell TR. Characterization of urinary metabolites from [1,2-methoxy-13C]-2-methoxyethanol in mice using 13C nuclear magnetic resonance spectroscopy. Chem Res Toxicol. 1992;5:553–60. doi: 10.1021/tx00028a015. [DOI] [PubMed] [Google Scholar]
- Vere RA. Aviation fuels. In: Hobson GD, editor. Modern petroleum technology. Chichester, UK: John Wiley & Son; 2003. pp. 723–71. part 2. [Google Scholar]


