Abstract
Acute myelogenous leukemia (AML) is a disease more common in the elderly than the young. It is increasingly recognized that conventional cytotoxic chemotherapies used in children and young adults may not be appropriate in older adults because of diverse host- and disease-biology factors. This review highlights some of the most promising new treatment options that are being evaluated for older patients with AML. These options include CPX-351 (Celator Pharmaceuticals Inc), a unique liposomal formulation of a fixed ratio of cytarabine and daunorubicin; timed sequential therapy with the CDK inhibitor alvocidib (flavopiridol; sanofi-aventis/NCI); the second-generation purine nucleoside analog clofarabine; the farnesyltransferase inhibitor tipifarnib (Johnson & Johnson Pharmaceutical Research and Development LLC); and the DN methyltransferase inhibitors decitabine and azacitidine.
Keywords: Acute myeloid leukemia, chemotherapy, elderly (older) adult, molecular predictor, poor-risk factor
Introduction
Acute myeloid leukemia in older adults: General considerations
A discussion of the management of acute myeloid leukemia (AML) is incomplete without reference to the age of the patient. The 5-year overall survival (OS) rate for AML in the US is 23.8% for the period of 1999 to 2005; however, this figure does not reveal the large degree of age-related heterogeneity that exists – the OS rate for adult patients of < 65 years of age is 36.9% compared with 4.7% for patients of ≥ 65 years [1]. The large differences in age-related outcomes are particularly important given that, in the period from 2002 to 2006, the median age of a patient at diagnosis of AML in the US was 67 years, with an incidence of 1.7 per 100,000 in individuals of < 65 years compared with 16.0 per 100,000 in those of ≥ 65 years [1].
The reasons for the age-related disparities in outcomes following AML diagnosis are multifactorial and include healthcare systems, and patient and disease factors. One study of Medicare patients (n = 2657) in the early 1990s demonstrated that 30% of patients over the age of 65 underwent chemotherapy in the 2 years following AML diagnosis. The median OS was 7 months in individuals treated with chemotherapy compared with 1 month in those who were not [2]. Determining which patients are offered chemotherapy and the timing of that chemotherapy relative to the time of diagnosis is affected not only by objective patient parameters, but also by the individual perspective of a physician regarding who is suitable for chemotherapy, with implications for survival [3,4].
AML biology in older patients differs from that observed in younger cohorts. Older patients with AML are more likely to have experienced an antecedent hematological disorder, such as myelodysplastic syndrome (MDS), with an incidence of up to 30 to 60% in some clinical trials series, or have received cytotoxic chemotherapy for prior malignancies [4]. So-called ‘favorable cytogenetics’ are less common than unfavorable cytogenetics (particularly abnormalities in chromosomes 5, 7 and 11), which are observed more often with increasing patient age [5,6]. When compared with cytogenetically identical younger patients with AML, older patients have a lower rate of complete remission (CR), and shorter disease-free survival (DFS) and OS rates [7]. Molecular differences in disease state have also been observed between older and younger patients. Microarray data suggest that overexpression of the RAS, SRC and TNF genes, and the subsequent increased pathway activation, are more common in older patients with AML; the increased activity of these signaling pathways results in a decreased sensitivity to chemotherapy agents such as anthracyclines [8].
Attempts have been made to formalize the characterization of elderly patients based on risk stratification models, including various combinations of age, cytogenetics, performance status, WBC count, secondary AML (including MDS and treatment-related AML) and medical comorbidity [5,6,9-12]. Such efforts have been hampered by the lack of an accepted age cutoff for when a patient with AML should be considered ‘elderly’ (eg, age 50 compared with age 70). In this regard, chronological age alone may be a suboptimal surrogate for the diversity of host physiology and leukemia biology factors that should guide treatment decisions.
Lack of a standard of care for elderly patients
In addition to significant heterogeneity in host and disease factors described in the preceding section, the lack of a current defined standard of care in this patient demographic makes comparison studies challenging. After 30 years, the ‘7+3’ regimen, consisting of the administration of standard dose cytarabine (ara-C; 100 to 200 mg/m2/day) for 7 days by continuous infusion with the addition of an anthracycline (typically either idarubicin [12 mg/m2] or daunorubicin [30 to 60 mg/m2]) for 3 of the 7 days, remains widely used in the US as an induction therapy for AML in all age groups [13]. This classical approach has been modified in terms of the dose and duration of cytarabine and/or anthracycline administration, as well as with the addition of etoposide in many countries outside of the US. Whether this treatment is appropriate in the elderly is unclear. For example, a review of adults (n = 968) enrolled in five Southwest Oncology Group (SWOG) trials demonstrated a 30-day mortality rate, following induction of chemotherapy, of 3% in patients of ≤ 55 years of age with an ECOG (Eastern Cooperative Oncology Group) performance status of 0 to 1, compared with 35% for patients of ≥ 65 years of age with a performance status of 2 and 82% for patients ≥ 75 years with a performance status of 3. In addition to early mortality from intensive treatment, significant and persistent morbidity often occurs, even in patients responding to such therapy [5].
The combination of early treatment-related mortality and late morbidity, particularly in patients with a poor performance status, and inherent drug resistance has stimulated the clinical investigation of new agents and approaches for older individuals with AML, based on both disease and host biology. This review discusses a selection of the approaches under clinical investigation for older patients with AML (Table 1).
Table 1.
Selected agents in clinical trials for the treatment of older adults with AML.
| Agent (Developing company) |
Structure | Development status | Cellular target/mechanism | ClinicalTrials.gov identifier |
|---|---|---|---|---|
|
| ||||
| CPX-351 (Celator Pharmaceuticals Inc) |
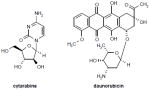
|
Phase II | 5:1 molar ratio of cytarabine:daunorubicin Cytarabine competes with cytidine for incorporation into DNA (inhibiting DNA synthesis) and also inhibits DNA polymerase (resulting in a decrease in DNA replication and repair). Daunorubicin, an intercalator and a topoisomerase II inhibitor (prevents DNA replication and inhibits protein synthesis) and generates oxygen free radicals, resulting in the cytotoxic lipid peroxidation of cell membrane lipids. |
NCT00788892 |
|
| ||||
| Alvocidib (sanofi-aventis/NCI) |
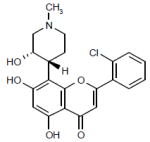
|
Phase II | CDK inhibitor that inhibits cell cycle progression and transcription. | NCT00407966 |
|
| ||||
| Clofarabine |
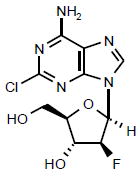
|
Launched | A purine nucleoside analog that inhibits DNA synthesis by two mechanisms: inhibition of DNA polymerase α and ribonucleotide reductase (ie, inhibits the repair of DNA damage). | NCT00373529 |
| NCT01041703 | ||||
|
| ||||
| Tipifarnib (Johnson & Johnson Pharmaceuti al Research and Development LLC) |
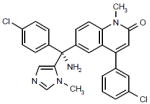
|
Phase II | Inhibits farnesyltransferase, resulting in the inhibition of multiple proteins involved in signaling, transcription and the cytoskeleton. | NCT00602771 |
|
| ||||
| Azacitidine |
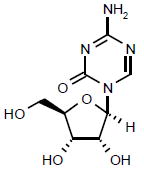
|
Launched | Inhibits DNMT-1, resulting in the inhibition of gene- promoter region hypermethylation and subsequently reinstates gene expression. | NCT00313586 |
|
| ||||
| Decitabine |
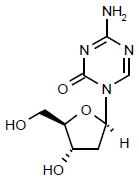
|
Launched | Inhibits DNMT-1, resulting in the inhibition of gene- promoter region hypermethylation and subsequently reinstates gene expression. | NCT00313586 |
|
| ||||
| Laromustine (Vion Pharmaceutials Inc) |
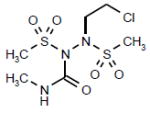
|
Phase III | A sulfonylhydrazine alkylating agent that causes DNA crosslinking and strand breaks, chromosomal aberrations, disruption of DNA synthesis, and inhibition of the DNA repair enzyme 06- alkylguanine-DNA alkyl transferase. | NCT00354276 |
| NCT00655395 | ||||
|
| ||||
| Voreloxin (Sunesis Pharmaceuticals Inc) |
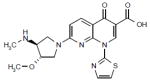
|
Phase II | A quinolone derivate that causes DNA intercalation and topoisomerase II inhibition, resulting in replication-dependent DNA damage, irreversible G2 arrest and subsequently apoptosis. | NCT00607997 |
|
| ||||
| Gemtuzumab |
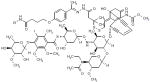
|
Launched | Humanized mAb to CD33 linked to the cytotoxic agent N-acetyl-γ calicheamicin 1,2- dimethyl hydrazine dichloride. a antitumor antibiotics that associates with the minor groove of DNA and, following reduction with glutathione causes sequence-specific, double-stranded DNA cleavage. | NCT00927498 |
| NCT00091234 | ||||
| NCT00006122 | ||||
DNMT-1 DNA methyltransferase
Anthracycline dosing: A new look at established anti-leukemia agents
During the past three decades, traditional chemotherapy regimens have been enhanced by increasing the dose of cytarabine administered. This approach has been successful in subsets of younger adults (aged < 50 to 55), but has demonstrated unacceptable toxicities without clear therapeutic advantages in older adults. A series of studies have examined the feasibility and efficacy of increasing the doses of anthracyclines as an alternative approach to treatment intensification in the older age groups [14-16]. Löwenberg et al demonstrated that doubling the dose of daunorubicin during induction therapy for ‘fit’ patients with AML aged ≥ 60 years led to an improvement in the CR rate from 54 to 64%, with achievement of CR following a single induction cycle in 52% of the individuals in the high-dose group compared with 35% of those in the conventional-dose group [15]. High-dose daunorubicin (90 mg/m2 qd for 3 days) yielded an improvement in the 2-year OS and event-free survival (EFS) rates in patients of ≤ 65 years of age, but had no significant impact on OS and EFS in patients with adverse cytogenetics, independent of age. In contrast, the study by Fernandez et al of high-dose daunorubicin in younger adults (< 60 years) demonstrated increases in both CR rate (71% compared with 57% for individuals on the standard dose) and OS (23.7 compared with 15.3 months) [14]. However, there was no apparent benefit for patients aged 50 to 60 years, or for those patients with unfavorable cytogenetics or mutations in the receptor tyrosine kinase Flt-3.
The Acute Leukemia French Association (ALFA) 9801 study compared a high-dose regimen of daunorubicin (80 mg/m2/day for 3 days) with two regimens of idarubicin (12 mg/m2/day for either 3 or 4 days); each of the three regimens were administered with a standard dose of cytarabine (200 mg/m2/day for 7 days) as induction therapy for adults aged ≥ 50 years [16]. Low mortality during the induction phase (3 to 8%) was associated with all arms of the study. The CR rate after the first induction course was equivalent in all three arms (61, 70 and 67% for daunorubicin, 3-day idarubicin and 4-day idarubicin, respectively), as were the rates of EFS (23.5% at 2 years and 18% at 4 years) and OS (median = 17 months, 28% at 2 years and 26.5% at 4 years). The addition of IL-2 maintenance therapy had no positive impact on any survival parameter in any of the three arms. The 3-day idarubicin regimen was the most effective in patients with adverse cytogenetics (CR = 74, 55 and 48% for 3-day idarubicin, 4-day idarubicin and daunorubicin, respectively). However, a patient age of ≥ 60 years and the presence of adverse cytogenetics remained poor prognostic factors in the multivariate analysis for all three arms [16].
CPX-351 (Celator Pharmaceuticals Inc; Table 1), a liposomal formulation encapsulating cytarabine and daunorubicin in a synergistic 5:1 molar ratio in vivo [17], accumulates in bone marrow and is preferentially taken up by leukemic cells. A phase I clinical trial of CPX-351 in adults with relapsed or refractory acute leukemia established a recommended phase II dose of 101 units/m2 – cytarabine (101 mg/m2) plus daunorubicin (44 mg/m2) – administered on days 1, 3 and 5, and demonstrated a decreased frequency and intensity of oral mucosal, gastrointestinal and skin toxicities when compared to expected toxicities from standard induction chemotherapy [18]. Clinical activity of CPX-351 was observed in this poor-risk population, with a CR/CRp rate of 23% (11 out of 44 patients) following first salvage therapy. Efficacy was particularly evident for patients with first relapse AML who were < 60 years of age (CR = 57%), but also in 20% of first relapse patients aged > 60 years. A randomized phase IIb trial to compare CPX-351 with the traditional 7+3 regimen (2:1 randomization of CPX-351 to 7+3 consisting of cytarabine [100 mg/m2/day for 7 days] plus daunorubicin [45 to 60 mg/m2/day for 3 days]) in adults aged 60 to 75 years with newly diagnosed AML is ongoing [19]. Preliminary data from this trial suggest that CPX-351 has excellent tolerability, with low induction mortality (3% for CPX-351 compared with 7% for 7+3); a similar incidence of adverse events was observed in both treatment arms [19].
Old concepts and new agents: Timed sequential therapy with alvocidib
Alvocidib (flavopiridol, HMR-1275; sanofi-aventis/NCI; Table 1) is a semi-synthetic flavonoid derived from the stem bark of Dysoxylum binectariferum [20]. Alvocidib induces cell cycle arrest at the G1 and G2 phases by inhibiting CDK1, 2, 4 and 7 [21], as well as the CDK9/cyclin T (P-TEFb) complex that phosphorylates and activates RNA polymerase II, thereby inhibiting mRNA transcription [22]. When alvocidib is administered simultaneously with S-phase-dependent agents, such as cytarabine, the alvocidib-induced G1 and G2 arrest leads to cytotoxic antagonism; however, sequential administration of these agents leads to synergistic cytotoxicity [23]. Exposure of primary leukemic marrow blasts to alvocidib, followed by drug withdrawal in vitro, had a dual priming effect of initial cytoreduction, followed by the recruitment of surviving cells into S phase and a resultant increased sensitivity to cytarabine, a mechanism reminiscent of timed sequential therapy (TST) [24].
Clinical trials of TST with alvocidib (flavopiridol) followed by cytarabine and mitoxantrone (FLAM) in adults with all stages of AML are ongoing. A recent phase II trial of FLAM enrolled adults (n = 45; median age = 61 years; range = 22 to 72 years) with newly diagnosed, poor-risk AML, of whom 82% had secondary AML, 73% had adverse cytogenetics and 69% with ≥ two poor-risk features (independent of age) [25]. Patients received a 1-h infusion of alvocidib (50 mg/m2 qd, iv) on days 1 to 3, followed by a 72-h continuous infusion of cytarabine (667 mg/m2 every 24 h) beginning on day 6 and mitoxantrone (40 mg/m2 iv bolus) 12 h after completing the cytarabine infusion on day 9 [25]. Treatment-related toxicities compared favorably with other intensive approaches, with a 4% induction mortality rate in the ≥ 60 years-of-age group. CR was observed in a total of 30 patients (67%) and in 13 of 24 (54%) patients aged ≥ 60 years, with a median OS of 12.6 months. Of the patients who exhibited a CR, 12 (40%) received myeloablative allogeneic bone-marrow transplantation (BMT) at first CR; all 12 patients were < 65 years of age. The median OS for all 45 patients in the trial was 7.4 months, with an OS of 5.8 months (range = 0.6 to 31) for those aged ≥ 60 years. For the 30 patients with CR, the median OS and DFS had not been reached (67% had survived after 12.5 to 31 months and 58% were in CR after 11.4 to 30 months), with a median follow-up period of 22 months. Ongoing development of this regimen includes the comparison of different methods of alvocidib administration in order to define an optimal delivery strategy for further comparative trials in patients with newly diagnosed, poor-risk AML. Additional trials aim to define those older adults who will benefit most from this protocol, the optimal manner to consolidate those patients who obtain CR and how best to salvage those individuals who do not.
Clofarabine
The use of first-generation deoxyadenosine analogs (eg, fludarabine) for the treatment of AML was limited by renal and neurotoxicity at the doses required for single-agent efficacy [26]. Clofarabine (Colar; 2-chloro-2’-fluoro-deoxy-9-β-d-arabinofuranosyladenine; Table 1) is a second-generation purine nucleoside analog that impedes DNA synthesis by inhibiting ribonucleotide reductase activity and chain elongation by DNA polymerase α [27]. Clofarabine has demonstrated efficacy in acute leukemia, with an MTD of 40 mg/m2/day for 5 days; the DLT for clofarabine is reversible hepatotoxicity without neurotoxicity [28].
In a phase II clinical trial of single-agent clofarabine, previously untreated, older patients with AML (n = 112; median age = 71 years; range = 60 to 88 years) with at least one unfavorable risk factor (ie, age ≥ 70 years, ECOG performance status = 2, secondary AML or non-favorable karyotype) received clofarabine (30 mg/m2/day iv) for 5 days [29]. Reinduction or consolidation (a total of six cycles was allowed) therapy was administered 28 days after the first cycle at a dose of 20 mg/m2/day for 5 days. The overall response rate was 46% (CR and CRp = 38% and 8%, respectively), with a median CR duration of 56 weeks and an all-patient median OS of 41 weeks. The all-cause 30-day and 60-day mortality rates were 10 and 16%, respectively [29]. These data compare favorably with that of traditional cytotoxic therapy. An ECOG trial is planned that will formally compare clofarabine and 7+3 induction in randomized older adults with AML (ClinicalTrials.gov identifier: NCT01041703).
Finally, a trial combining the administration of clofarabine (30 mg/m2/day iv) for 5 days with or without cytarabine (20 mg/m2/day sc) for 14 days in patients aged ≥ 60 years has also been reported [30]. Inclusion criteria included newly diagnosed AML and high-risk MDS. Higher CR rates were observed in those patients receiving both clofarabine and cytarabine (63% of 54 patients treated with the combination compared with 31% of 16 patients treated with clofarabine alone), but were not sufficient to demonstrate a significant difference in OS (11.4 months compared with 5.8 months for combination and single-agent treatments, respectively; p = 0.1) [30].
Tipifarnib
Tipifarnib (Johnson & Johnson Pharmaceutical Research and Development LLC; Table 1) is an orally available inhibitor of the enzyme farnesyltransferase, which post-translationally modifies a wide range of proteins involved in signal transduction, cytoskeletal integrity and mitosis (reviewed in reference [31]). Interruption of the farnesylation process prevents full maturation of the target proteins that, in turn, leads to inhibition of cell proliferation and an increase in apoptotic cell death.
As a single agent, tipifarnib exhibits modest activity in elderly adults with poor-risk AML. A phase II clinical trial of tipifarnib (600 mg po, bid) administered for 21 days, followed by a rest period of up to 42 days, was conducted in adults (n = 158; median age = 74 years; range = 34 to 85 years) with untreated, poor-risk AML (93% ≥ 65 years of age, 75% with secondary AML and 47% with adverse cytogenetics) [32]. In this trial, 14 and 9% of patients achieved CR and partial responses, respectively; the overall response rate was 23%. While the median OS for all 158 patients was 5.3 months, the median OS for those patients achieving CR was 18.3 months [32]. More recently, a phase III trial of single-agent tipifarnib compared with best supportive care, including hydroxyurea (HU), in patients (n = 457) aged ≥ 70 years with newly diagnosed AML who were deemed 'not fit' for conventional chemotherapy was conducted in Europe and Canada [33]. Although CR, with DFS rates of 8 months and OS rates of 22 months was achieved in 8% of those patients randomized to the tipifarnib arm compared with no CR in the supportive care/HU arm, the tipifarnib treatment did not demonstrate a statistically significant survival advantage [33].
In an attempt to increase CR rate and duration, tipifarnib has been combined with other anti-leukemic agents in vitro [34]. In primary AML marrow blasts, tipifarnib inhibited signaling downstream of the farnesylated small G-protein Rheb and synergistically enhanced etoposide-induced antiproliferative effects. These findings led to a phase I clinical trial of tipifarnib (300 to 600 mg po, bid for 14 or 21 days) plus etoposide (100 to 200 mg po, qd on days 1 to 3 and 8 to 10 for each cycle) in adults (n = 84) over the age of 70 years (median age = 77 years; range = 70 to 91 years) who were not candidates for conventional therapy [34]. The majority of patients (79%) had more than one high-risk feature in addition to advanced age. DLTs (mainly grade 3 mucositis) occurred with the 21-day tipifarnib regimen. The 30-day mortality rate was 6 and 21% for the 14-day and 21-day tipifarnib regimens, respectively. CRs were achieved in 16 of the 54 patients (30%) receiving the 14-day tipifarnib regimen, but in 5 of the 30 patients (17%) receiving the 21-day tipifarnib regimen [34]. Based on these results, a phase II, multicenter, randomized trial of tipifarnib (600 mg po, bid for 14 days) plus etoposide (100 mg po, qd on days 1 to 3 and 8 to 10) in adults of ≥ 70 years of age with newly diagnosed AML is ongoing (NCT00602771).
By examining the gene expression profile of marrow blasts from patients undergoing treatment with single-agent tipifarnib [32], Raponi et al [35] demonstrated that the expression ratio of two genes – RASGRP1, which encodes a Ras-activating guanine nucleotide exchange factor and APTX, the gene encoding the DNA repair protein aprataxin – could both positively and negatively predict patient response to single-agent tipifarnib [35]. Studies are being designed to determine if this two-gene signature could be used prospectively to select patients for tipifarnib-based AML therapy; such selection would be based on the RASGRP1:APTX ratio expressed by the blast population of the diagnostic bone marrow examination of the patient.
DNA methyltransferase inhibitors
Epigenetic changes in the methylation state of promoter regions can lead to transcriptional silencing of tumor suppression genes in a variety of cancers, including AML. Methylation of cytosine by the enzyme DNA methyltransferase (DNMT) allows the recruitment of transcriptional repression complexes via specific methyl-binding proteins, leading ultimately to the structural repression of transcription of a gene by the formation of inactive heterochromatin [36]. DNMT inhibitors, already approved for use in the treatment of MDS, are being evaluated for the treatment of newly diagnosed AML in the elderly.
Decitabine (Dacogen, 5-aza-2’-deoxycytidine; Table 1) is a cytosine nucleoside analog that is incorporated into DNA in S phase and irreversibly inhibits DNMT-1, thereby reversing DNA hypermethylation. A phase II, multicenter, single-arm, clinical trial of decitabine (20 mg/m2/day iv) administered for 5 days in 4-week cycles was conducted in patients (n = 55) aged > 60 years (mean age = 74 years; range = 61 to 87 years) with newly diagnosed AML who were considered ineligible for standard chemotherapy [37]. The median number of cycles was three and 64% of patients had three or more cycles. The 30-day mortality rate was 7%, the CR rate was 24% and the median time to achieve CR was 4.5 monthly cycles. The median OS for all patients was 7.7 months, with a median OS of 14 months for those patients achieving CR.
A subgroup analysis of the elderly patients (n = 113) in a previous phase III clinical trial of the DNMT-1 inhibitor azacitidine (Vidaza, 5-azacytidine; Table 1) in patients with high-risk MDS who met the WHO criteria for AML was recently reported [38]. The median age of these patients was 70 years (range = 50 to 83) and the average bone marrow blast count was 23% (only 3 of the 113 patients had a count of > 30%). The trial compared azacitidine (75 mg/m2/day sc) with conventional care (ie, best supportive care only, low-dose cytarabine, or an anthracycline plus cytarabine-based intensive chemotherapy) selected by the individual physician prior to randomization. The median OS for patients randomized to azacitidine was 24.5 months compared with 16.0 months for conventional care (OS = 13.4, 17.0 and 14.2 months for best supportive care, low-dose cytarabine and intensive chemotherapy, respectively). The 2-year OS rates were 50% for the azacitidine treatment and 16% for conventional care (2-year OS = 0, 31.8 and 25.0 months for best supportive care, low-dose cytarabine and intensive chemotherapy, respectively). These differences in OS were not reflected in the morphological CR rates: 18% for azacitidine and 16% for conventional care (0, 15 and 55% for best supportive care, low-dose cytarabine and intensive chemotherapy, respectively) [38]. This subgroup analysis requires prospective validation, but the wider applicability of these results in selected patients with low blast-count AML, in addition to elderly patients presenting with newly diagnosed AML with bone marrow blasts of > 30%, remains to be determined.
Conclusion
AML is a heterogeneous disease in terms of both pathogenesis and pathophysiology. Therefore, it is unsurprising that a single approach of aggressive induction chemotherapy may not provide optimal treatment, particularly for older patients in whom the principles of potentially curative approaches for younger patients may not hold true. However, there are exceptions to the general rule that older patients with AML have a poor prognosis with currently available treatment options (eg, in acute promyelocytic leukemia [39], core-binding-factor leukemias [40] or cytogenetically normal AML with nucleophosmin [NPM1] expression [41]). Nonetheless, patient selection with ‘risk-of-treatment’ stratification is crucial, as it is clear that, as age increases and performance status decreases, the initial harm caused by intensive cytotoxic induction may outweigh any potential benefit of the treatment. In certain cases, such as a 65-year-old patient with good performance status but high risk disease, cytotoxic chemotherapy (eg, CPX-351 or alvocidib-based TST) may be the most appropriate treatment; however, in other cases, such as an 80-year-old patient with poor performance status, less intensive approaches may maximize both quantity and quality of life, with a minimum of iatrogenic harm. As the number of older patients with AML achieving CR increases, research should focus on how to best address potential curative strategies. It remains an open question if allogeneic BMT with non-myeloablative preparative regimens and/or suppression of high-grade GvHD will change the long-term survival outlook for older adults with AML [42-44]. The ongoing development of molecular predictors of response should provide the opportunity to design an individualized treatment plan for older patients with AML.
References
-
••
of outstanding interest
-
•
of special interest
- 1.Surveillance Epidemiology and End Results cancer statistics review. National Cancer Institute; Bethesda, MS, US: 2009. seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 2.Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med. 2002;162(14):1597–1603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 3.Juliusson G, Billström R, Gruber A, Hellström-Lindberg E, Höglunds M, Karlsson K, Stockelberg D, Wahlin A, Aström M, Arnesson C, Brunell-Abrahamsson U, et al. Attitude towards remission induction for elderly patients with acute myeloid leukemia influences survival. Leukemia. 2006;20(1):42–47. doi: 10.1038/sj.leu.2404004. [DOI] [PubMed] [Google Scholar]
- 4.Sekeres MA, Elson P, Kalaycio ME, Advani AS, Copelan EA, Faderl S, Kantarjian HM, Estey E. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28–36. doi: 10.1182/blood-2008-05-157065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. doi: 10.1182/blood-2005-09-3724. A thoughtful and important retrospective analysis of performance status, hematological and cytogenetic factors on presentation, multi-drug resistance and treatment outcome with regard to age in patients (n = 968) with AML in SWOG clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, Wheatley K, Burnett AK, Goldstone AH Medical Research Council Adult Leukemia Working Party. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): Analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 7.Büchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Müller-Tidow C, Braess J, Spiekermann K, Kienast J, Staib P, Grüneisen A, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: A study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27(1):61–69. doi: 10.1200/JCO.2007.15.4245. [DOI] [PubMed] [Google Scholar]
- 8•.Rao AV, Valk PJ, Metzeler KH, Acharya CR, Tuchman SA, Stevenson MM, Rizzieri DA, Delwel R, Buske C, Bohlander SK, Potti A, et al. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(33):5580–5586. doi: 10.1200/JCO.2009.22.2547. Microarray evidence that in some cases, AML in older (> 55 years) adults represents a different disease from that observed in younger (< 45 years) adults. Also demonstrates distinct signaling pathway-activation profiles and anthracycline sensitivity. [DOI] [PubMed] [Google Scholar]
- 9.Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, Wierda W, Ferrajoli A, Kornblau S, Pierce S, Albitar M, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136(4):624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 10•.Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, Garcia-Manero G, Wierda W, Pierce S, Shan J, Estey E. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer. 2006;106(5):1090–1098. doi: 10.1002/cncr.21723. Aimed to determine negative prognostic factors, using multivariate analysis, to allow risk-stratification of older patients with AML prior to treatment decisions Advanced age, unfavorable cytogenetics, poor performance status, longer duration of antecedent hematological disorder and baseline abnormal organ function were all identified as independent predictors of negative outcomes. [DOI] [PubMed] [Google Scholar]
- 11.Malfuson JV, Etienne A, Turlure P, de Revel T, Thomas X, Contentin N, Terré C, Rigaudeau S, Bordessoule D, Vey N, Gardin C, et al. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica. 2008;93(12):1806–1813. doi: 10.3324/haematol.13309. [DOI] [PubMed] [Google Scholar]
- 12.Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, Moorman AV, Burnett AK. United Kingdom National Cancer Research Institute Haematological Oncology Clinical Studies Group and Acute Myeloid Leukaemia Subgroup: Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145(5):598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 13.Rai KR, Holland JF, Glidewell OJ, Weinberg V, Brunner K, Obrecht JP, Preisler HD, Nawabi IW, Prager D, Carey RW, Cooper MR, et al. Treatment of acute myelocytic leukemia: A study by Cancer and Leukemia Group B. Blood. 1981;58(6):1203–1212. [PubMed] [Google Scholar]
- 14.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, Racevskis J, Dewald GW, Ketterling RP, Bennett JM, Rowe JM, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Maertens J, Jongen-Lavrencic M, von Lilienfeld-Toal M, Biemond BJ, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 16.Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, Bourhis JH, Reman O, Turlure P, Contentin N, de Revel T, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: Results of the ALFA-9801 study. J Clin Oncol. 2010;28(5):808–814. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 17.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: Controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 18.Feldman EJ, Lancet J, Kolitz JE, Ritchie E, List AF, Asatiani E, Curcio TJ, Burton M, Fricano M, Swenson C, Mayer LD, et al. Phase I study of a liposomal carrier (CPX-351) containing a synergistic, fixed molar ratio of cytarabine (ara-C) and daunorubicin (DNR) in advanced leukemias. Blood. 2008;112(11) Abs 2984. [Google Scholar]
- 19.Lancet JE, Feldman EJ, Kolitz JE, Tallman MS, Hogge DE, Komrokji RS, Chiarella MT, Louie AC. Phase IIb randomized study of CPX-351 vs. conventional cyatarbine + daunorubicin in newly diagnosed AML patients aged 60-75: Safety report. Blood. 2009;114(22) Abs 1033. [Google Scholar]
- 20.Naik RG, Kattige SL, Bhat SV, Alreja BB, de Souza NJ, Rupp RH. An antiinflammatory cum immunomodulatory piperidinylbenzopyranone from Dysoxylum binectariferum: Isolation, structure and total synthesis. Tetrahedron. 1988;44(7):2081–2086. [Google Scholar]
- 21.Senderowicz AM, Sausville EA. Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst. 2000;92(5):376–387. doi: 10.1093/jnci/92.5.376. [DOI] [PubMed] [Google Scholar]
- 22.Chao S, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276(34):31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 23.Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: The importance of sequence of administration. Cancer Res. 1997;57(16):3375–3380. [PubMed] [Google Scholar]
- 24.Karp JE, Ross DD, Yang W, Tidwell ML, Wei Y, Greer J, Mann DL, Nakanishi T, Wright JJ, Colevas AD. Timed sequential therapy of acute leukemia with flavopiridol: In vitro model for a phase I clinical trial. Clin Cancer Res. 2003;9(1):307–315. [PubMed] [Google Scholar]
- 25.Karp JE, Blackford A, Smith BD, Alino K, Seung AH, Bolanos-Meade J, Greer JM, Carraway HE, Gore SD, Jones RJ, Levis MJ, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010 doi: 10.1016/j.leukres.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warrell RP, Jr, Berman E. Phase I and II study of fludarabine phosphate in leukemia: Therapeutic efficacy with delayed central nervous system toxicity. J Clin Oncol. 1986;4(1):74–79. doi: 10.1200/JCO.1986.4.1.74. [DOI] [PubMed] [Google Scholar]
- 27.Parker WB, Shaddix SC, Chang CH, White EL, Rose LM, Brockman RW, Shortnacy AT, Montgomery JA, Secrist JA, 3rd, Bennett LL., Jr Effects of 2-chloro-9-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5’-triphosphate. Cancer Res. 1991;51(9):2386–2394. [PubMed] [Google Scholar]
- 28.Kantarjian H, Gandhi V, Cortes J, Verstovsek S, Du M, Garcia-Manero G, Giles F, Faderl S, O’Brien S, Jeha S, Davis J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102(7):2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 29.Kantarjian HM, Erba HP, Claxton D, Arellano M, Lyons RM, Kovascovics T, Gabrilove J, Craig M, Douer D, Maris M, Petersdorf S, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28(4):549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 30.Faderl S, Ravandi F, Huang X, Garcia-Manero G, Ferrajoli A, Estrov Z, Borthakur G, Verstovsek S, Thomas DA, Kwari M, Kantarjian HM. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112(5):1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karp JE, Lancet JE. Tipifarnib in the treatment of newly diagnosed acute myelogenous leukemia. Biologics. 2008;2(3):491–500. doi: 10.2147/btt.s3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancet JE, Gojo I, Gotlib J, Feldman EJ, Greer J, Liesveld JL, Bruzek LM, Morris L, Park Y, Adjei AA, Kaufmann SH, et al. A phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood. 2007;109(4):1387–1394. doi: 10.1182/blood-2006-04-014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harousseau JL, Martinelli G, Jedrzejczak WW, Brandwein JM, Bordessoule D, Masszi T, Ossenkoppele GJ, Alexeeva JA, Beutel G, Maertens J, Vidriales MB, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114(6):1166–1173. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 34.Karp JE, Flatten K, Feldman EJ, Greer JM, Loegering DA, Ricklis RM, Morris LE, Ritchie E, Smith BD, Ironside V, Talbott T, et al. Active oral regimen for elderly adults with newly diagnosed acute myelogenous leukemia: A preclinical and phase 1 trial of the farnesyltransferase inhibitor tipifarnib (R115777, Zarnestra) combined with etoposide. Blood. 2009;113(20):4841–4852. doi: 10.1182/blood-2008-08-172726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Raponi M, Lancet JE, Fan H, Dossey L, Lee G, Gojo I, Feldman EJ, Gotlib J, Morris LE, Greenberg PL, Wright JJ, et al. A 2-gene classifier for predicting response to the farnesyltransferase inhibitor tipifarnib in acute myeloid leukemia. Blood. 2008;111(5):2589–2596. doi: 10.1182/blood-2007-09-112730. The heterogeneity of patient and disease parameters of older adults with AML has stimulated much interest in personalized medicine approaches, in particular, the development of molecular predictive tools to guide decision making. This article describes the validation of a gene expression ratio as a biomarker of responsiveness to tipifarnib induction therapy. [DOI] [PubMed] [Google Scholar]
- 36.Gore SD. Combination therapy with DNA methyltransferase inhibitors in hematologic malignancies. Nat Clin Pract Oncol. 2005;2(Suppl 1):S30–S35. doi: 10.1038/ncponc0346. [DOI] [PubMed] [Google Scholar]
- 37.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 38•.Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–569. doi: 10.1200/JCO.2009.23.8329. A retrospective subgroup analysis from phase III clinical trial data that compared azacitidine with conventional care regimens (either best supportive care, low-dose cytarabine or an anthracycline plus cytarabine-based intensive chemotherapy) in older adult patients with AML with low marrow blast count (20% to 30%) who were considered unsuitable for intensive chemotherapy. This hypothesis-generating research now deserves prospective validation. [DOI] [PubMed] [Google Scholar]
- 39.Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood. 2009;114(25):5126–35. doi: 10.1182/blood-2009-07-216457. [DOI] [PubMed] [Google Scholar]
- 40.Prébet T, Boissel N, Reutenauer S, Thomas X, Delaunay J, Cahn JY, Pigneux A, Quesnel B, Witz F, Thépot S, Ugo V, et al. Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: A collaborative study of the French CBF-AML intergroup. J Clin Oncol. 2009;27(28):4747–4753. doi: 10.1200/JCO.2008.21.0674. [DOI] [PubMed] [Google Scholar]
- 41•.Becker H, Marcucci G, Maharry K, Radmacher MD, Mrózek K, Margeson D, Whitman SP, Wu YZ, Schwind S, Paschka P, Powell BL, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J Clin Oncol. 2010;28(4):596–604. doi: 10.1200/JCO.2009.25.1496. Personalized molecular-level risk-stratification of AML in older adults using nucleophosmin (NPM1) mutation status as a biomarker demonstrated that mutations in this gene were associated with better outcomes (ie, higher CR rates and longer survival), particularly in patients of = 70 years of age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R, Giralt S. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109(4):1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 43.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Kiss TL, Sabry W, Lazarus HM, Lipton JH. Blood and marrow transplantation in elderly acute myeloid leukaemia patients – Older certainly is not better. Bone Marrow Transplant. 2007;40(5):405–416. doi: 10.1038/sj.bmt.1705747. The authors make the astute point that stem cell transplantation has not traditionally been used in the older patient with AML, but that the recent development of less intensive, non-myeloablative approaches may offer an opportunity to study this method as a post-remission therapy. [DOI] [PubMed] [Google Scholar]


