Abstract
Recent experimental data on Parkinson's disease (PD) predicts the critical role of inflammation in the progression of neurodegeneration and the promising preventive effects of nonsteroidal anti-inflammatory drugs (NSAIDs). Previous studies suggest that NSAIDs minimize cyclooxygenase-2 (COX-2) activity and thereby attenuate free radical generation. Prostaglandin E2 (PGE2) is an important product of COX activity and plays an important role in various physiologic and pathophysiologic conditions through its EP receptors (EP1–EP4). Part of the toxic effect of PGE2 in the central nervous system has been reported to be through the EP1 receptor; however, the effect of the EP1 receptor in PD remains elusive. Therefore, in our pursuit to determine if deletion of the PGE2 EP1 receptor will attenuate 6-hydroxy dopamine (6-OHDA)-induced Parkinsonism, mice were given a unilateral 6-OHDA injection into the medial forebrain bundle. We found that apomorphine-induced contralateral rotations were significantly attenuated in the 6-OHDA-lesioned EP1−/− mice compared with the 6-OHDA-lesioned WT mice. Quantitative analysis showed significant protection of dopaminergic neurons in the substantia nigra pars compacta of the 6-OHDA-lesioned EP1−/− mice. To the best of our knowledge, this is the first in vivo study to implicate the PGE2 EP1 receptor in toxin-induced Parkinsonism. We propose the PGE2 EP1 receptor as a new target to better understand some of the mechanisms leading to PD.
Keywords: Mouse, Neurodegeneration, Neuroinflammation, Parkinson's disease, Parkinsonism, Prostaglandin, Tyrosine hydroxylase
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder primarily marked with the selective loss of dopamine in the basal ganglia and the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Although the etiology of PD is relatively unknown, some of the factors associated with the progression of the disease include environmental toxins, oxidative stress, mitochondrial dysfunction, and abnormal protein accumulation (Dauer and Przedborski 2003; Jenner 1998; Dawson and Dawson 2003; Schulz et al. 2000; Hoang et al. 2009; Calabrese et al. 2005; Hardy 2010; Cuervo et al. 2010).
Various neurotoxin-induced PD models are used to understand the neuropathology associated with PD (Javoy et al. 1976; Iancu et al. 2005; Blum et al. 2001; Dauer and Przedborski 2003; Schmidt and Ferger 2001; Shimohama et al. 2003; Gao et al. 2003; Chesselet and Richter 2011). A unilateral injection (in the substantia nigra, medial forebrain bundle, or striatum of one hemisphere) of 6-hydroxy dopamine (6-OHDA) is one widely used method to induce asymmetrical Parkinson's disease (Åkerud et al. 2001; Ghosh et al. 2007; Gerlach and Riederer 1996; Dauer and Przedborski 2003; Ungerstedt 1968). A 6-OHDA injection results in rapid neuronal death, motor deficits, and contralateral rotations in response to the dopaminergic D1/D2 receptor agonist, apomorphine, within one week after the lesion is produced (Ungerstedt 1976; Jeon et al. 1995).
Cyclooxygenases (COX-1 and COX-2), the rate limiting enzymes responsible for generation of prostaglandins, has multifaceted effects, and may aggravate the degenerative process through prostaglandin production, initiation of the pro-inflammatory cascade, or by the generation of reactive oxygen species (Tyurin et al. 2006; Hoozemans et al. 2002; Teismann et al. 2003; Esposito et al. 2007; Vijitruth et al. 2006; Mohan et al. 2012). Constitutively, COX-2 is present in low levels in DA neurons; however, it is up-regulated in patients and animal PD models (Feng et al. 2003; Wang et al. 2005). Under normal circumstances, COX-2 is functionally coupled with cPLA2 at post-synaptic neuronal membranes (Kaufmann et al. 1996; Takano et al. 2000; Balsinde et al. 1998), and thus participates in cPLA2-mediated neurodegeneration (Kishimoto et al. 2010). A recent study suggests that a unilateral 6-OHDA injection in rats upregulates cPLA2 and COX-2 (Lee et al. 2010), whereas another study shows that lack of COX-2 and nNOS activity prevents MPTP-induced DNA damage (Hoang et al. 2009).
The activity of COX-2 increases in the presence of toxic stimuli (Esposito et al. 2007; Anderson et al. 1996), thus augmenting the PGE2 levels. The increased production of PGE2 results in aggravated neuroinflammation and neurotoxicity (Kawano et al. 2006; Carrasco et al. 2007). PGE2 exerts its effect through its principal EP receptors (EP1, EP2, EP3, and EP4), which either initiate Ca2+ or a cAMP-signaling pathway (Ushikubi et al. 2000; Suzawa et al. 2000; Hata and Breyer 2004; Narumiya 2009; Sugimoto et al. 2000; Narumiya et al. 1999). Recent data suggest that EP1 receptor activation propagates neurotoxicity through activation of the Ca2+ pathway (Kawano et al. 2006; Carrasco et al. 2007). Interestingly, a study from the laboratory of our collaborator, Dr. Narumiya, shows that EP1−/− mice have elevated levels of extracellular dopamine in the striatum as compared with the WT mice which potentially implies that the PGE2-EP1 signaling suppresses the SNpc dopaminergic neuronal activity (Tanaka et al. 2009). Moreover, we have previously shown that deletion of the PGE2 EP1 receptor and use of the EP1 antagonist attenuates NMDA-induced and MCAO-induced brain damage and the use of the agonist exacerbates NMDA-induced brain damage (Ahmad et al. 2006; Ahmad et al. 2008). This reinforces the contention that deletion or pharmacologic blockade of EP1 receptor is neuroprotective (Doré 2006).
Although it has been reported that COX-2 is involved in the progression of 6-OHDA-induced PD, the role of PGE2 and its receptor, EP1, have not been investigated in neurotoxin-induced PD. Based on earlier studies showing the neurotoxic role of the EP1 receptor and, more interestingly, the hyperdopaminergic condition of the EP1−/− mice, we sought to determine whether EP1 deletion would protect mice from asymmetrical Parkinsonism. To test our hypothesis, C57BL/6 WT and EP1−/− mice were given a unilateral stereotaxic injection of 6-OHDA. On day 7, we monitored apomorphine-induced contralateral rotations; on day 8, the mice were euthanized, and the dopaminergic neurons in the SNpc of each genotype were counted. To the best of our knowledge, this is the first in vivo study that shows the neurotoxic potentials of the EP1 receptor in a 6-OHDA-induced mouse model of PD.
Materials and Methods
Unilateral Stereotaxic Injection of 6-OHDA
Young (20–25 g; 8–10-weeks-old) WT (N = 16) and EP1−/− (N = 13) mice were used in accordance with the National Institutes of Health guidelines for the use of experimental animals, and the protocol was approved by our Institutional Animal Care and Use Committees. Colonies of WT and EP1−/− C57BL/6 mice were maintained in our animal facility. To avoid any genetic drift these mice were back-crossed and genotyped before experiments. Overall, the EP1−/− mice develop normally and have no gross abnormalities in behavior, macroscopic anatomy, or biochemical or hematologic indices (Saleem et al. 2007; Watanabe et al. 1999). A unilateral stereotaxic injection of 3.6 μg 6-OHDA (kept on ice in darkness) in 1 μl 0.02 % ascorbic acid saline was given at a rate of 0.2 μl/min in the medial forebrain bundle at coordinates A: −1.2, L: −1.1 relative to the bregma, and V: 5.0 relative to the dura. The mice were allowed to survive for 8 days. The mice were housed under controlled temperature and humidity with a 12/12 h light/dark cycle, and food and water were provided ad libitum.
Apomorphine-Induced Contralateral Rotations
On day 7, all mice were tested for apomorphine-induced contralateral rotations. Given that apomorphine is a dopamine agonist, its administration produces contralateral rotations in unilateral nigrostriatal degenerations, thus serving as a marker of significant dopaminergic cell death (Ungerstedt 1976). The mice were monitored three times in successive sessions of 5 min each immediately after a 0.5 mg/kg SC apomorphine injection. From each group, one mouse died during the survival time. Following our preset exclusion criteria, that those mice which did not show apomorphine-induced rotations, would be eliminated, three mice from each group were excluded from further experiments.
Tyrosine Hydroxylase (TH) Immunohistochemistry
On day 8, the mice were deeply anesthetized with pento-barbital (35 mg/kg), and were transcardially perfused and fixed with 1 M PBS and 4 % paraformaldehyde (PFA), respectively. Harvested brains were kept in 4 % PFA overnight and then equilibrated with 30 % sucrose.
Every 4th coronal cryosection of 25 μm from the substantia nigra was processed for tyrosine hydroxylase (TH) immunohistochemistry, as previously described (Feng et al. 2002). After washing and quenching, these sections were briefly incubated with a blocking solution (5 % normal goat serum and 0.2 % Triton X100 in 1 M PBS) for 30 min, and were subsequently incubated overnight with a rabbit Polyclonal anti-TH antibody (1:250, catalog # NB300-109; Novus Biologicals, Littleton, CO). Thereafter, sections were incubated with biotinylated secondary antibody, and immunolabeling was visualized with an avidin–biotin–peroxidase detection kit (Vector Laboratories, Burlingame, CA, USA) and 3′,3-diaminobenzidine. Sections were counter-stained with cresyl violet and cover-slipped.
Tallying TH-Immunoreactive (TH-ir) Dopaminergic Neurons in the SNpc
The medial terminal nucleus of the accessory optic tract was used as a landmark to differentiate between the ventral tegmental area and the SN. To determine the relative index of neuronal profiles, all TH-IR round cell bodies (TH-IR dopaminergic cells) in the contralateral and ipsilateral SNpc were manually counted in each section at 40×, and the total number of TH-IR cell bodies was multiplied by 4 (we stained every fourth section), which gave us the total number of TH-IR profiles in the entire SNpc.
Statistical Analysis
Data were analyzed by One-way ANOVA followed by Newman-Keuls post test to determine the significant difference in the total number of TH-IR profiles among the groups. Apomorphin-induced rotation data were analyzed by Student's t test. Values of p < 0.05 were considered to be significant. All values are expressed as mean ± SEM.
Results
EP1−/− Mice Exhibit Declined Apomorphine-Induced Rotations
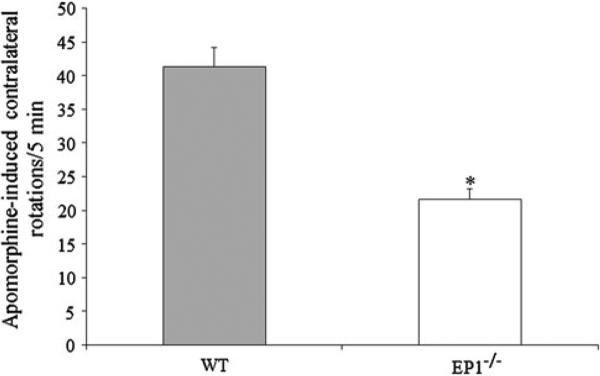
The subcutaneous injection of apomorphine resulted in contralateral rotations in 6-OHDA-treated mice. The data shows that this rotation was significantly attenuated in 6-OHDA-treated EP1−/− mice, and the EP1−/− mice were found to have 47.50 ± 8.96 % less contralateral rotations than the WT mice (Fig. 1).
Fig. 1.
EP1 deletion prevents rotational asymmetry in 6-OHDA-induced Parkinsonism: The C57BL/6 WT and EP1−/− mice were given a unilateral stereotaxic injection of 3.6 μg 6-OHDA in the medial forebrain bundle. On day 7, all mice were given a 0.5 mg/kg apomorphine injection SC, and were tested for apomorphine-induced contralateral rotations. The mice were monitored three times for a consecutive session of 5 min each immediately after the apomorphine injection. The figure shows that the rotations were significantly reduced by 47.50 ± 8.96 % in the EP1−/− mice. Data are presented as mean ± SEM. *p < 0.01 as compared with the WT group
Dopaminergic Neuronal Death was Attenuated in the EP1−/− Mice
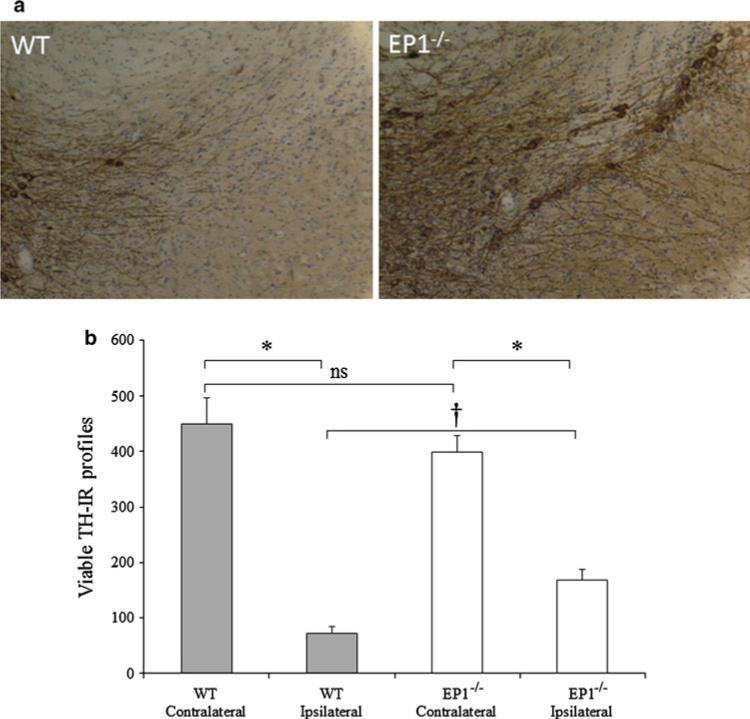
The unilateral injection of 6-OHDA led to the selective degeneration of dopaminergic neurons in the ipsilateral SNpc. The deletion of the EP1 receptor resulted in attenuation in dopaminergic neuronal death in the EP1−/− group compared with the WT group (Fig. 2a). The viable TH-IR cell body morphology significantly (p < 0.001) decreased to 18.73 ± 3.17 and 34.03 ± 4.71 % in the WT and EP1−/− mice, respectively. Thus, compared with the WT group, there were 47.68 ± 8.08 % more TH-IR cell body morphology in the EP1−/− group (p < 0.05) (Fig. 2b).
Fig. 2.
EP1 deletion prevents 6-OHDA-induced dopaminergic cell death: On day 8, the 6-OHDA-lesioned mice were transcardially perfused and fixed, and harvested brains were fixed overnight in PFA, then equilibrated with sucrose. Every 4th coronal cryosection of 25 μm from the substantia nigra was processed for TH immunohistochemistry. TH-IR profiles representing dopaminergic neurons in the ipsilateral and contralateral SNpc were manually counted in each section at ×40. a Representative sections at ×10 showing TH-IR profiles in the SNpc in WT ipsilateral (left panel), and EP1−/− ipsilateral (right panel) sections. b Histogram showing the total number of TH-IR profiles in each hemispheric SNpc of WT and EP1−/− mice. Quantification of the TH-IR profiles shows significant protection of the dopaminergic cells in the EP1−/− ipsilateral SNpc. There was 47.68 ± 8.08 % protection in the EP1−/− group as compared with the WT group. Data are presented as mean ± SEM. *p < 0.001 as compared with the WT or EP1−/− contralateral SNpc. †p < 0.05 as compared with the WT ipsilateral SNpc. There was no significant (ns) difference between the contralateral side of WT and EP1−/−
Discussion
We have previously shown that the EP1 receptor propagates NMDA-induced and stroke-induced brain damage (Ahmad et al. 2008; Ahmad et al. 2006). In this study, we wanted to determine if EP1 is also involved in PD, and whether the EP1−/− mice will be protected against 6-OHDA-induced Parkinsonism. We found that apomorphine-induced rotations were significantly attenuated in the EP1−/− mice. A quantitative analysis of TH-IR cell bodies in the SNpc showed a significant decrease in dopaminergic neuronal death in the ipsilateral SNpc of the EP1−/− mice compared with the WT mice. To the best of our knowledge, this is the first in vivo study that used EP1−/− mice and suggests a neurotoxic role of the EP1 receptor in 6-OHDA-induced Parkinsonism in mice.
Unilateral 6-OHDA-induced SNpc degeneration causes asymmetric and quantifiable motor behavior after the injection of DA receptor agonists (Blum et al. 2001; Ungerstedt 1968). One of the DA receptor agonists is apomorphine that at low dose results in stimulation of both D1 and D2 DA receptors. 6-OHDA lesioning results in significant death of dopaminergic cells containing DA receptors. This imbalance in DA receptor population results in more motor function on the contralateral side, which as a consequence results in asymmetrical rotations toward the contralateral side following apomorphine treatment (Ungerstedt 1968, 1976; Iancu et al. 2005). Given the role of inflammation in PD, it has been reported that the increased Ca2+-dependent PLA2 activity augments neuroinflammation in the putamen of a PD patient and 6-OHDA-lesioned rats (Lee et al. 2010; Ross et al. 2001). This could be due to up-regulation of COX-2, as it has been suggested by various reports that selective or nonselective inhibition of COX-2 provides a neuroprotective effect (Aubin et al. 1998; Mohanakumar et al. 2000; Nakayama et al. 1998; Scali et al. 2000). Likewise, COX-2 is also reported to play an important role in MPTP- and 6-OHDA-mediated toxicity (Hoang et al. 2009; Feng et al. 2003; Teismann et al. 2003; Przybyłkowski et al. 2004) and MPTP-induced DNA damage (Hoang et al. 2009; Teismann et al. 2003).
Interestingly, although COX activity results in significant prostaglandin production, most studies implicate COX catalytic activity and oxygen species formation in the progression of PD rather than the increased prostaglandin production. For example, Przybyłkowski et al. (2004) showed that prostaglandin production did not follow a COX-2 protein increase, and prostaglandin production was limited to the very early stage of injury (Przybyłkowski et al. 2004). Similarly, there are studies that propose the neuroprotective effect of NSAIDs to be through their free radical scavenging properties (Teismann and Ferger 2001; Aubin et al. 1998). These studies indicate that during the early phase of MPTP toxicity, the prostaglandin level increases along with COX; however, after a week, the prostaglandin level goes back to normal and the COX level still remains high. Thus, these studies conclude that the protective effect of NSAIDs against MPTP or 6-OHDA toxicity is due to their ability to quench free radicals rather than their effect in minimizing prostaglandin production and prostaglandin receptor activity.
In contrast, in this study, we have found that the apomorphine-induced rotation was significantly diminished in the EP1−/− mice injected with 6-OHDA. Similarly, the EP1−/− dopaminergic cells were significantly protected against 6-OHDA-induced toxicity. Thus, our rotational and immunohistochemical data implicate the PGE2 EP1 receptor in the progression of 6-OHDA-induced Parkinsonism. Moreover, using rat embryonic mesencephalon cell cultures, Carrasco et al. (2007) have shown that the EP1 receptor makes dopaminergic neurons more vulnerable to PGE2-induced neurotoxicity (Carrasco et al. 2007). Although the mechanism leading to the protection of dopaminergic neurons in EP1−/− mice remains to be determined, it is significant that under normal physiologic conditions, the basal level of dopamine is found to be higher in EP1−/− mice as compared with the WT counterparts (Tanaka et al. 2009). We are inclined to believe that this elevated level of dopamine restores the dopaminergic neurotransmission by directly stimulating the post-synaptic dopamine receptors, as has been shown by the therapeutic use of dopamine agonists and L-Dopa (McCormack and Di Monte 2003). Thus, 6-OHDA toxicity is prevented, and dopaminergic neuronal death is attenuated in EP1−/− mice. As a proof of this concept, this study proposes the EP1 receptor as a new target to better understand PD and a potential therapeutic target for effective intervention against PD.
Acknowledgments
This work was supported in part by grants from NIH NS046400, American Foundation for Parkinson Research, and The Parkinson Disease Foundation.
Contributor Information
Abdullah Shafique Ahmad, Department of Anesthesiology, University of Florida College of Medicine, PO Box 100159, Gainesville, FL 32610-0254, USA.
Takayuki Maruyama, Discovery Research Institute I, Ono Pharmaceutical Co Ltd, Mishima-gun, Osaka, Japan.
Shuh Narumiya, Department of Pharmacology, Faculty of Medicine, Kyoto University, Kyoto, Japan.
Sylvain Doré, Department of Anesthesiology, University of Florida College of Medicine, PO Box 100159, Gainesville, FL 32610-0254, USA; Department of Neurology, Psychiatry, and Neuroscience, and Center for Translational Research in Neurodegenerative Disease, University of Florida College of Medicine, 1275 Center Drive, Biomed Sci J493, PO Box 100159, Gainesville, FL 32610-0159, USA.
References
- Ahmad AS, Saleem S, Ahmad M, Doré S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol Sci. 2006;89(1):265–270. doi: 10.1093/toxsci/kfj022. [DOI] [PubMed] [Google Scholar]
- Ahmad AS, Kim YT, Ahmad M, Maruyama T, Doré S. Selective blockade of PGE2 EP1 receptor protects brain against experimental ischemia and excitotoxicity, and hippocampal slice cultures against oxygen-glucose deprivation. Neurotox Res. 2008;14(4):343–351. doi: 10.1007/BF03033858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of Glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci. 2001;21(20):8108–8118. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97(11):2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin N, Curet O, Deffois A, Carter C. Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem. 1998;71(4):1635–1642. doi: 10.1046/j.1471-4159.1998.71041635.x. [DOI] [PubMed] [Google Scholar]
- Balsinde J, Balboa MA, Dennis EA. Functional coupling between secretory phospholipase A2 and cyclooxygenase-2 and its regulation by cytosolic group IV phospholipase A2. Proc Natl Acad Sci. 1998;95(14):7951–7956. doi: 10.1073/pnas.95.14.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol. 2001;65(2):135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Lodi R, Tonon C, D'Agata V, Sapienza M, Scapagnini G, Mangiameli A, Pennisi G, Stella AM, Butterfield DA. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia. J Neurol Sci. 2005;233(1–2):145–162. doi: 10.1016/j.jns.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Carrasco E, Casper D, Werner P. PGE(2) receptor EP1 renders dopaminergic neurons selectively vulnerable to low-level oxidative stress and direct PGE(2) neurotoxicity. J Neurosci Res. 2007;85(14):3109–3117. doi: 10.1002/jnr.21425. [DOI] [PubMed] [Google Scholar]
- Chesselet M-F, Richter F. Modelling of Parkinson's disease in mice. Lancet Neurol. 2011;10(12):1108–1118. doi: 10.1016/s1474-4422(11). 70227-7. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Wong ESP, Martinez-Vicente M. Protein degradation, aggregation, and misfolding. Mov Disord. 2010;25(S1):S49–S54. doi: 10.1002/mds.22718. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03). 00568-3. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurode-generation in Parkinson's disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Doré S. GPCR antagonists as an alternative to COX-2 inhibitors: a case for the PGE2 EP1 receptor. Trends Pharmacol Sci. 2006;27(9):458–460. doi: 10.1016/j.tips.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson's disease. Exp Neurol. 2007;205(2):295–312. doi: 10.1016/j.exp. neurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Feng ZH, Wang TG, Li DD, Fung P, Wilson BC, Liu B, Ali SF, Langenbach R, Hong JS. Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl1, 2, 3, 6-tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci Lett. 2002;329(3):354–358. doi: 10.1016/s0304-3940(02)00704-8. [DOI] [PubMed] [Google Scholar]
- Feng Z, Li D, Fung PCW, Pei Z, Ramsden DB, Ho S-L. COX-2-deficient mice are less prone to MPTP-neurotoxicity than wild-type mice. NeuroReport. 2003;14(15):1927–1929. doi: 10.1097/00001756-200310270-00009. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J Neurosci. 2003;23(4):1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach M, Riederer P. Animal models of Parkinson's disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm. 1996;103(8):987–1041. doi: 10.1007/bf01291788. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-jB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci. 2007;104(47):18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. Genetic analysis of pathways to Parkinson's disease. Neuron. 2010;68(2):201–206. doi: 10.1016/j.neuron.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103(2):147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hoang T, Choi D-K, Nagai M, Wu D-C, Nagata T, Prou D, Wilson GL, Vila M, Jackson-Lewis V, Dawson VL, Dawson TM, Chesselet M-F, Przedborski S. Neuronal NOS and cyclooxygenase-2 contribute to DNA damage in a mouse model of Parkinson disease. Free Radical Biol Med. 2009;47(7):1049–1056. doi: 10.1016/j.freeradbiomed.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Janssen I, van Elk EJ, Rozemuller AJ, Eikelenboom P. The role of cyclo-oxygenase 1 and 2 activity in prostaglandin E(2) secretion by cultured human adult microglia: implications for Alzheimer's disease. Brain Res. 2002;951(2):218–226. doi: 10.1016/S0006-8993(02)03164-5. [DOI] [PubMed] [Google Scholar]
- Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav Brain Res. 2005;162(1):1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Javoy F, Sotelo C, Herbet A, Agid Y. Specificity of dopaminergic neuronal degeneration induced by intracerebral injection of 6-hydroxydopamine in the nigrostriatal dopamine system. Brain Res. 1976;102(2):201–215. doi: 10.1016/0006-8993(76)90877-5. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative mechanisms in nigral cell death in Parkinson's disease. Mov Disord. 1998;13(Suppl 1):24–34. [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4(2):131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A. 1996;93(6):2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12(2):225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Li RC, Zhang J, Klaus JA, Kibler KK, Doré S, Koehler RC, Sapirstein A. Cytosolic phospholipase A2 alpha amplifies early cyclooxygenase-2 expression, oxidative stress and MAP kinase phosphorylation after cerebral ischemia in mice. J Neuroinflammation. 2010;7:42. doi: 10.1186/1742-2094-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Bazinet R, Rapoport S, Bhattacharjee A. Brain arachidonic acid cascade enzymes are upregulated in a rat model of unilateral Parkinson disease. Neurochem Res. 2010;35(4):613–619. doi: 10.1007/s11064-009-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA. Effects of l-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J Neurochem. 2003;85(1):82–86. doi: 10.1046/j.1471-4159.2003.01621.x. [DOI] [PubMed] [Google Scholar]
- Mohan S, Ahmad AS, Glushakov A, Chambers C, Dore S. Putative role of prostaglandin receptor in intracerebral hemorrhage. Front Neurol. 2012;3:145. doi: 10.3389/fneur.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanakumar KP, Muralikrishnan D, Thomas B. Neuroprotection by sodium salicylate against 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-induced neurotoxicity. Brain Res. 2000;864(2):281–290. doi: 10.1016/s0006-8993(00)02189-2. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998;95(18):10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med. 2009;87(10):1015–1022. doi: 10.1007/s00109-009-0500-1. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Przybyłkowski A, Kurkowska-Jastrzȩbska I, Joniec I, Ciesielska A, Członkowska A, Członkowski A. Cyclooxygenases mRNA and protein expression in striata in the experimental mouse model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to mouse. Brain Res. 2004;1019(1–2):144–151. doi: 10.1016/j.brainres.2004.05.095. [DOI] [PubMed] [Google Scholar]
- Ross BM, Mamalias N, Moszczynska A, Rajput AH, Kish SJ. Elevated activity of phospholipid biosynthetic enzymes in substantia nigra of patients with Parkinson's disease. Neuroscience. 2001;102(4):899–904. doi: 10.1016/s0306-4522(00)00501-7. [DOI] [PubMed] [Google Scholar]
- Saleem S, Li R, Wei G, Doré S. Effects of EP1 receptor on cerebral blood flow in the middle cerebral artery occlusion model of stroke in mice. J Neurosci Res. 2007;85(11):2433–2440. doi: 10.1002/jnr.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scali C, Prosperi C, Vannucchi MG, Pepeu G, Casamenti F. Brain inflammatory reaction in an animal model of neuronal degeneration and its modulation by an anti-inflammatory drug: implication in Alzheimer's disease. Eur J Neurosci. 2000;12(6):1900–1912. doi: 10.1046/j.1460-9568.2000.00075.x. [DOI] [PubMed] [Google Scholar]
- Schmidt N, Ferger B. Neurochemical findings in the MPTP model of Parkinson's disease. J Neural Transm. 2001;108(11):1263–1282. doi: 10.1007/s007020100004. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267(16):4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Sawada H, Kitamura Y, Taniguchi T. Disease model: Parkinson's disease. Trends Mol Med. 2003;9(8):360–365. doi: 10.1016/S1471-4914(03)00117-5. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S, Ichikawa A. Distribution and function of prostanoid receptors: studies from knockout mice. Prog Lipid Res. 2000;39(4):289–314. doi: 10.1016/S0163-7827(00). 00008-4. [DOI] [PubMed] [Google Scholar]
- Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141(4):1554–1559. doi: 10.1210/en.141.4.1554. [DOI] [PubMed] [Google Scholar]
- Takano T, Panesar M, Papillon J, Cybulsky AV. Cyclooxygenases-1 and 2 couple to cytosolic but not group IIA phospholipase A2 in COS-1 cells. Prostaglandins Other Lipid Mediat. 2000;60(1–3):15–26. doi: 10.1016/s0090-6980(99)00033-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Furuyashiki T, Momiyama T, Namba H, Mizoguchi A, Mitsumori T, Kayahara T, Shichi H, Kimura K, Matsuoka T, Nawa H, Narumiya S. Prostaglandin E receptor EP1 enhances GABA-mediated inhibition of dopaminergic neurons in the substantia nigra pars compacta and regulates dopamine level in the dorsal striatum. Eur J Neurosci. 2009;30(12):2338–2346. doi: 10.1111/j.1460-9568.2009.07021.x. [DOI] [PubMed] [Google Scholar]
- Teismann P, Ferger B. Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson's disease. Synapse. 2001;39(2):167–174. doi: 10.1002/1098-2396(200102)39:2\167:aid-syn8[3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100(9):5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Osipov AN, Belikova NA, Basova LV, Kapralov AA, Bayir H, Kagan VE. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Differ. 2006;14(4):872–875. doi: 10.1038/sj.cdd.4402068. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxydopamine-induced degeneration of the nigrostriatal dopamine pathway: the turning syndrome. Pharmacol Ther B. 1976;2(1):37–40. doi: 10.1016/0306-039x(76). 90016-7. [DOI] [PubMed] [Google Scholar]
- Ushikubi F, Sugimoto Y, Ichikawa A, Narumiya S. Roles of prostanoids revealed from studies using mice lacking specific prostanoid receptors. Jpn J Pharmacol. 2000;83(4):279–285. doi: 10.1254/jjp.83.279. [DOI] [PubMed] [Google Scholar]
- Vijitruth R, Liu M, Choi D-Y, Nguyen X, Hunter R, Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson's disease. J Neuroinflammation. 2006;3(1):6. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Pei Z, Zhang W, Liu B, Langenbach R, Lee C, Wilson B, Reece JM, Miller DS, Hong JS. MPP?-induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J. 2005 doi: 10.1096/fj.04-2457fje. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Ushikubi F, Narumiya S, Sugimura T, Wakabayashi K. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59(20):5093–5096. [PubMed] [Google Scholar]