Abstract
Some epiphytic Hymenophyllaceae are restricted to lower parts of the host (<60 cm; 10–100 μmol photons m-2 s-1) in a secondary forest of Southern Chile; other species occupy the whole host height (≥10 m; max PPFD >1000 μmol photons m-2 s-1). Our aim was to study the photosynthetic light responses of two Hymenophyllaceae species in relation to their contrasting distribution. We determined light tolerance of Hymenoglossum cruentum and Hymenophyllum dentatum by measuring gas exchange, PSI and PSII light energy partitioning, NPQ components, and pigment contents. H. dentatum showed lower maximum photosynthesis rates (Amax) than H. cruentum, but the former species kept its net rates (An) near Amax across a wide light range. In contrast, in the latter one, An declined at PPFDs >60 μmol photons m-2 s-1. H. cruentum, the shadiest plant, showed higher chlorophyll contents than H. dentatum. Differences in energy partitioning at PSI and PSII were consistent with gas exchange results. H. dentatum exhibited a higher light compensation point of the partitioning of absorbed energy between photochemical Y(PSII) and non-photochemical Y(NPQ) processes. Hence, both species allocated energy mainly toward photochemistry instead of heat dissipation at their light saturation points. Above saturation, H. cruentum had higher heat dissipation than H. dentatum. PSI yield (YPSI) remained higher in H. dentatum than H. cruentum in a wider light range. In both species, the main cause of heat dissipation at PSI was a donor side limitation. An early dynamic photo-inhibition of PSII may have caused an over reduction of the Qa+ pool decreasing the efficiency of electron donation to PSI. In H. dentatum, a slight increase in heat dissipation due to acceptor side limitation of PSI was observed above 300 μmol photons m-2s-1. Differences in photosynthetic responses to light suggest that light tolerance and species plasticity could explain their contrasting vertical distribution.
Introduction
Although light energy is an essential resource for photosynthesis, both extreme low and high light intensity can limit plant performance [1]. Thus, sunlight is a key factor in determining plants distribution, since it is strictly related to the ability of such organisms to deal with its absorption, photochemical conversion, and harmless dissipation. When light energy is absorbed by chlorophyll molecules, these pigments reach a singlet excited state. To relax back to its ground form, the energy captured by them can basically have one of three fates: (1) it can be re-emitted as fluorescence; (2) it can be used to drive photochemical processes (e.g. photosynthesis, photorespiration, water-water cycle, etc.), or (3) it can be dissipated non-photochemically as heat [2]. These three processes have a competitive kinetics, in such a way that any increase in the efficiency of one will result in a decrease in the yield of the other two. Hence, changes in PSII chlorophyll fluorescence as well as in PSI absorbance provide important information about photochemical and non-photochemical efficiencies for energy dissipation [3–6]. For instance, heat dissipation at PSII level indicates photoprotective processes and/or photoinhibition according to its relaxing kinetic (i.e. qE: fast heat dissipation by xanthophyll cycle, or qI: sustained photoinhibition) [7, 8]. In analogy, heat dissipation by PSI may indicate a donor side limitation due to a shortage of electrons from the intersystem chain to reduce PSI or acceptor side limitation caused for a lack of electrons acceptors that restrain charge separation at PSI level [9]. Nevertheless, if harmless mechanisms of photoprotection (i.e. such as non-photochemical quenching) are insufficient to deal with an excess of absorbed energy and the prevention of photoinhibition, this excess will conduct to damaging free radicals formation (e.g. superoxide anion, hydrogen peroxide, hydroxyl radical, peroxyl radical and singlet oxygen), and to the subsequent photo-oxidative destruction of the photosynthetic apparatus [10, 11].
In natural environments, incident light varies over several orders of magnitude and on a broad time scale that can range from seasons to seconds. Thus, plants have developed a wide spectrum of biochemical and physiological responses to light that enable them to adjust their photosynthetic performance, and consequently, their net carbon gain and plant growth to their light environments [8, 10, 12, 13]. In this context, plants that have a greater capacity to avoid, use and/or dissipate the absorbed energy are frequently found in sunny habitats and they are known as sun plants. In contrast, plants that do not have the same ability are confined to shaded habitats and they are called shade plants [10, 14–16]. Shade plants are characterized by higher CO2 assimilation rates at low irradiances, lower light compensation and saturation points, low capacities for photoprotective pathways such thermal energy dissipation, lower chlorophyll a/b ratios and higher chlorophyll contents [10, 15]. The Hymenophyllaceae Link. is one of the most attractive, largest, and specious families of basal ferns that include more than 600 species. It exhibits a remarkable diversity in terms of plant morphology and habitat requirements [17]. The most conspicuous features of these plants are a mono or few cell layer fronds (to which they own the alternative name of “filmy ferns” or “filmies”), a highly reduced or absent cuticles, and the complete lack of differentiated epidermis and stomata [18]. These features suggest that filmy ferns have a limited control of gas exchange (i.e. CO2, O2 and water), and therefore, a restricted distribution. Although Hymenophyllaceae species are globally distributed, they are one of the main components of forests in tropical and temperate-humid regions, being recognized as an important indicator of these ecosystems conservation conditions [17–19]. These ferns diversity and abundance have been observed in the middle and lower strata of the forest, where pteridophytes are adapted to such environmental conditions [20–22]. From an ecophysiological point of view, filmy ferns are generally perceived as shade plants, inhabiting deep shade and constant humid places in the forest [23–26]. However, the latest studies carried out in tropical and temperate environments have reported that some filmy ferns are also able to inhabit the canopy, the top forest strata [22, 27, 28]. This suggests that some of these ferns are able to withstand high light intensities during significant periods of time.
In a secondary forest of Southern Chile, the diversity and abundance of filmy ferns decrease with the host tree height [22]. While some filmy species are restricted to their hosts lower heights (i.e. <60 cm), where light availability is very low, (i.e. 10–100 μmol photons m-2 s-1), other species occupy the whole host height range, (reaching heights ≥10 m). Light availability at this height near the canopy, although unfrequently can exceeds 500 μmol photons m-2 s-1 for at least a couple of hours during the day in spring and summer (Fig 1 and S1 Table). This filmy ferns vertical distribution in the host tree is, at least to some degree, explained by the microhabitat characteristics, particularly the relative humidity and canopy openness [22].
Fig 1. Light availability across a vertical gradient in the natural habitats of Hymenophyllaceae species in the Katalapi Park.
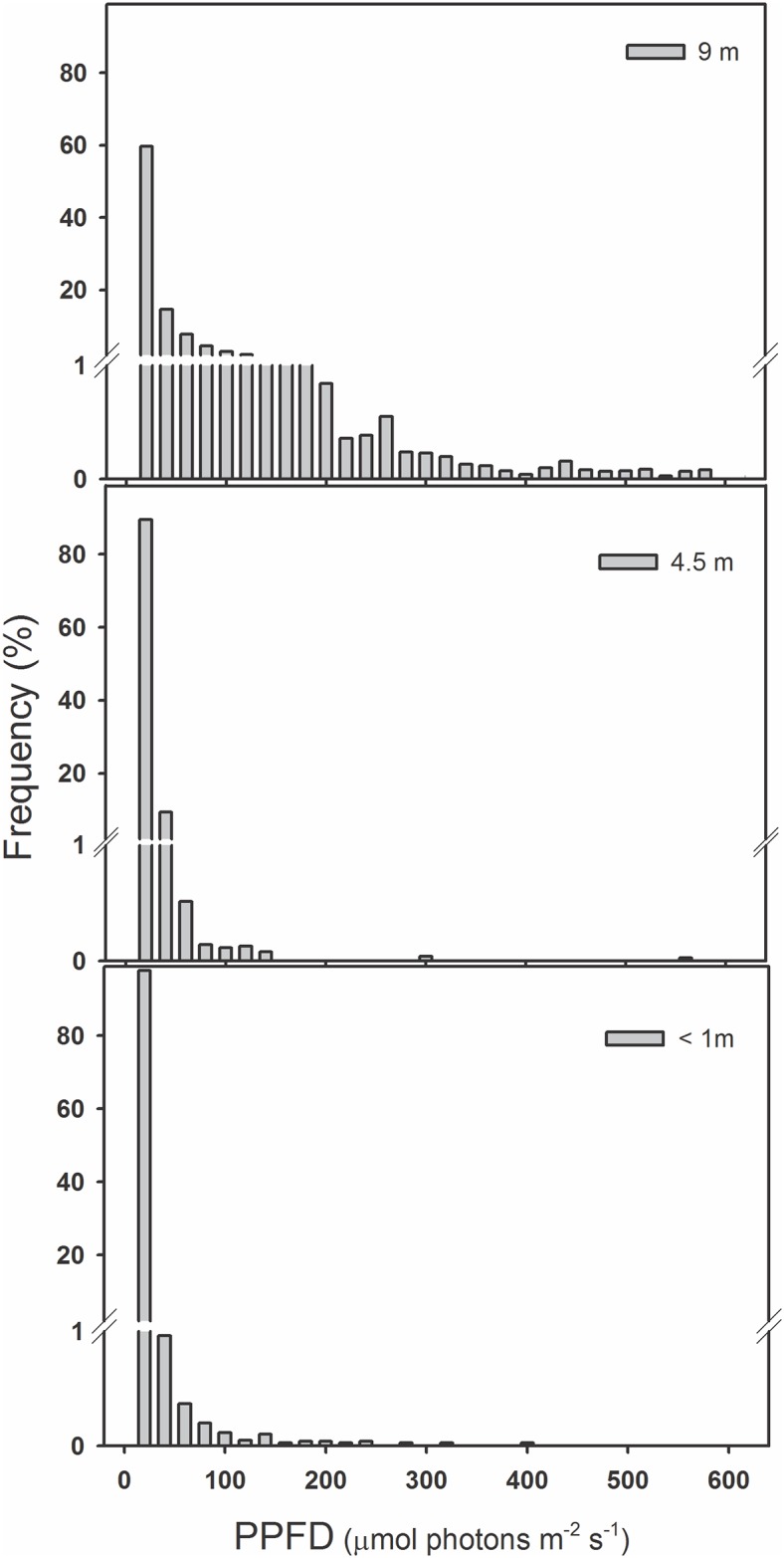
Frequency of observed photosynthetic photon flux density (PPFD, μmol photons m-2 s-1) measured at three trunk heights: <1, 4.5 and 9 m using data collected by two different data-loggers from 01 October 2010 until 01 February 2011.
The aim of this work was to study the photosynthetic light responses of two Hymenophyllaceae species and the relation of these with their contrasting distribution patterns in a secondary temperate rainforest of Southern Chile. For this purpose, we determined potential differences in light tolerance between Hymenoglossum cruentum (Cav) K. Presl. and Hymenophyllum dentatum (Cav.). H. cruentum is commonly found inhabiting the host lower heights (i.e. <60 cm) and/or most shaded places inside the forest, while H. dentatum is present along the whole host height range and/or in more light exposed sites. Therefore, we proposed that H. dentatum is more tolerant to high light intensities than H. cruentum, which is reflected in higher photosynthetic performance and better non-photochemical responses.
Materials and Methods
Study area and plant material
We compared light tolerance of two native epiphytic Hymenophyllaceae species: Hymenoglossum cruentum and Hymenophyllum dentatum (Fig 2). H. cruentum is an endemic Chilean species with plant height ranged 10–30 cm. Its fronds are entire and glabrous, with marginal sori placed on veins ends. In contrast, H. dentatum ranges 8–18 cm plant height. Its fronds are divided and have a dentate margin, hairy petiole, rachis and veins, and subaxilar sori [29].
Fig 2. Two epiphytic Hymenophyllaceae species with contrasting vertical distribution in a secondary temperate rainforest (Katalapi Park, Puerto Montt, Chile).

a) Hymenoglossum cruentum, and b) Hymenophyllum dentatum.
Plants were collected at the Katalapi Park (41°31’12.0”S—72°45’02.3”W), located in the Cordillera de Quillaipe, near Puerto Montt, Chile. This area is characterized by a temperate evergreen interior forest, dominated by Drimys winteri, Amomyrtus luma and A. meli, Laureliopsis philippiana, Nothofagus dombeyi and N. nitida, Raphythamnus spinosus, Weinmannia trichosperma, and several Proteaceae species. It presents a temperate-humid climate with strong oceanic influence [30], with a mean air temperature of 15°C and 1900 mm of annual rainfall. This study was duly authorized by the owner and Park Director (Ms Ana María Vliegenthart), and it did not involve endangered or protected species.
Fern species were collected from different levels of the forest according to their vertical abundance. Individuals of Hymenoglossum cruentum were collected from parts of host <60 cm heights, while individuals of Hymenophyllum dentatum were collected between 60–200 cm host heights. For each species, plant material corresponded to bark sections that contained groups of adult filmy ferns, which were obtained from several host species. After collection in the field plant material was immediately transported to a nursery in the Laboratory of Plant Physiology at the Universidad de Concepción (Concepción, Chile). Filmy species were kept in the nursery during six days to recover them from stress produced by transport. Therefore, the time between plant material collection and their photosynthetic performance measurements was less than 1 week. In this period, both species were exposed to similar conditions to those found in the field. We controlled relative humidity (70–90%) by watering plants 2–5 times per day during 2 min, using a semi-automatic fog type irrigation system (own fabrication). Light intensity was maintained <70 μmol photons m-2 s-1, using a black mesh on all walls and roof to block part of incoming sunlight. All measurements were performed in detached fully hydrated fronds (placed in distilled water overnight).
Vertical light gradient in the field
Photosynthetic photon flux densities (thereafter PPFD) were measured in the field to characterize vertical light gradients along trunk hosts (Table 1 and S1 Table). Two Quantum Smart sensors (S-LIA-M003, Hobo, Onset Computer Corporation, USA) were placed at three heights: <1m, 4.5 m and 9 m in trunks of two different stands of a well-grown secondary forest. Smart sensors were connected to an Energy logger (H22-001, Hobo Onset Computer Corporation, USA), and data were recorded every 15 min from October 01, 2010 to February 01, 2011 (i.e. Spring-summer period in the Southern Hemisphere).
Table 1. Vertical distribution of photosynthetic photon flux density (PPFD) in a secondary temperate rainforest of Southern Chile.
Values correspond to daily mean ± SE (n = 2). Measurements were carried out in January 2011 in the Katalapi Park.
| Trunk height (m) | Mean PPFD (μmol photons m-2 s-1) | Absolute maximum PPFD (μmol photons m-2 s-1) |
|---|---|---|
| < 1 | 32 ± 2 | 501 |
| 4.5 | 89 ± 4 | 548 |
| 9 | 623 ± 15 | 1,738 |
Gas exchange measurements
For each species, photosynthetic light responses were measured on fully expanded but non-reproductive fronds (i.e. without sori), of a similar size and among those that already developed in the field. Hence, expected differences in light tolerance between species are attributed to contrasting microhabitats where fronds developed.
Six detached fronds from different individuals were used to obtain photosynthetic responses to different PPFDs. An Infrared Gas Analyzer (Portable photosynthesis measuring system, GFS-3000, WALZ, Effeltrich, Germany) was programmed to expose fronds to a progressive stepwise increase in PPFD. Photosynthetic response curves were built with seventeen PPFD levels from 0 to 300 μmol photons m-2 s-1. Each frond was exposed 3 min to each level of PPFD, to stabilize the net rate of CO2 Assimilation (An) before each measurement. Therefore, the total time course for a whole light response curve was 51 min. An of each frond was measured at 15°C, 390 ppm CO2, and 95% relative humidity, to avoid suboptimal conditions produced by factors other than PPFD. This range of RH can be obtained in the GFS-3000 because it has and integrated H2O control via step motor for humidifying and drying with a range from 0 to nearly 100% RH preventing condensation (WALZ, Effeltrich, Germany). Before each measurement, fronds were hydrated for 24 h. An was fitted to a quadratic hyperbolic function using STATISTICA 7 Statsoft ® software and following Lambers et al. (2008) [31]. From each curve, light compensation (I c ), light saturation point (I s), and maximum rate of CO2 assimilation (A max ) were estimated. I c corresponds to the PPFD where the rate of CO2 assimilation is balanced by the rate of CO2 production in respiration and photorespiration; I s corresponds to the PPFD over which the rate of CO2 assimilation is maximal and insensitive to level of PPFD; and A max is the light-saturated rate of gross CO2 assimilation (net rate of CO2 assimilation + dark respiration) at infinitely high irradiance [31]. Fronds were darkened during 30 min before the beginning of photosynthetic response curves to obtain the Dark Respiration rate (R d ). In the case of H. cruentum, light steps used for fitting curves were limited to 50 μmol photons m-2 s-1 because above this PPFD, its net rate of CO2 assimilation abruptly dropped.
Given that H. dentatum fronds did not cover completely the area of the IRGA cuvette, we took a photograph of each frond inside the cuvette, and then we calculated its area by using Sigma scan® software. With this information we corrected An, Amax, and Rd measurements as way as to standardize these parameters to an area of 4 cm2, which was the area of the IRGA cuvette used for gas exchange measurements in both fern species. No area corrections were made for H. cruentum because their fronds covered completely the area of the IRGA cuvette.
Light energy partitioning at PSI and PSII
Simultaneous assessment of changes in P700 absorbance and PSII chlorophyll fluorescence were performed in four detached fronds of each filmy species using a Dual-PAM 100 measuring system (WALZ, Effeltrich, Germany). PPFD response curves were programmed using the scripting facility of the Dual-PAM 100 control software to expose each frond to successively increasing actinic light levels, with 3 min equilibration time at each level before measurements. These determinations were made at 15°C, kept constant by adding two metal collars around the Dual PAM-100 measuring heads and then connecting them to a cooling/heating circulator (Thermo Haake K15, Electron Corporation, Germany). Fully hydrated fronds were dark adapted during 30 min before each PPFD response curve. This dark adaptation is necessary to determine the intrinsic or maximal fluorescence of PSII (Fm), which is used in NPQ calculations [3]. Photochemical quenching (qL) was calculated according to Kramer et al. 2004 [9].
We assessed the photochemical light responses of these filmy ferns through the following parameters: For PSII we calculated: Y PSII, photochemical quantum yield of PSII; Y NPQ, regulated heat dissipation quantum yield, and Y NO, non-regulated heat dissipation quantum yield. For PSI we calculated: Y PSI, photochemical quantum yield of PSI, Y ND, non-photochemical quantum yield caused by a donor side limitation, and Y NA, non-photochemical quantum yield caused by an acceptor side limitation [9, 32]. Recordings and calculations were performed with the Dual-PAM 1.7 data analyses and control software (WALZ, Effeltrich, Germany). Calculations of ETRII (ETRII = 0.5 x Abs x Y(II) x PPFD) and ETRI (ETRI = 0.5 x Abs x Y(I) x PPFD) given by the instrument were corrected using the actual average absorbance of fully hydrated fronds (n = 4) of each filmy fern species. For this, the absorbance (Abs) was calculated using the automatic routine for measurement PAR-absorptivity image of Imaging PAM-mini (Walz, Effeltrich, Germany). This measurement requires successive illumination of the samples with red (R) and NIR light and the capture of each remission image. The absorbance is calculated by the equipment software pixel by pixel as follows: Abs = 1- R/NIR. The average values for absorbance were 0.58 ± 0.03 for H. cruentum and 0.44 ± 0.07 for H. dentatum (mean ± SE, n = 4).
NPQ components determination under photoinhibitory conditions
For each fern species, we determined fast and slow dark relaxing components of NPQ in five detached, adult, and fully expanded fronds of different individuals, which were previously dark adapted during 30 min to obtain the minimum and maximal fluorescence parameters (i.e. F0 and Fm, respectively). The measurements were made with a FMS II modulated fluorimeter (Hansatech Instruments Ltd, UK), whose probe was connected to a LD2/3 camera (Hansatech Instruments Ltd, UK), to expose fronds to a LS2 white light source (Hansatech Instruments Ltd, UK). Measurements were made at 15°C, kept constant using a cooling/heating circulator (Thermo Haake K15, Electron Corporation, Germany), that was connected to LD2/3 camera. Fast (NPQf) and slow (NPQs) relaxing components were obtained essentially as described by Walters and Horton (1991) [33], analyzing the kinetics of Fm recovery after actinic light has been turned off, where NPQs = (Fm-Fmr)/Fmr, and NPQf = (Fm-Fm’)-(Fm-Fmr). Fmr (value of Fm that would have been attained if only slowly relaxing quenching had been present) was obtained by extrapolation in a semi-logarithmic plot of maximum fluorescence yield versus time of data points recorded toward the end of the relaxation back to the time where the actinic light was removed. This graph was obtained after exposing 5 dark adapted fronds to a photoinhibitory treatment during 25 min, at an actinic light intensity of 1,500 μmol photons m-2 s-1, and 1h of recovery time in darkness.
Pigments Analyses
Approximately 0.1 g fresh tissue fronds per fern species (n = 3) were collected from plants growing in the nursery. Fresh samples were immediately frozen in liquid nitrogen and stored at -80°C until analysis. Samples were powdered, and immediately a spatula tip of CaCO3 was added before extracting with 1 mL 100% HPLC-grade acetone at 4 ◦C under dim light. The extract was spun down and the supernatant was filtered through 0.45 μm syringe filter. Pigments, including chlorophyll a and b, β-carotene (β-car) and α-carotene (α-car), neoxanthin (Neo), and xanthophyll cycle pigments, violaxanthin (V), antheraxanthin (A) and zeaxanthin (Z) contents were measured by a high performance liquid chromatography (HPLC) method described by García-Plazaola and Becerril (1999) [34], with instrumentation and HPLC conditions modified by Sáez et al. (2013) [35]. De-epoxidation state (DEPS) of the xanthophyll pool was calculated as follow: DEPS = (V+0.5A)/(V+A+Z).
Data analyses
Gas exchange parameters (i.e. Rd, Amax, Ic and Is) were compared between species using t tests. PPFD response curves were compared at two points, which corresponded to light saturation points (Is) of each species. At these points, differences between species for YPSII, YNPQ, YNO, YPSI, YND, YNA, ETRII and ETRI were assessed using t tests. Differences between species for NPQ components, pigments contents and ratios were assessed using t tests as well. Alternative non parametric tests were used when assumptions of normality and homoscedasticity where not met after transformations [36].
Results
Gas exchange responses
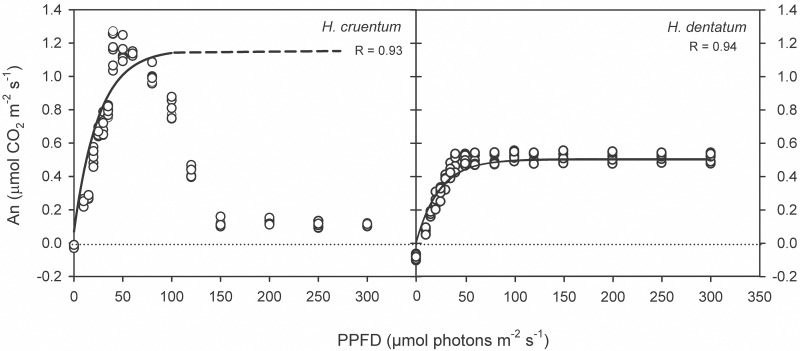
CO2 assimilation responses to an increase of photosynthetic photon flux density (PPFD) differed between species (Fig 3 and S2 Table). Hymenophyllum dentatum showed a mean Amax 2.2 times lower than Hymenoglossum cruentum (Table 2 and S3 Table; t = 30.1, P <0.0001).
Fig 3. Dependency of CO2 net assimilation rate (An, μmol m-2 s-1) on Photosynthetic Photon Flux Density (PPFD, μmol photons m-2 s-1) in two filmy fern species with contrasting vertical distribution: Hymenoglossum cruentum and Hymenophyllum dentatum.
All replicates were plotted in the graph (n = 6). A black line indicates the fit curve for photosynthetic responses to light following Lambers et al. (2008) [31]. A dashed line indicates the portion of the fit curve where observed values for H. cruentum diverted from those expected values.
Table 2. Photosynthetic performance of two filmy ferns with contrasting vertical distribution on tree trunks: Hymenoglossum cruentum and Hymenophyllum dentatum.
Parameters were obtained from gas exchange measurements: Rd, dark respiration; Amax, maximum CO2 assimilation rate; Ic, light compensation point; Is, light saturation point. Data are shown as mean values ± SE (n = 6).
| H. cruentum | H. dentatum | |
|---|---|---|
| Rd (μmol CO2 m-2 s-1) | 0.02 ± 0.01 | 0.08 ± 0.01*** |
| Amax (μmol CO2 m-2 s-1) | 1.16 ± 0.02 | 0.54 ± 0.01*** |
| Ic (μmol photons m-2 s-1) | 1.45 ± 0.07 | 4.9 ± 0.15*** |
| Is (μmol photons m-2 s-1) | 24.6 ± 0.07 | 40.5 ± 1.4** |
Levels of significance:
**, P <0.001;
***, P <0.0001.
H. dentatum kept its An near Amax over a wide range of PPFDs. In contrast, H. cruentum declined its An with an increase in PPFD, especially above 60 μmol photons m-2 s-1, where An sharply decreased to less than 10% of Amax (Fig 3; S2 and S3 Tables). Both species showed low Rd, with the lowest values recorded in fronds of H. cruentum (Table 2 and S3 Table; t = 9.5, P <0.0001). Light compensation point (Ic) was 3.2 times lower in fronds of H. cruentum than in fronds of H. dentatum (Table 2 and S3 Table; t = -21.2, P <0.0001). Similarly, light saturation point (Is) was 1.6 times lower in fronds of H. cruentum than in fronds of H. dentatum (Table 2 and S3 Table; Z = 2.9, P = 0.004).
Light Energy partitioning
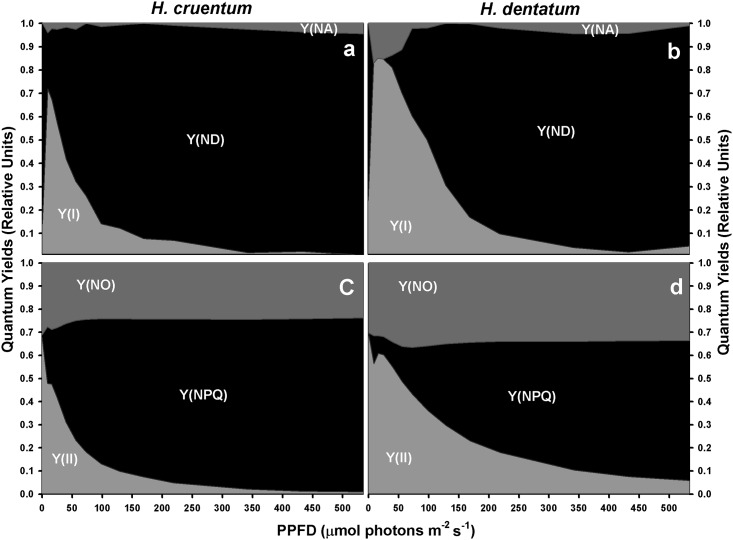
Even when the light response pattern of PSI and PSII yields was very similar between the two ferns species, the compensation points of photochemical and non-photochemical processes, as well as the specific values at the corresponding Is, were different between filmy species (Fig 4 and S4 Table). First, H. cruentum yields compensated at irradiances below 50 μmol photons m-2 s-1; whereas those of H. dentatum did near to 100 μmol photons m-2 s-1.
Fig 4. Changes in partitioning of absorbed excitation energy with increasing PPFD.
This was measured at PSI (a, b) and PSII (c, d) level in fronds of Hymenoglossum cruentum and Hymenophyllum dentatum. A dashed line indicates the portion of the curve where comparisons between species were made. These points correspond to the light saturation points of H. cruentum at 24.6 μmol photons m-2 s-1 (IsH.cru) and of H. dentatum at 40.5 μmol photons m-2 s-1 (IsH.den), both obtained from gas exchange measurements. Values are shown as mean ± SE (n = 4).
Secondly, H. cruentum was photochemically less effective than H. dentatum. This was reflected by its 18% at IsHcru (t = 8.1; P <0.001) and 24% at IsHden (t = -5.9; P <0.01) lower YPSII (Table 3 and S4 Table), as well as by its earlier YPSII decrease to values below 0.1 at irradiances about 200 μmol photons m-2 s-1 less than H. dentatum (Fig 4c and 4d, S4 Table).
Table 3. Changes in PSII and PSI components in fronds of Hymenoglossum cruentum and Hymenophyllum dentatum measured at their respective light saturation points (Is).
PSII parameters: YPSII, photochemical quantum yield of PSII; YNPQ, regulated heat dissipation quantum yield; YNO, non-regulated heat dissipation quantum yield; and ETRII, electron transport at the PSII. PSI parameters: YPSI, photochemical quantum yield of PSI; YND, non-photochemical quantum yield caused by a donor side limitation; YNA, non-photochemical quantum yield caused by an acceptor side limitation, and ETRI, electron transport rate at the PSI. Values correspond to mean ± SE (n = 4).
| Is | IsH.cru = 24.6 (μmol photons m-2 s-1) | IsH.den = 40.5 (μmol photons m-2 s-1) | ||
|---|---|---|---|---|
| Species | H. cruentum | H. dentatum | H. cruentum | H. dentatum |
| PSII | ||||
| YPSII | 0.42 ± 0.01 | 0.60 ± 0.03** | 0.31 ± 0.01 | 0.55 ± 0.04** |
| YNPQ | 0.30 ± 0.01 | 0.08 ± 0.02* | 0.43 ± 0.02 | 0.11 ± 0.04** |
| YNO | 0.28 ± 0.01 | 0.32 ± 0.01* | 0.26 ± 0.02 | 0.34 ± 0.01* |
| ETRII | 2.98 ± 0.05 | 3.1 ± 0.20ns | 3.64 ± 0.13 | 4.4 ± 0.44ns |
| PSI | ||||
| YPSI | 0.62 ± 0.04 | 0.85 ± 0.10ns | 0.44 ± 0.04 | 0.60 ± 0.14* |
| YND | 0.40 ± 0.05 | 0.00 ± 0.00* | 0.57 ± 0.05 | 0.06 ± 0.04** |
| YNA | 0.03 ± 0.03 | 0.15 ± 0.10ns | 0.02 ± 0.01 | 0.14 ± 0.04* |
| ETRI | 4.44 ± 0.23 | 3.51 ± 0.79ns | 5.24 ± 0.36 | 5.56 ± 1.39ns |
Levels of significance: ns, not significant;
*, P <0.05;
**, P <0.001.
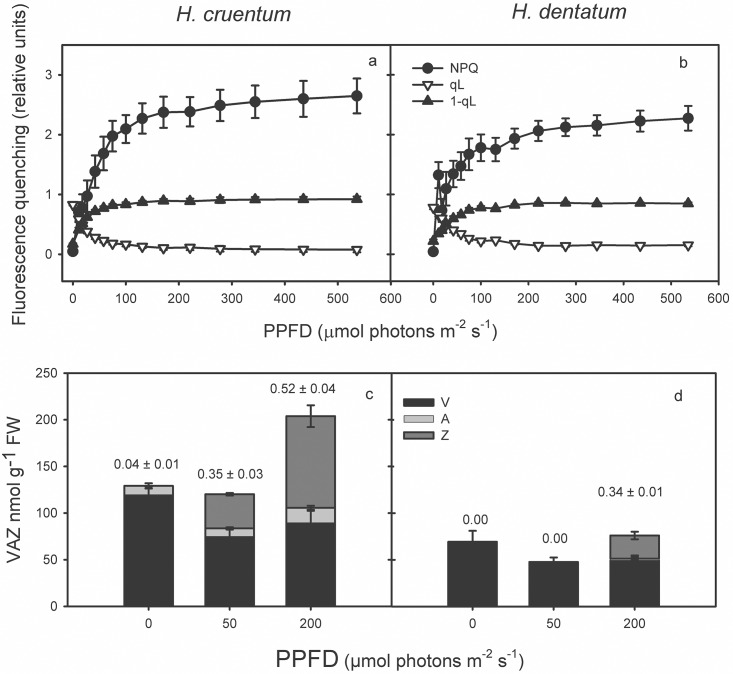
Such results coincide with the slightly lower proportion of open reaction centers of PSII (qL) in H. cruentum at saturating light intensities (Fig 5a and 5b, S5 Table). Hence, the redox state of the primary acceptor of PSII Qa-pool, estimated as 1-qL, was slightly higher in H. cruentum than in H. dentatum, being also saturated at lower PPFD.
Fig 5.
Light response curves of fluorescence derived quenching parameters NPQ, qL, and 1-qL, expressing non-photochemical quenching, the proportion of PSII open reaction centers (P680-Qa+) and the redox state of Qa, the primary acceptors of PSII for H. cruentum (a) and H. dentatum (b), respectively. Values correspond to the average of n = 3 ± SE. The xanthophyll cycle pool pigments and de-epoxidation state at three light points 0, 50 and 200 μmol photons m-2s-1 are shown for H. cruentum (c) and H. dentatum (d). Pigment determination was performed by HPLC. Values correspond to the average n = 3 ± SE, the numbers on top of each bar correspond to DEPS.
The discrepancies in the ability to use the absorbed light energy were consistent with the corresponding proportion of energy dissipated as heat. Specifically, H. cruentum was the species with higher YNPQ across the entire experimental light curve (Fig 4c and 4d). The maximum differences between the species were found at both saturating light intensities, being the YNPQ values of H. cruentum a 22% at IsHcru (Z = 2.3; P <0.05) and a 32% at IsHden (t = 7.1; P <0.001) higher than H. dentatum (Table 3). Besides its general higher thermal dissipation at PSII level, H. cruentum also exhibited higher PSI non-photochemical quenching (YND + YNA) at the lower irradiances (Fig 4). In spite of such concomitance, a striking result was the different limitations that cause PSI non-photochemical quenching in each filmy fern at light intensities below 70 μmol photons m-2s-1. In H. cruentum, the PSI thermal dissipation was always caused by a donor side limitation (Fig 4a). However, in H. dentatum such dissipation was mostly conditioned by an acceptor side limitation (Fig 4b). In fact, at IsHden, the H. cruentum YND was a 51% higher than YND in H. dentatum (t = 7.7; P <0.001); while the H. dentatum YNA was a 12% higher than H. cruentum (t = -2.8; P <0.05). The latter and coincident interspecific donor side limitation is consistent with the corresponding light-induced decreases in YPSII (Fig 4). Therefore, at any given PPFD, the excitation pressure of PSII and thermal energy dissipation tend to be higher for H. cruentum than H. dentatum (Fig 5a and 5b, S5 Table).
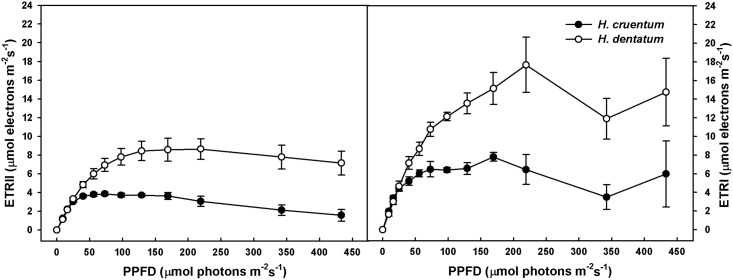
Concerning YNO values, these were slightly different between the two species across all the irradiances applied. Actually, at both saturating light intensities, H. dentatum exhibited a 4% at IsHcru (t = -2.7; P <0.05) and an 8% at IsHden (t = -3.4; P <0.05) higher values than H. cruentum (Table 3). Finally, and regarding ETRs, there were not interspecific differences in both ETRI and ETRII within the light saturation range for net photosynthesis of both species (from 24.6 to 40.5 μmol photons m-2 s-1, Table 3). However, above 50 μmol photons m-2 s-1 H. dentatum exhibited about 100% higher ETRI and ETRII than H. cruentum (Fig 6).
Fig 6. Light response curves of PSII (ETRII) and PSI (ETRI) electron transport rates in fronds of H. cruentum and H. dentatum.
Detached fronds were fully hydrated overnight and then dark adapted during 30 min. PPFD response curves were programmed using the scripting facility of the Dual-PAM 100 control software. Each frond was exposed to successively increasing actinic light levels (0 to 436 μmol photons m-2 s-1), with 3 min equilibration time at each light level before the application of saturating pulses. Values correspond to the mean ± SE (n = 4).
In both species ETRII were saturated at higher PPFD than net photosynthesis. This is a distinctive interspecific feature, where the ETRII of H. dentatum saturated at PPFDs above 100 μmol photons m-2 s-1 while the ETRII of H. cruentum saturated at about 50 μmol photons m-2 s-1. After saturation, ETRII tended to decrease in both species being remarkable in H. cruentum, reaching at the last light step about 50% of its maximal ETRII. In the case of ETRI, saturation was not clear in H. dentatum, which exhibited a sustained increase until near 225 μmol photons m-2 s-1 (Fig 6). ETRI in H. cruentum was saturated at about 50 μmol photons m-2 s-1 (Fig 6).
NPQ components under photoinhibition
Photoinhibition at 1,500 μmol photons m-2 s-1 for 1h rendered no differences for the total NPQ between filmy fern species (Table 4; t = 1.9, P = 0.097), neither in the separated comparisons of fast (t = 2, P = 0.079) nor in the slow NPQ components (t = 0.9, P = 0.405). Most of the NPQ measured in fronds of these species corresponded to the fast component (NPQf), being 84 and 91% of the total NPQ in H. dentatum and H. cruentum, respectively (Table 4 and S6 Table).
Table 4. Components of NPQ measured in fronds of Hymenoglossum cruentum and Hymenophyllum dentatum under photoinhibitory conditions.
NPQf corresponds to the fast dark relaxing component and NPQs corresponds to slow dark relaxing component. Values are shown as mean ± SE (n = 5).
| H. cruentum | H. dentatum | |
|---|---|---|
| NPQ | 3.59 ± 0.54 | 2.42 ± 0.32ns |
| NPQf | 3.25 ± 0.52 | 2.03 ± 0.312ns |
| NPQs | 0.34 ± 0.06 | 0.39 ± 0.03ns |
Levels of significance were ns, not significant.
Pigments contents
There were significant differences in Chlorophyll contents between filmy species (Table 5 and S6 Table). Chlorophyll a and b contents were higher in fronds of H. cruentum than in H. dentatum (t = 3.1, P = 0.038). However, Chl a:b ratio was similar in fronds of H. dentatum and in H. cruentum (t = -1.7, P = 0.165). Neoxantin (Neo), a xanthophyll associated to the light harvesting complex II (LHCII), was 86% higher in H. cruentum than H. dentatum (t = 3.2, P = 0.034). The content of β-carotene (β-car), a pigment preferentially associated to the core of reaction centers of both photosystems, showed non-significant differences between species (t = 0.3, P = 0.790). The higher ratio Neo/β-car exhibited by H. cruentum (Table 5 and S6 Table; t = 12.4, P = 0.0002) suggests a bigger proportion LHC/RC in this species than in H. dentatum. In addition, H. cruentum exhibited much higher contents of α-carotene than H. dentatum (t = 6.8, P = 0.002).
Table 5. Pigments content and their ratios of two Hymenophyllaceae with contrasting vertical distribution on the host tree trunk in a temperate rainforest of Southern Chile.
Values are shown as mean ± SE (n = 3).
| H. cruentum | H. dentatum | |
|---|---|---|
| Chlorophyll a (nmol g-1 FW) | 2,541 ± 241 | 1,403 ± 278* |
| Chlorophyll b (nmol g-1 FW) | 1014 ± 101 | 541 ± 123* |
| Total chlorophyll (nmol g-1 FW) | 3,555 ± 343 | 1,943 ± 401* |
| Chlorophyll a:b ratio | 2.51 ± 0.01 | 2.63 ± 0.07ns |
| α-carotene (nmol g-1 FW) | 115 ± 16 | 6 ± 2* |
| β-carotene (nmol g-1 FW) | 96 ± 10 | 90 ± 21ns |
| Neoxanthin (nmol g-1 FW) | 185 ± 15 | 99 ± 23* |
| Neo/β-car | 1.94 ± 0.04 | 1.11 ± 0.06** |
Levels of significance: ns, not significant;
*, P <0.05;
**, P <0.001.
Discussion
Our results confirm earlier findings which have characterized Hymenophyllaceae species as shade plants [23–26]. This was reflected by low light-saturated rates of net photosynthesis and low light compensation points in Hymenoglossum cruentum and Hymenophyllum dentatum. Although both fern species are adapted to low light intensities, H. dentatum has somehow higher light requirements than H. cruentum. This suggests that contrasting vertical distribution of both filmy species is related, at least in part, to differences in their light tolerance. Specifically, H. dentatum showed Amax values lower than H. cruentum (Table 2); however, it was able to maintain its An maximum value across a wide range of light intensities (Fig 3). Contrasting to this, the higher An values of H. cruentum declined abruptly after 60 μmol photons m-2 s-1 (Fig 3). Such difference between the photosynthetic behaviors of these filmy ferns is not fully understood, but we could speculate two nonexclusive explanations. First, it could be attributed to an earlier and more intense dynamic photoinhibition of H. cruentum at irradiances that exceed its optimum activity (24.6 μmol photons m-2 s-1). This is supported by the concomitant sharp increase in the fraction of energy dissipated as heat (YNPQ), which overpassed YPSII at 50 μmol photons m-2s-1, and also by the highest saturated NPQ observed in H. cruentum (Fig 5c and 5d). The idea that a chronic photo-inhibition is not likely comes from a photoinhibition assay. In this, the dark relaxation kinetics of NPQ was measured in H. cruentum and H. dentatum exhibiting about 80% and 90% of NPQ recovered within the fast relaxation kinetic component (NPQf), respectively. Therefore, both species were able to safely dissipate the excess absorbed energy, probably through xanthophyll dependent heat dissipation (Fig 5). The second possible explanation is that Amax was reduced because an increased CO2 diffusion limitation. Despite these species lack stomata, preliminary measurements and anatomy-based estimates suggest that Hymenophyllaceae do have a strong diffusional limitation to photosynthesis, which is associated to their lack of true mesophyll, presence of very thick cell walls and few chloroplasts with a disperse distribution inside cells (Jaume Flexas pers. comm.). The hydration level in poikilohydrous photosynthetic tissues may influences diffusion of CO2 [37]. In our case, measurements were done at the fully hydrated state. The light response curve took 51 minutes at 95% RH and 15°C inside the IRGA cuvette. This determined a VPD about 0.085 KPa, an order of magnitude lower than VPD experienced by plants in the field [38]. Therefore, the chance of dehydration during measurements is low, especially in H. cruentum that dehydrates at half of the dehydration rates of H. dentatum [39]. The high water status of the fronds in our experiment may have reduced CO2 diffusion since liquid phase diffusion of CO2 is four orders of magnitude lower than in gas phase [40]. It is likely that levels of internal CO2 of H. cruentum were enough to sustain low rates of photosynthesis at low light at the beginning of the measurement, but as the rate of CO2 assimilation increased with PPFD, internal CO2 concentration could have dropped to a limiting level, causing the abrupt drop of net photosynthesis. This hypothetical explanation concerning CO2 diffusion limitation has however, a contradictory result in our analyses of PSI yields. If CO2 limitations have caused this drop in An, then we would have expected a high PSI acceptor side limitation in H. cruentum. Nevertheless this was not the case (Table 3). A possible explanation to such contradictory result is that H. cruentum is able to use an alternative to NADP+ electron sink at high irradiance maintaining a high ETRII/net photosynthesis ratio. H. cruentum exhibited a sustained decrease of ETRII after reaching only 1.5 μmol electrons m-2 s-1 at 430 μmol photons m-2 s-1, increasing the ETRII/net photosynthesis ratio from about 4 to 15 ê/CO2. Considering this, it is likely that alternative electron sinks are also operative for H. dentatum which exhibited much higher ETRII/net photosynthesis ratio than H. cruentum (17 ê/CO2). The use of alternative electron sinks is a common physiological response in poikilohydric bryophytes [41]. Preliminary measurements of ETR under low oxygen (2%) near 150 μmol photons m-2 s-1 have shown a reduction of 30% of maximal relative ETR of H. cruentum (unpubl. data). This suggests oxygen as an electron alternative sink. Experiments to demonstrate the presence of alternative electron sinks in these two filmy ferns and their capacity to deal with the resulting reactive oxygen species are under way.
On the other side, low light compensation and saturation points of both filmy ferns (i.e. <50 μmol photons m-2 s-1) were consistent with those reported for Hymenophyllum tunbridgense and H. wilsonii, two British filmy species with contrasting distribution. According to the author, H. tunbridgense exhibited the lowest light compensation point, which coincides with its localized and sheltered distribution [42]. In a broad sense, differences in photosynthetic performance showed by H. dentatum and H. cruentum are characteristic of adaptation to sun and shade habitats [10], and were consistent with PPFD differences obtained in their respective habitats. Specifically, H. dentatum is able to tolerate moderate light intensities or even sunflecks (about 1,500 μmol photons m-2 s-1) at the upper parts of the host, while H. cruentum is restricted to shade sites in the forest (PPFD <100 μmol photons m-2 s-1; see Table 1 and Fig 1).
As was mentioned above, differences in light responses curves between H. cruentum and H. dentatum were consistent with the results found in photosynthetic performance (Fig 3). For instance, both filmy species were more efficient in dissipating the absorbed energy by photochemical reactions (YPSII) at their respective light saturation points (Table 3), but at higher irradiances, they change their strategy of photo-protection to heat dissipation (YNPQ) (Fig 4a and 4b). Regarding to PSI, the different initial pattern of donor and acceptor side limitations in both filmy ferns (Fig 4a and 4b), could be attributed to differences in their respective rubisco carboxylation speeds and/or rubisco contents. If this were true, it can be assume that H. cruentum would be the species that fulfills such physiological characteristics, having higher Amax at very low light intensities but lower An at higher irradiances (Fig 3). In the same way, the concomitant YND increase and YNA decrease in H. dentatum at irradiances that exceeds its photosynthetic saturating points, as well as its higher and longer maintained YPSI across a wide range of light intensities, suggests a possible participation of PSI cyclic electron flow in the maintenance of H. dentatum An during its exposure to permanent high or increasing light intensities (Fig 3b). Further studies should be conducted in order to understand the importance of PSI activity in these species adapted to light limiting conditions.
NPQ is primarily a measure of non-radiative dissipation of excitation energy and may be seen as essentially photo-protective path for higher plants and especially for poikilohydrous bryophytes and ferns [10, 39, 41]. Contrary to differences observed between species in YNPQ around their respective light saturation points, similar levels of total heat dissipation (NPQ) were observed under photoinhibitory conditions. Interestingly, most of NPQ recorded in fronds of both species corresponded to the fast NPQ component, which is involved in protection of PSII against over-excitation, indicating that although H. cruentum and H. dentatum are considered shade plants, they are able to manage excess of light energy absorbed, thus decreasing their probabilities to suffer photo-damage.
Chlorophyll contents are in line with the contrasting vertical distribution of filmy species (Table 5). H. cruentum, which inhabits the shadiest strata showed a higher chlorophyll a and b contents than H. dentatum, which could enable it maximizes the capture of limiting photons under low-light conditions [15, 25]. However, Chlorophyll a:b ratio in fronds of H. cruentum and in H. dentatum were similar. Both species showed Chl a:b ratio around 2.5, which is concordant with previously reported values for these species [22], and similar to those reported for shade Malayan ferns species [43]. A low Chl a:b ratio is generally indicative of a plants possessing a large proportion of Chl a/b-binding light-harvesting-complexes respect to reaction centers, an adaptation that is typical of plants adapted to low light environments [25, 44]. A high proportion Chl a/b-binding light-harvesting-complexes respect to reaction centers is also supported by a low Neo/β-car ratio observed in H. cruentum (Table 5). Neoxanthin is present in the LHCII while β-carotene is mainly in the reaction center core of both photosystems [45, 46]. According to the literature, higher plants do not present differences in Neo contents on chlorophyll basis. This is also the case in our filmy ferns; both have about 50 mmol per mol of total chlorophyll. Similar levels of β-carotene have been observed in shade leaves of angiosperms [47]. Interestingly, H. cruentum exhibited a significant higher content of α-carotene, which coincides with reported levels in deep shade species [48]. Nonetheless, β-carotene contents were not significantly different between both filmy species. Furthermore, H. cruentum had the highest xanthophyll cycle pigment pool and de-epoxidation level (Fig 5c and 5d). In general, this pattern is somehow different from angiosperms; because shade plants, usually exhibit lower β-carotene, lower xanthophyll cycle pigments pool and lower de-epoxidation state than sun plants [47, 48].
Ecophysiological studies with Hymenophyllaceae species are valuable empirical evidence of an evolutionary shift of adaptive strategy from typical vascular plant adaptation to the poikilohydry most typical of bryophytes [49]. Filmy ferns have unistratose leaves and are likely to be CO2 diffusion-limited at high irradiance [50]. In the case of bryophytes, sun-adapted species that suffer desiccation intermittently can maintain high rates of photosynthetic electron transport using oxygen as the only electron sink. This photoreduction of oxygen is linked with development of the high NPQ and associated photoprotection [51]. In this context, our results particularly invite considerations from two points of view: How do they highlight the different behavior of two filmies in the field, and what do they tell us about the adaptation and broad ecological niche of Hymenophyllaceae in general? For fundamental physical reasons, small ecto-hydric plants with monolayer leaves, in which external capillary water plays a physiologically essential role, are inherently best adapted to function at rather low light conditions [52, 53]. In our study, fronds of filmy ferns were always measured at full hydrated state, but in the field their vertical distribution also implies habitat differences explained by relative humidity [22]. Thus, it should be essential to assess light tolerance differences under wet conditions that represent this natural scenario. Thereby, we could know if photosynthetic performance and photoprotection responses observed in H. cruentum and H. dentatum underlie physiological responses against combined stresses of bright light and repeated cycles of drying and rewetting, especially at the upper strata of the forest.
Conclusions
Our photosynthetic characterization under full hydration state confirms that Hymenoglossum cruentum and Hymenophyllum dentatum are shade plants. However, their differences in photosynthetic performance and photoprotection responses suggest that they possess different levels of light tolerance, having H. dentatum more plasticity for using a wider range of light. This is consistent with the contrasting vertical distribution of these filmy ferns previously observed in the rainforest of Southern Chile.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Katalapi Park for allowing us to access the study area and for their facilities and logistic support. We also thank Carla Alvear and Mariela Mora for their technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by AS-A Programa de Atracción e Inserción de capital humano avanzado PAI-CONICYT 791100040 [http://www.conicyt.cl/pai/sobre-pai/que-es-pai/] to LAB; Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT 1090397 and FONDECYT 1120964 [http://www.conicyt.cl/fondecyt/category/concursos/fondecyt-regular/] to MJP; Beca de Doctorado Nacional de la Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) [http://www.conicyt.cl/becas-conicyt/2014/08/beca-doctorado-nacional-2015/]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Valladares F, Niinemets U. Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst. 2008; 39: 237–57. [Google Scholar]
- 2. de Bianchi S, Ballottari M, DallˊOsto L, Bassi R. Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans. 2010; 38: 651–660. 10.1042/BST0380651 [DOI] [PubMed] [Google Scholar]
- 3. Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. J Exp Bot. 2000; 51: 659–668. [DOI] [PubMed] [Google Scholar]
- 4. Bradbury M, Baker NR. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of Photosystem II electron acceptors and fluorescence emission from Photosystems I and II. Biochim Biophys Acta 1981; 635: 542–551. [DOI] [PubMed] [Google Scholar]
- 5. Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosyn Res. 1986; 10: 51–61. 10.1007/BF00024185 [DOI] [PubMed] [Google Scholar]
- 6. Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991; 42: 313–349. [Google Scholar]
- 7. Horton P, Hague A. Studies on the induction of chlorophyll fluorescence in isolated barley protoplasts: IV. Resolution of non-photochemical quenching. Biochim Biophys Acta 1988; 932: 107–117. [Google Scholar]
- 8. Muller P, Li XP, Niyogi KK. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001; 125: 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kramer DM, Johnson G, Kiirats O, Edwards GE. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosyn Res. 2004; 79: 209–218. [DOI] [PubMed] [Google Scholar]
- 10. Demmig-Adams B, Adams WW. Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992; 43: 599–626. [Google Scholar]
- 11. Osmond CB. What is photoinhibition? Some insights from comparisons of shade and sun plants In: Baker N, Bowyer JR, editors. Photoinhibition of photosynthesis: from molecular mechanisms to the field. BIOS Scientific Publishers Limited; 1993. pp. 1–24. [Google Scholar]
- 12. Pearcy RW. Sunflecks and Photosynthesis in Plant Canopy. Annu Rev Plant Physiol Plant Mol Biol. 1990; 41: 421–453. [Google Scholar]
- 13. Bailey S, Walters RG, Jansson S, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 2001; 213: 794–801. [DOI] [PubMed] [Google Scholar]
- 14. Björkman O. Carboxydismutase activity in shade-adapted and sun-adapted species of higher plants. Physiol Plantarum 1968; 21: 1–10. [Google Scholar]
- 15. Boardman NK. Comparative photosynthesis of sun and shade plants. Annu Rev Plant Physiol 1977; 28: 355–377. [Google Scholar]
- 16. Walter RG. Towards an understanding of photosynthetic acclimation. Light Stress in Plants: Mechanisms and interactions Special Issue. J Exp Bot. 2005; 56: 435–447. [DOI] [PubMed] [Google Scholar]
- 17. Dubuisson JY, Hennequin S, Rakotondrainibe F, Schneider H. Ecological diversity and adaptative tendencies in the tropical fern Trichomanes L. (Hymenophyllaceae) with special reference to climbing and epiphytic habits. Bot J Linn Soc. 2003; 142: 41–63. [Google Scholar]
- 18. Tryon RM, Tryon AF. Fern and allied plants, with special reference to tropical America. New York, Heidelberg, Berlin: Springer-Verlag; 1982. [Google Scholar]
- 19. Fuller GD. Filmy ferns as indicators of forest conditions. Bot Gazette 1925; 79: 232. [Google Scholar]
- 20. Hernández-Rosas J. Diversidad de grupos funcionales de plantas del dosel de un bosque húmedo tropical del alto Orinoco, Estado Amazonas, Venezuela. Ecotropicos 1999; 12: 33–48. [Google Scholar]
- 21. Andrade J, Graham E, Zotz G. Determinantes morfofisiológicos y ambientales de la distribución de epifitas en el dosel de bosques tropicales In: Cabrera M, editor. Fisiología ecológica en plantas; 2004. pp. 139–156. [Google Scholar]
- 22. Parra MJ, Acuña K, Corcuera LJ, Saldaña A. Vertical distribution of Hymenophyllaceae species among host tree microhabitats in a temperate rainforest in Southern Chile. J Veg Sci. 2009; 20: 588–595. [Google Scholar]
- 23. Gessner F. Die Assimilation der Hymenophyllaceen. Protoplasma 1940; 34: 102–116. [Google Scholar]
- 24. Richards PW, Evans GB. Biological Flora of the British Isles. Hymenophyllum. J. Ecol. 1972; 60: 245–268. [Google Scholar]
- 25. Johnson GN, Rumsey FJ, Headley AD, Sheffield E. Adaptations to extreme low light in the Trichomanes speciosum . New Phytol. 2000; 148: 423–431. [DOI] [PubMed] [Google Scholar]
- 26. Proctor MCF. Comparative ecophysiological measurements on the light responses, water relations and desiccation tolerance of the filmy fern Hymenophyllum wilsonii Hook. and H. Tunbridgense (L.) Smith. Ann Bot. 2003; 91: 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zotz G, Buche M. The epiphytic filmy ferns of a tropical lowland forest- species occurrence and habitat preferences. Ecotropica 2000; 6: 203–206. [Google Scholar]
- 28. Clement JP, Moffett MW, Shaw DC, Lara A. Crown structure and biodiversity in Fitzroya cupressoides, the giant conifers of Alerce Andino National Park, Chile. Selbyana 2001; 22: 76–88. [Google Scholar]
- 29. Rodríguez R. Pteridophyta In: Marticorena C, Rodríguez R, editores. Flora de Chile, Volumen 1 Concepción: Editora Aníbal Pinto S.A.; 1995. pp. 119–309. [Google Scholar]
- 30. di Castri F, Hajek E. Bioclimatología de Chile. Santiago: Editorial Universidad Católica de Chile; 1975. [Google Scholar]
- 31. Lambers H, Chapin FS III, Pons TL. Plant Physiological Ecology, 2nd ed. New York: Springer Science + Business Media; 2008. [Google Scholar]
- 32. Klughammer C, Schreiber U. Saturation Pulse method for assessment of energy conversion in PSI. PAN 2008; 1: 11–14. [Google Scholar]
- 33. Walters RG, Horton P. Resolution of components of non-photochemical chlorophyll fluorescence quenching in barley leaves. Photosyn Res. 1991; 27: 121–133. 10.1007/BF00033251 [DOI] [PubMed] [Google Scholar]
- 34. García-Plazaola JI, Becerril JM. A Rapid High-performance Liquid Chromatography Method to Measure Lipophilic Antioxidants in Stressed Plants: Simultaneous Determination of Carotenoids and Tocopherols. Phytochem Anal. 1999; 10: 307–313. [Google Scholar]
- 35. Sáez PL, Bravo LA, Latsague MI, Toneatti MJ, Sánchez-Olate M, Ríos DG. Light energy management in micropropagated plants of Castanea sativa, effects of photoinhibition. Plant Sci. 2013; 201: 12–24. 10.1016/j.plantsci.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 36. Dytham C. Choosing and Using Statistics: A Biologist's Guide. 2nd ed New York: Blackwell Publishing, 2005. [Google Scholar]
- 37. Rice SK, Giles L. The influence of water content and leaf anatomy on carbon isotope discrimination and photosynthesis in Sphagnum. Plant Cell Environ. 1996; 19: 118–124. [Google Scholar]
- 38. Saldaña A, Parra MJ, Flores-Bavestrello A, Corcuera LJ, Bravo LA. Effects of forest successional status on microenvironmental conditions, diversity, and distribution of filmy fern species in a temperate rainforest. Plant Species Biol. 2014; 29: 253–262. [Google Scholar]
- 39.Parra MJ. Distribución vertical de helechos película (Hymenophyllaceae) en un bosque templado lluvioso del sur de Chile: una aproximación ecofisiológica. Tesis de Doctorado en Ciencias Biológicas área Botánica, Universidad de Concepción; 2012.
- 40. Proctor MCF. Photosynthesis in Bryophytes and Early Land Plants. In Advances in Photosynthesis and Respiration Volume 37; 2014. pp 59–77. [Google Scholar]
- 41. Proctor MCF, Smirnoff N. Ecophysiology of photosynthesis in bryophytes: major roles for oxygen photoreduction and non-photochemical quenching? Physiol Plantarum 2011; 141: 130–140. [DOI] [PubMed] [Google Scholar]
- 42.Evans GB. Studies on the autecology of the British species of Hymenophyllum, H. wilsonii Hk and H. tunbrigense (L.) Sm. PhD Thesis, University of Wales; 1964.
- 43. Nasrulhaq-Boyce A, Haji Mohamed MA. Photosynthetic and respiratory characteristics of Malayan sun and shade ferns. New Phytol. 1987; 105: 81–88. [DOI] [PubMed] [Google Scholar]
- 44. Evans JR. Acclimation by the thylakoid membranes to growth irradiance and the partitioning of nitrogen between soluble and thylakoid proteins. Aust J Plant Physiol. 1988; 15: 93–106. [Google Scholar]
- 45. Trebst A. Function of β-Carotene and Tocopherol in Photosystem II. Z Naturforsch. 2003; 58C: 609–620.. [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Mao L, Hu X. Insight into the Structural Role of Carotenoids in the Photosystem I: A Quantum Chemical Analysis. Biophys J. 2004; 86: 3097–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Demmig-Adams B. Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol. 1998; 39: 474–482. [Google Scholar]
- 48. Matsubara S, Krause GH, Aranda J, Virgo A, Beisel KG, Jahns P, et al. Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct Plant Biol. 2009; 36: 20–36. [DOI] [PubMed] [Google Scholar]
- 49. Proctor MCF. Light and desiccation responses of some Hymenophyllaceae (filmy ferns) from Trinidad, Venezuela and New Zealand: poikilohydry in a light-limited but low-evaporation ecological niche. Ann Bot. 2012; 109: 1019–1026. 10.1093/aob/mcs012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Proctor MCF. Why do Polytrichaceae have lamellae? J Bryol. 2005; 27: 221–229. [Google Scholar]
- 51. Proctor MCF. The diversification of bryophytes in evolving environments In: Hanson DT, Rice SK editors. Photosynthesis of Bryophytes and Early Land Plants. Dordrecht: Springer Science + Business Media; 2014. pp 59–77. [Google Scholar]
- 52. Proctor MCF, Tuba Z. Poikilohydry and homoihidry: antithesis or spectrum of possibilities? New Phytol. 2002; 156: 327–349. [DOI] [PubMed] [Google Scholar]
- 53. Marschall M, Proctor M. Are bryophytes shade plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Ann Bot. 2004; 94: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.