Abstract
Amino acid oxidases (AAOs) catalyze the oxidative deamination of amino acids releasing ammonium and hydrogen peroxide. Several kinds of these enzymes have been reported. Depending on the amino acid isomer used as a substrate, it is possible to differentiate between l-amino acid oxidases and d-amino acid oxidases. Both use FAD as cofactor and oxidize the amino acid in the alpha position releasing the corresponding keto acid. Recently, a novel class of AAOs has been described that does not contain FAD as cofactor, but a quinone generated by post-translational modification of residues in the same protein. These proteins are named as LodA-like proteins, after the first member of this group described, LodA, a lysine epsilon oxidase synthesized by the marine bacterium Marinomonas mediterranea. In this review, a phylogenetic analysis of all the enzymes described with AAO activity has been performed. It is shown that it is possible to recognize different groups of these enzymes and those containing the quinone cofactor are clearly differentiated. In marine bacteria, particularly in the genus Pseudoalteromonas, most of the proteins described as antimicrobial because of their capacity to generate hydrogen peroxide belong to the group of LodA-like proteins.
Keywords: amino acid oxidases, quinone cofactor, flavoprotein, antimicrobial
1. Introduction
In a broad sense, amino acid oxidases (AAOs) can be described as enzymes that oxidize amino acids releasing ammonium and hydrogen peroxide. Two big groups of these enzymes are recognized depending on the chirality of the amino acid used as substrate. l-Amino acid oxidases, EC 1.4.3.2, (commonly abbreviated as LAAOs or LAOs) are flavoenzymes that oxidize l-amino acids releasing the corresponding α-keto acid in addition to ammonium and hydrogen peroxide. LAAOs are distributed in many biological groups. The best characterized members of this family have been studied in snake venoms [1]. Additionally, several enzymes with this activity have also been described in many other groups including bacteria [2]. d-Amino acid oxidases (DAAOs or DAOs), EC 1.4.3.3, are flavoproteins showing strict specificity for d-amino acids [3,4]. These enzymes are also broadly distributed in different groups of organisms with distinct physiological roles. Glycine is the unique amino acid that has no enantiomers. In Bacillus, a glycine oxidase has been described [5]. Recently, a novel class of AAOs has been reported. The marine bacterium Marinomonas mediterranea synthesizes LodA, a l-lysine epsilon-oxidase (EC 1.4.3.20) [6,7]. The cofactor of LodA is cysteine tryptophylquinone (CTQ) (Figure 1), a type of quinone cofactor generated by the post-translational modification of two residues in the same protein [8,9]. GoxA is an enzyme with sequence similarity to LodA and the same kind of cofactor, but it shows glycine oxidase activity [10,11]. The analysis of sequenced microbial genomes has revealed that, approximately, 1% of them contain genes encoding proteins similar to LodA and GoxA [12].
Figure 1.
Cysteine tryptophylquinone (CTQ) cofactor.
Enzymes with AAO activity are of great scientific interest because of the number of physiological activities displayed in different organisms. For example, the generation of hydrogen peroxide has been described from a physiological point of view as involved in antimicrobial processes. Among these processes, bacterial biofilm development [13], microbial competition related to biocontrol processes in fungi [14], protection of fish skin bacterial infections [15] and participation in the human immune system [16] are included. AAOs are also of interest in relation to their biotechnological applications [4,17]. Recently, their potential interest in medicine as antitumorals and as antimicrobials has increased [18,19]. In both cases, the hydrogen peroxide generated as product of the reaction plays a very important role.
LAAOs have been known for more than half a century. In many cases, when an enzyme showed that activity it was assumed to be a flavoprotein. However, it is important to point out that not in all the cases it has been demonstrated that the activity is due to an enzyme with a flavin cofactor. It is also important to bear in mind that the enzymatic activity of a protein may depend on the presence of other compounds, as it has been described for an enzyme whose activity can change from l-amino acid oxidase to monooxygenase by either mutation or change in the conditions of the assay [20]. Besides, it is well known that many enzymes may show latent promiscuous activities, and that the actual enzymatic activity could be different to the one initially described. As an example, an enzyme from Streptococcus oligofermentans was initially described as an l-amino acid oxidase [21] and lately reclassified as an aminoacetone oxidase involved in antioxidant mechanisms [22].
The aim of this review has been to study the phylogenetic relationship of all the enzymes described as amino acid oxidases, including the new family with quinone cofactor, in order to facilitate future work on novel enzymes with that activity. In the last section, the enzymes synthesized by marine microorganisms described as antimicrobial proteins will be discussed.
2. Phylogenetic Analysis of Proteins with Amino Acid Oxidase Activity
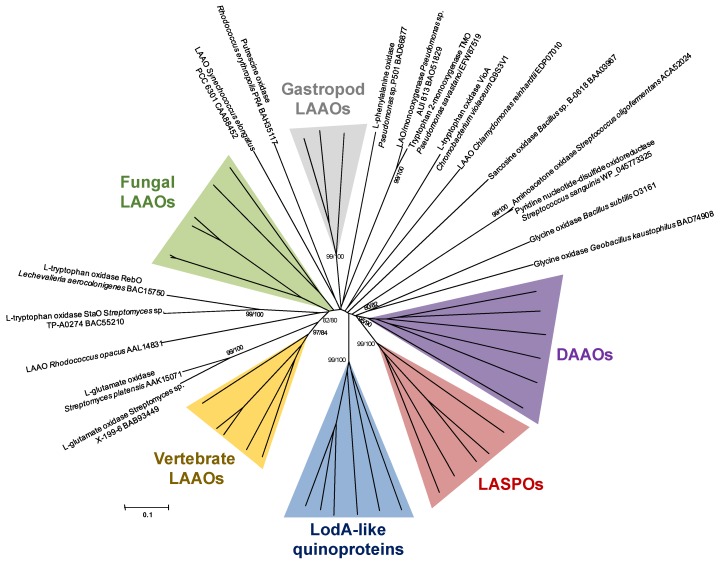
A bibliographic search has been performed in order to obtain representative sequences of proteins with amino acid oxidase activity. All the microbial proteins considered are shown in supplementary Table 1. Table S1 includes proteins characterized at the molecular level, as well as other enzymes in which only the enzymatic activity has been reported. In order to shed light on the evolutionary relationships of AAOs, a phylogenetic analysis has been performed that includes the microbial proteins for which the encoding gene has been cloned, plus other representative proteins from higher organisms (Figure 2). Those AAOs from higher organisms are listed in supplementary Table 2.
Figure 2.
Phylogenetic relationships of enzymes with amino acid oxidase activity. The tree was created by the Neighbor-Joining method integrated in the program MEGA6 [23]. Sequences were aligned using the program MUSCLE built in MEGA6. The evolutionary distances were computed using the p-distance method and are in the units of the number of amino acid differences per site. Numbers at branches indicate bootstrap values higher than 70% for both Neighbor-Joining and Maximum Likelihood trees. The colored groups are detailed in Figure 3 and Figure 4. LAAOs, l-amino acid oxidases; DAAO, d-amino acid oxidases; LASPOs, l-aspartate oxidases.
It has been possible to recognize several closely related groups which are named after their common characteristic. The proteins similar to LodA containing a quinone cofactor form a phylogenetic group clearly differentiated from all the other enzymes. Among the rest of enzymes with AAO activity, DAAOs from different organisms constitute a well-defined cluster. Another group clearly differentiated is the one containing l-aspartate oxidases. Regarding LAAOs, they constitute a broad group distributed in different organisms where they have evolved to meet different physiological functions. In the next sections, the different groups will be discussed separately.
2.1. AAOs with a Quinone Cofactor (LodA-Like Proteins)
l-Lysine ε-oxidase (LodA) synthesized by the melanogenic marine bacterium M. mediterranea was the first quinoprotein reported with LAAO activity [7]. LodA received a new number by the Enzyme Commission (EC 1.4.3.20) since it catalyzes the oxidative deamination of the amine group in epsilon position of l-Lys [6]. It has been demonstrated that the cofactor of LodA is cysteine tryptophylquinone (CTQ) (Figure 1), which is generated by post-translational modification of residues in the same protein [9,11]. In the post-translational modification of LodA, the flavoprotein LodB, encoded in the same operon, plays an important role [8,24]. Regarding its physiological function, it has been shown that LodA plays a role, mediated by the hydrogen peroxide generated, in the development and differentiation of microbial biofilms [13].
Another quinoprotein with AAO activity was identified in M. mediterranea. This is a novel glycine oxidase (GoxA) which shows important differences in terms of substrate range and sensitivity to inhibitors to other glycine oxidases previously described [10]. GoxA is more specific for glycine and structurally different since it also contains the cofactor CTQ [11]. Genome mining revealed genes encoding proteins similar to LodA/GoxA in a number of microbial genomes. They are mostly present in Bacteria, absent in Archaea and, in Eukarya, only detected in a small group of fungi of the class Agaromycetes [12]. This new family of proteins has been named as the LodA-like family of proteins. Sequence alignment of the LodA-like proteins allowed the detection of several conserved residues. All these proteins showed a Cys and a Trp that aligned with the residues that are forming part of the CTQ cofactor in LodA and GoxA. Thus, this observation strongly suggests that proteins of the LodA-like family may contain a CTQ quinone cofactor [12]. Apart from LodA and GoxA, various oxidases of this group have been characterized in marine bacteria as discussed later (Section 3).
2.2. d-Amino Acid Oxidases
d-Amino acid oxidases (EC 1.4.3.3) are FAD-containing proteins with a strict stereospecificity. They catalyze the oxidative deamination of neutral and basic d-amino acids to give α-keto acids, ammonium and hydrogen peroxide [3,25]. However, DAAOs show a negligible or no activity towards acidic d-amino acids, which are substrates for the closely related animal flavoproteins d-aspartate oxidases (DDO, also abbreviated as DASPO; EC 1.4.3.1) [26] (Figure 3B). DAAOs also cluster close to the glycine oxidases group (Figure 2). DAAO activity has been described in a wide variety of organisms such as bacteria [27], fungi [28], plants [29], nematodes [30], fishes [31], and mammals, including humans [32]. DAAOs participate in different physiological functions [33]. In yeast, they have a catabolic role since DAAOs allow yeast cells to grow on d-amino acids. In humans, DAAOs manage the levels of the neuromodulator d-serine of NMDA (N-methyl-d-aspartate) receptors, which are related with many pathological processes. However, the physiological role of bacterial DAAOs is still largely unknown [25]. DAAOs can be utilized for a broad range of applications, such as the determination of d-amino acids, production of building blocks for pharmaceuticals, synthesis of α-keto acids, and diagnosis and treatment of certain diseases [4,34].
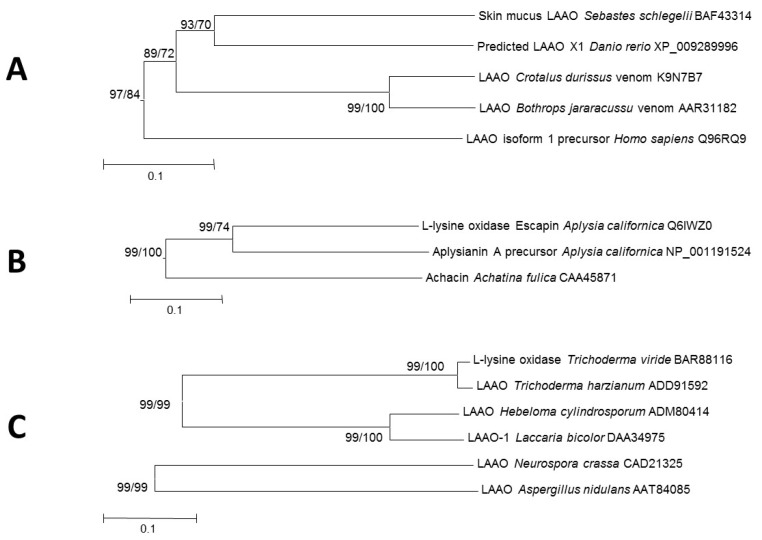
Figure 3.
Phylogenetic relationships of representative LodA-like proteins (A), d-amino acid oxidases (B) and l-aspartate oxidases (C); The tree was created by the Neighbor-Joining method integrated in the program MEGA6 [23]. Sequences were aligned using the program MUSCLE built in MEGA6. The evolutionary distances were computed using the p-distance method and are in the units of the number of amino acid differences per site. Numbers at branches indicate bootstrap values higher than 70% for both Neighbor-Joining and Maximum Likelihood trees. An asterisk indicates that this branch was not detected, or it had a value lower than 70%.
2.3. l-Aspartate Oxidases
l-Aspartate oxidases (LASPOs; EC 1.4.3.16) are flavoproteins encoded by the gene nadB that play an important role in NAD+ synthesis in the cells, as they catalyze the formation of iminoaspartate from l-aspartate, the first step in the de novo biosynthesis of NAD+ in many bacterial, archaeal and plant cells [35]. NadB forms a reversible multienzyme complex with NadA, which carries on the transformation leading to quinolinic acid, an intermediary in the NAD+ synthetic pathway. The high activity of NadB seems to imply an oxidative stress for the cells, due to the generation of hydrogen peroxide [36]. It is noteworthy that, under physiological conditions, LASPOs do not produce ammonia, since the final product of the pathway conserves the original N-atom of l-aspartic acid. In this feature, they do not conform to the classical definition of amino acid oxidases. Interestingly, NadB function is substituted by an NADP-dependent l-aspartate dehydrogenase in Thermotoga maritima which has no sequence similarities with any amino acid oxidase. Moreover, the gene encoding that enzyme is next to nadA in the genome, as described in Bacillus [37].
Sequence analysis reveals that LASPOs have some similarities with fumarate reductases and succinate dehydrogenases in terms of folding topology and conformation of the FAD-binding site and active center, suggesting a similar catalytic mechanism [38,39] and a common evolutionary origin [40]. This implies that l-aspartate oxidases are different from other flavin-dependent amino acid oxidases, such as the d-amino acid oxidases. This fact is reflected as a separate and defined cluster in the phylogenetic tree (Figure 2 and Figure 3C).
One of the similarities between LASPOs and the succinate dehydrogenase/fumarate reductase oxidoreductase family is their ability to use different electron acceptors [41]. Accordingly, NadB can use either molecular oxygen or fumarate as electron acceptors of the reaction, permitting aspartate oxidation in anaerobic conditions [42]. Other aspartate oxidase, such as those from archaea, are being widely proposed for bioanalytical methods due to their high thermostability [43,44]. For instance, the LASPO from Sulfolobus tokodaii, which has been recombinantly expressed, has a broad pH range of activity and shows a weak inhibition pattern. This enzyme not only has a high thermal stability but also binds tightly the FAD cofactor [43].
Although aspartate oxidases were proposed as potential drug targets, since they are absent in mammals [38], this research has not been followed, probably because prokaryotes may have alternative routes for NAD+ production [45]. On the other hand, the gene is considered as an antivirulence loci (AVL), either inactivated or lost in all pathogenic Shigella strains studied, as the presence of quinolinic acid is detrimental to its invasive process [46]. This pathoadaptive evolution is accompanied by a nicotinic acid auxotrophy that bypasses the need of quinolinic acid production in the NAD+ synthetic pathway.
2.4. LAAOs in Animals
The LAAOs from animals clusters in two different groups. One of them includes enzymes from vertebrates, and the other group includes gastropod enzymes (Figure 2). This observation is in agreement with previous studies that indicated that those enzymes may have evolved separately in the innate immune system of both groups [18,47].
2.4.1. LAAOs from Vertebrates
The phylogenetic analysis of AAOs revealed a cluster including enzymes from snake venoms, fishes and mammals (Figure 2 and Figure 4A). Venom LAAOs have been subject of study for many years and several reviews have been published dealing with their biochemical properties and their interest in pharmacology [1,48]. Venom LAAOs show preference towards hydrophobic amino acids such as l-Leu, l-Phe and l-Met [48]. Numerous biological effects, such as antiparasitic, antimicrobial, apoptotic, etc., have been reported for those enzymes [1,49].
Figure 4.
Phylogenetic relationships of representative l-amino acid oxidases in vertebrates (A), gastropods (B) and fungi (C). The tree was created by the Neighbor-Joining method integrated in the program MEGA6 [23]. Sequences were aligned using the program MUSCLE built in MEGA6. The evolutionary distances were computed using the p-distance method and are in the units of the number of amino acid differences per site. Numbers at branches indicate bootstrap values higher than 70% for both Neighbor-Joining and Maximum Likelihood trees.
The skin of the fish is a barrier preventing the infection by microorganisms. The mucus layer contains many peptides and proteins that play a role in that process. In this layer, LAAOs specific for l-lysine have been detected in different fishes such as the rockfish Sebastes schlegelii [15] and the great sculpin Myoxocephalus polyacanthocephalus [50]. The Sebastes LAAO has been used for the design of a biosensor for l-Lys [51]. LAAOs are not only present in the skin mucus but also in other tissues and the serum of fishes [52,53]. The induction of the LAAO of the Atlantic cod Gadus morhua after exposure of the fish to pathogenic bacteria supports its involvement in antibacterial defense [54].
Regarding mammals, the interleukin 4-induced gene (IL4I1) codes for an l-amino acid oxidase with strong preference for l-phenylalanine as substrate, which seems to act as a regulator of the immune system [55]. IL4I1 shows antimicrobial properties that have been associated to the hydrogen peroxide generated, the basification of the medium due to ammonia accumulation and the deprivation of the amino acid [16]. A LAAO has been detected in the milk of the mouse with substrate similarity to the human enzyme that has been proposed to participate in the protection against bacterial infections [56].
2.4.2. LAAOs in Gastropods
Several gastropods are known to produce LAAOs (Figure 4B). The sea hare Aplysia californica, synthesizes a LAAO named escapin that is present in a separate gland from its substrates (Lys and Arg). The mixing of the secretion of both glands at the time of the attack of the predator generates a defensive mechanism against them [57]. Escapin shows antimicrobial activity against a range of bacteria, even when it is recombinantly expressed [58]. It has been shown that the antimicrobial activity of escapin depends not only on the hydrogen peroxide generated, but also on chemical compounds generated during the oxidation of l-lysine [59,60]. Another sea hare (Aplysia punctata) synthesizes a very similar LAAO (93% identical to escapin) whose antitumoral activity has been characterized [61]. Achacin is a LAAO isolated and characterized as an antimicrobial protein from the skin mucus of the giant snail Achatina fulica [62]. The cytotoxicity of achacin is mediated by two different mechanisms: hydrogen peroxide generation and apoptosis induction mediated by l-amino acids depletion [63].
2.5. Fungal LAAOs
Different flavoproteins with l-amino acid oxidase activities have been described in fungi. The Neurospora crassa and Aspergillus nidulans enzymes do not cluster in the same branch as the others, in spite of being close to them in the phylogenetic tree (Figure 4C). It is proposed that the utilization of amino acids as nitrogen source is their major physiological function. This role is also assumed for LAAOs in other eukaryotic microorganisms such as the single-cell alga Chlamydomonas reinhardtii [64,65]. Accordingly, fungal LAAOs usually have a broad substrate range to be able to use distinct amino acids for growing. This is the case of the LAAO from Aspergillus nidulans and Neurospora crassa, which are induced under nitrogen-limiting conditions [66,67]. Similarly, LAAO synthesized by Laccaria bicolor and Hebeloma sp. are also involved in amino acid catabolism and over-expressed under limiting concentrations of nitrogen. In addition, these enzymes seem to perform an important ecological role since they catalyze nitrogen mineralization from amino acids [68].
Aspergillus fumigatus produces another LAAO of wide substrate specificity, although it shows a certain degree of substrate preference. The enzyme is more specific for hydrophobic aromatic amino acids, namely l-tyrosine and l-phenylalanine [69]. A. fumigatus LAAO can be applied in the resolution of some racemic dl-amino acids, yielding optically pure d-amino acids [70].
Not all LAAOs reported in fungi are of broad substrate range and play a metabolic function. The l-lysine α-oxidase from Trichoderma viride (LysOX) exhibits high substrate specificity for l-lysine [71]. Recently, the crystal structure of LysOX has revealed similar overall structure to that of snake venom LAAOs. However, some residues in the funnel to the active site as well as the residues involved in the substrate side-chain recognition are distinct [72]. LysOX is expected to be a potential anti-cancer agent since it inhibits growth of cancer cells but shows relatively low cytotoxicity for normal cells [72,73]. Furthermore, LysOX is stable over a wide range of pH and temperature, so it is an attractive candidate for an enzyme-based l-lysine sensor [74,75]. More recently, an extracellular LAAO with antimicrobial activity has been isolated from Trichoderma harzianum ETS 323. This oxidase shows the best substrate specificity constant for l-phenylalanine, although it is also active on l-Lys, l-Glu and l-Ala. It is proposed that membrane permeabilization and reactive oxidative species production are involved in the mechanism responsible for its antibacterial activity [76]. Notably, LysOX and T. harzianum LAAO may be related with the capability of different Trichoderma species as biocontrol agents.
In addition, other fungal LAAOs with substrate specificity have been described. For instance, l-lysine oxidase from induced Saccharomyces cerevisiae has been reported as potential biosensor for l-lysine [77]. Coprinus sp. SF-1 LAAO catalyzes specifically the oxidative deamination of l-tryptophan, although it can also catalyze its decarboxylation depending on conditions. This oxidase is supposed to be involved in the l-tryptophan metabolism of the fungus [78].
2.6. Other Bacterial LAAOs
There are many bacterial enzymes showing LAAO activity that do not form a defined clustered or cannot be included in any of the previously described groups. In many cases, bacterial enzymes are more similar to proteins from eukaryotes than to other bacterial LAOs. An example would be glutamate oxidases from actinobacteria which are closely relate to LAAOs from animals. This observation suggests that these enzymes have an old evolutionary origin and that they have evolved to meet different physiological functions in distinct groups. In this section, we will discuss separately the most relevant features of the enzymes selected.
The flavoprotein glycine oxidase (GO, EC 1.4.3.19) catalyzes the oxidative deamination of some primary and secondary small-sized amines and d-amino acids to yield the corresponding α-keto acid, ammonium and hydrogen peroxide [79,80]. Glycine oxidase in Bacillus subtilis (ThiO), encoded by the yjbR gene, is involved in the biosynthesis of the thiazole moiety of thiamine [5]. In addition, another glycine oxidase has been described in the marine thermophilic bacterium Geobacillus kaustophilus (GoxK) [81]. This protein shares a similar substrate range and structure with the glycine oxidase from B. subtilis. Regarding applications, glycine oxidase can be used as a biosensor for glycine in biological samples [82], and in the degradation of the herbicide glyphosate [83,84]. Flavoproteins with sarcosine oxidase activity (SOX, EC 1.5.3.1), like the one from Bacillus sp. B-0618 (MSOX), exhibit substrate similarity with glycine oxidases since it catalyzes the oxidative demethylation of sarcosine (N-methylglycine) and other secondary amino acids (N-methyl-l-alanine, N-ethylglycine, and l-proline) [85]. However, MSOX does not cluster in the same group with glycine oxidases (Figure 2).
The hydrogen peroxide generated by a LAAO activity was described as a factor responsible for the competition of the oral Streptococcus oligofermentans, involved in the initial steps of dental biofilm formation, against Streptococcus mutans, one of the main caries-causing pathogens. The gene responsible for this action was cloned and its activity characterized as being held on 7 different amino acids, l-aspartic acid being the one with a higher rate of hydrogen peroxide production [21]. However, latter studies revealed that this flavoprotein is an aminoacetone oxidase (Aao) with a role in the antioxidant defence of S. oligofermentans against cellular ROS generation [22]. Phylogenetic analyses of several Streptococcus strains revealed that the gene in S. oligofermentans was acquired by horizontal gene transfer from a source closely related to Streptococcus sanguinis as a mechanism of adaptation to the aerobic conditions in the oral cavity [86]. In fact, BLAST analysis of S. oligofermentans aao gene product reveals that the most similar proteins are annotated as hypothetical or as pyridine nucleotide-disulfide oxidoreductases, such as in the case of S. sanguinis. In agreement with that, structure-function relationships of SoAAO set apart this protein from the oxidase/dehydrogenase class of flavoproteins [87].These enzymes have been investigated for their role in diverse processes such as epoxyalkanes degradation in Xanthobacter [88], as part of a redox system based on coenzyme A in Staphylococcus aureus [89] or as catalyzing the disulfide bond formation in Chromobacterium violaceun FK228 anticancer depsipeptide [90].
Rhodococcus opacus synthesizes a LAAO with broad substrate range that makes it interesting in some biotechnological applications such as the solving of racemic mixtures of amino acids [91]. The structure of the enzyme has been determined, what has contributed to a better understanding of its molecular mechanism of action [92]. Other LAAOs with broad substrate range have been cloned from different Rhodococcus strains [93]. As far as we know, no physiological function has been proposed for these enzymes, since they have been characterized in terms of their possible biotechnological applications. Other enzymes with broad substrate range were described in Bacillus carotarum [94], Cellulomonas [95], Morganella morganii [96] and Corynebacterium [97]. An example of LAAO with high substrate specificity is the glutamate oxidase synthesized by Streptomyces [98,99]. The physiological function of this extracellular enzyme is unknown. From the biotechnological point of view it is used as part of biosensors for the determination of glutamate [100].
Several LAAOs have been described in bacteria of the genus Pseudomonas. The strain Pseudomonas sp. AIU 813 synthesizes an enzyme with oxidase activity on l-Lys, ornithine and l-Arg [101]. This flavoprotein was lately reclassified as an l-amino acid/monooxygenase since the second activity was higher than the first [20]. Interestingly, the capacity to act as an oxidase or as a decarboxilating monooxygenase is shared by other enzymes such as the PAO (l-phenylalanine oxidase) from Pseudomonas P501 which is able to show different activities depending on the substrate [102,103]. Pseudomonas savastanoi TMO (tryptophan monooxygenase) does not show oxidase activity, but it can be revealed by mutagenesis [104].
VioA from Chromobacterium violaceum encodes an enzyme with l-tryptophan 2′,3′-oxidase activity that is involved in the synthesis of violacein [105]. An enzyme with the same enzymatic activity also participates in the synthesis of other secondary metabolites such as rebeccamycin and staurosporine produced by actimomycetes [106,107]. Interestingly the enzymes from the actinobacteria are not phylogenetically close to the one synthesized by Chromobacterium (Figure 2).
LAAOs with preference for basic amino acids have been described in cyanobacteria [108]. In Synechococus PCC 7942 that enzyme has been related to the use of Arg as nitrogen source [109].
3. Amino Acid Oxidases with Antimicrobial Activity in Marine Bacteria
The colonization of submerged surfaces is of great importance in marine environments. On the one hand, it can generate the biofouling of surfaces. On the other hand, bacteria associated with surfaces are important factors inducing the settlement and metamorphosis of larvae of higher organisms [110]. The genus Pseudoalteromonas has been recognized as an important component of the surface of many different marine surfaces [111,112]. Marinomonas mediterranea is a bacterium that forms part of the microbiota of the seagrass Posidonia oceanica [113]. From both, Pseudoalteromonas and Marinomonas, proteins with antibacterial activity were reported [114,115]. As described above, LodA is an amino acid oxidase with quinone cofactor. The autolytic protein of the marine bacterium Pseudoalteromonas tunicata (AlpP) also possesses lysine oxidase activity and high sequence similarity with LodA [13]. LodA and AlpP are involved in biofilm formation and mediate differentiation, dispersal, and phenotypic variation among the dispersed cells [13]. In two non-marine bacteria, Caulobacter crescentus and Chromobacterium violaceum, LodA-like proteins also generate hydrogen peroxide related with biofilm differentiation in those microorganisms, but the substrate of the activity was not reported [13].
Interestingly, other LodA-like proteins in the genus Pseudoalteromonas have been described as having antimicrobial activity. Two different strains of Pseudoalteromonas flavipulchra synthesize antimicrobial proteins of the Lod-A like group. P. flavipulchra JG1 produces an extracellular antibacterial protein (PfaP) with high similarity to LodA and AlpP [116]. The AAO of the strain C2 was reported to have a broad substrate range oxidizing l-Lys, l-Met, l-Glu, l-Leu, l-Gln, l-Tyr and l-Phe (Table S1.). The hydrogen peroxide generated by its catalysis mediates its antibacterial activity [117]. Another antibacterial LAAO with similar broad substrate range (l-Met, l-Gln, l-Leu, l-Phe, l-Glu, l-Trp, etc.) has been described in several Pseudoalteromonas luteoviolacea strains but the genes encoding those enzymes have not been reported yet [118]. As far as we know, the only case of LAAO activity not related to a quinoprotein in the genus Pseudoalteromonas has been describe in the strain B3, in which it has been associated to a flavoprotein with similarity to LASPOs [119,120] (Figure 3C). Genome mining of the genomes of Pseudoalteromonas strains, and other marine bacteria (data not shown) has revealed that they contain homologues to the gene encoding LASPO (nadB) as well as homologues to nadA. It remains to be determined whether these LASPOs participate in NAD synthesis or have evolved to meet an antimicrobial role. In the genomes of marine bacteria it is also possible to detect other genes encoding flavoproteins, some of them with similarity to amino oxidases. However, to the best of our knowledge, it has not been determined the actual enzymatic activity of the product of those genes or their possible antimicrobial activity.
The data available indicate that in the genomes of marine bacteria of the genus Pseudoalteromonas and Marinomonas it is common the presence of genes encoding proteins similar to LodA [12]. Although LodA-like proteins have been mainly described in marine bacteria, genes encoding proteins similar to them are present in approximately 1% of the microbial genomes sequenced, including microorganisms present in different environments. In the case of the freshwater Rheinheimera aquatica GR5, a protein with a peptide fragment with high similarity to LodA and AlpP was identified. This is an antimicrobial protein due to the hydrogen peroxide generation in the specific oxidation of l-Lys [121]. Nevertheless, it is important to bear in mind that in some microorganisms it is possible to detect several lodA-like genes [12]. Thus far, it has been shown that two of those genes in Marinomonas code for proteins with, respectively, l-lysine ε-oxidase and glycine oxidase activity. This observation suggests that they could have different, perhaps complementary physiological functions. LodA-like proteins may also constitute a reservoir of novel enzymatic activities of potential biotechnological interest.
Supplementary Files
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Izidoro L.F., Sobrinho J.C., Mendes M.M., Costa T.R., Grabner A.N., Rodrigues V.M., da Silva S.L., Zanchi F.B., Zuliani J.P., Fernandes C.F., et al. Snake venom l-amino acid oxidases: Trends in pharmacology and biochemistry. Biomed. Res. Int. 2014;2014:196754. doi: 10.1155/2014/196754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu Z., Qiao H. Advances in non-snake venom l-amino acid oxidase. Appl. Biochem. Biotechnol. 2012;167:1–13. doi: 10.1007/s12010-012-9611-1. [DOI] [PubMed] [Google Scholar]
- 3.Pollegioni L., Molla G., Sacchi S., Rosini E., Verga R., Pilone M. Properties and applications of microbial d-amino acid oxidases: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008;78:1–16. doi: 10.1007/s00253-007-1282-4. [DOI] [PubMed] [Google Scholar]
- 4.Pollegioni L., Molla G. New biotech applications from evolved d-amino acid oxidases. Trends Biotechnol. 2011;29:276–283. doi: 10.1016/j.tibtech.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Settembre E.C., Dorrestein P.C., Park J.H., Augustine A.M., Begley T.P., Ealick S.E. Structural and mechanistic studies on ThiO, a glycine oxidase essential for thiamin biosynthesis in Bacillus subtilis. Biochemistry. 2003;42:2971–2981. doi: 10.1021/bi026916v. [DOI] [PubMed] [Google Scholar]
- 6.Gomez D., Lucas-Elio P., Sanchez-Amat A., Solano F. A novel type of lysine oxidase: l-Lysine-epsilon-oxidase. Biochim. Biophys. Acta. 2006;1764:1577–1585. doi: 10.1016/j.bbapap.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Lucas-Elio P., Gomez D., Solano F., Sanchez-Amat A. The antimicrobial activity of marinocine, synthesized by Marinomonas mediterranea, is due to hydrogen peroxide generated by its lysine oxidase activity. J. Bacteriol. 2006;188:2493–2501. doi: 10.1128/JB.188.7.2493-2501.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chacon-Verdu M., Gomez D., Solano F., Lucas-Elio P., Sanchez-Amat A. LodB is required for the recombinant synthesis of the quinoprotein l-lysine-ε-oxidase from Marinomonas mediterranea. Appl. Microbiol. Biotechnol. 2014;98:2981–2989. doi: 10.1007/s00253-013-5168-3. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki S., Nakano S., Matsui D., Akaji S., Inagaki K., Asano Y. X-ray crystallographic evidence for the presence of the cysteine tryptophylquinone cofactor in l-lysine epsilon-oxidase from Marinomonas mediterranea. J. Biochem. 2013;154:233–236. doi: 10.1093/jb/mvt070. [DOI] [PubMed] [Google Scholar]
- 10.Campillo-Brocal J.C., Lucas-Elio P., Sanchez-Amat A. Identification in Marinomonas mediterranea of a novel quinoprotein with glycine oxidase activity. Microbiologyopen. 2013;2:684–694. doi: 10.1002/mbo3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chacon-Verdu M., Campillo-Brocal J.C., Lucas-Elio P., Davidson V.L., Sanchez-Amat A. Characterization of recombinant biosynthetic precursors of the cysteine tryptophylquinone cofactors of l-lysine-epsilon-oxidase and glycine oxidase from Marinomonas mediterranea. Biochim. Biophys. Acta. 2015;1854:1123–1131. doi: 10.1016/j.bbapap.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Campillo-Brocal J.C., Chacon-Verdu M.D., Lucas-Elío P., Sanchez-Amat A. Distribution in microbial genomes of genes similar to lodA and goxA which encode a novel family of quinoproteins with amino acid oxidase activity. BMC Genomics. 2015;16:231. doi: 10.1186/s12864-015-1455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai-Prochnow A., Lucas-Elio P., Egan S., Thomas T., Webb J.S., Sanchez-Amat A., Kjelleberg S. Hydrogen peroxide linked to lysine oxidase activity facilitates biofilm differentiation and dispersal in several Gram-negative bacteria. J. Bacteriol. 2008;190:5493–5501. doi: 10.1128/JB.00549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang C.A., Cheng C.H., Lo C.T., Liu S.Y., Lee J.W., Peng K.C. A novel l-amino acid oxidase from Trichoderma harzianum ETS 323 associated with antagonism of Rhizoctonia solani. J. Agric. Food Chem. 2011;59:4519–4526. doi: 10.1021/jf104603w. [DOI] [PubMed] [Google Scholar]
- 15.Kitani Y., Kikuchi N., Zhang G., Ishizaki S., Shimakura K., Shiomi K., Nagashima Y. Antibacterial action of l-amino acid oxidase from the skin mucus of rockfish Sebastes schlegelii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008;149:394–400. doi: 10.1016/j.cbpb.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Puiffe M.L., Lachaise I., Molinier-Frenkel V.R., Castellano F. Antibacterial properties of the mammalian l-amino acid oxidase IL4I1. PLoS ONE. 2013;8: doi: 10.1371/journal.pone.0054589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollegioni L., Motta P., Molla G. l-Amino acid oxidase as biocatalyst: A dream too far? Appl. Microbiol. Biotechnol. 2013;97:9323–9341. doi: 10.1007/s00253-013-5230-1. [DOI] [PubMed] [Google Scholar]
- 18.Kasai K., Ishikawa T., Nakamura T., Miura T. Antibacterial properties of l-amino acid oxidase: Mechanisms of action and perspectives for therapeutic applications. Appl. Microbiol. Biotechnol. 2015;99:1–11. doi: 10.1007/s00253-015-6844-2. [DOI] [PubMed] [Google Scholar]
- 19.Rosini E., Pollegioni L., Ghisla S., Orru R., Molla G. Optimization of d-amino acid oxidase for low substrate concentrations—Towards a cancer enzyme therapy. FEBS J. 2009;276:4921–4932. doi: 10.1111/j.1742-4658.2009.07191.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsui D., Im D.H., Sugawara A., Fukuta Y., Fushinobu S., Isobe K., Asano Y. Mutational and crystallographic analysis of l-amino acid oxidase/monooxygenase from Pseudomonas sp. AIU 813: Interconversion between oxidase and monooxygenase activities. FEBS Open Bio. 2014;4:220–228. doi: 10.1016/j.fob.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong H., Chen W., Shi W., Qi F., Dong X. SO-LAAO, a novel l-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J. Bacteriol. 2008;190:4716–4721. doi: 10.1128/JB.00363-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou P., Liu L., Tong H., Dong X. Role of operon aaoSo-mutT in antioxidant defense in Streptococcus oligofermentans. PLoS ONE. 2012;7: doi: 10.1371/journal.pone.0038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sehanobish E., Chacon-Verdu M.D., Sanchez-Amat A., Davidson V.L. Roles of active site residues in LodA, a cysteine tryptophylquinone dependent epsilon-lysine oxidase. Arch. Biochem. Biophys. 2015;579:26–32. doi: 10.1016/j.abb.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi S., Abe K., Kera Y. Bacterial d-amino acid oxidases: Recent findings and future perspectives. Bioengineered. 2015;6:237–241. doi: 10.1080/21655979.2015.1052917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Aniello A., Vetere A., Petrucelli L. Further study on the specificity of d-amino acid oxidase and of d-aspartate oxidase and time course for complete oxidation of d-amino acids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1993;105:731–734. doi: 10.1016/0305-0491(93)90113-J. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi S., Furukawara M., Omae K., Tadokoro N., Saito Y., Abe K., Kera Y. A highly stable d-amino acid oxidase of the thermophilic bacterium Rubrobacter xylanophilus. Appl. Environ. Microbiol. 2014;80:7219–7229. doi: 10.1128/AEM.02193-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caligiuri A., DArrigo P., Rosini E., Pedrocchi-Fantoni G., Tessaro D., Molla G., Servi S., Pollegioni L. Activity of yeast d-amino acid oxidase on aromatic unnatural amino acids. J. Mol. Catal. B Enzym. 2008;50:93–98. doi: 10.1016/j.molcatb.2007.09.014. [DOI] [Google Scholar]
- 29.Gholizadeh A., Kohnehrouz B.B. Molecular cloning and expression in Escherichia coli of an active fused Zea mays L. d-amino acid oxidase. Biochemistry (Mosc.) 2009;74:137–144. doi: 10.1134/S0006297909020035. [DOI] [PubMed] [Google Scholar]
- 30.Katane M., Saitoh Y., Seida Y., Sekine M., Furuchi T., Homma H. Comparative characterization of three d-aspartate oxidases and one d-amino acid oxidase from Caenorhabditis elegans. Chem. Biodivers. 2010;7:1424–1434. doi: 10.1002/cbdv.200900294. [DOI] [PubMed] [Google Scholar]
- 31.Sarower G.M., Okada S., Abe H. Molecular characterization of d-amino acid oxidase from common carp Cyprinus carpio and its induction with exogenous free d-alanine. Arch. Biochem. Biophys. 2003;420:121–129. doi: 10.1016/j.abb.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Molla G., Sacchi S., Bernasconi M., Pilone M.S., Fukui K., Pollegioni L. Characterization of human d-amino acid oxidase. FEBS Lett. 2006;580:2358–2364. doi: 10.1016/j.febslet.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 33.Pollegioni L., Piubelli L., Sacchi S., Pilone M.S., Molla G. Physiological functions of d-amino acid oxidases: From yeast to humans. Cell. Mol. Life Sci. 2007;64:1373–1394. doi: 10.1007/s00018-007-6558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoronenkova S.V., Tishkov V.I. d-Amino acid oxidase: Physiological role and applications. Biochemistry (Mosc) 2008;73:1511–1518. doi: 10.1134/S0006297908130105. [DOI] [PubMed] [Google Scholar]
- 35.Nasu S., Wicks F.D., Gholson R.K. l-Aspartate oxidase, a newly discovered enzyme of Escherichia coli, is the B protein of quinolinate synthetase. J. Biol. Chem. 1982;257:626–632. [PubMed] [Google Scholar]
- 36.Korshunov S., Imlay J.A. Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol. Microbiol. 2010;75:1389–1401. doi: 10.1111/j.1365-2958.2010.07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z., Savchenko A., Yakunin A., Zhang R., Edwards A., Arrowsmith C., Tong L. Aspartate dehydrogenase, a novel enzyme identified from structural and functional studies of TM1643. J. Biol. Chem. 2003;278:8804–8808. doi: 10.1074/jbc.M211892200. [DOI] [PubMed] [Google Scholar]
- 38.Bossi R.T., Negri A., Tedeschi G., Mattevi A. Structure of FAD-bound l-aspartate oxidase: Insight into substrate specificity and catalysis. Biochemistry. 2002;41:3018–3024. doi: 10.1021/bi015939r. [DOI] [PubMed] [Google Scholar]
- 39.Mattevi A., Tedeschi G., Bacchella L., Coda A., Negri A., Ronchi S. Structure of l-aspartate oxidase: Implications for the succinate dehydrogenase/fumarate reductase oxidoreductase family. Structure. 1999;7:745–756. doi: 10.1016/S0969-2126(99)80099-9. [DOI] [PubMed] [Google Scholar]
- 40.Tedeschi G., Nonnis S., Strumbo B., Cruciani G., Carosati E., Negri A. On the catalytic role of the active site residue E121 of E. coli l-aspartate oxidase. Biochimie. 2010;92:1335–1342. doi: 10.1016/j.biochi.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Sakuraba H., Satomura T., Kawakami R., Yamamoto S., Kawarabayasi Y., Kikuchi H., Ohshima T. l-Aspartate oxidase is present in the anaerobic hyperthermophilic archaeon Pyrococcus horikoshii OT-3: Characteristics and role in the de novo biosynthesis of nicotinamide adenine dinucleotide proposed by genome sequencing. Extremophiles. 2002;6:275–281. doi: 10.1007/s00792-001-0254-3. [DOI] [PubMed] [Google Scholar]
- 42.Marinoni I., Nonnis S., Monteferrante C., Heathcote P., Hartig E., Bottger L.H., Trautwein A.X., Negri A., Albertini A.M., Tedeschi G. Characterization of l-aspartate oxidase and quinolinate synthase from Bacillus subtilis. FEBS J. 2008;275:5090–5107. doi: 10.1111/j.1742-4658.2008.06641.x. [DOI] [PubMed] [Google Scholar]
- 43.Bifulco D., Pollegioni L., Tessaro D., Servi S., Molla G. A thermostable l-aspartate oxidase: A new tool for biotechnological applications. Appl. Microbiol. Biotechnol. 2013;97:7285–7295. doi: 10.1007/s00253-013-4688-1. [DOI] [PubMed] [Google Scholar]
- 44.Mutaguchi Y., Ohmori T., Sakuraba H., Yoneda K., Doi K., Ohshima T. Visible wavelength spectrophotometric assays of l-aspartate and d-aspartate using hyperthermophilic enzyme systems. Anal. Biochem. 2011;409:1–6. doi: 10.1016/j.ab.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Lin H., Kwan A.L., Dutcher S.K. Synthesizing and salvaging NAD: Lessons learned from Chlamydomonas reinhardtii. PLoS Genet. 2010;6: doi: 10.1371/journal.pgen.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Martino M.L., Fioravanti R., Barbabella G., Prosseda G., Colonna B., Casalino M. Molecular evolution of the nicotinic acid requirement within the Shigella/EIEC pathotype. Int. J. Med. Microbiol. 2013;303:651–661. doi: 10.1016/j.ijmm.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Hughes A.L. Origin and diversification of the l-amino oxidase family in innate immune defenses of animals. Immunogenetics. 2010;62:753–759. doi: 10.1007/s00251-010-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du X.Y., Clemetson K.J. Snake venom l-amino acid oxidases. Toxicon. 2002;40:659–665. doi: 10.1016/S0041-0101(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 49.Calderon L.A., Sobrinho J.C., Zaqueo K.D., de Moura A.A., Grabner A.N., Mazzi M.C.V., Marcussi S., Nomizo A., Fernandes C.F.C., Zuliani J.P., et al. Antitumoral activity of snake venom proteins: New trends in cancer therapy. Biomed. Res. Int. 2014;2014:203639. doi: 10.1155/2014/203639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagashima Y., Tsukamoto C., Kitani Y., Ishizaki S., Nagai H., Yanagimoto T. Isolation and cDNA cloning of an antibacterial l-amino acid oxidase from the skin mucus of the great sculpin Myoxocephalus polyacanthocephalus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009;154:55–61. doi: 10.1016/j.cbpb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Endo H., Hayashi Y., Kitani Y., Ren H., Hayashi T., Nagashima Y. Optical enzyme sensor for determining l-lysine content using l-lysine oxidase from the rockfish Sebastes schlegeli. Anal. Bioanal. Chem. 2008;391:1255–1261. doi: 10.1007/s00216-008-1847-9. [DOI] [PubMed] [Google Scholar]
- 52.Kitani Y., Ishida M., Ishizaki S., Nagashima Y. Discovery of serum l-amino acid oxidase in the rockfish Sebastes schlegeli: Isolation and biochemical characterization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010;157:351–356. doi: 10.1016/j.cbpb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Wang F., Li R., Xie M., Li A. The serum of rabbitfish (Siganus oramin) has antimicrobial activity to some pathogenic organisms and a novel serum l-amino acid oxidase is isolated. Fish. Shellfish Immunol. 2011;30:1095–1108. doi: 10.1016/j.fsi.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Kitani Y., Fernandes J.M.O., Kiron V. Identification of the atlantic cod l-amino acid oxidase and its alterations following bacterial exposure. Dev. Comp. Immunol. 2015;50:116–120. doi: 10.1016/j.dci.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Boulland M.L., Marquet J., Molinier-Frenkel V.R., Moller P., Guiter C., Lasoudris F., Copie-Bergman C., Baia M., Gaulard P., Leroy K., et al. Human IL4I1 is a secreted l-phenylalanine oxidase expressed by mature dendritic cells that inhibits T-lymphocyte proliferation. Blood. 2007;110:220–227. doi: 10.1182/blood-2006-07-036210. [DOI] [PubMed] [Google Scholar]
- 56.Sun Y., Nonobe E., Kobayashi Y., Kuraishi T., Aoki F., Yamamoto K., Sakai S. Characterization and expression of l-amino acid oxidase of mouse milk. J. Biol. Chem. 2002;277:19080–19086. doi: 10.1074/jbc.M200936200. [DOI] [PubMed] [Google Scholar]
- 57.Johnson P.M., Kicklighter C.E., Schmidt M., Kamio M., Yang H., Elkin D., Michel W.C., Tai P.C., Derby C.D. Packaging of chemicals in the defensive secretory glands of the sea hare Aplysia californica. J. Exp. Biol. 2006;209:78–88. doi: 10.1242/jeb.01972. [DOI] [PubMed] [Google Scholar]
- 58.Yang H., Johnson P.M., Ko K.C., Kamio M., Germann M.W., Derby C.D., Tai P.C. Cloning, characterization and expression of escapin, a broadly antimicrobial FAD-containing l-amino acid oxidase from ink of the sea hare Aplysia californica. J. Exp. Biol. 2005;208:3609–3622. doi: 10.1242/jeb.01795. [DOI] [PubMed] [Google Scholar]
- 59.Ko K.C., Wang B., Tai P.C., Derby C.D. Identification of potent bactericidal compounds produced by escapin, an l-amino acid oxidase in the ink of the sea hare Aplysia californica. Antimicrob. Agents Chemother. 2008;52:4455–4462. doi: 10.1128/AAC.01103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko K.C., Tai P.C., Derby C.D. Mechanisms of action of escapin, a bactericidal agent in the ink secretion of the sea hare Aplysia californica: Rapid and long-lasting DNA condensation and involvement of the OxyR-regulated oxidative stress pathway. Antimicrob. Agents Chemother. 2012;56:1725–1734. doi: 10.1128/AAC.05874-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butzke D., Hurwitz R., Thiede B., Goedert S., Rudel T. Cloning and biochemical characterization of APIT, a new l-amino acid oxidase from Aplysia punctata. Toxicon. 2005;46:479–489. doi: 10.1016/j.toxicon.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Ehara T., Kitajima S., Kanzawa N., Tamiya T., Tsuchiya T. Antimicrobial action of achacin is mediated by l-amino acid oxidase activity. FEBS Lett. 2002;531:509–512. doi: 10.1016/S0014-5793(02)03608-6. [DOI] [PubMed] [Google Scholar]
- 63.Kanzawa N., Shintani S., Ohta K., Kitajima S., Ehara T., Kobayashi H., Kizaki H., Tsuchiya T. Achacin induces cell death in HeLa cells through two different mechanisms. Arch. Biochem. Biophys. 2004;422:103–109. doi: 10.1016/j.abb.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Perez-Alegre M., Franco A.R. Resistance to l-methionine-S-sulfoximine in Chlamydomonas reinhardtii is due to an alteration in a general amino acid transport system. Planta. 1998;207:20–26. doi: 10.1007/s004250050451. [DOI] [Google Scholar]
- 65.Vallon O., Bulte L., Kuras R., Olive J., Wollman F.A. Extensive accumulation of an extracellular l-amino-acid oxidase during gametogenesis of Chlamydomonas reinhardtii. Eur. J. Biochem. 1993;215:351–360. doi: 10.1111/j.1432-1033.1993.tb18041.x. [DOI] [PubMed] [Google Scholar]
- 66.Davis M.A., Askin M.C., Hynes M.J. Amino acid catabolism by an areA-regulated gene encoding an l-amino acid oxidase with broad substrate specificity in Aspergillus nidulans. Appl. Environ. Microbiol. 2005;71:3551–3555. doi: 10.1128/AEM.71.7.3551-3555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sikora L., Marzluf G.A. Regulation of l-amino acid oxidase and of d-amino acid oxidase in Neurospora crassa. Mol. Gen. Genet. 1982;186:33–39. doi: 10.1007/BF00422908. [DOI] [PubMed] [Google Scholar]
- 68.Nuutinen J.T., Timoneni S. Identification of nitrogen mineralization enzymes, l-amino acid oxidases, from the ectomycorrhizal fungi Hebeloma spp. and Laccaria bicolor. Mycol. Res. 2008;112:1453–1464. doi: 10.1016/j.mycres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 69.Singh S., Gogoi B.K., Bezbaruah R.L. Optimization of medium and cultivation conditions for l-amino acid oxidase production by Aspergillus fumigatus. Can. J. Microbiol. 2009;55:1096–1102. doi: 10.1139/W09-068. [DOI] [PubMed] [Google Scholar]
- 70.Singh S., Gogoi B., Bezbaruah R. Racemic resolution of some dl-amino acids using Aspergillus fumigatus l-amino acid oxidase. Curr. Microbiol. 2011;63:94–99. doi: 10.1007/s00284-011-9955-8. [DOI] [PubMed] [Google Scholar]
- 71.Kusakabe H., Kodama K., Kuninaka A., Yoshino H., Misono H., Soda K. A new antitumor enzyme, l-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J. Biol. Chem. 1980;255:976–981. [PubMed] [Google Scholar]
- 72.Amano M., Mizuguchi H., Sano T., Kondo H., Shinyashiki K., Inagaki J., Tamura T., Kawaguchi T., Kusakabe H., Imada K., et al. Recombinant expression, molecular characterization and crystal structure of antitumor enzyme, l-lysine-α-oxidase from Trichoderma viride. J. Biochem. 2015;157:549–559. doi: 10.1093/jb/mvv012. [DOI] [PubMed] [Google Scholar]
- 73.Lukasheva E.V., Berezov T.T. l-Lysine alpha-oxidase: Physicochemical and biological properties. Biochemistry (Mosc) 2002;67:1152–1158. doi: 10.1023/A:1020967408229. [DOI] [PubMed] [Google Scholar]
- 74.Chauhan N., Narang J., Sunny, Pundir C.S. Immobilization of lysine oxidase on a gold-platinum nanoparticles modified Au electrode for detection of lysine. Enzyme Microb. Technol. 2013;52:265–271. doi: 10.1016/j.enzmictec.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Pohlmann A., Stamm W.W., Kusakabe H., Kula M.R. Enzymatic determination of l-lysine by flow-injection techniques. Anal. Chim. Acta. 1990;235:329–335. doi: 10.1016/S0003-2670(00)82091-7. [DOI] [Google Scholar]
- 76.Yang C.A., Cheng C.H., Liu S.Y., Lo C.T., Lee J.W., Peng K.C. Identification of antibacterial mechanism of l-amino acid oxidase derived from Trichoderma harzianum ETS 323. FEBS J. 2011;278:3381–3394. doi: 10.1111/j.1742-4658.2011.08262.x. [DOI] [PubMed] [Google Scholar]
- 77.Akyilmaz E., Erdogan A., Ozturk R., Yasa I. Sensitive determination of l-lysine with a new amperometric microbial biosensor based on Saccharomyces cerevisiae yeast cells. Biosens. Bioelectron. 2007;22:1055–1060. doi: 10.1016/j.bios.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 78.Furuya Y., Sawada H., Hirahara T., Ito K., Ohshiro T., Izumi Y. A novel enzyme, l-tryptophan oxidase, from a basidiomycete, Coprinus sp. SF-1: Purification and characterization. Biosci. Biotechnol. Biochem. 2000;64:1486–1493. doi: 10.1271/bbb.64.1486. [DOI] [PubMed] [Google Scholar]
- 79.Nishiya Y., Imanaka T. Purification and characterization of a novel glycine oxidase from Bacillus subtilis. FEBS Lett. 1998;438:263–266. doi: 10.1016/S0014-5793(98)01313-1. [DOI] [PubMed] [Google Scholar]
- 80.Job V., Marcone G.L., Pilone M.S., Pollegioni L. Glycine oxidase from Bacillus subtilis. Characterization of a new flavoprotein. J. Biol. Chem. 2002;277:6985–6993. doi: 10.1074/jbc.M111095200. [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Martinez I., Navarro-Fernandez J., Garcia-Carmona F., Takami H., Sanchez-Ferrer A. Characterization and structural modeling of a novel thermostable glycine oxidase from Geobacillus kaustophilus HTA426. Proteins. 2008;70:1429–1441. doi: 10.1002/prot.21690. [DOI] [PubMed] [Google Scholar]
- 82.Rosini E., Piubelli L., Molla G., Frattini L., Valentino M., Varriale A., D'Auria S., Pollegioni L. Novel biosensors based on optimized glycine oxidase. FEBS J. 2014;281:3460–3472. doi: 10.1111/febs.12873. [DOI] [PubMed] [Google Scholar]
- 83.Nicolia A., Ferradini N., Molla G., Biagetti E., Pollegioni L., Veronesi F., Rosellini D. Expression of an evolved engineered variant of a bacterial glycine oxidase leads to glyphosate resistance in alfalfa. J. Biotechnol. 2014;184:201–208. doi: 10.1016/j.jbiotec.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 84.Yao P., Lin Y., Wu G., Lu Y., Zhan T., Kumar A., Zhang L., Liu Z. Improvement of glycine oxidase by DNA shuffling, and site-saturation mutagenesis of F247 residue. Int. J. Biol. Macromol. 2015;79:965–970. doi: 10.1016/j.ijbiomac.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 85.Trickey P., Wagner M.A., Jorns M.S., Mathews F.S. Monomeric sarcosine oxidase: Structure of a covalently flavinylated amine oxidizing enzyme. Structure. 1999;7:331–345. doi: 10.1016/S0969-2126(99)80043-4. [DOI] [PubMed] [Google Scholar]
- 86.Boggs J.M., South A.H., Hughes A.L. Phylogenetic analysis supports horizontal gene transfer of l-amino acid oxidase gene in Streptococcus oligofermentans. Infect. Genet. Evol. 2012;12:1005–1009. doi: 10.1016/j.meegid.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Molla G., Nardini M., Motta P., D'Arrigo P., Panzeri W., Pollegioni L. Aminoacetone oxidase from Streptococcus oligofermentans belongs to a new three-domain family of bacterial flavoproteins. Biochem. J. 2014;464:387–399. doi: 10.1042/BJ20140972. [DOI] [PubMed] [Google Scholar]
- 88.Swaving J., de Bont J.A., Westphal A., de K.A. A novel type of pyridine nucleotide-disulfide oxidoreductase is essential for NAD+- and NADPH-dependent degradation of epoxyalkanes by Xanthobacter strain Py2. J. Bacteriol. 1996;178:6644–6646. doi: 10.1128/jb.178.22.6644-6646.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.delCardayre S.B., Davies J.E. Staphylococcus aureus coenzyme A disulfide reductase, a new subfamily of pyridine nucleotide-disulfide oxidoreductase. Sequence, expression, and analysis of cdr. J. Biol. Chem. 1998;273:5752–5757. doi: 10.1074/jbc.273.10.5752. [DOI] [PubMed] [Google Scholar]
- 90.Wang C., Wesener S.R., Zhang H., Cheng Y.Q. An FAD-dependent pyridine nucleotide-disulfide oxidoreductase is involved in disulfide bond formation in FK228 anticancer depsipeptide. Chem. Biol. 2009;16:585–593. doi: 10.1016/j.chembiol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Geueke B., Hummel W., Bommarius A. l-Amino Acid Oxidase from Rhodococcus Species. 6461841 B2. U.S. Patent. 2002 Oct 8;
- 92.Faust A., Niefind K., Hummel W., Schomburg D. The structure of a bacterial l-amino acid oxidase from Rhodococcus opacus gives new evidence for the hydride mechanism for dehydrogenation. J. Mol. Biol. 2007;367:234–248. doi: 10.1016/j.jmb.2006.11.071. [DOI] [PubMed] [Google Scholar]
- 93.Isobe K., Satou S., Matsumoto E., Yoshida S., Yamada M., Hibi M., Ogawa J. Characterization and application of a l-specific amino acid oxidase from Rhodococcus sp. AIU LAB-3. J. Biosci. Bioeng. 2013;115:613–617. doi: 10.1016/j.jbiosc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Brearley G.M., Price C.P., Atkinson T., Hammond P.M. Purification and partial characterisation of a broad-range l-amino acid oxidase from Bacillus carotarum 2Pfa isolated from soil. Appl. Microbiol. Biotechnol. 1994;41:670–676. doi: 10.1007/BF00167283. [DOI] [Google Scholar]
- 95.Braun M., Kim J.M., Schmid R.D. Purification and some properties of an extracellular l-amino acid oxidase from Cellulomonas cellulans AM8 isolated from soil. Appl. Microbiol. Biotechnol. 1992;37:594–598. doi: 10.1007/BF00240732. [DOI] [Google Scholar]
- 96.Bouvrette P., Luong J.H. T. Purification and further characterization of an l-phenylalanine oxidase from Morganella morganii. Appl. Biochem. Biotechnol. 1994;48:61–74. doi: 10.1007/BF02796163. [DOI] [Google Scholar]
- 97.Coudert M. Charcterization and physiological function of a soluble l-amino acid oxidase in Corynebacterium. Arch. Microbiol. 1975;102:151–153. doi: 10.1007/BF00428360. [DOI] [PubMed] [Google Scholar]
- 98.Arima J., Sasaki C., Sakaguchi C., Mizuno H., Tamura T., Kashima A., Kusakabe H., Sugio S., Inagaki K. Structural characterization of l-glutamate oxidase from Streptomyces sp. X-119–6. FEBS J. 2009;276:3894–3903. doi: 10.1111/j.1742-4658.2009.07103.x. [DOI] [PubMed] [Google Scholar]
- 99.Arima J., Tamura T., Kusakabe H., Ashiuchi M., Yagi T., Tanaka H., Inagaki K. Recombinant expression, biochemical characterization and stabilization through proteolysis of an l-glutamate oxidase from Streptomyces sp. X-119–6. J. Biochem. 2003;134:805–812. doi: 10.1093/jb/mvg206. [DOI] [PubMed] [Google Scholar]
- 100.Batra B., Pundir C.S. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens. Bioelectron. 2013;47:496–501. doi: 10.1016/j.bios.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 101.Isobe K., Sugawara A., Domon H., Fukuta Y., Asano Y. Purification and characterization of an l-amino acid oxidase from Pseudomonas sp. AIU 813. J. Biosci. Bioeng. 2012;114:257–261. doi: 10.1016/j.jbiosc.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 102.Koyama H. Oxidation and oxygenation of l-amino acids catalyzed by a l-phenylalanine oxidase (Deaminating and decarboxylating) from Pseudomonas Sp. P-501. J. Biochem. 1984;96:421–427. doi: 10.1093/oxfordjournals.jbchem.a134853. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki H., Higashi Y., Asano M., Suguro M., Kigawa M., Maeda M., Katayama S., Mukouyama E.B., Uchiyama K. Sequencing and expression of the l-phenylalanine oxidase gene from Pseudomonas sp. P-501. Proteolytic activation of the proenzyme. J. Biochem. 2004;136:617–627. doi: 10.1093/jb/mvh169. [DOI] [PubMed] [Google Scholar]
- 104.Sobrado P., Fitzpatrick P.F. Analysis of the role of the active site residue Arg98 in the flavoprotein tryptophan 2-monooxygenase, a member of the l-amino oxidase family. Biochemistry. 2003;42:13826–13832. doi: 10.1021/bi035299n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Genet R., Benetti P.H., Hammadi A., Menez A. l-Tryptophan 2′,3′-oxidase from Chromobacterium violaceum. Substrate specificity and mechanistic implications. J. Biol. Chem. 1995;270:23540–23545. doi: 10.1074/jbc.270.40.23540. [DOI] [PubMed] [Google Scholar]
- 106.Kameya M., Onaka H., Asano Y. Selective tryptophan determination using tryptophan oxidases involved in bis-indole antibiotic biosynthesis. Anal. Biochem. 2013;438:124–132. doi: 10.1016/j.ab.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 107.Nishizawa T., Aldrich C., Sherman D.H. Molecular analysis of the rebeccamycin l-amino acid oxidase from Lechevalieria aerocolonigenes ATCC 39243. J. Bacteriol. 2005;187:2084–2092. doi: 10.1128/JB.187.6.2084-2092.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gau A.E., Heindl A.F., Nodop A.F., Kahmann U.F., Pistorius E.K. l-Amino acid oxidases with specificity for basic l-amino acids in cyanobacteria. Z. Naturforsch. C Biosci. 2007;62:273–284. doi: 10.1515/znc-2007-3-419. [DOI] [PubMed] [Google Scholar]
- 109.Bockholt R., Scholten-Beck G.F., Pistorius E.K. Construction and partial characterization of an l-amino acid oxidase-free Synechococcus PCC 7942 mutant and localization of the l-amino acid oxidase in the corresponding wild type. Biochim. Biophys. Acta. 1996;1307:111–121. doi: 10.1016/0167-4781(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 110.Qian P.Y., Lau S.C.K., Dahms H.U., Dobretsov S., Harder T. Marine biofilms as mediators of colonization by marine macroorganisms: Implications for antifouling and aquaculture. Mar. Biotechnol. 2007;9:399–410. doi: 10.1007/s10126-007-9001-9. [DOI] [PubMed] [Google Scholar]
- 111.Holmstrom C., Egan S., Franks A., McCloy S., Kjelleberg S. Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol. Ecol. 2002;41:47–58. doi: 10.1016/S0168-6496(02)00239-8. [DOI] [PubMed] [Google Scholar]
- 112.Shikuma N.J., Pilhofer M., Weiss G.L., Hadfield M.G., Jensen G.J., Newman D.K. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science. 2014;343:529–533. doi: 10.1126/science.1246794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Celdran D., Espinosa E., Sanchez-Amat A., Atucha A. Effects of epibiotic bacteria on leaf growth and epiphytes of seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 2012;456:21–27. doi: 10.3354/meps09672. [DOI] [Google Scholar]
- 114.James S.G., Holmstrom C., Kjelleberg S. Purification and characterization of a novel antibacterial protein from the marine bacterium D2. Appl. Environ. Microbiol. 1996;62:2783–2788. doi: 10.1128/aem.62.8.2783-2788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lucas-Elio P., Hernandez P., Sanchez-Amat A., Solano F. Purification and partial characterization of marinocine, a new broad-spectrum antibacterial protein produced by Marinomonas mediterranea. Biochim. Biophys. Acta. 2005;1721:193–203. doi: 10.1016/j.bbagen.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 116.Yu M., Wang J., Tang K., Shi X., Wang S., Zhu W.M., Zhang X.H. Purification and characterization of antibacterial compounds of Pseudoalteromonas flavipulchra JG1. Microbiology. 2012;158:835–842. doi: 10.1099/mic.0.055970-0. [DOI] [PubMed] [Google Scholar]
- 117.Chen W.M., Lin C.Y., Chen C.A., Wang J.T., Sheu S.Y. Involvement of an l-amino acid oxidase in the activity of the marine bacterium Pseudoalteromonas flavipulchra against methicillin-resistant Staphylococcus aureus. Enzyme Microb. Technol. 2010;47:52–58. doi: 10.1016/j.enzmictec.2010.03.008. [DOI] [Google Scholar]
- 118.Gomez D., Espinosa E., Bertazzo M., Lucas-Elio P., Solano F., Sanchez-Amat A. The macromolecule with antimicrobial activity synthesized by Pseudoalteromonas luteoviolacea strains is an l-amino acid oxidase. Appl. Microbiol. Biotechnol. 2008;79:925–930. doi: 10.1007/s00253-008-1499-x. [DOI] [PubMed] [Google Scholar]
- 119.Yu Z., Zhou N., Qiao H., Qiu J. Identification, cloning, and expression of l-amino acid oxidase from marine Pseudoalteromonas sp. B3. Sci. World J. 2014;2014:979858. doi: 10.1155/2014/979858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu Z., Wang J., Lin J., Zhao M., Qiu J. Exploring regulation genes involved in the expression of l-amino acid oxidase in Pseudoalteromonas sp. Rf-1. PLoS ONE. 2015;10: doi: 10.1371/journal.pone.0122741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen W.M., Lin C.Y., Sheu S.Y. Investigating antimicrobial activity in Rheinheimera sp. due to hydrogen peroxide generated by l-lysine oxidase activity. Enzyme Microb. Technol. 2010;46:487–493. doi: 10.1016/j.enzmictec.2010.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.