Abstract
In vitro cell cultures are an important tool for obtaining insights into cellular processes in an isolated system and a supplement to in vivo animal experiments. While primary dissociated cultures permit a single homogeneous cell population to be studied, there is a clear need to explore the function of brain cells in a three-dimensional system where the main architecture of the cells is preserved. Thus, organotypic brain slice cultures have proven to be very useful in investigating cellular and molecular processes of the brain in vitro. This review summarizes (1) the historical development of organotypic brain slices focusing on the membrane technology, (2) methodological aspects regarding culturing procedures, age of donors or media, (3) whether the cholinergic neurons serve as a model of neurodegeneration in Alzheimer’s disease, (4) or the nigrostriatal dopaminergic neurons as a model of Parkinson’s disease and (5) how the vascular network can be studied, especially with regard to a synthetic blood–brain barrier. This review will also highlight some limits of the model and give an outlook on future applications.
Keywords: organotypic, whole-brain cultures, dopaminergic, cholinergic, vascular
INTRODUCTION
In vitro cell cultures are an important technique for studying large quantities of homogeneous cells in an isolated environment. Thus, the in vitro culturing of primary dissociated neurons, astrocytes or oligodendrocytes, or endothelial cells has become an essential method employed by many neuroscientists. Especially also with a view to the increasing number of animal research experiments, in vitro cultures permit the number of experiment animals and their suffering to be markedly reduced. Primary in vitro cell cultures allow survival, morphology, function as well as the influence of toxic or protective chemicals to be studied. However, isolated cells do not reflect the nature of the organism due to the isolation and lack of contact with other cells. Thus, over the last decades organotypic cultures have been found to be an important step forward in simulating more in vivo-like situations. Organotypic cultures allow several aspects of structural and synaptic organization of the original tissue to be preserved. This review will summarize historical and methodological aspects of organotypic cultures and discuss whether cultures containing dopaminergic or cholinergic neurons can serve as in vitro models of Parkinson’s or Alzheimer’s disease, respectively.
The term “organotypic” was first published in 1954 in a report on differentiation of the chick embryo eye (Reinbold, 1954), followed by a report on the lung and heart (Loffredo and Sampaolo, 1956) and the intestine (Monesi, 1960). The first description of CNS tissue focused on rat hypophysis (Bousquet and Meunier, 1962) and was followed by the pioneering work of Crain (1966 and 1972) on the development of “organotypic” bioelectric activities in CNS tissues during maturation. Interestingly, the first detailed description of brain tissue was published using organotypic cerebellum (Wolf, 1970; Hauw et al., 1972).
A first technical description was given by Boyd (1971) in a report on a chamber for organotypic cultures used to grow large volumes of tissue. This chamber was further modified and optimized as a tissue plate (Ansevin and Lipps, 1973). The breakthrough was made by Gähwiler’s group, who cultured organotypic brain slices using the roller tube technique (Gähwiler, 1981a,b; Gähwiler, 1988; Braschler et al., 1989; Gähwiler et al., 1997, 2001; Victorov et al., 2001). The method was modified and optimized by Stoppini et al. (1991), who found that organotypic brain slices survive well when cultured on semipermeable membranes. Meanwhile, this method has been used and adapted by several research groups including ours (Bergold and Casaccia-Bonnefil, 1997; Noraberg, 2004; De Simoni and Yu, 2006; Marx, 2010; Ullrich et al., 2011; Ullrich and Humpel, 2011; Shamir and Ewald, 2014). As an attractive alternative the in oculo model was developed, which allows three-dimensional tissue grafts in the anterior eye chamber to be studied.
(a) Organotypic tissue slices in the anterior eye chamber
The anterior chamber of the eye is an easily accessible site, and it has been well documented that grafting of brain tissue into the lateral angle between the cornea and the iris provides a perfect environment for survival and growth. This in oculo model (Hoffer et al., 1974; Olson et al., 1985) allows various brain tissues (e.g. the hippocampus, cerebellum, locus coeruleus, substantia nigra, cortex) to be studied in total isolation. The anterior surface of the rodent iris is highly vascularized, which supports survival of transplanted brain tissues. This model allows tissue growth, trophic effects and interactions of different brain areas to be studied. Indeed, models of neuronal pathways have been constructed, such as e.g. the nigrostriatal dopamine, coeruleospinal or the cholinergic septohippocampal pathways. Thus, this in oculo model allows isolated brain tissues to be investigated in vivo directly in the rodent eye. The tissue can be directly followed by simple stereomicroscope observation, each animal can be given grafts in both eyes, vision is not disturbed and the whole procedure is rapid and simple so that a large number of animals can be generated. The major disadvantage of this model is, however, that it is still a severe animal experiment and does not reduce the number of animal experiments. Moreover, in some situations the nerve fibers innervating the iris hamper or stimulate the in oculo grafts.
(b) Roller tube technique
Initially, organotypic brain slice cultures were established using the roller tube technique. The brain slices are placed on coverslips in a drop of plasma to which thrombin is added to make the plasma coagulate, and thereby “glue” the brain slice to the coverslip. With proper use of plasma and thrombin very few slices are lost by falling off, – yet they may still to some extent disorganize, die and disappear.
(c) Semipermeable membrane technique
The semipermeable membrane technique is a modification of the roller tube technique. In contrast to the roller tube technique, slices are placed on a semipermeable membrane and medium is added below the membrane. Lack of or delayed attachment and falling off is not a problem for brain slices grown by this technique, given that the inserts with the semipermeable membrane are kept in regular incubators, and only moved and handled at medium change. The membrane technology has the big advantage that it employs two compartments separated by a permeable membrane. Cells can be cultured in the lower compartment and slices cultured on the upper membrane. The size of the pores in the membrane determines which substrates/cells can diffuse into the slice or whether slices can be directly co-cultured with other cells, e.g. forming a blood–brain barrier (BBB) (see below). Usually, slices are never fully soaked in medium but are covered with only a small film of medium at the upper surface.
METHODOLOGICAL ASPECTS USING THE MEMBRANE TECHNIQUE
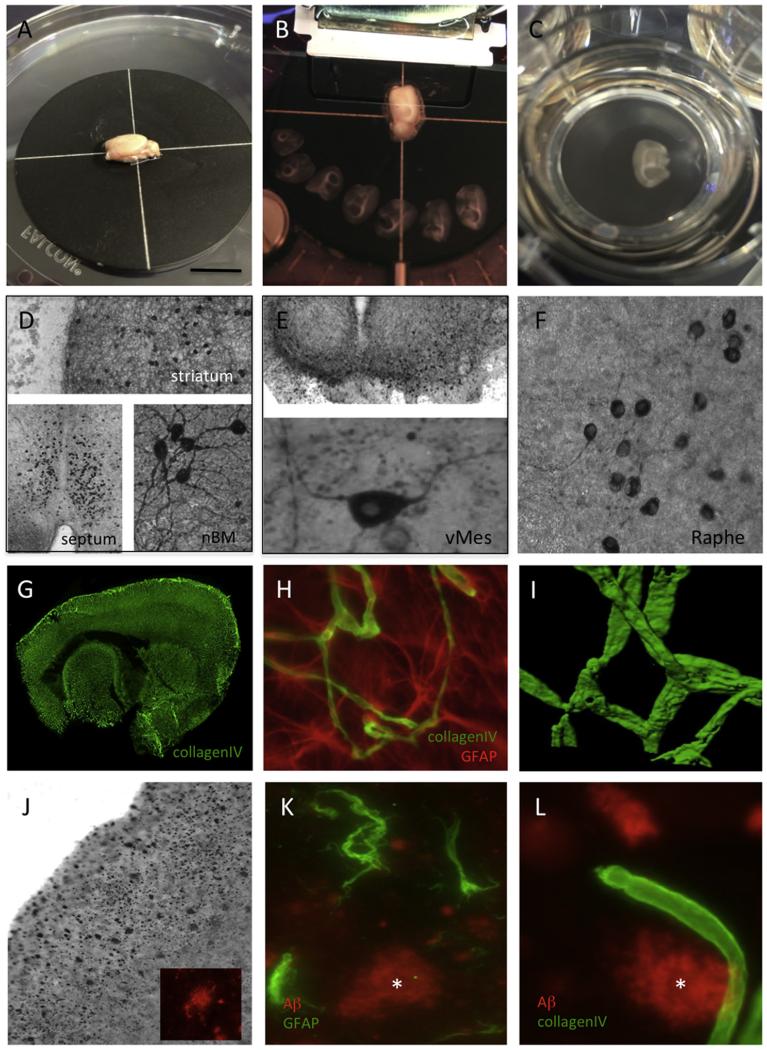
A short technical description is given in the following section focusing on the membrane technology (Fig. 1). The animals (e.g. postnatal P5-P10) are rapidly sacrificed, the head briefly placed in 70% ethanol and the brains dissected. The brains are glued (e.g. Glue Loctite) to the chuck of a water-cooled vibratome (e.g. Leica VT1000A) and trimmed close with a commercial shave razor. Under aseptic conditions, 100- to 400-μm-thick whole-brain (sagittal or coronal) sections are cut and collected in sterile medium. The organotypic slices are carefully placed in a 0.4-μm membrane insert (Millipore PICM03050) in a 6-well plate. Optional slices can also be first placed on a sterile 0.4-μm pore extramembrane (Millipore HTTP02500). Brain slices (1–3 per well depending on size) are cultured in 6-well plates (Greiner) at 37 °C and 5% CO2 and are incubated for minimum two weeks with the medium changed once or twice per week. Slices are usually cultured with or without growth factors to support survival of specific neurons. At the end of the experiment, slices are fixed for 3 h at 4 °C in 4% paraformaldehyde (PAF)/10 mM phosphate-buffered saline (PBS) and then stored in PBS/sodium azide at 4 °C until use. Alternatively, brain slices can also be cut into 200- to 400-μm-thick sections using a Mac Illwain tissue chopper, with six to eight slices cultured on the membrane.
Fig. 1.
Organotypic brain slices are prepared from whole postnatal or adult brains (A), and 100- to 400-μm-thick sections are cut with a vibratome (B) and placed in an insert with 0.4-μm semipermeable pores (C). Cholinergic neurons stained for choline acetyltransferase+ neurons were found in the striatum, septum and basal nucleus of Meynert after incubation with 10 ng/ml nerve growth factor (NGF) for two weeks (D). Dopaminergic tyrosine hydroxylase+ neurons survive well in the ventral mesencephalon (vMES) when incubated with 10 ng/ml glial cell line-derived neurotrophic factor (GDNF) for two weeks (E). Immunostainings for tryptophane hydroxylase show raphe neurons after two weeks in culture (F). Brain slices display a strong vascular network, as seen by collagen IV staining (Alexa-488, green) (G). The vascular system is in direct interaction with astrocytes co-stained for collagen IV (Alexa-488, green) and glial fibrillary acidic protein (GFAP, Alexa-546, red) (H). High-power confocal microscopy shows a collagen IV+ (Alexa-488, green) vessel in the organotypic slices after two weeks in culture (I). Adult whole-brain organotypic slices (110-μm thick) of the APP_SweDI Alzheimer mouse model were cultured for two weeks, stained for beta-amyloid using Alexa-546 (red) (J, K, L) and co-stained with GFAP (Alexa-488, green) for reactive astrocytes (K) or collagen IV using Alexa-488 (green) (L). Note that sections in Figure D-F&J were stained using the chromogenic substance DAB, while the sections in G–L underwent fluorescent staining. Scale bar in A = 980 μm (A–C), 300 μm (D-striatum), 600 μm (D-septum), 90 μm (D-nBM); 600 μm (E upper), 20 μm (E lower), 70 μm (F), 250 μm (G), 60 μm (H), 30 μm (I), 240 μm (J), 36 μm (K) and 24 μm (L).
Medium to culture organotypic brain slices
We usually add 1.2 ml/well of the well-established culture medium according to Stoppini et al. (1991): 50% MEM/HEPES (Gibco), 25% heat-inactivated horse serum (Gibco/Lifetech, Austria), 25% Hanks’ solution (Gibco), 2 mM NaHCO3 (Merck, Austria), 6.5 mg/ml glucose (Merck, Germany), 2 mM glutamine (Merck, Germany), pH 7.2. Horse serum has a positive influence on tissue flattening, providing positive survival promoting effects on neurons, astroglia or microglia in organotypic brain slices. However, in some cases the medium must be modified (Kim et al., 2013). Initially, Annis et al. (1990) reported on a chemically defined medium for organotypic slice cultures and often there is a need to further optimize or adapt the medium for specific conditions, e.g. when using glucose–oxygen deprivation or serum-deprivation or when culturing slices from adult donors.
Age of donors for organotypic slice cultures
Donor age is very important for organotypic slice cultures. It is well known and established that tissue or cells from embryonic donors survive better and also increase in size.
(a) Embryonic donors >E14
Using in oculo transplants Henschen et al. (1985) showed that E14 tissue increases to eightfold its initial size, while E16 increases to threefold its initial size and E17 increases to twice its initial size. There are clear indications that primary dissociated neurons are well established from embryonic donors and survive well. While brain slices from embryonic donors also survive well on membrane inserts, usually organotypic brain slices are derived from postnatal donors due to their higher maturity.
(b) Postnatal donors (<P12)
For organotypic brain cultures postnatal day 10–12 donors are recommended because of better morphology, increased survival and more stable/homogeneous susceptibility in lesion models. Plenz and Kitai (1996) developed cortex striatum mesencephalon (triple) organotypic cultures from rat postnatal day 0–2 brain and modified the “roller tube technique” by embedding slices in a plasma/thrombin clot on a Millicell membrane on a cover slip. Organotypic slice cultures from the mesencephalon, striatum, hippocampus and cerebellum were prepared from late fetal (E21) to P7 rats and cultured for three to 60 days using the roller tube technique (Ostergaard et al., 1990). In our hands, we noticed that P8–P10 brains provide a perfect time window for establishing brain slice cultures on membranes that survive well, even for several months (Marksteiner and Humpel, 2008).
(c) Adult donors
In my opinion, there is a clear need to culture brain tissue from adult donors. Unfortunately, not many papers have been published on intact functional adult organotypic slices. Most authors who claim to successfully use adult slices investigate mainly processes in acute very short-living adult slices (Lossi et al., 2009). At any rate, for long-term cultures the culture conditions need to be optimized for adult organotypic slices. Kim et al. (2013) claimed to culture adult hippocampal slices in serum-free medium. Wilhelmi et al. (2002) used a CSF-like medium and reported good culturing of adult hippocampal tissue for at least six days. We ourselves have good experience in culturing slices from adult mice. However, one needs to be very careful to culture thin (approx. 100–120 μm) sections. Using such 110-μm-thin adult sections from transgenic Alzheimer mice we were able to show that beta-amyloid plaques are still evident and surrounded by reactive astrocytes and microglia (Humpel, 2015). However, we were not able to prolong the survival of sensitive neurons (such as e.g. dopaminergic or cholinergic neurons), even when incubated with growth factors. Indeed, there is a clear need to develop and characterize adult organotypic brain sections, either for the purpose of studying slices from transgenic animals (Duff et al., 2002; Quadros et al., 2003; Mewes et al., 2012) or, more importantly, slices from human postmortem or biopsy brains (Eugene et al., 2014).
Acute versus long-term cultures
When performing experiments with brain slices the question arises: when to do the analysis? In general, slices can be studied immediately after dissection (acute) or after having been grown for longer times to chronological adult age and maturation. The analysis of acute slice experiments (not culturing) has the advantage of providing insights into cellular or molecular processes of rapidly sacrificed animals and may display a near in vivo situation. For these experiments, slices must not be cultured, but endogenously released (toxic) molecules are normally washed out before the experiment starts. Usually, electrophysiology or release experiments can be performed or experiments after short incubation with stimuli, where slices are then extracted by e.g. sonication or lysis and then further analyzed. For this purpose slice thickness and survival are not relevant. Moreover, slices from adult donors or even postmortem tissue can be processed. However, in the case of organotypic brain slice cultures these slices need to be cultured for at least ten to 14 days to guarantee that they are not activated by endogenous release of e.g. calcium or glutamate and that reactive astrogliosis is minimized. Further, developing slices need time for maturation and stabilization of intrinsic axonal projections. Only such “resting non-activated” brain slices are useful for further investigation.
Flattening as a means of macroscopic survival
The organotypic sections attach to the membranes a few days after being transferred to the membrane inserts and are fully attached to the membrane after two weeks in vitro. This is important because the slices flatten and become transparent, which is an important macroscopic sign that the slices are healthy. However, using the lack of flattening alone and measuring the thickness of the cultured slices as a criterion for lack of slice culture survival after set-up appears complicated. More importantly, the general change in color and transparency from whitish-opaque at the time of set-up to a transparent gray during the first week is an important criterion for evaluating whether the slices are well-cultured. Non-surviving cultures or parts that do not survive remain whitish-opaque. Furthermore, outgrowth of cells from the edge of the living slices is another important criterion for evaluating good slices. Thick and not flattened slices should normally be withdrawn from the experiment. In our hands we observed a time window of postnatal 8–12 days, during which slices flatten down very well. The differences observed in “flattening out” of the brain slice cultures of different donor age can be explained by developmental stage differences in growth ability and texture of the slices. However, we recently (Humpel, 2015) started to culture slices from adult animals and sectioned 110-μm-thick slices, some of which display good functional activity. Anyone who wants to measure tissue slice thickness can consult the report by Guy et al. (2011).
Survival of cells in slices
The survival of cells in the organotypic slice cultures is the most important criterion to consider. In general, the older the animal, the less tissue survives and the greater the cell death is. While this is not the principal problem for astrocytes or endothelial cells, neuronal survival is the major challenge. Several parameters influence the survival of neurons, such as tissue age, medium composition including growth factors and serum, thinning of the tissue slice, preparation speed, sterility, health of the donor animals, etc. The lack of thinning is the most important first macroscopic criterion of cell death or necrosis. However, to get more information on cellular viability, tissue slices must be counterstained with cell death-specific agents. Several fluorescent dyes are commercially available to directly study the viability of cells in living slices under the inverse fluorescence microscope. The most frequently used dyes are propidiumiodine, ethidiumbromide, SYTOX dyes, Hoechst dyes, acridinorange or DAPI or annexin V (see for more details Lossi et al., 2009). The advantage of these “live cell stainings” is that the slices can be investigated directly under the microscope and can be further cultured. However, all these dyes are not specific for a particular cell type and do not give information on neuronal survival. In order to study cell-specific death or apoptosis, slices need to be fixed (usually 3 h 4% PAF) and then counterstained for cell-specific markers (e.g. microtubuli associated protein-2 for neurons, glial fibrillary acidic protein for astrocytes or CD11b for microglia or laminin for vessels). In some cases it is very useful to investigate apoptotic cell death. Several different specific apoptotic markers are available, such as e.g. cleaved caspases or PARP-1, FADD, proto-oncogenes or mitochondrial enzymes (see for details Lossi et al., 2009). There are several examples of published papers investigating apoptotic cell death in organotypic brain slices, such as e.g. after stimulation with phencyclidine (Timpe et al., 2014), microRNAs (Irmady et al., 2014), berberine (Simões Pires et al., 2014), manganese (Xu et al., 2014) or iron (Dixon et al., 2012), tunicamycin (Leggett et al., 2012), palmitoylethanolamide (Scuderi et al., 2012), cathepsins (Ceccariglia et al., 2011), prostaglandins (Koch et al., 2010) or PARP-2 inhibitors (Moroni et al., 2009). Further, a nice work shows that in the organotypic postnatal mouse cerebellar cortex the anti-apoptotic protein BCL-2 is regulated by autophagy modulating neuronal survival (Lossi et al., 2010). Thus, many papers have been published on exploring necrosis or apoptosis in organotypic brain slices, however, cannot be completely reviewed here without being complete.
Applications using organotypic brain slices
Organotypic brain slice cultures offer many possibilities to study many types of brain cells in vitro. This review will highlight only a few possibilities from the many publications showing the strong potency of these in vitro cultures. Several applications have been reported, such as e.g. repeated multi-electrophysiological recordings and stimulations (Egert et al., 1998; Jahnsen et al., 1999; Karpiak and Plenz, 2002; Dong and Buonomano, 2005), or gene transfer techniques (Ridoux et al., 1995; Thomas et al., 1998; Murphy and Messer, 2001), retrograde tracing using fluorescent dyes (Ullrich and Humpel, 2011), or long-term live imaging (Gogolla et al., 2006). Organotypic brain slices can be analyzed using all common neurobiological methods. Slices can be detached from the membranes and extracted (e.g. by sonification or lysis) for use for ELISAs, RT-PCR, HPLC etc. PAF-fixed slices are easy to handle and free-floating; they can be immunohistochemically stained (chromogenic or fluorescent), transferred to glass slides and cover slipped or directly analyzed under an inverted microscope (Ullrich et al., 2011). Slices can also be analyzed by in situ hybridization, although this is a bit tricky because sometimes only fresh (unfixed) slices can be used (Gerfin-Moser and Monyer, 2002; Ullrich et al., 2011).
In our research group we usually conduct neuroprotection and neurotoxicity assays. Organotypic brain slices can be easily used to test neuroprotective molecules such as e.g. growth factors or neuroactive drugs (Sundstrom et al., 2005; Drexler et al., 2010). Usually, brain slices are incubated from the beginning of culturing with the respective neuroprotective drugs for e.g. two weeks and then analyzed. Organotypic brain slices are also well-established models for neurotoxological screenings (Noraberg, 2004). In such an experiment slices are cultured for at least two weeks under optimal conditions (if necessary with growth factors) to guarantee a stable well-established non-inflamed and non-reactive model. Then we usually withdraw the growth factor for three days and subsequently add an exogenous degenerative toxic stimulus before incubating for three to 14 days. We have observed that organotypic brain slices need markedly higher doses of a toxic stimulus than do primary cells.
AXOTOMY, LOSS OF TARGET AND SYNAPTOGENESIS AND THE HIPPOCAMPUS
Several brain areas have been cultured as organotypic brain slices: cortex (Giesing et al., 1975), striatum (Ostergaard et al., 1995), substantia nigra (Whetsell et al., 1981; Kida, 1986), raphe (Jonakait et al., 1988; Hochstrasser et al., 2011), locus coeruleus (Knöpfel et al., 1989), the basal forebrain (Robertson et al., 1997) and the suprachiasmatic nucleus (Wray et al., 1993) as well as several others, such as the hypothalamus, thalamus, supraoptic nucleus or olfactory system.
The most explored brain area in organotypic cultures is the hippocampus. In 1973, LaVail and Wolf (1973) reported for the first time on the postnatal development of mouse dentate gyrus. Several research groups have explored the morphology, histogenesis and ultrastructure as well as the functional role of the hippocampal formation (Zhabotinski et al., 1979; Gähwiler, 1981a; Beach et al., 1982; Gähwiler and Hefti, 1984; Zimmer and Gähwiler, 1987; del Rio et al., 1991; Buchs et al., 1993; Muller et al. 1993): the hippocampal organotypic formation served as a model for studying neurodegeneration (oxygen glucose deprivation, oxidative stress, posttrauma, anoxia, asphyxia, hypothermia, hypoglycemia, ischemia, epileptogenics, ethanol), neurotoxicity (N-methyl-d-aspartate (NMDA) toxicity, metals), infections, as well as neuroinflammation and neuroprotection. Furthermore, spine morphology, dendritic growth, mossy fiber sprouting and synaptic plasticity including long-term potentiation, neurogenesis and stem cells have been explored in the organotypic hippocampus.
Although in organotypic brain slice cultures the cells maintain their connections, they lose their target innervation because the slices are an axotomized system. This axotomy is the major disadvantage of the slice culture system, because axotomy causes neuronal cell death. Especially embryonic or neonatal brains are very sensitive for axotomy, because they are dependent on their targets and the supply of target-derived neurotrophic factors. In mature brains axotomy may lead to regenerative responses without any severe neuronal death, due to local production and secretion of growth factors. Clearly some of the neurons in the cut and cultured slices maintain their axonal connections to other neurons within the given tissue slice, just as they and other neurons loose normal afferent connections from more distant areas and levels not included in the slice. Loss of afferent connections to neurons within the cultured slices, combined with the loss of efferent connectivity to normal (outside) target areas, elicits a reorganization and expansion of intrinsic axons to “denervated” intrinsic terminal fields. Definitely, the addition of exogenous growth factors is recommended for specific subsets of neurons, such as e.g. nerve growth factor (NGF) for cholinergic neurons (see below). The need for growth factor supplements for a specific tissue and neuronal population needs to be determined experimentally, possibly also a combination of growth factors. We also experienced that some neuronal populations, e.g. serotonergic neurons, also survive without growth factor addition. However, not all neurons in a brain slice are axotomized, e.g. cholinergic interneurons in the striatum can be studied as an isolated non-axotomized system. These neurons will lose synaptic innervations from e.g. cortex or mesencephalon and be functionally dysregulated.
However, on the other hand, axotomy also allows reactive synaptogenesis and neuronal sprouting in organotypic brain slice cultures to be studied, such as e.g. mossy fiber reorganization in hippocampal slice cultures (Zimmer and Gähwiler, 1984; Gähwiler, 1981a). In fact, the hippocampus is a brain region of specific interest for the study of synapse formation, especially mossy fiber sprouting. The pioneering work of Stoppini et al. (1993) showed neurite outgrowth and reactive synaptogenesis in one- to three-week old hippocampal organotypic cultures. They (Stoppini et al., 1993) observed a thin scar within six days of lesion formation, the presence of numerous degenerative and regenerative processes after one day and many new functional synaptic contacts and complete recovery of transmission within three to six days. These data were extended by Robain et al. (1994), who showed that mossy fibers expanded their terminal fields and invaded the CA3 region and dentate gyrus. Muller et al. (1994) found that the sprouting reaction was triggered by the expression of neuronal cell adhesion molecules, playing an important role in neuronal sprouting and synapse regeneration. Such an axotomy slice model also allows new innervations to be studied in co-culture models. del Río et al. (1996) found that Cajal-Retzius cells survive in long-term single hippocampal cultures, but that fewer cells survive when coupled to the entorhinal cortex, more likely simulating an in vivo situation. Taken together, all these experiments nicely show that long-term organotypic slice cultures are an attractive potent model for studying reactive synaptogenesis and neuronal plasticity, cellular atrophy and age-related processes (Bahr, 1995).
NEURODEGENERATION OF CHOLINERGIC NEURONS AS A MODEL FOR ALZHEIMER’S DISEASE?
Cell death of cholinergic neurons is the central hallmark of Alzheimer’s disease. Cholinergic neurons are located in distinct areas of the brain, and neurons located in the septum/diagonal band of Broca project to the hippocampus, while neurons located in the basal nucleus of Meynert innervate the whole cortex. In the striatum the cholinergic neurons are mainly large interneurons. Already in 1983, Keller et al. (1983) reported the presence of cholinergic cells and nerve fibers in organotypic cultures of the septum and hippocampus. This was further developed and characterized by Gähwiler et al. (1990), who showed for the first time that NGF is required to maintain cholinergic septal organotypic neurons. We ourselves focused on the cholinergic neurons of the nucleus basalis of Meynert and verified the important role of NGF for cholinergic neurons, thus supporting the view that organotypic brain slices may be a potent tool for studying neurodegeneration of cholinergic neurons linking to Alzheimer’s disease (Weis et al., 2001; Humpel and Weis, 2002).
NGF is an example of how in vitro experiments can revolutionize a whole scientific field (Levi-Montalcini et al., 1995). The trophic effect of NGF was first shown in spinal cord ganglia in vitro (Crain and Peterson, 1974; Sedel et al., 1999), and the first effects of NGF on cholinergic neurons also in vitro (Honegger and Lenoir, 1982). These important in vitro experiments have led to many in vivo works. It is well-established that NGF is the most potent neuroprotective molecule to support the survival of cholinergic neurons in organotypic brain slice cultures. In our hands cholinergic neurons of the nucleus basalis of Meynert survive well when incubated from the beginning with 10 ng/ml NGF, and we find approx. 100 neurons/slice (Weis et al., 2001). However, when slices are incubated without NGF, nearly 10 neurons/slice are found, but do not look healthy.
Finally, the organotypic slice model, especially the hippocampal formation, has served as a good model for studying beta-amyloid toxicity as a model for Alzheimer’s disease. Several groups have studied cytochemical changes (Suh et al., 2008; Frozza et al., 2009) and apoptotic cell death (Allen et al., 1995; Chong et al., 2006) after beta-amyloid toxicity, protective effects mediating oxidative stress (Bruce et al., 1996; Clapp-Lilly et al., 2001), the modulating effects of different pro-inflammatory stimuli (Harris-White et al., 1998), intracellular pathways (Nassif et al., 2007; Tardito et al., 2007) as well as tau phosphorylation (Johansson et al., 2006). Prasanthi et al. (2011) showed that endoplasmatic reticulum stress-mediated transcriptional activation in organotypic adult rabbit hippocampal slices triggered with 27-hydroxycholesterol. Schrag et al. (2008) found that neurons in organotypic slices from adult dwarf mice are resistant to beta-amyloid induced tau-hyperphosphorylation and changes in apoptosis-regulatory protein levels. Using rat cortical neurons in culture and entorhinal-hippocampal organotypic slices, Alberdi et al. (2010) found that beta-amyloid oligomers significantly induced intracellular Ca2+ and apoptotic cell death through a mechanism requiring NMDA and AMPA receptor activation. In organotypic hippocampal slice cultures it was shown (Kreutz et al., 2011) that ganglioside GM1 exhibited a neuroprotective activity on beta-amyloid-induced apoptosis. Finally, we showed for the first time that organotypic brain slices develop beta-amyloid “plaque-like deposits” when incubated for several weeks under low acidic pH with apolipoprotein E4 (Marksteiner and Humpel, 2008).
DOPAMINERGIC NEURONAL CO-CULTURES AS A MODEL FOR PARKINSON’S DISEASE?
The major advantage of organotypic brain slices is that it permits cells from two or more functionally related brain areas to be cultured simultaneously. A first publication on co-cultures of organotypic tissue reported the innervation of fetal rodent skeletal muscle by spinal cord (Peterson and Crain, 1970). Many other co-cultures have been studied meanwhile, including septo-hippocampal, cortico-striatal, cortico-spinal, cortico-thalamic and entorhinal-hippocampal (Woodhams and Atkinson, 1996). The most studied co-culture system, however, is the striatonigral system, because it plays an important role in Parkinson’s disease. Such co-cultures allow the long-distance nerve fiber growth and connectivity between neuronal populations and brain areas to be studied and characterized.
Cell death of dopaminergic neurons is the central hallmark of Parkinson’s disease. Dopaminergic neurons are located in the ventral mesencephalon (vMes) and neurons of the substantia nigra project into the dorsal striatum (nigrostriatal pathway), while neurons of the ventral tegmental area project into the ventral striatum (meso-limbic pathway). Organotypic brain slices of the vMes and the striatum are well-established, and several exciting papers describe the nigrostriatal pathway in slices. Survival of dopaminergic neurons in the substantia nigra in organotypic brain slices was already reported in 1982 (Hendelman et al., 1982) and further characterized in 1989 (Jaeger et al., 1989). Pioneering work has been done by Zimmer’s groups, who detailed the survival and nerve fiber growth of dopaminergic nigrostriatal neurons (Ostergaard et al., 1990, 1991). We ourselves characterized mesencephalic dopamine neurons and observed that glial cell line-derived neurotrophic factor (GDNF) was essential for survival and nerve fiber growth (Schatz et al., 1999), which was verified by others (Jaumotte and Zigmond, 2005; af Bjerkén et al., 2007). This work provided the basis for further developing and characterizing the nigrostriatal nerve fiber innervation (Heine and Franke, 2014) and developing organotypic slices as an in vitro model for Parkinson’s disease (Stahl et al., 2009; Ullrich and Humpel, 2009b; Cavaliere et al., 2010; Daviaud et al., 2014).
The striatonigral tract is of special interest, because it degenerates in Parkinson’s disease. It has been reported that outgrowth of dopamine fibers from the mesencephalon occurs irrespective of the age of the donor rats, and a pronounced innervation of dopamine nerve fibers into the striatum has been seen (Ostergaard et al., 1990). The distance between the mesencephalon and the striatum was between 0.5 and 2.0 mm at the end of culturing. Thus, the dopaminergic fibers from the vMes could extend over a long distance, and it was reported that the maximum distance covered between striatonigral co-slices was 5.7 mm (Ostergaard et al., 1990). Franke et al. (2003) reported that in mesencephalic/striatal co-slices an extensive fiber bridge was observed in the co-cultures and that dopaminergic neurons develop their typical innervation pattern. Snyder-Keller et al. (2001) showed that the striatal patch/matrix organization was maintained in organotypic slice cultures taken from E19-P4 rats. We ourselves showed in a previous work that cultures of mesencephalic/striatal co-slices exhibit a large number of surviving dopamine neurons in the presence of GDNF and that intense fiber innervation is seen in striatal slices (Schatz et al., 1999; Zassler et al., 2003, 2005). Using sagittal brain slices we ourselves showed for the first time that dopamine neurons survive although the striatonigral pathway is not functional (Ullrich et al., 2011).
THE VASCULAR SYSTEM IN ORGANOTYPIC SLICES
Brain capillaries constitute the BBB and innervate all areas of the brain. A first description of the vasculature of organotypic brain slices was given in 1975 (Wolff et al., 1975). Subsequently, Renkawek et al. (1976) characterized brain capillaries in organotypic cultures using relatively unselective butyryl cholinesterase stainings. We ourselves were one of the first to demonstrate at the cellular level that organotypic brain slices contain a strong network of laminin-positive brain capillaries (Moser et al., 2003, 2004). Laminin is a well-established basement membrane marker and excellently stains the vascular structures of the brain. We demonstrated that capillaries survive well in organotypic sections without any circulation (Moser et al., 2003). Although the capillaries are no longer functional and do not display any blood flow, it is likely that they express and secrete a cocktail of various molecules that may indeed also influence other cells in the slices including nerve fiber innervations (Moser et al., 2003; Kovács et al., 2011). Meanwhile brain vessels in organotypic cultures have been well studied, and especially the neurovascular unit and the interaction of endothelial cells with pericytes is coming under intense investigation (Camenzind et al., 2010; Chip et al., 2013, 2014; Morin-Brureau et al., 2013; Zehendner et al., 2013; Mishra et al., 2014).
The testing of pro- or anti-angiogenic growth factors is important when studying angiogenesis and revascularization in organotypic slices (Morin-Brureau et al., 2011). Especially two growth factors are of particular interest, when exploring the vascular network: vascular endothelial growth factor (VEGF) and fibroblast-growth factor-2 (FGF-2, bFGF). VEGF and its tyrosine kinase receptors (VEGFR-1, flt-1 and VEGFR-2, flk-1/KDR) are key mediators of angiogenesis. They are usually expressed during embryonic development but are downregulated in the adult. Kremer et al. (1997) investigated for the first time the time-dependent expression of VEGFR-2 in cerebral slice cultures and found that VEGF and hypoxia upregulated VEGFR-2 expression. This was verified by Rosenstein et al. (1998), who found significant angiogenic effects after VEGF application in a dose-responsive manner in fetal, newborn and adult rat cortical slices, which was abolished by a VEGF neutralizing antibody. After VEGF application, explants from adult donors had enlarged, dilated vessels that appeared to be an expansion of the existing network (Rosenstein et al., 1998). Further, they found that these slice culture vessels expressed both VEGF receptors (Rosenstein et al., 1998). Interestingly, the same group showed that VEGF had a neurotrophic effect in fetal organotypic cortex explants, and it was suggested that VEGF has neuroprotective activity independent of a vascular component (Rosenstein et al., 2003). The effect of FGF-2 on the vascular network was contradictory. While Rosenstein et al. (1998) found that all FGF-2 treated slice cultures exhibited substantially fewer vascular profiles, Bendfeldt et al. (2007) showed that FGF-2 maintained blood vessels and preserved the composition of tight junctions in neonatal mouse brain slices. However, while moderate FGF-2 concentrations (0.5–5 ng/ml) markedly increased the number of vessels, an excess of FGF-2 (50 ng/ml) reduced the vessel density. This again here clearly points to the need to perform dose- as well as time-dependent experiments in testing the effects of exogenous stimuli in brain slice cultures.
There is a clear need to develop fast and simple in vitro models for a high-throughput screening of pro-angiogenic factors or angiogenic inhibitors (Staton et al., 2004). So far the most useful angiogenic assays include in vivo Matrigel plug and sponge and corneal neovascularization, the chick chorioallantoic membrane and aortic arch assays, the in vitro cellular (proliferation, migration, tube formation) and organotypic (aortic ring) assays (Auerbach et al., 2003; Staton et al., 2009). Most pro- or anti-angiogenic drugs have been tested in co-cultures of endothelial cells and pericytes or smooth muscle cells forming a tubular network, but organotypic brain slice cultures have to our knowledge not yet extensively used for screening pharmacological drugs. We ourselves used this model to investigate whether brain vessels degenerate, sprout or can grow over a lesion site. Using e.g. laminin-counterstained brain slices, we overlay this vascular network on a 6 × 6 grid in Photoshop and quantify the vascular network by counting the crossings in the 6 × 6 grid (Moser et al., 2003). Indeed, using such a model we previously showed that in adult brain cultures of adult transgenic Alzheimer mice, substance P and calcium channel blockers induced angiogenesis (Daschil et al., 2015). Further, we demonstrated that brain vessels in different organotypic brain slices can grow back together when exogenously stimulated (Ullrich and Humpel, 2009a).
Another important innovative approach is to co-culture brain endothelial cells with organotypic brain slices and build up an in vitro BBB. Indeed, Duport et al. (1998) developed and characterized such a model 15 years ago when they overlaid organotypic brain slices on an endothelial monolayer growing on permeable membranes and concluded that this model possesses characteristics of a BBB in situ including tightness. However, this model seems to be very complex and tricky, and we ourselves have never succeeded in setting up such a complex in vitro model, nor are we aware of other groups using such a system. Indeed, the development of a simple BBB model using only brain capillary endothelial cells (BCECs) is a very tricky and complex model and must include tight junctions and a close layer of BCEC to guarantee an electrically tight junction resistance. Thus, although this was powerful pioneering work, much more work is needed before this “slice-BBB model” can serve for further pharmacological use.
HOW COMPARABLE ARE BRAIN SLICES WITH THE IN VIVO SITUATION?
The main question arises: how close are in vitro models to the in vivo situation? This is a complex question because another question may also be asked, namely how close are in vivo murine models to the human situation? In my opinion, in vitro models can help us gain more mechanistic insights, which then must be proven in vivo in the animal model. Vice versa, results in animal models must be proven in postmortem human material and finally in human imaging and therapeutic/diagnostic approaches. Primary as well as organotypic models definitely have their advantages and disadvantages. Both in vitro models may demonstrate proof of principle, which must subsequently be proven in vivo. Regarding organotypic brain slice cultures, the complex three-dimensional architecture is partly maintained, while pathways are largely disconnected but could also be re-established. Thus, as compared to primary single-cell cultures, such an organotypic slice culture model is at least closest to an in vivo situation.
However, at this point another question arises: do we want to study development or a mature adult situation. It is well known that developing neurons have different characteristics (dependence on growth factor, receptor expression, protein expression …) than do mature adult neurons. Thus, it needs to be proven whether and, if so, when cultured neurons derived from postnatal donors develop a mature phenotype and display the same molecular and cellular pattern as a mature adult neuron. Regarding dissociated neurons, this question can be neglected because primary dissociated neurons cannot be cultured for several weeks. However, organotypic brain slice cultures can be cultured for long times, and the question arises whether slices derived from postnatal donors and cultured for more than two months can represent a mature adult situation. Clearly, much more work is needed to fully answer this question. On the other hand, a similarly critical question suggests itself, namely whether a one-year-old mouse compares with the mature adult situation of a 40-year-old human.
OUTLOOK
Taken together, the organotypic slice cultures are a potent in vitro system for studying many of the brain’s cells. However, there are several challenging options for further improving this model. (1) There is a clear need to reconstruct axotomized neuronal pathways to establish functional pathways. It will be necessary to improve growth factor applications and to target the inputs into the target regions. Brain slices could become a potent means of studying nerve regeneration across longer distances, and it is important to test the bridging of various substances, such as e.g. tubes of polyglycolic acid-collagen (Kiyotani et al., 1996) or other biomaterials, including growth factor-releasing scaffold nanostructures (Breen et al., 2009). (2) The development of adult organotypic brain slices (including human tissue) is one of the primary goals, since most researchers want to study changes in disease models and correlate to an adult situation. Whether the postnatally derived slices represent only a developing model and in no way compare with a mature adult situation is still the subject of discussion. (3) To overcome this problem long-term cultures are necessary. However, maintaining cultures over several months is time-consuming and keeping them under sterile conditions is tricky. (4) There is a definite need to couple brain slices and a BBB; such a complex model will allow the entry of substances directly into the brain to be studied and may simulate an in vivo situation even better. (5) As a perspective, it would be highly attractive to couple the slices and the vascular system with a tube perfusion system; this would permit the simulation of blood flow and the continuous supply of needed substances. Such a model would also allow the release of neurotransmitters or cytokines from brain slices to be measured. (6) Brain slices may also serve as diagnostic tools; e.g. coupling slices and electrode arrays or biochips (Maher et al., 1999; Kristensen et al., 2001) may provide direct and fast information on a cell type; brain slices could be directly perfused with human body fluids, such as e.g. cerebrospinal fluids or plasma. (7) Brain slices could be coupled with stem cells to study neurogenesis, or neurogenesis could be stimulated by e.g. excitotoxic cell death chemicals (Daviaud et al., 2013; Mazzone et al., 2013). This could also be done to build up whole functional brain areas. Initial pioneering work establishing cerebral organoids was recently published by Lancaster et al. (2013). Moreover, exogenous cells modified, manipulated or genetically engineered could further improve the slice model. Choi et al. (2014) recently established a three-dimensional human neural cell culture model of Alzheimer’s disease.
In conclusion, organotypic slice cultures are an innovative and potent in vitro method that permits several cell types of the brain to be studied in a complex network. Slices can be cultured as single slices or as whole-brain sagittal slices. Further improvement and new techniques might make it possible to prepare whole functional brain models, possibly forming a complex artificial brain including a BBB. Such a complex brain culture system might provide an excellent model for studying neurodegenerative brain diseases, including e.g. Alzheimer’s and Parkinson’s disease. Finally, organotypic brain slice cultures markedly reduce the number of severe animal experiments contributing to the 3Rs (reduce, refine, replace).
Acknowledgments
I would like to express my thanks to Kathrin Kniewallner for help with the confocal microscopic pictures, Karin Albrecht (technician) for excellent preparation of the organotypic slice cultures and Monika Greil (technician) for immunostainings. This study was supported by the Austrian Science Fund (P24734-B24) and by a EU project (BrainMatTrain, Nr. 676408).
Abbreviations
- BBB
blood–brain barrier
- BCECs
brain capillary endothelial cells
- FGF-2
fibroblast-growth factor-2
- GDNF
glial cell line-derived neurotrophic factor
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- NGF
nerve growth factor
- NMDA
N-methyl-d-aspartate
- PBS
phosphate-buffered saline
- VEGF
vascular endothelial growth factor
- vMes
ventral mesencephalon
REFERENCES
- af Bjerkén S, Boger HA, Nelson M, Hoffer BJ, Granholm AC, Strömberg I. Effects of glial cell line-derived neurotrophic factor deletion on ventral mesencephalic organotypic tissue cultures. Brain Res. 2007;1133:10–19. doi: 10.1016/j.brainres.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47(3):264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Allen YS, Devanathan PH, Owen GP. Neurotoxicity of beta-amyloid protein: cytochemical changes and apoptotic cell death investigated in organotypic cultures. Clin Exp Pharmacol Physiol. 1995;22:370–371. doi: 10.1111/j.1440-1681.1995.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Annis CM, Edmond J, Robertson RT. A chemically-defined medium for organotypic slice cultures. J Neurosci Methods. 1990;32:63–70. doi: 10.1016/0165-0270(90)90072-n. [DOI] [PubMed] [Google Scholar]
- Ansevin KD, Lipps BV. Tissue plate: a new technique for long-termorganotypic culture. In Vitro. 1973;8:483–488. doi: 10.1007/BF02615951. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- Bahr BA. Long-term hippocampal slices: a model system for investigating synaptic mechanisms and pathologic processes. J Neurosci Res. 1995;42(3):294–305. doi: 10.1002/jnr.490420303. [DOI] [PubMed] [Google Scholar]
- Beach RL, Bathgate SL, Cotman CW. Identification of cell types in rat hippocampal slices maintained in organotypic cultures. Brain Res. 1982;255:3–20. doi: 10.1016/0165-3806(82)90071-2. [DOI] [PubMed] [Google Scholar]
- Bendfeldt K, Radojevic V, Kapfhammer J, Nitsch C. Basic fibroblast growth factor modulates density of blood vessels and preserves tight junctions in organotypic cortical cultures of mice: a new in vitro model of the blood–brain barrier. J Neurosci. 2007;27(12):3260–3267. doi: 10.1523/JNEUROSCI.4033-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergold PJ, Casaccia-Bonnefil P. Preparation of organotypic hippocampal slice cultures using the membrane filter method. Methods Mol Biol. 1997;72:15–22. doi: 10.1385/0-89603-394-5:15. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Meunier JM. Organotypic culture, on natural and artificial media, of fragments of the adult rat hypophysis. C R Seances Soc Biol Fil. 1962;156:65–67. [PubMed] [Google Scholar]
- Boyd WH. A chamber for organotypic culture; adapted for growing large volumes of tissue. Stain Technol. 1971;46:85–87. doi: 10.3109/10520297109067827. [DOI] [PubMed] [Google Scholar]
- Braschler UF, Iannone A, Spenger C, Streit J, Lüscher HR. A modified roller tube technique for organotypic cocultures of embryonic rat spinal cord, sensory ganglia and skeletal muscle. J Neurosci Methods. 1989;29:121–129. doi: 10.1016/0165-0270(89)90023-x. [DOI] [PubMed] [Google Scholar]
- Breen A, O’Brien T, Pandit A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng Part B Rev. 2009;15:201–214. doi: 10.1089/ten.TEB.2008.0527. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Malfroy B, Baudry M. Beta-amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci U S A. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchs PA, Stoppini L, Muller D. Structural modifications associated with synaptic development in area CA1 of rat hippocampal organotypic cultures. Brain Res Dev Brain Res. 1993;71:81–91. doi: 10.1016/0165-3806(93)90108-m. [DOI] [PubMed] [Google Scholar]
- Camenzind RS, Chip S, Gutmann H, Kapfhammer JP, Nitsch C, Bendfeldt K. Preservation of transendothelial glucose transporter 1 and P-glycoprotein transporters in a cortical slice culture model of the blood-brain barrier. Neuroscience. 2010;170(1):361–371. doi: 10.1016/j.neuroscience.2010.06.073. [DOI] [PubMed] [Google Scholar]
- Cavaliere F, Vicente ES, Matute C. An organotypic culture model to study nigro-striatal degeneration. J Neurosci Methods. 2010;188:205–212. doi: 10.1016/j.jneumeth.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Ceccariglia S, D’Altocolle A, Del Fa’ A, Pizzolante F, Caccia E, Michetti F, Gangitano C. Cathepsin D plays a crucial role in the trimethyltin-induced hippocampal neurodegeneration process. Neuroscience. 2011;174:160–170. doi: 10.1016/j.neuroscience.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Chip S, Nitsch C, Wellmann S, Kapfhammer JP. Subfield-specific neurovascular remodeling in the entorhino-hippocampal-organotypic slice culture as a response to oxygen–glucose deprivation and excitotoxic cell death. J Cereb Blood Flow Metab. 2013;33(4):508–518. doi: 10.1038/jcbfm.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chip S, Zhu X, Kapfhammer JP. The analysis of neurovascular remodeling in entorhino-hippocampal organotypic slice cultures. J Vis Exp. 2014;92:e52023. doi: 10.3791/52023. http://dx.doi.org/10.3791/52023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006;281:20315–20325. doi: 10.1074/jbc.M601016200. [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Smith MA, Perry G, Harris PL, Zhu X, Duffy LK. Melatonin acts as mantioxidant and pro-oxidant in an organotypic slice culture model of Alzheimer’s disease. NeuroReport. 2001;12:1277–1280. doi: 10.1097/00001756-200105080-00044. [DOI] [PubMed] [Google Scholar]
- Crain SM. Development of “organotypic” bioelectric activities in central nervous tissues during maturation in culture. Int Rev Neurobiol. 1966;9:1–43. doi: 10.1016/s0074-7742(08)60135-x. [DOI] [PubMed] [Google Scholar]
- Crain SM, Bornstein MB. Organotypic bioelectric activity in cultured reaggregates of dissociated rodent brain cells. Science. 1972;176:182–184. doi: 10.1126/science.176.4031.182. [DOI] [PubMed] [Google Scholar]
- Crain SM, Peterson ER. Enhanced afferent synaptic functions in fetal mouse spinal cord-sensory ganglion explants following NGF-induced ganglion hypertrophy. Brain Res. 1974;79(1):145–152. doi: 10.1016/0006-8993(74)90574-5. [DOI] [PubMed] [Google Scholar]
- Daschil N, Kniewallner KM, Obermair GJ, Hutter-Paier B, Windisch M, Marksteiner J, Humpel C. L-Type calcium channel blockers and substance P induce angiogenesis of cortical vessels associated with beta-amyloid plaques in an Alzheimer mouse model. Neurobiol Aging. 2015 doi: 10.1016/j.neurobiolaging.2014.12.027. http://dx.doi.org/10.1016/j.neurobiolaging.2014.12.027. S0197-4580(14)00844-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviaud N, Garbayo E, Schiller PC, Perez-Pinzon M, Montero-Menei CN. Organotypic cultures as tools for optimizing central nervous system cell therapies. Exp Neurol. 2013;248:429–440. doi: 10.1016/j.expneurol.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Daviaud N, Garbayo E, Lautram N, Franconi F, Lemaire L, Perez-Pinzon M, Montero-Menei CN. Modeling nigrostriatal degeneration in organotypic cultures, a new ex vivo model of Parkinson’s disease. Neuroscience. 2014;256:10–22. doi: 10.1016/j.neuroscience.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni A, Yu LM. Preparation of organotypic hippocampal slice cultures: interface method. Nat Protoc. 2006;1:1439–1445. doi: 10.1038/nprot.2006.228. [DOI] [PubMed] [Google Scholar]
- del Rio JA, Heimrich B, Soriano E, Schwegler H, Frotscher M. Proliferation and differentiation of glial fibrillary acidic protein-immunoreactive glial cells in organotypic slice cultures of rat hippocampus. Neuroscience. 1991;43:335–347. doi: 10.1016/0306-4522(91)90298-3. [DOI] [PubMed] [Google Scholar]
- del Río JA, Heimrich B, Supèr H, Borrell V, Frotscher M, Soriano E. Differential survival of Cajal-Retzius cells in organotypic cultures of hippocampus and neocortex. J Neurosci. 1996;16(21):6896–6907. doi: 10.1523/JNEUROSCI.16-21-06896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Buonomano DV. A technique for repeated recordings in cortical organotypic slices. J Neurosci Methods. 2005;146:69–75. doi: 10.1016/j.jneumeth.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Drexler B, Hentschke H, Antkowiak B, Grasshoff C. Organotypic cultures as tools for testing neuroactive drugs – link between in-vitro and in-vivo experiments. Curr Med Chem. 2010;17:4538–4550. doi: 10.2174/092986710794183042. [DOI] [PubMed] [Google Scholar]
- Duff K, Noble W, Gaynor K, Matsuoka Y. Organotypic slice cultures from transgenic mice as disease model systems. J Mol Neurosci. 2002;19:317–320. doi: 10.1385/JMN:19:3:317. [DOI] [PubMed] [Google Scholar]
- Duport S, Robert F, Muller D, Grau G, Parisi L, Stoppini L. An in vitro blood-brain barrier model: cocultures between endothelial cells and organotypic brain slice cultures. Proc Natl Acad Sci U S A. 1998;95:1840–1845. doi: 10.1073/pnas.95.4.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egert U, Schlosshauer B, Fennrich S, Nisch W, Fejtl M, Knott T, Müller T, Hämmerle H. A novel organotypic long-term culture of the rat hippocampus on substrate-integrated multielectrode arrays. Brain Res Brain Res Protoc. 1998;2:229–242. doi: 10.1016/s1385-299x(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Eugene E, Cluzeaud F, Cifuentes-Diaz C, Fricker D, Le Duigou C, Clemenceau S, Baulac M, Poncer JC, Miles R. An organotypic brain slice preparation from adult patients with temporal lobe epilepsy. J Neurosci Methods. 2014;235:234–244. doi: 10.1016/j.jneumeth.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Schelhorn N, Illes P. Dopaminergic neurons develop axonal projections to their target areas in organotypic co-cultures of the ventral mesencephalon and the striatum/prefrontal cortex. Neurochem Int. 2003;42:431–439. doi: 10.1016/s0197-0186(02)00134-1. [DOI] [PubMed] [Google Scholar]
- Frozza RL, Horn AP, Hoppe JB, Simão F, Gerhardt D, Comiran RA, Salbego CG. A comparative study of beta-amyloid peptides Abeta1-42 and Abeta25-35 toxicity in organotypic hippocampal slice cultures. Neurochem Res. 2009;34:295–303. doi: 10.1007/s11064-008-9776-8. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Morphological differentiation of nerve cells in thin organotypic cultures derived from rat hippocampus and cerebellum. Proc R Soc Lond B Biol Sci. 1981a;211:287–290. doi: 10.1098/rspb.1981.0007. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981b;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic cultures of neural tissue. Trends Neurosci. 1988;11:484–489. doi: 10.1016/0166-2236(88)90007-0. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Hefti F. Guidance of acetylcholinesterase-containing fibers by target tissue in co-cultured brain slices. Neuroscience. 1984;40:235–243. doi: 10.1016/0306-4522(84)90088-5. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Rietschin L, Knöpfel T, Enz A. Continuous presence of nerve growth factor is required for maintenance of cholinergic septal neurons in norganotypic slice cultures. Neuroscience. 1990;36:27–31. doi: 10.1016/0306-4522(90)90348-8. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Thompson SM, Muller D. Preparation and maintenance of organotypic slice cultures of CNS tissue. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0611s09. Chapter 6: Unit 6.11. [DOI] [PubMed] [Google Scholar]
- Gerfin-Moser A, Monyer H. In situ hybridization on organotypic slice cultures. Int Rev Neurobiol. 2002;47:125–134. doi: 10.1016/s0074-7742(02)47058-4. [DOI] [PubMed] [Google Scholar]
- Giesing M, Neumann G, Egge H, Zilliken F. Lipid metabolism of developing central nervous tissues in organotypic cultures. I. Lipid distribution and fatty acid profiles of the medium for rat brain cortex in vitro. Nutr Metab. 1975;19:242–250. doi: 10.1159/000175671. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, DePaola V, Caroni P. Long-term live imaging of neuronal circuits in organotypic hippocampal slice cultures. Nat Protoc. 2006;1:1223–1226. doi: 10.1038/nprot.2006.169. [DOI] [PubMed] [Google Scholar]
- Guy Y, Rupert AE, Sandberg M, Weber SG. A simple method for measuring organotypic tissue slice culture thickness. J Neurosci Methods. 2011;199:78–81. doi: 10.1016/j.jneumeth.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-White ME, Chu T, Balverde Z, Sigel JJ, Flanders KC, Frautschy SA. Effects of transforming growth factor-beta (isoforms 1–3) on amyloid-beta deposition, inflammation, and cell targeting in organotypic hippocampal slice cultures. J Neurosci. 1998;18:10366–10374. doi: 10.1523/JNEUROSCI.18-24-10366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw JJ, Berger B, Escourolle R. Presence of synapses in organotypic culture in vitro of human cerebellum. C R Acad Sci Hebd Seances Acad Sci D. 1972;274:264–266. [PubMed] [Google Scholar]
- Heine C, Franke H. Organotypic slice co-culture systems to study axon regeneration in the dopaminergic system ex vivo. Methods Mol Biol. 2014;1162:97–111. doi: 10.1007/978-1-4939-0777-9_8. [DOI] [PubMed] [Google Scholar]
- Hendelman WJ, Marshall KC, Ferguson R, Carrière S. Catecholamine neurons of the central nervous system in organotypic culture. Dev Neurosci. 1982;5:64–76. doi: 10.1159/000112662. [DOI] [PubMed] [Google Scholar]
- Henschen A, Hoffer B, Olson L. Spinal cord grafts in oculo: survival, growth, histological organization and electrophysiological characteristics. Exp Brain Res. 1985;60(1):38–47. doi: 10.1007/BF00237016. [DOI] [PubMed] [Google Scholar]
- Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience. 2011;184:128–138. doi: 10.1016/j.neuroscience.2011.03.070. [DOI] [PubMed] [Google Scholar]
- Hoffer B, Seiger A, Ljungberg T, Olson L. Electrophysiological and cytological studies of brain homografts in the anterior chamber of the eye: maturation of cerebellar cortex in oculo. Brain Res. 1974;79(2):165–184. doi: 10.1016/0006-8993(74)90409-0. [DOI] [PubMed] [Google Scholar]
- Honegger P, Lenoir D. Nerve growth factor (NGF) stimulation of cholinergic telencephalic neurons in aggregating cell cultures. Brain Res. 1982;55(2):229–238. doi: 10.1016/0165-3806(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Humpel C. Organotypic vibrosections from whole brain adult Alzheimer mice (overexpressing amyloid-precursor-protein with the Swedish-Dutch-Iowa mutations) as a model to study clearance of beta-amyloid plaques. Front Aging Neurosci. 2015;7:47. doi: 10.3389/fnagi.2015.00047. http://dx.doi.org/10.3389/fnagi.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C, Weis C. Nerve growth factor and cholinergic CNS neurons studied in organotypic brain slices. Implication in Alzheimer’s disease? J Neural Transm Suppl. 2002;62:253–263. doi: 10.1007/978-3-7091-6139-5_23. [DOI] [PubMed] [Google Scholar]
- Irmady K, Jackman KA, Padow VA, Shahani N, Martin LA, Cerchietti L, Unsicker K, Iadecola C, Hempstead BL. Mir-592 regulates the induction and cell death-promoting activity of p75NTR in neuronal ischemic injury. J Neurosci. 2014;34(9):3419–3428. doi: 10.1523/JNEUROSCI.1982-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger C, Gonzalo Ruiz A, Llinás R. Organotypic slice cultures of dopaminergic neurons of substantia nigra. Brain Res Bull. 1989;22:981–991. doi: 10.1016/0361-9230(89)90010-5. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Kristensen BW, Thiébaud P, Noraberg J, Jakobsen B, Bove M, Martinoia S, Koudelka-Hep M, Grattarola M, Zimmer J. Coupling of organotypic brain slice cultures to silicon-based arrays of electrodes. Methods. 1999;18:160–172. doi: 10.1006/meth.1999.0769. [DOI] [PubMed] [Google Scholar]
- Jaumotte JD, Zigmond MJ. Dopaminergic innervation of forebrain by ventral mesencephalon in organotypic slice co-cultures: effects of GDNF. Brain Res Mol Brain Res. 2005;134:139–146. doi: 10.1016/j.molbrainres.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Johansson S, Jämsä A, Vasänge M, Winblad B, Luthman J, Cowburn RF. Increased tau phosphorylation at the Ser396 epitope after amyloid beta-exposure in organotypic cultures. NeuroReport. 2006;17:907–911. doi: 10.1097/01.wnr.0000221844.35502.29. [DOI] [PubMed] [Google Scholar]
- Jonakait GM, Schotland S, Ni L. Development of serotonin, substance P and thyrotrophin-releasing hormone in mouse medullary raphe grown in organotypic tissue culture: developmental regulation by serotonin. Brain Res. 1988;473:336–343. doi: 10.1016/0006-8993(88)90863-3. [DOI] [PubMed] [Google Scholar]
- Karpiak VC, Plenz D. Preparation and maintenance of organotypic cultures for multi-electrode array recordings. Curr Protoc Neurosci. 2002 doi: 10.1002/0471142301.ns0615s19. Chapter 6: Unit 6.15. [DOI] [PubMed] [Google Scholar]
- Keller F, Rimvall K, Waser PG. Choline acetyltransferase in organotypic cultures of rat septum and hippocampus. Neurosci Lett. 1983;42:273–278. doi: 10.1016/0304-3940(83)90274-4. [DOI] [PubMed] [Google Scholar]
- Kida E. Ultrastructural properties of rat substantia nigra in organotypic culture. Neuropatol Pol. 1986;24:145–159. [PubMed] [Google Scholar]
- Kim H, Kim E, Park M, Lee E, Namkoong K. Organotypic hippocampal slice culture from the adult mouse brain: a versatile tool for translational neuropsychopharmacology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;41:36–43. doi: 10.1016/j.pnpbp.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Kiyotani T, Teramachi M, Takimoto Y, Nakamura T, Shimizu Y, Endo K. Nerve regeneration across a 25-mm gap bridged by a polyglycolic acid-collagen tube: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 1996;740:66–74. doi: 10.1016/s0006-8993(96)00848-7. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Rietschin L, Gähwiler BH. Organotypic co-cultures of rat locus coeruleus and hippocampus. Eur J Neurosci. 1989;1:678–689. doi: 10.1111/j.1460-9568.1989.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Koch H, Huh SE, Elsen FP, Carroll MS, Hodge RD, Bedogni F, Turner MS, Hevner RF, Ramirez JM. Prostaglandin E2-induced synaptic plasticity in neocortical networks of organotypic slice cultures. J Neurosci. 2010;30(35):11678–11687. doi: 10.1523/JNEUROSCI.4665-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács R, Papageorgiou I, Heinemann U. Slice cultures as a model to study neurovascular coupling and blood brain barrier in vitro. Cardiovasc Psychiatry Neurol. 2011;2011:646958. doi: 10.1155/2011/646958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer C, Breier G, Risau W, Plate KH. Up-regulation of flk-1/vascular endothelial growth factor receptor 2 by its ligand in a cerebral slice culture system. Cancer Res. 1997;57(17):3852–3859. [PubMed] [Google Scholar]
- Kreutz F, Frozza RL, Breier AC, de Oliveira VA, Horn AP, Pettenuzzo LF, Netto CA, Salbego CG, Trindade VM. Amyloid-beta induced toxicity involves ganglioside expression and is sensitive to GM1 neuroprotective action. Neurochem Int. 2011;59(5):648–655. doi: 10.1016/j.neuint.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Kristensen BW, Noraberg J, Thiebaud P, Koudelka-Hep M, Zimmer J. Biocompatibility of silicon-based arrays of electrodes coupled to organotypic hippocampal brain slice cultures. Brain Res. 2001;896:1–17. doi: 10.1016/s0006-8993(00)03304-7. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail JH, Wolf MK. Postnatal development of the mouse dentate gyrus in organotypic cultures of the hippocampal formation. Am J Anat. 1973;137:47–65. doi: 10.1002/aja.1001370105. [DOI] [PubMed] [Google Scholar]
- Leggett C, McGehee DS, Mastrianni J, Yang W, Bai T, Brorson JR. Tunicamycin produces TDP-43 cytoplasmic inclusions in cultured brain organotypic slices. J Neurol Sci. 2012;317(1–2):66–73. doi: 10.1016/j.jns.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R, Dal Toso R, della Valle F, Skaper SD, Leon A. Update of the NGF saga. J Neurol Sci. 1995;130(2):119–127. doi: 10.1016/0022-510x(95)00007-o. [DOI] [PubMed] [Google Scholar]
- Loffredo Sampaolo C, Sampaolo G. Organotypic cultures of chick embryo lung; some histologic and histochemical aspects. Boll Soc Ital Biol Sper. 1956;32:797–801. [PubMed] [Google Scholar]
- Lossi L, Alasia S, Salio C, Merighi A. Cell death and proliferation in acute slices and organotypic cultures of mammalian CNS. Prog Neurobiol. 2009;88:221–245. doi: 10.1016/j.pneurobio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Lossi L, Gambino G, Salio C, Merighi A. Autophagy regulates the post-translational cleavage of BCL-2 and promotes neuronal survival. ScientificWorldJournal. 2010;10:924–929. doi: 10.1100/tsw.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MP, Pine J, Wright J, Tai Y-C. The neurochip: a new multielectrode device for stimulating and recording from cultured neurons. J Neurosci Methods. 1999;87:45–56. doi: 10.1016/s0165-0270(98)00156-3. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Humpel C. Beta-amyloid expression, release and extracellular deposition in aged rat brain slices. Mol Psychiatry. 2008;13:939–952. doi: 10.1038/sj.mp.4002072. [DOI] [PubMed] [Google Scholar]
- Marx U. Organotypic tissue culture for substance testing. J Biotechnol. 2010;148:1–2. doi: 10.1016/j.jbiotec.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Mazzone GL, Mladinic M, Nistri A. Excitotoxic cell death induces delayed proliferation of endogenous neuroprogenitor cells in organotypic slice cultures of the rat spinal cord. Cell Death Dis. 2013;4:e902. doi: 10.1038/cddis.2013.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewes A, Franke H, Singer D. Organotypic brain slice cultures of adult transgenic P301S mice–a model for tauopathy studies. PLoS One. 2012;7:e45017. doi: 10.1371/journal.pone.0045017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, O’Farrell FM, Reynell C, Hamilton NB, Hall CN, Attwell D. Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nat Protoc. 2014;9:323–336. doi: 10.1038/nprot.2014.019. [DOI] [PubMed] [Google Scholar]
- Monesi V. Differentiation of argyrophil and argentaffin cells in organotypic cultures of embryonic chick intestine. J Embryol Exp Morphol. 1960;8:302–313. [PubMed] [Google Scholar]
- Morin-Brureau M, Lebrun A, Rousset MC, Fagni L, Bockaert J, de Bock F, Lerner-Natoli M. Epileptiform activity induces vascular remodeling and zonula occludens 1 downregulation in organotypic hippocampal cultures: role of VEGF signaling pathways. J Neurosci. 2011;31:10677–10688. doi: 10.1523/JNEUROSCI.5692-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Brureau M, De Bock F, Lerner-Natoli M. Organotypic brain slices: a model to study the neurovascular unit micro-environment in epilepsies. Fluids Barriers CNS. 2013;10:11. doi: 10.1186/2045-8118-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Formentini L, Gerace E, Camaioni E, Pellegrini-Giampietro DE, Chiarugi A, Pellicciari R. Selective PARP-2 inhibitors increase apoptosis in hippocampal slices but protect cortical cells in models of post-ischaemic brain damage. Br J Pharmacol. 2009;157(5):854–862. doi: 10.1111/j.1476-5381.2009.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser KV, Schmidt-Kastner R, Hinterhuber H, Humpel C. Brain capillaries and cholinergic neurons persist in organotypic brain slices in the absence of blood flow. Eur J Neurosci. 2003;18:85–94. doi: 10.1046/j.1460-9568.2003.02728.x. [DOI] [PubMed] [Google Scholar]
- Moser KV, Reindl M, Blasig I, Humpel C. Brain capillary endothelial cells proliferate in response to NGF, express NGF receptors and secrete NGF after inflammation. Brain Res. 2004;1017:53–60. doi: 10.1016/j.brainres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Muller D, Buchs PA, Stoppini L. Time course of synaptic development in hippocampal organotypic cultures. Brain Res Dev Brain Res. 1993;71:93–100. doi: 10.1016/0165-3806(93)90109-n. [DOI] [PubMed] [Google Scholar]
- Muller D, Stoppini L, Wang C, Kiss JZ. A role for polysialylated neural cell adhesion molecule in lesion-induced sprouting in hippocampal organotypic cultures. Neuroscience. 1994;61(3):441–445. doi: 10.1016/0306-4522(94)90424-3. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Messer A. Gene transfer methods for CNS organotypic cultures: a comparison of three nonviral methods. Mol Ther. 2001;3:113–121. doi: 10.1006/mthe.2000.0235. [DOI] [PubMed] [Google Scholar]
- Nassif M, Hoppe J, Santin K, Frozza R, Zamin LL, Simão F, Horn AP, Salbego C. Beta-amyloid peptide toxicity in organotypic hippocampal slice culture involves Akt/PKB, GSK-3beta, and PTEN. Neurochem Int. 2007;50:229–235. doi: 10.1016/j.neuint.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Noraberg J. Organotypic brain slice cultures: an efficient and reliable method for neurotoxicological screening and mechanistic studies. Altern Lab Anim. 2004;32:329–337. doi: 10.1177/026119290403200403. [DOI] [PubMed] [Google Scholar]
- Olson L, Backlund EO, Freed W, Herrera-Marschitz M, Hoffer B, Seiger A, Strömberg I. Transplantation of monoamine-producing cell systems in oculo and intracranially: experiments in search of a treatment for Parkinson’s disease. Ann N Y Acad Sci. 1985;457:105–126. doi: 10.1111/j.1749-6632.1985.tb20801.x. [DOI] [PubMed] [Google Scholar]
- Ostergaard K, Schou JP, Zimmer J. Rat ventral mesencephalon grown as organotypic slice cultures and co-cultured with striatum, hippocampus, and cerebellum. Exp Brain Res. 1990;82:547–565. doi: 10.1007/BF00228796. [DOI] [PubMed] [Google Scholar]
- Ostergaard K, Schou JP, Gähwiler BH, Zimmer J. Tyrosine hydroxylase immunoreactive neurons in organotypic slice cultures of the rat striatum and neocortex. Exp Brain Res. 1991;83:357–365. doi: 10.1007/BF00231159. [DOI] [PubMed] [Google Scholar]
- Ostergaard K, Finsen B, Zimmer J. Organotypic slice cultures of the rat striatum: an immunocytochemical, histochemical and in situ hybridization study of somatostatin, neuropeptide Y, nicotinamide adenine dinucleotide phosphate-diaphorase, and enkephalin. Exp Brain Res. 1995;103:70–84. doi: 10.1007/BF00241966. [DOI] [PubMed] [Google Scholar]
- Peterson ER, Crain SM. Innervation in cultures of fetal rodent skeletal muscle by organotypic explants of spinal cord from different animals. Z Zellforsch Mikrosk Anat. 1970;106:1–21. doi: 10.1007/BF01027714. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Organotypic cortex-striatum-mesencephalon cultures: the nigrostriatal pathway. Neurosci Lett. 1996;209:177–180. doi: 10.1016/0304-3940(96)12644-6. [DOI] [PubMed] [Google Scholar]
- Prasanthi JR, Larson T, Schommer J, Ghribi O. Silencing GADD153/CHOP gene expression protects against Alzheimer’s disease-like pathology induced by 27-hydroxycholesterol in rabbit hippocampus. PLoS One. 2011;6:e26420. doi: 10.1371/journal.pone.0026420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Quadros A, Patel N, Crescentini R, Crawford F, Paris D, Mullan M. Increased TNFalpha production and Cox-2 activity in organotypic brain slice cultures from APPsw transgenic mice. Neurosci Lett. 2003;353:66–68. doi: 10.1016/j.neulet.2003.08.076. [DOI] [PubMed] [Google Scholar]
- Reinbold R. Organotypic differentiation of the eye of the chick embryo in vitro. C R Seances Soc Biol Fil. 1954;148:1493–1495. [PubMed] [Google Scholar]
- Renkawek K, Murray MR, Spatz M, Klatzo I. Distinctive histochemical characteristics of brain capillaries in organotypic culture. Exp Neurol. 1976;50:194–206. doi: 10.1016/0014-4886(76)90246-6. [DOI] [PubMed] [Google Scholar]
- Ridoux V, Robert J, Perricaudet M, Mallet J, Le Gal La.Salle G. Adenovirus mediated gene transfer in organotypic brain slices. Neurobiol Dis. 1995;2:49–54. doi: 10.1006/nbdi.1995.0005. [DOI] [PubMed] [Google Scholar]
- Robain O, Barbin G, Billette de Villemeur T, Jardin L, Jahchan T, Ben-Ari Y. Development of mossy fiber synapses in hippocampal slice culture. Brain Res Dev Brain Res. 1994;80(1–2):244–250. doi: 10.1016/0165-3806(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Robertson RT, Baratta J, Kageyama GH, Ha DH, Yu J. Specificity of attachment and neurite outgrowth of dissociated basal forebrain cholinergic neurons seeded on to organotypic slice cultures of forebrain. Neuroscience. 1997;80:741–752. doi: 10.1016/s0306-4522(97)00067-5. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Silverman WF, Krum JM. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95(12):7086–7091. doi: 10.1073/pnas.95.12.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23(35):11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz DS, Kaufmann WA, Saria A, Humpel C. Dopamine neurons in a simple GDNF-treated meso-striatal organotypic co-culture model. Exp Brain Res. 1999;127:270–278. doi: 10.1007/s002210050796. [DOI] [PubMed] [Google Scholar]
- Schrag M, Sharma S, Brown-Borg H, Ghribi O. Hippocampus of Ames dwarf mice is resistant to beta-amyloid-induced tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus. 2008;18(3):239–244. doi: 10.1002/hipo.20387. [DOI] [PubMed] [Google Scholar]
- Scuderi C, Valenza M, Stecca C, Esposito G, Carratù MR, Steardo L. Palmitoylethanolamide exerts neuroprotective effects in mixed neuroglial cultures and organotypic hippocampal slices via peroxisome proliferator-activated receptor-alpha. J Neuroinflammation. 2012;9:49. doi: 10.1186/1742-2094-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel F, Béchade C, Triller A. Nerve growth factor (NGF) induces motoneuron apoptosis in rat embryonic spinal cord in vitro. Eur J Neurosci. 1999;11:3904–3912. doi: 10.1046/j.1460-9568.1999.00814.x. [DOI] [PubMed] [Google Scholar]
- Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões Pires EN, Frozza RL, Hoppe JB, Menezes Bde M, Salbego CG. Berberine was neuroprotective against an in vitro model of brain ischemia: survival and apoptosis pathways involved. Brain Res. 2014;1557:26–33. doi: 10.1016/j.brainres.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Snyder-Keller A, Costantini LC, Graber DJ. Development of striatal patch/matrix organization in organotypic co-cultures of perinatal striatum, cortex an substantia nigra. Neuroscience. 2001;103:97–109. doi: 10.1016/s0306-4522(00)00535-2. [DOI] [PubMed] [Google Scholar]
- Stahl K, Skare Ø , Torp R. Organotypic cultures as a model of Parkinson s disease. A twist to an old model. ScientificWorldJournal. 2009;9:811–821. doi: 10.1100/tsw.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton CA, Stribbling SM, Tazzyman S, Hughes R, Brown NJ, Lewis CE. Current methods for assaying angiogenesis in vitro and in vivo. Int J Exp Pathol. 2004;85:33–48. doi: 10.1111/j.0959-9673.2004.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton CA, Reed MW, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol. 2009;90:195–221. doi: 10.1111/j.1365-2613.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. Lesion-induced neurite sprouting and synapse formation in hippocampal organotypic cultures. Neuroscience. 1993;57(4):985–994. doi: 10.1016/0306-4522(93)90043-f. [DOI] [PubMed] [Google Scholar]
- Suh EC, Jung YJ, Kim YA, Park EM, Lee KE. A beta 25–35 induces presynaptic changes in organotypic hippocampal slice cultures. Neurotoxicology. 2008;29:691–699. doi: 10.1016/j.neuro.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Sundstrom L, Morrison B, 3rd, Bradley M, Pringle A. Organotypic cultures as tools for functional screening in the CNS. Drug Discov Today. 2005;10:993–1000. doi: 10.1016/S1359-6446(05)03502-6. [DOI] [PubMed] [Google Scholar]
- Tardito D, Gennarelli M, Musazzi L, Gesuete R, Chiarini S, Barbiero VS, mRydel RE, Racagni G, Popoli M. Long-term soluble Abeta1-40 activates CaM kinase II in organotypic hippocampal cultures. Neurobiol Aging. 2007;28:1388–1395. doi: 10.1016/j.neurobiolaging.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Thomas A, Kim DS, Fields RL, Chin H, Gainer H. Quantitative analysis of gene expression in organotypic slice-explant cultures by particle-mediated gene transfer. J Neurosci Methods. 1998;84:181–191. doi: 10.1016/s0165-0270(98)00117-4. [DOI] [PubMed] [Google Scholar]
- Timpe JM, Wang CZ, Kim J, Johnson KM. Alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptor activation protects against phencyclidine-induced caspase-3 activity by activating voltage-gated calcium channels. J Neurosci Res. 2014;92(12):1785–1791. doi: 10.1002/jnr.23446. [DOI] [PubMed] [Google Scholar]
- Ullrich C, Humpel C. The pro-apoptotic substance thapsigargin selectively stimulates re-growth of brain capillaries. Curr Neurovasc Res. 2009a;6:171–180. doi: 10.2174/156720209788970063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C, Humpel C. Rotenone induces cell death of cholinergic neurons in an organotypic co-culture brain slice model. Neurochem Res. 2009b;34:2147–2153. doi: 10.1007/s11064-009-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C, Humpel C. Mini-ruby is rapidly taken up by neurons and astrocytes in organotypic brain slices. Neurochem Res. 2011;36:1817–1823. doi: 10.1007/s11064-011-0499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C, Daschil N, Humpel C. Organotypic vibrosections: novel whole sagittal brain cultures. J Neurosci Methods. 2011;201:131–141. doi: 10.1016/j.jneumeth.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorov IV, Lyjin AA, Aleksandrova OP. A modified roller method for organotypic brain cultures: free-floating slices of postnatal rat hippocampus. Brain Res Brain Res Protoc. 2001;7:30–37. doi: 10.1016/s1385-299x(00)00059-3. [DOI] [PubMed] [Google Scholar]
- Weis C, Marksteiner J, Humpel C. Nerve growth factor and glial cell line-derived neurotrophic factor restore the cholinergic phenotype in organotypic brain slices of the basal nucleus of Meynert. Neuroscience. 2001;102:129–138. doi: 10.1016/s0306-4522(00)00452-8. [DOI] [PubMed] [Google Scholar]
- Whetsell WO, Jr, Mytilineou C, Shen J, Yahr MD. The development of the dog nigrostriatal system in organotypic culture. J Neural Transm. 1981;52:149–161. doi: 10.1007/BF01249600. [DOI] [PubMed] [Google Scholar]
- Wilhelmi E, Schöder UH, Benabdallah A, Sieg F, Breder J, Reymann KG. Organotypic brain-slice cultures from adult rats: approaches for a prolonged culture time. Altern Lab Anim. 2002;30:275–283. doi: 10.1177/026119290203000304. [DOI] [PubMed] [Google Scholar]
- Wolf MK. Anatomy of cultured mouse cerebellum. II. Organotypic migration of granule cells demonstrated by silver impregnation of normal and mutant cultures. J Comp Neurol. 1970;140:281–298. doi: 10.1002/cne.901400304. [DOI] [PubMed] [Google Scholar]
- Wolff JR, Goerz C, Bär T, Güldner FH. Common morphogenetic aspects of various organotypic microvascular patterns. Microvasc Res. 1975;10:373–395. doi: 10.1016/0026-2862(75)90040-0. [DOI] [PubMed] [Google Scholar]
- Woodhams PL, Atkinson DJ. Regeneration of entorhino-dentate projections in organotypic slice cultures: mode of axonal regrowth and effects of growth factors. Exp Neurol. 1996;140:68–78. doi: 10.1006/exnr.1996.0116. [DOI] [PubMed] [Google Scholar]
- Wray S, Castel M, Gainer H. Characterization of the suprachiasmatic nucleus in organotypic slice explant cultures. Microsc Res Tech. 1993;25:46–60. doi: 10.1002/jemt.1070250108. [DOI] [PubMed] [Google Scholar]
- Xu B, Wang F, Wu SW, Deng Y, Liu W, Feng S, Yang TY, Xu ZF. Alpha-synuclein is involved in manganese-induced ER stress via PERK signal pathway in organotypic brain slice cultures. Mol Neurobiol. 2014;49(1):399–412. doi: 10.1007/s12035-013-8527-2. [DOI] [PubMed] [Google Scholar]
- Zassler B, Weis C, Humpel C. Tumor necrosis factor-alpha triggers cell death of sensitized potassium chloride-stimulated cholinergic neurons. Mol Brain Res. 2003;113:78–85. doi: 10.1016/s0169-328x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Zassler B, Dechant G, Humpel C. Urea enhances the nerve growth factor-induced neuroprotective effect on cholinergic neurons in organotypic rat brain slices. Neuroscience. 2005;130:317–323. doi: 10.1016/j.neuroscience.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Zehendner CM, Wedler HE, Luhmann HJ. A novel in vitro model to study pericytes in the neurovascular unit of the developing cortex. PLoS One. 2013;8:e81637. doi: 10.1371/journal.pone.0081637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhabotinski YM, Chumasov EI, Chubakov AR, Konovalov HV. Development of synaptic structure and function in organotypic cultures of the rat hippocampus. Neuroscience. 1979;4:913–920. doi: 10.1016/0306-4522(79)90175-1. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Gähwiler BH. Cellular and connective organization of slice cultures of the rat hippocampus and fascia dentata. J Comp Neurol. 1984;228:432–446. doi: 10.1002/cne.902280310. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Gähwiler BH. Growth of hippocampal mossy fibers: a lesion and coculture study of organotypic slice cultures. J Comp Neurol. 1987;264:1–13. doi: 10.1002/cne.902640102. [DOI] [PubMed] [Google Scholar]