Abstract
Blue laser imaging is a new system for image-enhanced endoscopy using laser light. Blue laser imaging utilizes two monochromatic lasers (410 and 450 nm) instead of xenon light. A 410 nm laser visualizes vascular microarchitecture, similar to narrow band imaging, and a 450 nm laser provides white light by excitation. According to three recently published reports, the diagnostic ability of polyp characterization using blue laser imaging compares favorably with narrow band imaging. No published data are available to date regarding polyp detection with blue laser imaging. However, blue laser imaging has the possibility to increase the detection of colorectal polyps by depicting brighter and clearer endoscopic images, even at a distant view, compared with first-generation image-enhanced endoscopy. A clinical trial to compare the detection between blue laser imaging and xenon light is warranted.
Keywords: image enhanced endoscopy, blue laser imaging, magnification colonoscopy, colorectal neoplasm, colon polyp, colorectal cancer
Introduction
Gastrointestinal cancers are a leading cause of death around the world. The introduction of gastrointestinal endoscopy has revolutionized the way we detect and treat gastrointestinal cancers, particularly in patients with early stage disease. Since the early 1960s, white light endoscopy has been widely used for the detection and characterization of colorectal polyps [Wolff and Shinya, 1971, 1973]. In the 1990s, magnification endoscopy using the dye spray technique attracted colonoscopists’ attention [Kudo, 1993; Axelrad et al. 1996; Togashi et al. 1999]. In the 2000s, however, it was replaced by hardware-based image-enhanced endoscopy (IEE) [Machida et al. 2004] because the dye-based technique is complicated and inconvenient, whereas hardware-based techniques enable the colonoscopist to easily obtain an enhanced image by simply pushing a button. The original concept of IEE also includes dye-based techniques [Kaltenbach et al. 2008b], but hardware-based techniques are most commonly used. IEE can be used throughout the gastrointestinal tract to improve the discrimination of the type of lesions and their malignant potential. Recently, hardware-based IEE has played an important role in not only the detection, but also in the characterization of colorectal polyps.
First-generation hardware-based IEE systems include narrow band imaging (NBI) [Machida et al. 2004], flexible spectral-imaging color enhancement (FICE) [Togashi et al. 2009] and i-SCAN [Hoffman et al. 2010a], all of which were initially released in the early 2000s. These systems do not involve injection of any dye and rely solely on hardware-based technology. However, first-generation IEEs have critical drawbacks, e.g. dark images at a distant view and low-resolution images. Thus, first-generation NBI does not demonstrate a higher adenoma detection rate than white light endoscopy in large clinical trials [Rex and Helbig, 2007; Kaltenbach et al. 2008a; Uraoka et al. 2008; Adler et al. 2009]. As for other hardware-based IEE techniques, one large clinical trial failed to demonstrate any objective advantage of the FICE technique over conventional high-resolution endoscopy in terms of improved adenoma detection rates [Aminalai et al. 2010], although a relatively small clinical trial (one arm: 100) in a single center showed a significantly higher adenoma detection rate in high-definition colonoscopy with i-SCAN, compared with standard resolution endoscopy [Hoffman et al. 2010b].
In 2013, blue laser imaging (BLI) was released by the Fujifilm Corporation. BLI has successfully achieved a bright and clear image even at a distant view [Osawa and Yamamoto, 2014]. These capabilities may lead to improved detection of polyps and more accurate diagnoses. The vast majority of gastroenterologists are not familiar with BLI due to general lack of availability of this equipment. The Japanese government approved reimbursement for BLI through the national health insurance system. Since introducing BLI to our medical center in 2013, we have used BLI for routine colonoscopy. In this review article, we describe the principles of BLI, tips for the use of BLI, polyp detection and polyp characterization using BLI.
Principles of BLI
First, we review the principles of NBI in generating endoscopic images similar to BLI. NBI uses optical filters to change the wavelength of the transmitted light, which in turn targets the microvessels of the mucosa. NBI utilizes two kinds of light: blue light (wavelength of 415 ± 30 nm) and green light (wavelength 540 ± 30 nm). The blue light penetrates the superficial epithelium and visualizes microstructures in the mucosa as well as the submucosa. This selective narrowing of the blue and green light, and the omission of red light, increases the fidelity of the images and improves visualization of microvessels as well as surface patterns resembling pit patterns.
The word ‘laser’ is an acronym for ‘light amplification by stimulated emission of radiation’. Laser light is characterized by spatial and temporal coherence, resulting in clearer images for vessel/surface microstructure of the mucosal surface through ‘further narrowing’. BLI uses two monochromatic lasers (410 ± 10 nm and 450 ± 10 nm) instead of xenon light to obtain image enhanced endoscopy, as shown in Figure 1. The 410 ± 10 nm laser is vital to visualize the vascular microarchitecture on the mucosal surface. The 450 ± 10 nm laser provides white light by excitation. This laser stimulates the white light phosphor in the tip of the endoscope to produce broadband white light, providing the standard view obtained with conventional xenon light sources. The brightness of the white light is controlled by the laser output power. In addition, longer wavelength light such as a 450 ± 10nm laser is less absorbed by small vessels and reaches the deeper layers in the tissue, thus depicting larger blood vessels in the deeper layers.
Figure 1.
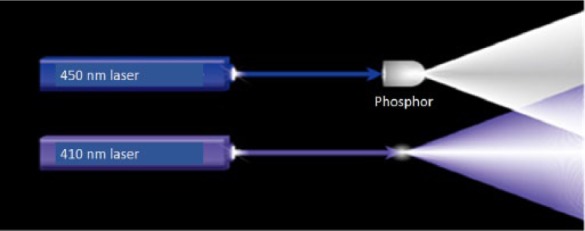
Laser illumination using two lasers and white light phosphor.
A 410 nm laser looks blue light. A 450 nm laser stimulates the white light phosphor in the tip of the scope to produce the broadband white light.
Tips for the use of BLI
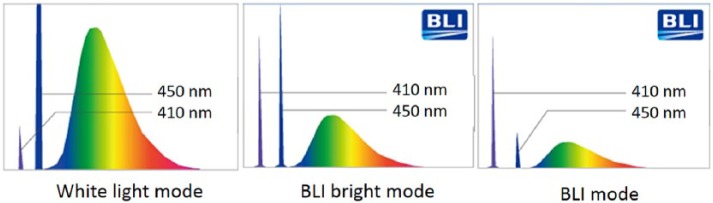
The BLI system utilizes three modes: BLI mode, BLI-bright mode and white light mode. To alter the mode, the colonoscopist simply selects the desired mode on the operating portion of the colonoscope with a button. Figure 2 shows the light spectrum in each mode. The intensity balance of the monochromatic lasers differs in each mode, enabling high contrast images of blood vessels over a wide range from distant images to close-up images, even with magnification.
Figure 2.
Light spectrum in each mode.
Note: white light mode contains 410 nm laser light.
1. BLI mode
The BLI laser power ratio is raised to maximize the contrast of the microvascular pattern seen. In this mode, the vascular pattern as well as the surface pattern can be most clearly visualized on the display. The BLI mode is dedicated to close-up views or magnified views.
2. BLI-bright mode
A brighter view is accomplished using the BLI high-contrast image by controlling the power ratio of the BLI laser and the white light laser. In contrast to conventional IEE, both the vascular pattern and the surface pattern are enhanced while maintaining the brightness, even at a distant view. The BLI-bright mode may be suitable for detection, whereas the BLI mode is suitable for more detailed magnified observations.
3. White light mode
Different from conventional white light generated by a xenon light source, white light mode using the BLI system uses a 410 ± 10 nm laser to enhance vascular images. Thus, the vascular microstructure is more clearly depicted using the white light mode compared with that obtained using xenon light.
Recommended approach
It is important to match the mode and application properly. At the beginning, perform an overall observation of the lesion using the white light mode with normal magnification. As mentioned above, white light mode may provide a clearer image than that using xenon light. Next, the BLI-bright mode without magnification is applied. The extent of the lesion should be evaluated using this mode. Then, use the BLI-bright mode to identify the region with advanced histological atypia based on changes in color tone and superficial structure. Finally, apply the BLI mode to obtain magnified images of the surface pattern and vascular pattern by focusing on the region identified. An example of such a region is shown in Figure 3 (adenoma) and Figure 4 (nonadenoma).
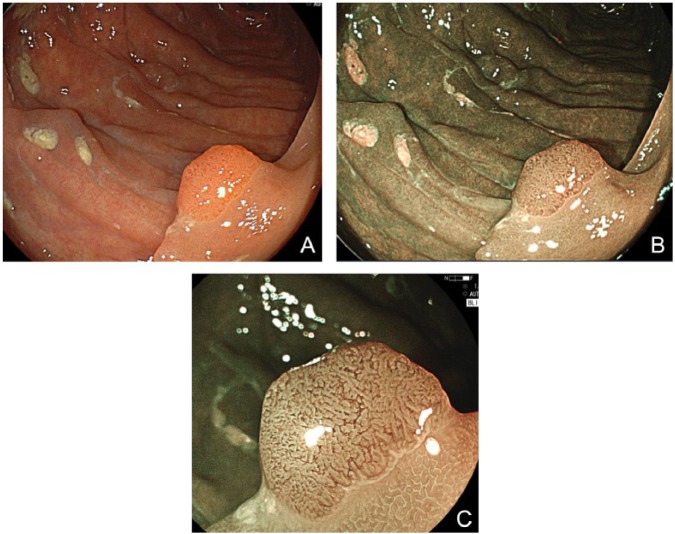
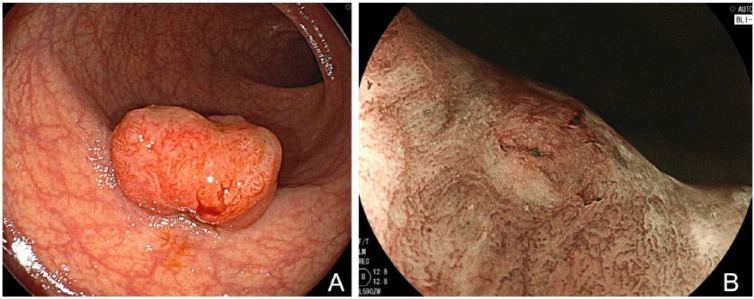
Figure 3.
(A) White-light mode. A sessile adenoma measuring 6 mm in size is clearly identified, but its superficial structure is not depicted clearly. (B) BLI-bright mode. Surrounding normal mucosa is depicted clearly and brightly even in a distant view. The vessel pattern of the polyp as well as the surface pattern is clearly depicted without magnification. (C) BLI mode. Magnified view shows a regular structure, suggesting a low-grade adenoma.
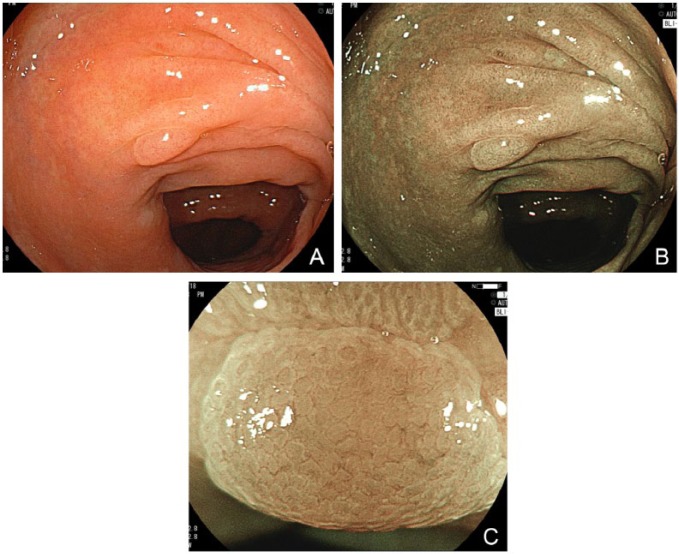
Figure 4.
(A) White-light mode. A hyperplastic polyp measuring 6 mm shows a pale appearance. (B) BLI-bright mode. Demarcation line of the polyp is clearly seen in a distant view. (C) BLI mode. Magnified view does not show any distinct structure, but only small vessels are observed on the polyp surface, suggesting a non-neoplastic polyp.
Polyp detection using BLI
Similar to previous clinical trials investigating the detectability of NBI, a recent clinical trial using the latest generation NBI colonoscopes (designated the 190 series or Exera III) has failed to demonstrate an increased detection rate of colon polyps [Rex et al. 2015]. No data on the detection of polyps using BLI is available to date. It has been reported recently that BLI-bright mode improves the visibility of colorectal polyps [Yoshida et al. 2015], suggesting the possibility of a high yield using BLI-blight mode. Also, in the latest report making a direct comparison between BLI and NBI, only BLI-bright maintained adequate brightness and contrast up to 40 mm with significantly longer observable distances compared with NBI and BLI modes, although the new NBI system was not used [Kaneko et al. 2014]. In our experience, BLI-bright mode, as well as white light mode, allows a bright and clear image to be obtained, even at a distant view. Therefore, BLI may increase the detection rate of colorectal polyps in a future clinical trial. At present, a multicenter randomized clinical trial to compare the detection rate of BLI-blight mode and xenon white light is ongoing in Japan. Final results should be available in the spring of 2016.
Polyp characterization using BLI
So far, only two reports of polyp characterization using BLI have been published by the same group [Yoshida et al. 2014a, 2014b]. Sufficient evidence is still lacking in this field. According to these reports, the diagnostic accuracy of BLI with no magnification for differentiating between neoplastic and non-neoplastic polyps <10 mm in diameter is 95.2%, which is greater than that using white light (83.2%). In addition, the diagnostic accuracy of BLI magnification in the NBI classification was 74.0 % (77/104), similar to that of NBI magnification (77.8%). The authors concluded that the diagnostic effectiveness of this method is similar to that of NBI magnification.
Indeed, our reading test on an image library of 80 small (⩽5 mm) colorectal polyps demonstrated similar results regarding the diagnostic ability of BLI with magnification. High diagnostic sensitivity (⩽96% by all three raters) was achieved using BLI mode [Togashi et al. 2014a]. Furthermore, the sensitivity of endoscopic diagnosis using white light images (84–94%) compared favorably with that of BLI, implying that white light mode also contains a 410 ± 10 nm laser to enhance vascular imaging. This finding is not consistent with previously published data. Further research is required to validate these observations.
BLI facilitates discrimination of adenoma from invasive cancer. BLI magnification by laser source could predict invasion depth of colorectal neoplasms [Yoshida et al. 2014a]. In our experience [Togashi et al. 2014b], clinical impact of this method was equivalent to that of NBI magnification. So far only one report is available in this field. Figure 5 represents a typical mucosal lesion, whereas Figure 6 represents deeply invasive cancer.
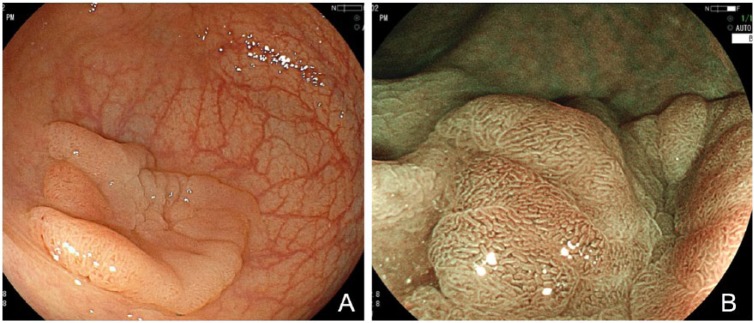
Figure 5.
(A) White-light mode. Laterally spreading tumor measuring 28 mm in maximum size is clearly identified in a plain image. (B) BLI mode. Magnified view shows regularly arranged structure, suggesting a low-grade tubular adenoma.
Figure 6.
(A). White-light mode. Protruding tumor measuring 18 mm shows an irregular surface in a plain image. (B) BLI mode. Magnified view shows an irregular vascular pattern and an avascular area can be seen partially. Surface pattern also shows irregularity. These finding definitely indicates substantially invasive cancer.
Conclusion
A new hardware-based IEE system, the BLI system, provides endoscopic images similar to NBI, since BLI uses a 410 ± 10 nm monochromatic laser to enhance the vascular images. The diagnostic ability of BLI is almost equivalent to that of NBI, although conclusive evidence is still lacking. Based on our preliminary data, the white light generated using the BLI system may allow more accurate discrimination of adenomas from nonadenomatous lesions compared with conventional white light. Further studies are required to validate these observations.
Acknowledgments
We appreciate the dedicated support from Jinko Kobayashi, Sumie Suzuki and Sanae Tanaka at Aizu Medical Center Fukushima Medical University.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Kazutomo Togashi, Aizu Medical Center Fukushima Medical University – Coloproctology, Aizuwakamatsu, Fukushima, Japan.
Daiki Nemoto, Aizu Medical Center Fukushima Medical University – Coloproctology, Aizuwakamatsu, Fukushima, Japan.
Kenichi Utano, Aizu Medical Center Fukushima Medical University – Coloproctology, Aizuwakamatsu, Fukushima, Japan.
Noriyuki Isohata, Aizu Medical Center Fukushima Medical University – Coloproctology, Aizuwakamatsu, Fukushima, Japan.
Kensuke Kumamoto, Aizu Medical Center Fukushima Medical University – Coloproctology, Aizuwakamatsu, Fukushima, Japan.
Shungo Endo, Aizu Medical Center Fukushima Medical University – Coloproctology, Aizuwakamatsu, Fukushima, Japan.
Alan K. Lefor, Jichi Medical University – Surgery, Shimotsuke, Tochigi, Japan
References
- Adler A., Aschenbeck J., Yenerim T., Mayr M., Aminalai A., Drossel R., et al. (2009) Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: a prospective randomized trial. Gastroenterology 136: 410–416 e411; quiz 715. [DOI] [PubMed] [Google Scholar]
- Aminalai A., Rosch T., Aschenbeck J., Mayr M., Drossel R., Schroder A., et al. (2010) Live image processing does not increase adenoma detection rate during colonoscopy: a randomized comparison between FICE and conventional imaging (Berlin Colonoscopy Project 5, BECOP-5). Am J Gastroenterol 105: 2383–2388. [DOI] [PubMed] [Google Scholar]
- Axelrad A., Fleischer D., Geller A., Nguyen C., Lewis J., Al-Kawas F., et al. (1996) High-resolution chromoendoscopy for the diagnosis of diminutive colon polyps: implications for colon cancer screening. Gastroenterology 110: 1253–1258. [DOI] [PubMed] [Google Scholar]
- Hoffman A., Kagel C., Goetz M., Tresch A., Mudter J., Biesterfeld S., et al. (2010a) Recognition and characterization of small colonic neoplasia with high-definition colonoscopy Using i-SCAN is as precise as chromoendoscopy. Dig Liver Dis 42: 45–50. [DOI] [PubMed] [Google Scholar]
- Hoffman A., Sar F., Goetz M., Tresch A., Mudter J., Biesterfeld S., et al. (2010b) High definition colonoscopy combined with i-SCAN is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy 42: 827–833. [DOI] [PubMed] [Google Scholar]
- Kaltenbach T., Friedland S., Soetikno R. (2008a) A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut 57: 1406–1412. [DOI] [PubMed] [Google Scholar]
- Kaltenbach T., Sano Y., Friedland S., Soetikno R. and American Gastroenterological Association (2008b) American Gastroenterological Association (AGA) Institute technology assessment on image-enhanced endoscopy. Gastroenterology 134: 327–340. [DOI] [PubMed] [Google Scholar]
- Kaneko K., Oono Y., Yano T., Ikematsu H., Odagaki T., Yoda Y., et al. (2014) Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI). Endosc Int Open 2: E212–E219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo S. (1993) Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 25: 455–461. [DOI] [PubMed] [Google Scholar]
- Machida H., Sano Y., Hamamoto Y., Muto M., Kozu T., Tajiri H., et al. (2004) Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy 36: 1094–1098. [DOI] [PubMed] [Google Scholar]
- Osawa H., Yamamoto H. (2014) Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig Endosc 26(Suppl. 1): 105–115. [DOI] [PubMed] [Google Scholar]
- Rex D., Clodfelter R., Rahmani F., Fatima H., James-Stevenson T., Tang J.et al. (2015) Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial. Gastrointest Endosc. 5 May 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Rex D., Helbig C. (2007) High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology 133: 42–47. [DOI] [PubMed] [Google Scholar]
- Togashi K., Kashida H., Yamamoto H., Nemoto D., Hewett D. (2014a) Validation of narrow band imaging nice classification system for endoscopic diagnosis of small colorectal polyps using blue laser imaging. Gastrointest Endosc 79: AB462 [Google Scholar]
- Togashi K., Konishi F., Ishizuka T., Sato T., Senba S., Kanazawa K. (1999) Efficacy of magnifying endoscopy in the differential diagnosis of neoplastic and non-neoplastic polyps of the large bowel. Dis Colon Rectum 42: 1602–1608. [DOI] [PubMed] [Google Scholar]
- Togashi K., Nemoto D., Isohata N., Otani T., Utano K., Endo S., et al. (2014b) Use of blue laser imaging for identification of colorectal cancer with deep submucosal invasion. Gastrointest Endosc 79: AB476 [Google Scholar]
- Togashi K., Osawa H., Koinuma K., Hayashi Y., Miyata T., Sunada K., et al. (2009) A Comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc 69: 734–741. [DOI] [PubMed] [Google Scholar]
- Uraoka T., Saito Y., Matsuda T., Sano Y., Ikehara H., Mashimo Y., et al. (2008) Detectability of colorectal neoplastic lesions using a narrow-band imaging system: a pilot study. J Gastroenterol Hepatol 23: 1810–1815. [DOI] [PubMed] [Google Scholar]
- Wolff W., Shinya H. (1971) Colonofiberoscopy. JAMA 217: 1509–1512. [PubMed] [Google Scholar]
- Wolff W., Shinya H. (1973) Polypectomy via the fiberoptic colonoscope. Removal of neoplasms beyond reach of the sigmoidoscope. N Engl J Med 288: 329–332. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Hisabe T., Hirose R., Ogiso K., Inada Y., Konishi H., et al. (2015) Improvement in the visibility of colorectal polyps by using blue laser imaging. Gastrointest Endosc. 4 April 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yoshida N., Hisabe T., Inada Y., Kugai M., Yagi N., Hirai F., et al. (2014a) The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol 49: 73–80. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Yagi N., Inada Y., Kugai M., Okayama T., Kamada K., et al. (2014b) Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc 26: 250–258. [DOI] [PubMed] [Google Scholar]