Abstract
Background:
Management guidelines from the American Association for the Study of Liver Diseases/American College of Gastroenterology/American Gastroenterology Association published in 2012 for nonalcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) recommend weight loss, vitamin E and pioglitazone as effective therapies for the treatment of biopsy-confirmed NASH. However, little is known about how physicians in the US diagnose NASH or whether published guidelines are being followed.
Methods:
We assessed current diagnostic and treatment patterns of the management of NAFLD and NASH among academic gastroenterologists and hepatologists in the US using a standardized survey developed to collect information regarding respondents’ practice environments, diagnostic techniques, and medication usage in patients with NAFLD/NASH.
Results:
We invited 482 gastroenterologists and hepatologists, predominantly from academic centers, of whom 163 completed the survey. Only 24% of providers routinely perform liver biopsy, predominantly among patients with elevated serum aminotransferases. Vitamin E is prescribed regularly by 70% while only 14% routinely prescribe pioglitazone. Despite recommendations to the contrary, ~25% prescribe pioglitazone or vitamin E without biopsy confirmation of NASH. Metformin is used as frequently as pioglitazone despite its proven lack of efficacy in NASH. Overall, 40–73% adhere to published guidelines, depending on the specific question. There was no significant difference seen in adherence to guidelines between gastroenterologists and hepatologists.
Conclusion:
This survey suggests that clinical practice patterns among gastroenterologists and hepatologists for the management of NASH frequently diverge from published practice guidelines. Although liver biopsy remains the gold standard to diagnose NASH, less than 25% of respondents routinely require it to make the diagnosis of NASH. We conclude that NASH is underdiagnosed in gastroenterology and hepatology practices, highlighting the need to refine noninvasive diagnostic tools.
Keywords: liver biopsy, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, pioglitazone, practice guidelines, practice patterns, diagnosis, treatment
Introduction
The prevalence of nonalcoholic fatty liver disease (NAFLD) is estimated to be approximately 33% of the general population and >40% among middle aged Americans, while the prevalence of nonalcoholic steatohepatitis (NASH) approaches 3–5% in the general population and up to 12% in older patients and in diabetics [Vernon et al. 2011; Williams et al. 2011]. The most recent diagnosis and management guidelines for NAFLD and NASH were published in 2012 [Chalasani et al. 2012].
A survey of practice patterns regarding NAFLD in France was also published in 2012, indicating growing awareness of the condition but also some discrepancies between guidelines and actual practice [Ratziu et al. 2012]. The French survey, which exclusively polled gastroenterologists and hepatologists, found an over-reliance on elevated aminotransferases to suspect NASH and prompt liver biopsy, rather than the risk factors of obesity, diabetes and advanced age in the setting of steatosis. This is despite the fact that the majority of patients with NAFLD have normal alanine aminotransferase (ALT) values [Ratziu et al. 2010; Fracanzani et al. 2008; Mofrad et al. 2003]. In contrast, 90% of providers were comfortable using noninvasive fibrosis markers and 73% of respondents followed these patients themselves rather than deferring to a general practitioner [Ratziu et al. 2012]. Respondents prescribed pharmacologic therapy for NAFLD/NASH in only about 20% of their patients [Ratziu et al. 2012].
There are currently no existing data about the practices of gastroenterologists and hepatologists in the US in regard to NAFLD/NASH. Furthermore, little is known about the adherence to current guidelines in clinical practice and whether there are differences between gastroenterologists and hepatologists in the implementation of these guidelines.
Materials and methods
We sought to assess the current diagnostic and treatment patterns of NAFLD and NASH among gastroenterologists and hepatologists in the US by employing a 23 question survey. We collected information regarding respondents’ practice environments, the use of invasive and noninvasive diagnostic techniques, the role of diet, exercise and selected medications in the treatment of NAFLD and NASH, and the frequency of referral for bariatric surgery. The survey is provided in the supplement.
Using the American Association for the Study of Liver Diseases (AASLD) membership directory and publicly available email addresses, we compiled a database of 482 physicians at US academic medical centers with gastroenterology and hepatology training programs. The goal was to select a group of physicians with the most expertise in liver disease. Using a personalized survey link sent via email to solicit anonymous responses, data were collected from 18 March 2014 through 14 April 2014. One reminder email was sent halfway through this period to those who had not yet responded. A link to the survey was also published on the AASLD member-to-member survey service on 18 October 2013 and responses were collected through 14 April 2014. Safeguards were in place to assure that respondents only entered data once.
We compared the responses of providers with general gastroenterology–hepatology practices with those who strictly specialize in hepatology, and compared overall patterns with the established guidelines from AASLD, the American College of Gastroenterology (ACG) and the American Gastroenterology Association (AGA). For the purposes of this manuscript, the former group are referred to as ‘gastroenterologists’ and the latter as ‘hepatologists.’ Fisher’s exact test was used for comparisons between groups, with a two-tailed p value < 0.05 deemed significant.
Results
A total of 135 of 482 surveyed physicians responded to the email link (a response rate of 28%) and an additional 28 responded via the AASLD website link. All responses were anonymous. No distinction was made in the analysis between those who responded via the web link or the email invitation.
Expertise of those responding to the survey
Table 1 provides the details of respondents’ practices and exposure to NAFLD/NASH patients. The vast majority of providers are in academic medical practices, with approximately 57% exclusively seeing hepatology patients. 62% of respondents report seeing between 5–20 patients with NAFLD per month and 35% see more than 20 per month. Among these, 51.9% report seeing <5 biopsy confirmed NASH patients/month. The majority ( 31.5% ) see 5–10 and 10.5% and 6.2% see 10–20 and >20 per month. Hepatologists are more likely to see biopsy confirmed NASH patients each month compared with general gastroenterologists (22% of hepatologists see more than 10 such patients versus 11% of gastroenterologists), but this difference was not statistically significant.
Table 1.
Surveyed providers’ practice information.
| Member of AASLD | 93% |
| Staff/attending physician | 96% |
| Academic, gastroenterology and hepatology | 39% |
| Academic, hepatology Only | 57% |
| Private practice, gastroenterology and hepatology | 2.5% |
| Private practice, hepatology only | 1.2% |
| Number of patients with NAFLD seen monthly | |
| <5 | 3% |
| 5–10 | 25% |
| 10–20 | 37% |
| >20 | 35% |
| Number of patients with biopsy confirmed NASH seen monthly | |
| <5 | 52% |
| 5–10 | 31% |
| 10–20 | 11% |
| >20 | 6% |
AALSD, American Association for the Study of Liver Diseases; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Use of liver biopsy
Overall, only 24% of respondents routinely perform liver biopsy in patients with presumed NAFLD, but a statistically significantly higher proportion of hepatologists do so compared with gastroenterologists (31% versus 15%, p < 0.05). The majority of providers (57%), hepatologists and gastroenterologists alike, rely on elevated serum liver enzymes to determine need for liver biopsy (Figure 1).
Figure 1.
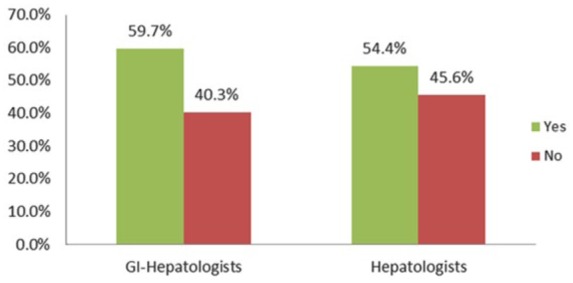
Are elevated liver enzymes required for liver biopsy?
GI, gastrointestinal.
Patterns of treatment for NASH
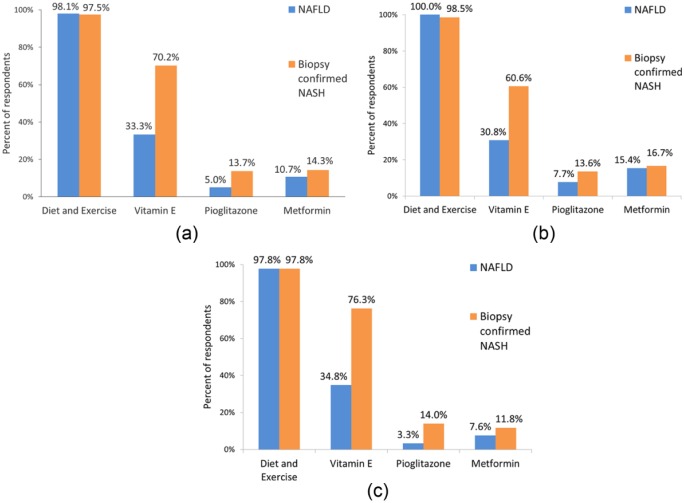
There is near universal consensus among providers in recommending diet and exercise to patients with biopsy confirmed NASH (98%). However, data were not collected on how many provided a structured plan for lifestyle modification. With respect to pharmacologic therapies, vitamin E and piogli-tazone are used by 70% and 14% of respondents, respectively. Hepatologists are significantly more likely to prescribe vitamin E than gastroenterologists (76% versus 61%, p < 0.04%), but similar proportions of both groups use pioglitazone (12% versus 17%). Somewhat surprisingly, metformin is still being used as a primary therapy for NASH by 14% of physicians surveyed despite guideline recommendations to the contrary (Figure 2).
Figure 2.
Treatment recommendations: (a) all respondents; (b) gastroenterologists; and (c) hepatologists.
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
The risks of pharmacologic treatment and current guidelines suggest that currently available drugs should be limited to those with biopsy confirmed NASH. However, most of those surveyed do not regularly perform liver biopsy to guide therapy. Of hepatologists and gastroenterologists, only 47% and 42%, respectively, always require biopsy confirmed NASH prior to starting vitamin E. In contrast, prescribers of pioglitazone are more likely to confirm NASH histologically prior to initiating therapy (74% of hepatologists and 60% of gastroenterologists).
Vitamin E is the most commonly prescribed medication for NASH across all respondents. Among those who prescribe vitamin E, significant proportions do so in patients without histologic confirmation of NASH. Whereas some appropriately exclude patients with diabetes (34%), cirrhosis 20.7% or both (9%) for whom there is less evidence and potentially increased risk, 45% prescribe vitamin E to all patients. In those not prescribing vitamin E, 70% attribute this to a concern for risks and 24% feel it is ineffective. There appears to be broad awareness of the potential side effects of vitamin E. Respondents noted they discussed the risk of cardiovascular events (78.5%), mortality (51.9%), prostate cancer (48.1%), stroke (42.2%) and bleeding (31.9%) with patients prior to starting vitamin E. Based on this, comments of individual respondents noted that because of a concern for potential risks, patients deemed to be high risk for cardiovascular disease (CVD), stroke and prostate cancer (age, male sex) were not offered vitamin E.
The use of pioglitazone to treat patients with NASH is more limited across respondents. When it is prescribed, 67% overall limit its use to those with biopsy confirmed NASH. The most common subgroups excluded are obese and cirrhotic patients (30% and 32%); interestingly, 9% exclude patients with diabetes. 46% of prescribers do not exclude any particular group of patients from pioglitazone treatment. There appears to be good awareness of potential side effects and in those who avoid its use, it is due to these potential risks. Interestingly, when pioglitazone is prescribed, it is given for a limited amount of time in 60% and not as a chronic therapy.
Use of bariatric surgery in clinical practice
A quarter of providers do not refer patients with NAFLD to bariatric surgery. Of those that do, only 24% have referred more than 5 patients for this procedure in the last year (Figure 3). Gastroenterologists are less likely than hepatologists to refer patients with NAFLD or NASH for consideration of bariatric surgery (65% referred at least one patient in the preceding 12 months, compared with 83% of hepatologists, p = 0.014%). Approximately half of all surveyed providers feel comfortable recommending it to patients with compensated NASH cirrhosis (55%).
Figure 3.
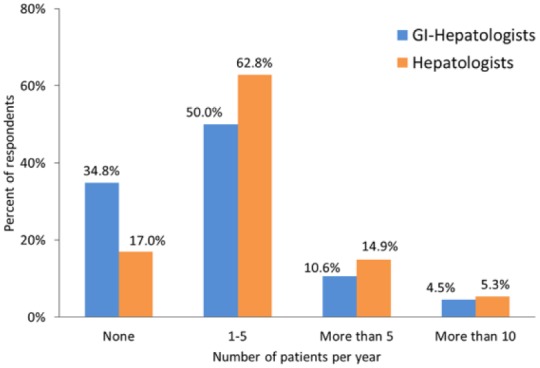
Annual number of patients with NAFLD or NASH referred for bariatric surgery.
GI, gastrointestinal; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
A summarized comparison of responses by gastroenterologists and hepatologists to those survey questions with guideline-based answers can be found in Table 2.
Table 2.
Comparison of responses by gastroenterologists and hepatologists to those survey questions with guideline-based answers.
| Selected guideline related questions | Guideline based answer | GI-hepatologists adherent to guideline | Hepatologists adherent to guideline | p value |
|---|---|---|---|---|
| Are elevated liver enzymes required for you to consider liver biopsy in patients with fatty liver identified on imaging? | No | 27/67 (40%) | 41/90 (46%) | 0.52 |
| If you use vitamin E, do you require biopsy confirmed NASH before starting therapy? | Yes | 23/55 (42%) | 42/90 (47%) | 0.61 |
| If you use pioglitazone, do you require biopsy confirmed NASH before starting therapy? | Yes | 22/37 (59%) | 36/49 (73%) | 0.25 |
| Are there are subgroups of patients to whom you do not prescribe vitamin E? | Yes – diabetics and cirrhotics | 33/56 (59%) | 40/77 (52%) | 0.48 |
GI, gastrointestinal; NASH, nonalcoholic steatohepatitis.
Discussion
While the prevalence and diagnostic features of the spectrum of NAFLD are increasingly well recognized, the optimal approach to the evaluation and management of NAFLD appears to be evolving. A multi-society panel of experts, derived from and endorsed by AASLD/AGA/ACG developed guidelines in 2012 to assist practitioners in applying a consistent approach to the evaluation and management of patients with NAFLD [Chalasani et al. 2012]. In this survey of academic gastroenterologists and hepatologists, we found that adherence to practice guidelines for NAFLD/NASH is weak for some fundamental aspects of the recommendations and varies widely between practitioners.
It is important to consider how reported adherence to the AASLD/AGA/ACG guidelines for NAFLD compares with adherence to other comparable sets of guidelines in other disciplines. For comparison, the adherence rate of hemoglobin A1C testing and treatment modification to guidelines in type 2 diabetics is 7–39% [Lian and Liang, 2014]. When lipid lowering guidelines recommended annual low-density lipoprotein (LDL) measurements and LDL goals of <100 mg/dl for patients with coronary artery disease, one study found approximately 60% of patients in a large cohort had LDL checked appropriately and approximately 25% were at their LDL goal [Sloan et al. 2001]. How much of this variance is related to appropriate personalized medicine where the care provider makes an informed decision to depart from practice guidelines for appropriate reasons versus a lack of awareness of guidelines is unknown. In addition, some variance from guidelines is to be expected as guidelines are evidence based and such evidence rarely addresses every possible clinical scenario.
Given the practice patterns of the respondents, this survey appears to have appropriately targeted those with significant expertise and exposure to NAFLD. For most questions regarding the management of NAFLD/NASH, there was no statistically significant difference seen between adherence to guidelines by gastroenterologists and hepatologists. This indicates that in most cases, the lack of adherence is not related to the degree of training in hepatology or a lack of awareness of guidelines. This is despite the fact that hepatologists tend to see more biopsy confirmed NASH patients each month than gastroenterologists. For a few questions, however, there were significant practice differences: hepatologists perform biopsies more often on patients suspected of having NASH, they prescribe vitamin E more frequently, and refer more patients for bariatric surgery than gastroenterologists. In regard to performing biopsies, this finding is similar to that seen by Ratziu and colleagues in France, who found that hepatology specialized respondents more aggressively pursued the distinction between steatosis and steatohepatitis and performed liver biopsy more often than gastroenterologists [Ratziu et al. 2012]. The absence of any approved pharmacotherapy for NAFLD and NASH may have had an impact on the perceived value of liver biopsy, as most management recommendations are independent of histological findings for patients with NAFLD (e.g. diet and lifestyle modification) [Chalasani et al. 2012]. It is also possible that noninvasive indices of liver disease, such as ultrasound and magnetic resonance elastography, have attenuated enthusiasm for liver biopsy in some patients.
Liver biopsy appears to be performed less often than needed for making decisions about treatment by both gastroenterologists and hepatologists. These data suggest this may be due to an over-reliance on elevated aminotransferases to identify patients who would be appropriate for biopsy and the perception that biopsy may not change management. This is evidenced by the fact that many providers prescribe medications such as vitamin E and pioglitazone without confirming NASH. Furthermore, this may explain the overwhelming preference providers have for using vitamin E over pioglitazone, as vitamin E is believed to be safer and not associated with weight gain. Its perceived safety may also explain why many providers prescribe it to patient populations outside of the recommendations, such as in diabetics or cirrhotics. The high degree of tolerability and low risk of initiating vitamin E supplementation is likely to have attenuated enthusiasm for performing liver biopsy prior to initiating vitamin E supplementation.
The use of vitamin E in patients without biopsy confirmed disease, including those with diabetes or cirrhosis, is nonetheless somewhat surprising because the survey suggests that most prescribers are aware of the potential side effects of vitamin E supplementation. However, a subset of respondents seems to avoid vitamin E unnecessarily. Specific safety concerns include bleeding risk due to an association of vitamin E with increased risk of hemorrhagic stroke (0.8 more per 1000 treated persons, or 22% relative risk increase), countered by a decreased risk of ischemic stroke (2.1 fewer per 1000 treated persons, or 10% relative risk reduction) [Schurks et al. 2010]. The potential association between vitamin E use and increased mortality has also received substantial attention [Miller et al. 2005]. However, the frequently referenced meta-analysis by Miller and colleagues has several limitations including the inclusion of trials using multiple formulations and dosages of vitamin E and trials of vitamin E in combination with other supplements. The use of different methodologies in more recent meta-analyses have yielded conflicting results, with no increased mortality seen from vitamin E use [Gerss and Kopcke, 2009; Berry et al. 2009; Curtis et al. 2014]. In certain populations, however, vitamin E appears to have the potential for serious side effects, such as the development or worsening of heart failure in those with known CVD or diabetes [Marchioli et al. 2006; Lonn et al. 2005]. Among healthy men, vitamin E has been shown to slightly but significantly increase the absolute risk of development of prostate cancer (1.6 per 1000 person-years) [Klein et al. 2011]. Data from the survey suggest that, while respondents seem aware of potential risks, the understanding of the magnitude of the risk may be overstated, particularly with respect to overall mortality risk related to vitamin E use.
Most gastroenterologists and hepatologists surveyed do not regularly prescribe pioglitazone for the treatment of NASH. Weight gain is the most common unwanted side effect and can be substantial in some patients [Sanyal et al. 2010]. Provided it is not given to patients with established heart failure [New York Heart Association (NYHA) class II, III or IV], the risk of significant heart failure exacerbation is no higher than placebo [Sanyal et al. 2010] or metformin [Breunig et al. 2014]. Other increased risks such as that of bladder cancer are low or nonexistent as suggested by a recent analysis of 1 million patients spanning 5.9 million person-years over a 4–7.4 year follow up [Levin et al. 2014]. While pioglitazone remains the best studied compound in patients with NASH in the presence of diabetes or cirrhosis, our data show that there is reluctance to use pioglitazone in these subgroups of patients that may benefit.
The use of metformin as frequently as pioglitazone is surprising given its lack of efficacy for this indication and the recommendation against its use as a treatment for NASH [Chalasani et al. 2012]. Metformin does not appear to have much effect on NAFLD/NASH independent of its potential for weight loss in regard to normalization of aminotransferases, insulin sensitization or improvement in liver histology [Shields et al. 2009; Haukeland et al. 2009; Omer et al. 2010]. However, it may have a role in chemoprevention of hepatocellular carcinoma [Donadon et al. 2010; Lai et al. 2012; Zhang et al. 2012; Chen et al. 2013].
Bariatric surgery has been shown to improve survival in two large studies; this improvement in survival is specifically related to reduced death from CVD and cancer – the most common causes of death in patients with NASH [Adams et al. 2007; Sjostrom et al. 2007]. Bariatric surgery can substantially improve or reverse metabolic comorbidities associated with NAFLD and available data suggest it is also effective in reversing or improving NASH [Mathurin et al. 2009; Mummadi et al. 2008]. However, our survey indicates that a quarter of providers do not refer potentially eligible patients with NAFLD for bariatric surgery. While there is concern for mild worsening of fibrosis after bariatric surgery, more recent work has shown that fibrosis may also improve [Caiazzo et al. 2014; Lassailly et al. 2015]. Current guidelines do not formally recommend bariatric surgery as a treatment for NASH per se, but they do note that patients with noncirrhotic NASH who have established indications for bariatric surgery should be considered. The cornerstone of treatment of NAFLD is lifestyle intervention and careful management of related comorbidities with the intention of ultimately reducing mortality related to CVD. Based on this we should consider bariatric surgery in appropriate patients because it has been shown to reduce both cardiovascular and cancer related death overall.
The limitations of our survey include the self-reported nature of the data, as well as the possibility of differences in practice between respondents and nonrespondents. The 28% response rate, however, is adequate, as similar surveys generally elicit a 20% response [Dykema et al. 2013]. Referral bias may be playing a role in the increased use of liver biopsy by hepatologists compared with gastroenterologists, as the former are more likely to offer NAFLD/NASH clinical trials to patients. The questions also by their nature do not account for unique patient circumstances that inevitably arise. Finally, there may be differences in interpretation of the guidelines and available data accounting for some of the nonadherence that is seen.
Conclusion
In a survey of academic gastroenterologists and hepatologists to identify practice patterns regarding the diagnosis and management of NAFLD and NASH, we found that real life practices often differ substantially from published guidelines. Liver biopsy is recommended infrequently, suggesting that NASH is underdiagnosed, even in highly specialized practices. Pioglitazone is used much less often than vitamin E and vitamin E is often prescribed to patient groups that lack supporting data or avoided unnecessarily. As new therapeutic options become available, accurately diagnosing and staging NASH will become even more important.
Patients with advanced NASH often have normal aminotransferases and are thus often left undiagnosed, since most providers use the presence of abnormal liver chemistries to perform a biopsy. The most important divergence in clinical practice identified here is the failure to regularly incorporate liver biopsy to diagnose NASH. It is clear from recently published literature that the natural history of patients diagnosed with NASH and fibrosis is distinct from those diagnosed with non-NASH/NAFLD. Not only does an accurate histological diagnosis allow the practitioner to provide valuable prognostic information, it opens the opportunity to offer emerging therapy to those with more advanced disease.
The best approach to improve adherence to best practice is to continue to increase awareness of the disease. This can be achieved through publications identifying gaps in knowledge and in clinical practice and through educational programs to increase awareness of NASH as a distinct entity from non-NASH NAFLD. Ongoing biomarker development will speed this process and at some point allow us to avoid the need for biopsy in most instances. Until then with increased awareness of emerging therapies, providers may be more willing to undergo the risk of liver biopsy if they have more to offer their patients. Box 1 summarizes current knowledge of the prevalence and treatment of NAFLD and NASH, and our findings on real life practice patterns.
Box 1.
Summary.
| Current knowledge |
| - The prevalence of nonalcoholic steatohepatitis (NASH) is increasing and accounts for a growing number of patients with cirrhosis or hepatocellular cancer. |
| - Liver biopsy is the Gold Standard method to diagnose NASH. |
| - Treatment options are limited, though vitamin E and pioglitazone improve liver injury related to NASH and are recommended by practice guidelines. |
| New findings |
| - Even physicians with significant expertise in liver disease are reluctant to recommend liver biopsy for patients in the absence of elevations in liver chemistries when suspecting NASH. |
| - Patients are being treated with pioglitazone or vitamin E often without biopsy confirmation of NASH. |
| - Patient selection for treatment often does not follow published guidelines. |
Acknowledgments
We are grateful to the American Association for the Study of Liver Diseases for posting our survey on its website.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Mary E. Rinella, Associate Professor of Medicine, Division of Gastroenterology and Hepatology, Northwestern University Feinberg School of Medicine, 676 N. St. Clair, Arkes Pavillion 14-012, Chicago, IL 60611, USA.
Zurabi Lominadze, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Rohit Loomba, Division of Gastroenterology and Hepatology, University of California San Diego, La Jolla CA, USA.
Michael Charlton, Intermountain Medical Center, Salt Lake City, Utah, USA.
Brent A. Neuschwander-Tetri, Division of Gastroenterology and Hepatology, St Louis University, St Louis, Missouri, USA
Stephen H. Caldwell, Gastroenterology and Hepatology, University of Virginia, Charlottesville, Virginia, USA
Kris Kowdley, Division of Gastroenterology and Hepatology, Swedish Hospital Medical Center, Seattle Washington, USA.
Stephen A. Harrison, Division of Gastroenterology and Hepatology, Brooke Army Medical Center, San Antonio, Texas, USA
References
- Adams T., Gress R., Smith S., Halverson R., Simper S., Rosamond W., et al. (2007) Long-term mortality after gastric bypass surgery. N Engl J Med 357: 753–761. [DOI] [PubMed] [Google Scholar]
- Berry D., Wathen J., Newell M. (2009) Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clin Trials 6: 28–41. [DOI] [PubMed] [Google Scholar]
- Breunig I., Shaya F., McPherson M., Snitker S. (2014) Development of heart failure in medicaid patients with type 2 diabetes treated with pioglitazone, rosiglitazone, or metformin. J Manag Care Pharm 20: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo R., Lassailly G., Leteurtre E., Baud G., Verkindt H., Raverdy V., et al. (2014) Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg 260: 893–898; discussion 898–899. [DOI] [PubMed] [Google Scholar]
- Chalasani N., Younossi Z., Lavine J., Diehl A., Brunt E., Cusi K., et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- Chen H., Shieh J., Chang C., Chen T., Lin J., Wu M., et al. (2013) Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 62: 606–615. [DOI] [PubMed] [Google Scholar]
- Curtis A., Bullen M., Piccenna L., McNeil J. (2014) Vitamin e supplementation and mortality in healthy people: a meta-analysis of randomised controlled trials. Cardiovasc Drugs Ther 28: 563–573. [DOI] [PubMed] [Google Scholar]
- Donadon V., Balbi M., Mas M., Casarin P., Zanette G. (2010) Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int 30: 750–758. [DOI] [PubMed] [Google Scholar]
- Dykema J., Jones N., Piche T., Stevenson J. (2013) Surveying clinicians by web: current issues in design and administration. Eval Health Prof 36: 352–381. [DOI] [PubMed] [Google Scholar]
- Fracanzani A., Valenti L., Bugianesi E., Andreoletti M., Colli A., Vanni E, et al. (2008) Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 48: 792–798. [DOI] [PubMed] [Google Scholar]
- Gerss J., Kopcke W. (2009) The questionable association of vitamin E supplementation and mortality – inconsistent results of different meta-analytic approaches. Cell Mol Biol 55(Suppl.): OL1111–OL1120. [PubMed] [Google Scholar]
- Haukeland J., Konopski Z., Eggesbo H., von Volkmann H., Raschpichler G., Bjoro K., et al. (2009) Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol 44: 853–860. [DOI] [PubMed] [Google Scholar]
- Klein E., Thompson I., Jr., Tangen C., Crowley J., Lucia M., Goodman P., et al. (2011) Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306: 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S., Chen P., Liao K., Muo C., Lin C., Sung F. (2012) Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol 107: 46–52. [DOI] [PubMed] [Google Scholar]
- Lassailly G., Caiazzo R., Buob D., Pigeyre M., Verkindt H., Labreuche J., et al. (2015) Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 149: 379–388. [DOI] [PubMed] [Google Scholar]
- Levin D., Bell S., Sund R., Hartikainen S., Tuomilehto J., Pukkala E., et al. (2014) Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia 58: 493–504. [DOI] [PubMed] [Google Scholar]
- Lian J., Liang Y. (2014) Diabetes management in the real world and the impact of adherence to guideline recommendations. Curr Med Res Opin 30: 2233–2240. [DOI] [PubMed] [Google Scholar]
- Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J., et al. (2005) Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293: 1338–1347. [DOI] [PubMed] [Google Scholar]
- Marchioli R., Levantesi G., Macchia A., Marfisi R., Nicolosi G., Tavazzi L., et al. (2006) Vitamin E increases the risk of developing heart failure after myocardial infarction: results from the GISSI-Prevenzione trial. J Cardiovasc Med 7: 347–350. [DOI] [PubMed] [Google Scholar]
- Mathurin P., Hollebecque A., Arnalsteen L., Buob D., Leteurtre E., Caiazzo R., et al. (2009) Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 137: 532–540. [DOI] [PubMed] [Google Scholar]
- Miller E., 3rd, Pastor-Barriuso R., Dalal D., Riemersma R., Appel L., Guallar E. (2005) Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142: 37–46. [DOI] [PubMed] [Google Scholar]
- Mofrad P., Contos M., Haque M., Sargeant C., Fisher R., Luketic V., et al. (2003) Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37: 1286–1292. [DOI] [PubMed] [Google Scholar]
- Mummadi R., Kasturi K., Chennareddygari S., Sood G. (2008) Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 6: 1396–1402. [DOI] [PubMed] [Google Scholar]
- Omer Z., Cetinkalp S., Akyildiz M., Yilmaz F., Batur Y., Yilmaz C., et al. (2010) Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 22: 18–23. [DOI] [PubMed] [Google Scholar]
- Ratziu V., Bellentani S., Cortez-Pinto H., Day C., Marchesini G. (2010) A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 53: 372–384. [DOI] [PubMed] [Google Scholar]
- Ratziu V., Cadranel J., Serfaty L., Denis J., Renou C., Delassalle P., et al. (2012) A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol 57: 376–383. [DOI] [PubMed] [Google Scholar]
- Sanyal A., Chalasani N., Kowdley K., McCullough A., Diehl A., Bass N., et al. (2010) Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurks M., Glynn R., Rist P., Tzourio C., Kurth T. (2010) Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 341: c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields W., Thompson K., Grice G., Harrison S., Coyle W. (2009) The effect of metformin and standard therapy versus standard therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): a pilot trial. Therap Adv Gastroenterol 2: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom L., Narbro K., Sjostrom C., Karason K., Larsson B., Wedel H., et al. (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752. [DOI] [PubMed] [Google Scholar]
- Sloan K., Sales A., Willems J., Every N., Martin G., Sun H., et al. (2001) Frequency of serum low-density lipoprotein cholesterol measurement and frequency of results < or=100 mg/dl among patients who had coronary events (Northwest VA Network Study). Am J Cardiol 88: 1143–1146. [DOI] [PubMed] [Google Scholar]
- Vernon G., Baranova A., Younossi Z. (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34: 274–285. [DOI] [PubMed] [Google Scholar]
- Williams C., Stengel J., Asike M., Torres D., Shaw J., Contreras M., et al. (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140: 124–131. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zheng Z., Shi R., Su Q., Jiang Q., Kip K. (2012) Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 97: 2347–2353. [DOI] [PubMed] [Google Scholar]