Abstract
Faecal incontinence, defined as the involuntary loss of solid or liquid stool, is a common problem affecting 0.8–8.3% of the adult population. Individuals suffering from faecal incontinence often live a restricted life with reduced quality of life. The present paper is a clinically oriented review of the pathophysiology, evaluation and treatment of faecal incontinence.
First-line therapy should be conservative and usually include dietary adjustments, fibre supplement, constipating agents or mini enemas. Biofeedback therapy to improve external anal sphincter function can be offered but the evidence for long-term effect is poor. There is good evidence that colonic irrigation can reduce symptoms and improve quality of life, especially in patients with neurogenic faecal incontinence. Surgical interventions should only be considered if conservative measures fail. Sacral nerve stimulation is a minimally invasive procedure with high rate of success. Advanced surgical procedures should be restricted to highly selected patients and only performed at specialist centres. A stoma should be considered if other treatment modalities fail.
Keywords: faecal incontinence, conservative treatment, quality of life
Introduction
Epidemiology of faecal incontinence
Faecal incontinence (FI) can be defined as the recurrent uncontrolled passage of faecal material in a person with a developmental age of at least 4 years. The prevalence of FI in the adult population has been estimated at 0.8–6.2% [Pretlove et al. 2006; Damon et al. 2006]. Recent data from the North America, however, indicate a much higher prevalence of 8.3% even in noninstitutionalized adults [Ditah et al. 2014]. The prevalence of FI increases with age from approximately 3% in the age group from 20 to 29 years to 16% in people aged 70 or over [Ditah et al. 2014]. In nursing home residents the prevalence of FI may be as high as 47% [Nelson et al. 1998]. The risk of FI is independently associated with older age, diabetes, loose stools and three or more bowel movements per day [Ditah et al. 2014]. It is controversial whether FI is associated with female sex or not [Ditah et al. 2014; Nelson et al. 1998].
FI has serious consequences for social actives and quality of life [Drossman et al. 1993; Crowell et al. 2007]. Furthermore, FI is a cause of significant cost due to diagnostics, treatment, care and reduced ability to work [Bharucha et al. 2005; Xu et al. 2012].
The literature on FI is extensive and multiple treatment modalities exist. The present paper is a clinically oriented review of the pathophysiology, evaluation and treatment of FI.
Physiology of anal continence
Anal continence depends on complex interactions among a number of factors. The internal anal sphincter muscle (IAS) is a continuation of the circular rectal smooth muscle layer. Its main function is to generate most of the anal resting pressure, thereby preventing FI at rest [Lestar et al. 1989]. The IAS is under reflex control. The external anal sphincter muscle (EAS) is composed of striated muscle cells and is partially under voluntary control. The EAS contributes to the anal resting pressure but its main function is to generate the anal squeeze pressure [Lestar et al. 1989]. The puborectalis muscle forms a sling around the upper part of the anal canal. The tone of the puborectalis muscle creates the anorectal angle, which prevents movement of faeces from the rectum to the anal canal between defecations. In contrast to the rectum, the anal canal is densely innervated by sensory nerve cells. Anal sensibility is necessary for the person to contract the EAS if defecation is to be postponed. The anal sampling reflex, including short-lasting rectal contraction and relaxation of the upper part of the anal canal, allows the subject to sense the content of the rectum [Duthie and Bennett, 1963].
Normal compliance of the rectal wall is necessary for the rectum to function as a reservoir. Impaired rectal sensation may cause faecal retention and thereby FI while a hypersensate or hyperreactive rectum may cause urge symptoms and FI. Colorectal transit time may be used to determine the transport time to the different segments of the colon [Abrahamsson et al. 1988]. If transit is fast, stools may become loose or liquid and difficult to retain.
Any factor described above may be disturbed and thereby make the subject incontinent. If no major structural or neurological abnormalities are found, FI is defined as primary or functional/idiopathic [Bharucha et al. 2006]. If the underlying pathology can be identified, FI is defined as secondary to the specific condition.
FI secondary to other disorders
FI is a common consequence of a number of conditions and diseases, some of which are shown in Table 1. Congenital, traumatic or iatrogenic defects of the anal canal are well known causes of FI. Especially, sphincter lesions resulting from vaginal delivery may result in FI. Many patients with neurological disorders affecting the brain, spinal cord or peripheral nervous system have FI because of impaired anal sphincter control, reduced or absent anorectal sensibility or abnormal anorectal reflexes. Patients with diabetes may have neuropathy of the anal canal and some have chronic diarrhoea. In patients with various connective diseases, notable scleroderma, there is myopathy and atrophy of the IAS. Rectal surgery may compromise the reservoir function of the rectum as seen in patients with low anterior resection syndrome. The same can be seen after radiotherapy towards the pelvic organs and in some patients with inflammatory bowel disease or irritable bowel syndrome. A large rectocele or rectal intersuception may cause FI or soiling, as stool can be retained (stool trapping) and later leak through the anal canal [Melchior et al. 2015]. Usually, a full-wall rectal prolapse will compromise the IAS and EAS and cause FI [Faucheron et al. 2015].
Table 1.
Common causes of faecal incontinence.
| Anal sphincter dysfunction | Congenital anorectal malformations |
| Radiation therapy | |
| Obstetric anal sphincter injury | |
| Anal surgery | |
| Perianal fistulas | |
| Sexual abuse | |
| Rectal disorders | Inflammatory bowel disease |
| Radiation therapy | |
| Rectocele | |
| Rectal intersusception | |
| Rectal prolapse | |
| Faecal impaction | |
| Neurological disorders | Spinal cord lesions |
| Stroke | |
| Multiple sclerosis | |
| Spina bifida | |
| Diabetic neuropathy | |
| Obstetric nerve damage | |
| Myopathy | Systemic scleroderma |
| Fast colorectal transit time | Chronic diarrhoea |
| Irritable bowel syndrome | |
| Psychological | Encopresis |
| Dementia |
If FI is secondary to other disorders, the underlying disorder should be treated if possible. In many cases this is, however, not possible and specific treatment should be attempted as described later in this paper.
Idiopathic (functional) FI
According to the ROME III criteria, functional FI is defined as the recurrent uncontrolled passage of faecal material in a person with a developmental age of at least 4 years and one of the following: abnormal functioning of normally innervated intact muscles; minor abnormalities of sphincter structure or innervation; normal or disordered bowel habits (i.e. faecal retention or diarrhoea); and psychological causes [Bharucha et al. 2006]. Also according to the ROME criteria, the following have to be excluded: abnormal innervation caused by lesions within the brain, spinal cord, sacral nerve roots, or generalized peripheral or autonomic neuropathy; anal sphincter abnormalities associated with a multisystem disease; and structural or neurological causes believed to be the primary cause of FI.
Idiopathic FI is associated with a weakened pelvic floor as indicated by reduced anal pressures and increased distensibility of the anal canal [Sorensen et al. 2014]. Damage to the pudendal nerve during childbirth is considered an important aetiological factor for idiopathic FI [Kiff and Swash 1984].
Evaluation of FI
Patient history
A careful patient history is essential in the evaluation of FI. The nature and type of FI should be characterized and include onset, frequency, duration, diurnal variation, stool consistency, previous management, coexisting urinary incontinence, relation to food intake and physical activity, and the impact on social activities and quality of life. It is important to describe whether the patient has urge FI (FI preceded by a strong desire to defecate) or passive FI (FI not preceded by desire to defecate). It is also of clinical relevance to determine whether the patient has incontinence to substantial amounts of solid or liquid stools or whether it is soiling/seepage of small amounts of liquid stools.
The number of childbirths, any history of obstetric anal sphincter injuries, previous anorectal surgery, and other anal or perianal trauma must be elucidated. As neurological disorders and systemic disorders such as diabetes mellitus and connective tissue disorders often cause FI, these should be mentioned if present. Recent onset of FI can be the first sign of neoplasia or inflammation and therefore requires further endoscopic evaluation according to national recommendations.
The patient history should be supported by patient-reported outcome measures (PROMs) to quantify patient symptoms and convert individual experiences into data. Thereby, grading of the severity of FI is possible and treatment can be monitored for quality control and for scientific purposes. Several different PROMs for FI exist. Most of the scores have been subjected to limited validation. The most widely used scoring tools are the Wexner incontinence score [Jorge and Wexner, 1993], the St Marks FI grading system [Vaizey et al. 1999], the Fecal Incontinence Quality of Life Scale [Rockwood et al. 2000] and the International Consultation on Incontinence Questionnaire – Bowel Symptoms (ICIQ-B) [Cotterill et al. 2011].
The Wexner incontinence score is probably the most widely used to assess the severity of FI. The score consist of five items (incontinence of solid stool, liquid stool, and flatus, the need to wear a pad and lifestyle alterations), which are summarized over 4 weeks. For each item, a score from 0 to 4 is assigned, depending on the frequency from always/daily (4) to never (0). The sum of the five items gives the total score, which ranges from 0 indicating full continence, to 20 indicating complete incontinence.
A bowel habit diary can be used for daily registration of bowel habits. Traditionally this has been done on paper forms, but current initiatives are undertaken to convert bowel habit diaries in to smartphone apps.
Physical examination
Physical examination of the perineum and the anal canal is mandatory [Dobben et al. 2007]. The passive closure of the anal canal and perianal skin erosions can be assessed with inspection. With digital rectal examination ‘the educated finger’ provides a rough impression of the anal resting and squeeze pressures [Hallan et al. 1989; Mimura et al. 2004; Eckardt and Kanzler, 1993]. Furthermore, coordinated relaxation of the pelvic floor muscles at straining can be accessed digitally. In patients with coexisting symptoms of difficult rectal evacuation the patient should be asked to sit on a commode and perform a Valsalva manoeuvre in order to reveal a rectal prolapse.
Endoanal ultrasonography and anorectal physiology tests
The integrity of the anal sphincter can be examined in detail with endoanal ultrasonography [Sultan et al. 1994; Law et al. 1991]. This provides a clear presentation of the IAS and EAS which can reveal structural causes of anal sphincter dysfunction [Felt-Bersma and Cazemier, 2006]. Endoanal ultrasonography has a high degree of sensitivity and specificity and it correlates well with manometric findings [Bordeianou et al. 2008]. The examination is easy to perform and 3D images can be saved for training and supervision.
Anal physiology testing often done in severe FI and before sophisticated treatment modalities are introduced. However, the prognostic value of each test has been questioned and it should be emphasized that an anal physiology test cannot stand alone in the clinical decision making [Hill et al. 2006].
Anal manometry determines the anal resting pressure and anal squeeze pressure. It can either be done by water perfused [McHugh and Diamant, 1987] or solid state catheters.
High-resolution manometry of the anal canal provides detailed spatial information about anal pressures. The technique is increasingly used but it remains to be established whether the rather expensive equipment provides better guidance towards therapy than standard manometry. Rectal capacity and compliance can be tested with a rectal balloon gradually filled with either air or water. Rectal sensibility is also assessed during balloon filling as the patient reports ‘first detectable sensation’, ‘sensation of urge to defecate’ and ‘maximum tolerable rectal volume’. The barostat is considered the golden standard for assessment of rectal compliance, but the procedure is very time consuming [Vanhoutvin et al. 2009]. A new ‘quick-barostat’ may solve this problem [Sauter et al. 2014].
The threshold for anal mucosal sensation can be determined with neurophysiology tests [Felt-Bersma et al. 1997] and the pudendal nerve terminal motor latency time can reveal possible traction neuropathy [Kiff and Swash, 1984]. Colonic transit times by use of radiopaque markers and a single abdominal X-ray can reveal coexisting constipation [Abrahamsson et al. 1988]. Dynamic magnetic resonance imaging [Melchior et al. 2015], conventional defaecography or a transperineal ultrasonography is indicated if a large rectocele or rectal intussusception is suspected.
Conservative treatment of FI
Conservative therapy is first-line treatment for patients with FI. Conservative therapy is defined as any nonsurgical, noninvasive intervention improving faecal continence or as a treatment to prevent further deterioration over time. Several conservative treatment options are available, but the durability over time is often poor and a more invasive approach can be necessary [Tjandra et al. 2008]. A treatment algorithm for FI has been proposed by the International Consultation on Incontinence [Abrams et al. 2010]. Recently, we have published that conservative management of FI in a specialist nurse-led programme is effective and appreciated by the patients [Duelund-Jakobsen et al. 2015]
Behavioural and medical therapy
The first step in the treatment of FI is usually regulation of diet, fluid intake and bowel habits. Patients must be advised to avoid food or drinks that cause loose stool and frequent bowel movements. Fibre and bulking agents, such as natural psyllium, methyl cellulose or synthetic polycarbophil, may augment stool consistency and have documented effect against FI [Bliss et al. 2001; Sze and Hobbs, 2009].
An anal plug can be attempted in patients with passive FI, especially those with soiling of small amounts of liquid stools. Even though most patients cannot tolerate the use of anal plugs on a permanent basis, they may be useful for occasional use [Deutekom and Dobben, 2012].
Constipating agents, such as loperamide, are frequently used and the efficacy has been documented against FI associated with loose stool [Lauti et al. 2008]. Loperamide is an opiate derivate that acts by increasing transit time through the small intestine and the proximal colon, thereby promoting absorption and improving stool consistency [Ooms et al. 1984; Ruppin, 1987]. Furthermore, loperamide impairs the rectoanal inhibitory reflex and increases the anal resting pressure [Hanauer, 2008; Musial et al. 1992; Ruppin, 1987; Read et al. 1982].
Laxatives, mini enemas and suppositories can be useful, especially in those with coexisting evacuation disorders.
Biofeedback therapy assists the patient in learning to improve contraction of the EAS. The exercises are performed with a manometric probe in the anal canal. The probe is attached to a visual or verbal amplifier that gives response proportional to the pressure delivered during squeeze. Individualized training programmes lasting 6–8 weeks with twice-daily exercises at home followed by an evaluation is common practice. Patients are encouraged to continue the strengthening exercises lifelong. The mechanism of action for biofeedback therapy is still not fully understood [Papachrysostomou and Smith, 1994]. A recent Cochrane review concluded that the current literature does not allow a definitive assessment of the possible role of biofeedback therapy in the management of FI [Norton and Cody, 2012].
Transanal irrigation
Transanal irrigation can reduce episodes of FI. The patient usually administers the enema daily or every other day thereby obtaining a state of ‘pseudo-continence’ because the distal colon and the rectum are without stools. A number of specialized catheters can be used. Usually, body-tempered water is used. The procedure is simple and safe for long-term treatment [Christensen et al. 2009]. A study using a scintigraphy model demonstrated that in patients with idiopathic FI, transanal irrigation results in almost complete emptying of the rectosigmoid and descending colon [Christensen et al. 2003]. Medium-term follow up has shown that 51% of patients have a successful outcome after 21 months [Christensen et al. 2009]. The most firm evidence for transanal irrigation is, however, among patients with FI and evacuation disorder as a consequence of spinal cord injury [Christensen et al. 2006].
Surgical treatment of FI
Injectable bulking agents
Patients with passive incontinence such as seepage and soiling or mild FI without significant EAS defects are candidates for treatment with bulking agents. A number of different biomaterials including autologous fat, silicone, cross-linked collagen, dextranomer in hyaluronic acid gel, carbon coated beads and others have been used for injection into the submucosa or the intersphincteric space to augment the anal sphincter and improve continence.
A systematic review of efficacy and safety of injectables was published in 2011 [Hussain et al. 2011]. A pooled analysis on a total of 1070 patients concluded that continence improved in 70% during the early postoperative period but in only 42% at 12-month follow up. A Cochrane review from 2013 identified five randomized studies and concluded that no evidence of long-term outcome was available [Maeda et al. 2013]. A multicentre trial randomized 206 patients in a 2:1 ratio to receive either submucosal injections of dextranomer in stabilized hyaluronic acid gel or sham injections. At 6 months, 52% of the patients in the treatment arm reached the primary endpoint of 50% reduction in FI episodes. Surprisingly, 31% of the patients in the sham injection group also responded to treatment [Graf et al. 2011]. In 2014 the results from 83 out of 115 patients in the treatment arm were updated and the success rate was 63% [La Torre and de la Portilla, 2013].
In 2011, Ratto and colleagues introduced a new procedure with implant of Gatekeeper. This is a self-expandable prosthesis placed in the intersphincteric space of the anal canal. Preliminary results from 14 patients showed a significant decrease in episodes of major FI while quality of life significantly improved. No complications were reported [Ratto et al. 2011] but long-term results are needed.
Neuromodulation
Neuromodulation is an attractive therapeutic option when conservative measures fail. Neuromodulation has to some extent obviated the use of overlapping sphincter repair and more extensive surgical procedures which had high morbidity and poor long-term efficacy [Rongen et al. 2003; Glasgow and Lowry, 2012]. Treatment of FI by neuromodulation is mainly conducted by sacral nerve stimulation (SNS) using the implantable Interstim therapy system (Medtronic, Minneapolis, MN, USA) or lately tibial neuromodulation using a peripheral electrode with the Urgent PC neuromodulation system (Uroplasty Ltd, Manchester, UK). Both modalities are adopted from treatment of urinary incontinence [McGuire et al. 1983; Stoller, 1999]. SNS was introduced for idiopathic FI in 1995 [Matzel et al. 1995]. Tibial neuromodulation was first applied for treatment of FI in 2003 [Shafik et al. 2003]. The mechanism of action remains to be established, but direct or peripheral stimulation of sacral roots modulating afferent or efferent central pathways controlling colorectal motility and perception is proposed [Gourcerol et al. 2011].
Sacral nerve stimulation
The procedure is done under local or general anaesthesia. An electrode is placed through a sacral foramen S2–S4 (preferably S3) to stimulate the sacral nerve roots. The procedure is composed of two stages; first, a percutaneous nerve evaluation (PNE) test that is performed with one or more test leads. Alternatively, a permanent lead with an extension cable is used and connected to an external pulse generator. If FI is significantly reduced during the PNE test, usually by at least 50% [Duelund-Jakobsen et al. 2012], a permanent electrode is implanted and a pulse generator is placed in a gluteal pocket. The pulse generator is accessible for programming by external telemetry.
The result of the test period has a high predictive value for subsequent successful permanent implantation. Whereas no demographic data predicted a positive PNE test, the results of external sphincter evaluation appear to be a predictor [Maeda et al. 2010; Hornung et al. 2014]. Normal pudendal terminal motor latency and low stimulation amplitude have been identified as predictors for successful SNS [Duelund-Jakobsen et al. 2014; Gallas et al. 2011].
Several studies have documented that SNS effectively reduces episodes of FI and improves quality of life in the short term [Hollingshead et al. 2011; Wexner et al. 2010; Tjandra et al. 2008]. Even though the effect appears to be maintained at medium- and long-term follow up [Duelund-Jakobsen et al. 2012; Altomare et al. 2015; Michelsen et al. 2010; Mellgren et al. 2011], some loss of efficacy and adverse events (pain/discomfort) have been reported [Maeda et al. 2011].
Posterior tibial nerve modulation
The posterior tibial nerve is a mixed sensory motor nerve with afferent fibres originating from the lumbosacral dorsal roots (L4–S3). Stimulation is delivered transcutaneously using a plaster electrode (TTNS) or percutaneously using a needle electrode (PTNS). The electrode is placed above the medial malleolus, and a ground surface electrode placed on the ipsilateral leg. Various treatment protocols have been applied ranging from 4 weeks to 12 weeks, with scheduled stimulation sessions from daily to once per week. A comparison between stimulation regimes showed significantly better efficacy with more frequent stimulation sessions [Thomas et al. 2013].
The main indication is urge FI but treatment has often been offered quite liberally on a trial and error basis. Two randomized controlled trials have evaluated the effects of peripheral neuromodulation versus sham but results were conflicting [George et al. 2013; Leroi et al. 2012].
Only uncontrolled data are available from TTNS and generally the effect was smaller than reported for PTNS and perhaps not clinically relevant [Leroi et al. 2012; Eleouet et al. 2010; Queralto et al. 2006]. Accordingly, a randomized controlled trial did not show significant reduction in incontinence episodes with TTNS compared with sham [Leroi et al. 2012].
In a randomized trial, results from SNS and PTNS did not differ significantly [Thin et al. 2015]. Thus, the role of posterior tibial nerve modulation is unclear, but an ongoing large British randomized study of PTNS versus sham may help define it.
Sphincteroplasty
Sphincteroplasty to reconstruct defects in the EAS has been the standard treatment of FI after documented external sphincter defects. Short-term improvement in FI has been reported in up to 86% of patients [Cheung and Wald, 2004; Barisic et al. 2006]. After 3 months, almost two-thirds of the patients reported excellent or good results with improved quality of life. However, several retrospective studies show a deterioration of the functional outcome in the long term. After 5–10 years, only 25–40% of the patients are continent [Lehto et al. 2013; Bravo Gutierrez et al. 2004].
Antegrade continence enema
Antegrade colonic irrigation through an appendicostomy was first described in children with FI by Malone and colleagues in 1990 [Malone et al. 1990]. The method aims at avoiding FI by scheduled controlled emptying of the colon. Antegrade irrigation is well established for FI in children with anorectal malformations or neurological disorders [Levitt et al. 1997]. Data on adults are fewer but long-term results appear to be good with a rate of success around 75% [Lefevre et al. 2006; Poirier et al. 2007]. Appendicostomy stenosis, leakage of mucus or intestinal content from the stoma and surgical site infections are common complications, which may require surgical revision.
Laparoscopic ventral rectopexy
Laparoscopic ventral rectopexy has been introduced against high-grade rectal intussusception with outcomes comparable to those for full thickness rectal prolapse [Gosselink et al. 2015]. Studies are still few, observational in design and with 1-year follow up only. Complications include comparable chronic pelvic pain and mesh erosion as reported from treatment of full thickness rectal prolapse [Evans et al. 2105; Faucheron et al. 2015].
Radiofrequency energy
Treatment of FI by means of temperature-controlled radiofrequency energy administered to the anal canal was first described in 2002 and named the SECCA procedure [Takahashi et al. 2002]. The technique is simple and the radiofrequency energy is delivered to the sphincters with an anoscope. A recent review concluded that with appropriate patient selection improvement of FI symptoms and quality of life persist for at least 6 months and seems to continue for a further 5 years [Frascio et al. 2014]. However, the number of patients treated is small and only 39 patients were followed for 5 years. A recently published animal study found that SECCA caused restructuring of the IAS and EAS rather than fibrosis and scarring [Herman et al. 2015].
Neosphincter procedures
Patients with severe FI who do not achieve satisfactory results on any of the treatment regimens described above can be considered for neosphincter procedures such as the gracilis muscle transposition (dynamic graciloplasty), implantation of an artificial bowel sphincter or the magnetic anal sphincter. The surgical techniques for these procedures are complex and should only be carried out in selected centres.
Graciloplasty is performed with the patient’s gracilis muscle to create a new sphincter around the anus. To sustain muscle tone, an electrode is placed in the gracilis muscle and connected to a stimulator implanted in the abdominal wall. In a systematic review the success rate of graciloplasty ranged from 42% to 85% [Chapman et al. 2002]. Complications are common and include pain, surgical site infections, and problems related to the electronic device. Hospitalization or surgery were required in 42% of the patients [Matzel et al. 2001].
The artificial bowel sphincter consists of an inflatable cuff placed around the anal canal, a reservoir balloon and a pump positioned in the labia or scrotum connecting the cuff and the balloon. The cuff is filled with fluid to maintain continence and emptied when there is a need to defecate. The rate of success is very variable and adverse events are common. A single centre study from 2011 included 52 patients and the follow up was over 5 years. Fifty percent of the patients required revision, mainly due to a leaking cuff, and 27% were definitively explanted, mainly due to device-related infection.
At the end of follow up, 67.3% had an active device and there were significant improvements in both Wexner incontinence and quality of life scores. The authors concluded that with careful patient selection and meticulous surgical technique this method may still be considered an option for patients with severe FI [Wong et al. 2011b].
The magnetic anal sphincter consists of a ring of titanium beads with internal magnetic cores. It is placed around the EAS. During defecation the beads separate to allow stool passing. Data on the method are very few. The artificial bowel sphincter and the magnetic anal sphincter were compared in a prospective but nonrandomized study with only 10 patients included in each group. Both groups obtained a significant improvement in Wexner incontinence score and quality of life. There were no significant differences in postoperative complications and revision/explanation rates [Lehur et al. 2010; Wong et al. 2011a].
Colostomy
Colostomy is the final option to treat FI, if other therapies fail or if the patient is not suitable for the previously described conservative or surgical procedures. Patients are often reluctant to have a colostomy, assuming that quality of life with a stoma is poor. Several studies show that colostomy formation actually improves quality of life in most. A cross-sectional survey showed that general quality of life and FI-related quality of life scores were significantly improved in patients who had received a stoma due to FI compared with other patients with FI [Colquhoun et al. 2006]. In another retrospective study, 80% of patients with FI stated that they would choose to have the stoma again [Norton et al. 2005].
Treatment algorithm for management of FI
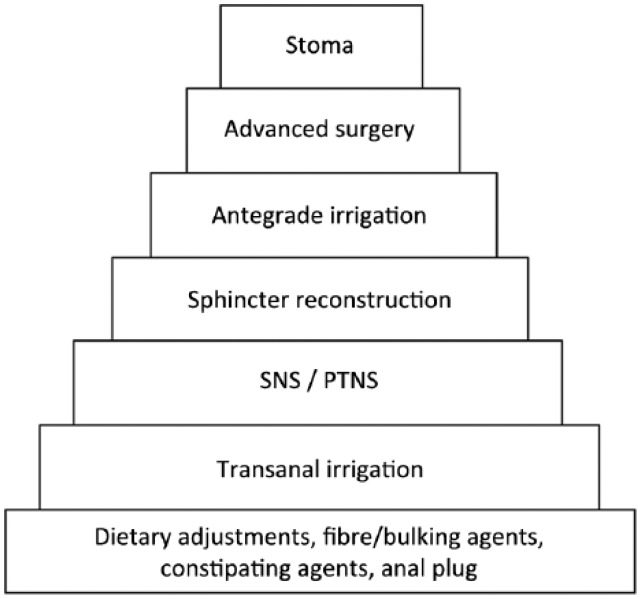
Standard management of FI follows a stepwise approach to achieve patient satisfaction with the most minimal therapeutic intervention (Figure 1). Detailed history and physical examination are mandatory and in most patients treatment should be preceded by endoscopy.
Figure 1.
Management algorithm of faecal incontinence. SNS, sacral nerve stimulation; PTNS, percutaneously using a needle electrode.
In 2010 we implemented a specialist nurse-led programme for the treatment of FI. A colorectal specialist performed a preadmission assessment of referrals and nearly 80% were seen and managed by the specialist nurses without consulting a doctor. Treatment efficacy and patient satisfaction were high. The concept of specialist nurse-led clinics reduced the number of patients requiring evaluation by a colorectal surgeon. We recommend that standard conservative treatment should have been attempted before patients are referred to consult a colorectal specialist [Duelund-Jakobsen et al. 2015]. Anorectal physiology tests and endoanal ultrasound should be reserved for patients not responding to initial conservative treatment.
Dietary advice, dietary fibre supplement and constipating agents are usually tried as first-line therapy. Some patients may benefit from an anal plug, mini enema or biofeedback. Colonic irrigation is especially effective in patients with neurological disorders causing FI but can be attempted in other cases too. Injections of bulging agents can be offered to selected patients with passive incontinence but long-term results are not convincing. SNS is a well documented treatment for FI with good long-term results and complications are at an acceptable level. The role of posterior tibial nerve modulation is unresolved. The SECCA procedure is not a standard procedure in many centres and further research is needed before its place in the treatment algorithm can be established. Complex surgical interventions such as gracilis muscle transposition or artificial sphincter implantation should only be considered in highly selected patients and they are only offered at a few centres internationally. Finally, a colostomy should be considered in patients with severe FI not responding to continence regimes described above (Figure 1).
Acknowledgments
All authors contributed equally to this review.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Jakob Duelund-Jakobsen, Pelvic Floor Unit, Department of Surgery P, Aarhus University Hospital, Tage-Hansens Gade 2, 8000 Aarhus C, Denmark.
Jonas Worsoe, Pelvic Floor Unit, Department of Surgery P, Aarhus University Hospital, Denmark.
Lilli Lundby, Pelvic Floor Unit, Department of Surgery P, Aarhus University Hospital, Denmark.
Peter Christensen, Pelvic Floor Unit, Department of Surgery P, Aarhus University Hospital, Denmark.
Klaus Krogh, Neurogastroenterology Unit, Department of Hepatology and Gastroenterology, Aarhus University Hospital, Denmark.
References
- Abrahamsson H., Antov S., Bosaeus I. (1988) Gastrointestinal and colonic segmental transit time evaluated by a single abdominal x-ray in healthy subjects and constipated patients. Scand J Gastroenterol Suppl 152: 72–80. [DOI] [PubMed] [Google Scholar]
- Abrams P., Andersson K., Birder L., Brubaker L., Cardozo L., Chapple C., et al. Members of Committees and Fourth International Consultation on Incontinence (2010) Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn 29: 213–240. [DOI] [PubMed] [Google Scholar]
- Altomare D., Giuratrabocchetta S., Knowles C., Munoz Duyos A., Robert-Yap J., Matzel K. and European SNS Outcome Study Group (2015) Long-term outcomes of sacral nerve stimulation for faecal incontinence. Br J Surg 102: 407–415. [DOI] [PubMed] [Google Scholar]
- Barisic G., Krivokapic Z., Markovic V., Popovic M. (2006) Outcome of overlapping anal sphincter repair after 3 months and after a mean of 80 months. Int J Colorectal Dis 21: 52–56. [DOI] [PubMed] [Google Scholar]
- Bharucha A., Wald A., Enck P., Rao S. (2006) Functional anorectal disorders. Gastroenterology 130: 1510–1518. [DOI] [PubMed] [Google Scholar]
- Bharucha A., Zinsmeister A., Locke G., Seide B., McKeon K., Schleck C., et al. (2005) Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology 129: 42–49. [DOI] [PubMed] [Google Scholar]
- Bliss D., Jung H., Savik K., Lowry A., LeMoine M., Jensen L., et al. (2001) Supplementation with dietary fiber improves fecal incontinence. Nurs Res 50: 203–213. [DOI] [PubMed] [Google Scholar]
- Bordeianou L., Lee K., Rockwood T., Baxter N., Lowry A., Mellgren A., et al. (2008) Anal resting pressures at manometry correlate with the Fecal Incontinence Severity Index and with presence of sphincter defects on ultrasound. Dis Colon Rectum 51: 1010–1014. [DOI] [PubMed] [Google Scholar]
- Bravo Gutierrez A., Madoff R., Lowry A., Parker S., Buie W., Baxter N. (2004) Long-term results of anterior sphincteroplasty. Dis Colon Rectum 47: 727–731; discussion 731–732. [DOI] [PubMed] [Google Scholar]
- Chapman A., Geerdes B., Hewett P., Young J., Eyers T., Kiroff G., et al. (2002) Systematic review of dynamic graciloplasty in the treatment of faecal incontinence. Br J Surg 89: 138–153. [DOI] [PubMed] [Google Scholar]
- Cheung O., Wald A. (2004) Review article: the management of pelvic floor disorders. Aliment Pharmacol Therapeut 19: 481–495. [DOI] [PubMed] [Google Scholar]
- Christensen P., Bazzocchi G., Coggrave M., Abel R., Hultling C., Krogh K., et al. (2006) A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology 131: 738–747. [DOI] [PubMed] [Google Scholar]
- Christensen P., Krogh K., Buntzen S., Payandeh F., Laurberg S. (2009) Long-term outcome and safety of transanal irrigation for constipation and fecal incontinence. Dis Colon Rectum 52: 286–292. [DOI] [PubMed] [Google Scholar]
- Christensen P., Olsen N., Krogh K., Bacher T., Laurberg S. (2003) Scintigraphic assessment of retrograde colonic washout in fecal incontinence and constipation. Dis Colon Rectum 46: 68–76. [DOI] [PubMed] [Google Scholar]
- Colquhoun P., Kaiser R., Jr, Efron J., Weiss E., Nogueras J., Vernava A., 3rd, et al. (2006) Is the quality of life better in patients with colostomy than patients with fecal incontinence? World J Surg 30: 1925–1928. [DOI] [PubMed] [Google Scholar]
- Cotterill N., Norton C., Avery K., Abrams P., Donovan J. (2011) Psychometric evaluation of a new patient-completed questionnaire for evaluating anal incontinence symptoms and impact on quality of life: the ICIQ-B. Dis Colon Rectum 54: 1235–1250. [DOI] [PubMed] [Google Scholar]
- Crowell M., Schettler V., Lacy B., Lunsford T., Harris L., DiBaise J., et al. (2007) Impact of anal incontinence on psychosocial function and health-related quality of life. Dig Dis Sci 52: 1627–1631. [DOI] [PubMed] [Google Scholar]
- Damon H., Guye O., Seigneurin A., Long F., Sonko A., Faucheron J., et al. (2006) Prevalence of anal incontinence in adults and impact on quality-of-life. Gastroenterol Clin Biol 30: 37–43. [DOI] [PubMed] [Google Scholar]
- Deutekom M., Dobben A. (2012) Plugs for containing faecal incontinence. Cochrane Database Syst Rev 4: CD005086. [DOI] [PubMed] [Google Scholar]
- Ditah I., Devaki P., Luma H., Ditah C., Njei B., Jaiyeoba C., et al. (2014) Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005–2010. Clin Gastroenterol Hepatol 12: 636–43.e1–2. [DOI] [PubMed] [Google Scholar]
- Dobben A., Terra M., Deutekom M., Gerhards M., Bijnen A., Felt-Bersma R., et al. (2007) Anal inspection and digital rectal examination compared to anorectal physiology tests and endoanal ultrasonography in evaluating fecal incontinence. Int J Colorectal Dis 22: 783–790. [DOI] [PubMed] [Google Scholar]
- Drossman D., Li Z., Andruzzi E., Temple R., Talley N., Thompson W., et al. (1993) U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Digest Dis Sci 38: 1569–1580. [DOI] [PubMed] [Google Scholar]
- Duelund-Jakobsen J., Haas S., Buntzen S., Lundby L., Boje G., Laurberg S. (2015) Nurse led clinics can manage faecal incontinence effectively: results from a tertiary referral centre. Colorectal Dis 17: 710–715. [DOI] [PubMed] [Google Scholar]
- Duelund-Jakobsen J., van Wunnik B., Buntzen S., Lundby L., Baeten C., Laurberg S. (2012) Functional results and patient satisfaction with sacral nerve stimulation for idiopathic faecal incontinence. Colorectal Dis 14: 753–759. [DOI] [PubMed] [Google Scholar]
- Duelund-Jakobsen J., van Wunnik B., Buntzen S., Lundby L., Laurberg S., Baeten C. (2014) Baseline factors predictive of patient satisfaction with sacral neuromodulation for idiopathic fecal incontinence. Int J Colorectal Dis 29: 793–798. [DOI] [PubMed] [Google Scholar]
- Duthie H., Bennett R. (1963) The relation of sensation in the anal canal to the functional anal sphincter: a possible factor in anal continence. Gut 4: 179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt V., Kanzler G. (1993) How reliable is digital examination for the evaluation of anal sphincter tone? Int J Colorectal Dis 8: 95–97. [DOI] [PubMed] [Google Scholar]
- Eleouet M., Siproudhis L., Guillou N., Le Couedic J., Bouguen G., Bretagne J. (2010) Chronic posterior tibial nerve transcutaneous electrical nerve stimulation (TENS) to treat fecal incontinence (FI). Int J Colorectal Dis 25: 1127–1132. [DOI] [PubMed] [Google Scholar]
- Evans C., Stevenson A., Sileri P., Mercer-Jones M., Dixon A., Cunningham C., et al. (2015) A multicenter collaboration to assess the safety of laparoscopic ventral rectopexy. Dis Colon Rectum 58: 799–807. [DOI] [PubMed] [Google Scholar]
- Faucheron J., Trilling B., Girard E., Sage P., Barbois S., Reche F. (2015) Anterior rectopexy for full-thickness rectal prolapse: technical and functional results. World J Gastroenterol 21: 5049–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felt-Bersma R., Cazemier M. (2006) Endosonography in anorectal disease: an overview. Scand J Gastroenterol Suppl 243: 165–174. [DOI] [PubMed] [Google Scholar]
- Felt-Bersma R., Poen A., Cuesta M., Meuwissen S. (1997) Anal sensitivity test: what does it measure and do we need it? Cause or derivative of anorectal complaints. Dis Colon Rectum 40: 811–816. [DOI] [PubMed] [Google Scholar]
- Frascio M., Mandolfino F., Imperatore M., Stabilini C., Fornaro R., Gianetta E., et al. (2014) The SECCA procedure for faecal incontinence: a review. Colorectal Dis 16: 167–172. [DOI] [PubMed] [Google Scholar]
- Gallas S., Michot F., Faucheron J., Meurette G., Lehur P., Barth X., et al. (2011) Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: results of trial stimulation in 200 patients. Colorectal Dis 13: 689–696. [DOI] [PubMed] [Google Scholar]
- George A., Kalmar K., Sala S., Kopanakis K., Panarese A., Dudding T., et al. (2013) Randomized controlled trial of percutaneous versus transcutaneous posterior tibial nerve stimulation in faecal incontinence. Br J Surg 100: 330–338. [DOI] [PubMed] [Google Scholar]
- Glasgow S., Lowry A. (2012) Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum 55: 482–490. [DOI] [PubMed] [Google Scholar]
- Gosselink M., Joshi H., Adusumilli S., van Onkelen R., Fourie S., Hompes R., et al. (2015) Laparoscopic ventral rectopexy for faecal incontinence: equivalent benefit is seen in internal and external rectal prolapse. J Gastrointest Surg 19: 558–563. [DOI] [PubMed] [Google Scholar]
- Gourcerol G., Vitton V., Leroi A., Michot F., Abysique A., Bouvier M. (2011) How sacral nerve stimulation works in patients with faecal incontinence. Colorectal Dis 13: e203–e211. [DOI] [PubMed] [Google Scholar]
- Graf W., Mellgren A., Matzel K., Hull T., Johansson C., Bernstein M. and NASHA Dx Study Group (2011) Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet 377: 997–1003. [DOI] [PubMed] [Google Scholar]
- Hallan R., Marzouk D., Waldron D., Womack N., Williams N. (1989) Comparison of digital and manometric assessment of anal sphincter function. Br J Surg 76: 973–975. [DOI] [PubMed] [Google Scholar]
- Hanauer S. (2008) The role of loperamide in gastrointestinal disorders. Rev Gastroenterol Disord 8: 15–20. [PubMed] [Google Scholar]
- Herman R., Berho M., Murawski M., Nowakowski M., Rys J., Schwarz T., et al. (2015) Defining the histopathological changes induced by nonablative radiofrequency treatment of faecal incontinence – a blinded assessment in an animal model. Colorectal Dis 17: 433–440. [DOI] [PubMed] [Google Scholar]
- Hill K., Fanning S., Fennerty M., Faigel D. (2006) Endoanal ultrasound compared to anorectal manometry for the evaluation of fecal incontinence: a study of the effect these tests have on clinical outcome. Dig Dis Sci 51: 235–240. [DOI] [PubMed] [Google Scholar]
- Hollingshead J., Dudding T., Vaizey C. (2011) Sacral nerve stimulation for faecal incontinence: results from a single centre over a 10-year period. Colorectal Dis 13: 1030–1034. [DOI] [PubMed] [Google Scholar]
- Hornung B., Carlson G., Mitchell P., Klarskov N., Lose G., Telford K., et al. (2014) Anal acoustic reflectometry predicts the outcome of percutaneous nerve evaluation for faecal incontinence. Br J Surg 101: 1310–1316. [DOI] [PubMed] [Google Scholar]
- Hussain Z., Lim M., Stojkovic S. (2011) Systematic review of perianal implants in the treatment of faecal incontinence. Br J Surg 98: 1526–1536. [DOI] [PubMed] [Google Scholar]
- Jorge J., Wexner S. (1993) Etiology and management of fecal incontinence. Dis Colon Rectum 36: 77–97. [DOI] [PubMed] [Google Scholar]
- Kiff E., Swash M. (1984) Slowed conduction in the pudendal nerves in idiopathic (neurogenic) faecal incontinence. Br J Surg 71: 614–616. [DOI] [PubMed] [Google Scholar]
- La Torre F., de la Portilla F. (2013) Long-term efficacy of dextranomer in stabilized hyaluronic acid (NASHA/Dx) for treatment of faecal incontinence. Colorectal Dis 15: 569–574. [DOI] [PubMed] [Google Scholar]
- Lauti M., Scott D., Thompson-Fawcett M. (2008) Fibre supplementation in addition to loperamide for faecal incontinence in adults: a randomized trial. Colorectal Dis 10: 553–562. [DOI] [PubMed] [Google Scholar]
- Law P., Kamm M., Bartram C. (1991) Anal endosonography in the investigation of faecal incontinence. Br J Surg 78: 312–314. [DOI] [PubMed] [Google Scholar]
- Lefevre J., Parc Y., Giraudo G., Bell S., Parc R., Tiret E. (2006) Outcome of antegrade continence enema procedures for faecal incontinence in adults. Br J Surg 93: 1265–1269. [DOI] [PubMed] [Google Scholar]
- Lehto K., Hyoty M., Collin P., Huhtala H., Aitola P. (2013) Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis 28: 653–658. [DOI] [PubMed] [Google Scholar]
- Lehur P., McNevin S., Buntzen S., Mellgren A., Laurberg S., Madoff R. (2010) Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum 53: 1604–1610. [DOI] [PubMed] [Google Scholar]
- Leroi A., Siproudhis L., Etienney I., Damon H., Zerbib F., Amarenco G., et al. (2012) Transcutaneous electrical tibial nerve stimulation in the treatment of fecal incontinence: a randomized trial (CONSORT 1a). Am J Gastroenterol 107: 1888–1896. [DOI] [PubMed] [Google Scholar]
- Lestar B., Penninckx F., Kerremans R. (1989) The composition of anal basal pressure. An in vivo and in vitro study in man. Int J Colorectal Dis 4: 118–122. [DOI] [PubMed] [Google Scholar]
- Levitt M., Soffer S., Pena A. (1997) Continent appendicostomy in the bowel management of fecally incontinent children. J Pediatr Surg 32: 1630–1633. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Lundby L., Buntzen S., Laurberg S. (2011) Suboptimal outcome following sacral nerve stimulation for faecal incontinence. Br J Surg 98: 140–147. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Laurberg S., Norton C. (2013) Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev 2: CD007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y., Norton C., Lundby L., Buntzen S., Laurberg S. (2010) Predictors of the outcome of percutaneous nerve evaluation for faecal incontinence. Br J Surg 97: 1096–1102. [DOI] [PubMed] [Google Scholar]
- Malone P., Ransley P., Kiely E. (1990) Preliminary report: the antegrade continence enema. Lancet 336: 1217–1218. [DOI] [PubMed] [Google Scholar]
- Matzel K., Madoff R., LaFontaine L., Baeten C., Buie W., Christiansen J., et al. and Dynamic Graciloplasty Therapy Study Group (2001) Complica-tions of dynamic graciloplasty: incidence, management, and impact on outcome. Dis Colon Rectum 44: 1427–1435. [DOI] [PubMed] [Google Scholar]
- Matzel K., Stadelmaier U., Hohenfellner M., Gall F. (1995) Electrical stimulation of sacral spinal nerves for treatment of faecal incontinence. Lancet 346: 1124–1127. [DOI] [PubMed] [Google Scholar]
- McGuire E., Zhang S., Horwinski E., Lytton B. (1983) Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol 129: 78–79. [DOI] [PubMed] [Google Scholar]
- McHugh S., Diamant N. (1987) Anal canal pressure profile: a reappraisal as determined by rapid pullthrough technique. Gut 28: 1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior C., Bridoux V., Touchais O., Savoye-Collet C., Leroi A. (2015) MRI defaecography in patients with faecal incontinence. Colorectal Dis 17: O62–O69. [DOI] [PubMed] [Google Scholar]
- Mellgren A., Wexner S., Coller J., Devroede G., Lerew D., Madoff R., et al. and SNS Study Group (2011) Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 54: 1065–1075. [DOI] [PubMed] [Google Scholar]
- Michelsen H., Thompson-Fawcett M., Lundby L., Krogh K., Laurberg S., Buntzen S. (2010) Six years of experience with sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 53: 414–421. [DOI] [PubMed] [Google Scholar]
- Mimura T., Kaminishi M., Kamm M. (2004) Diagnostic evaluation of patients with faecal incontinence at a specialist institution. Digest Surg 21: 235–241; discussion 241. [DOI] [PubMed] [Google Scholar]
- Musial F., Enck P., Kalveram K., Erckenbrecht J. (1992) The effect of loperamide on anorectal function in normal healthy men. J Clin Gastroenterol 15: 321–324. [DOI] [PubMed] [Google Scholar]
- Nelson R., Furner S., Jesudason V. (1998) Fecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum 41: 1226–1229. [DOI] [PubMed] [Google Scholar]
- Norton C., Burch J., Kamm M. (2005) Patients’ views of a colostomy for fecal incontinence. Dis Colon Rectum 48: 1062–1069. [DOI] [PubMed] [Google Scholar]
- Norton C., Cody J. (2012) Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev 7: CD002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms L., Degryse A., Janssen P. (1984) Mechanisms of action of loperamide. Scand J Gastroenterol Suppl 96: 145–155. [PubMed] [Google Scholar]
- Papachrysostomou M., Smith A. (1994) Effects of biofeedback on obstructive defecation–reconditioning of the defecation reflex? Gut 35: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier M., Abcarian H., Nelson R. (2007) Malone antegrade continent enema: an alternative to resection in severe defecation disorders. Dis Colon Rectum 50: 22–28. [DOI] [PubMed] [Google Scholar]
- Pretlove S., Radley S., Toozs-Hobson P., Thompson P., Coomarasamy A., Khan K. (2006) Prevalence of anal incontinence according to age and gender: a systematic review and meta-regression analysis. Int Urogynecol J Pelvic Floor Dysfunct 17: 407–417. [DOI] [PubMed] [Google Scholar]
- Queralto M., Portier G., Cabarrot P., Bonnaud G., Chotard J., Nadrigny M., Lazorthes F. (2006) Preliminary results of peripheral transcutaneous neuromodulation in the treatment of idiopathic fecal incontinence. Int J Colorectal Dis 21: 670–672. [DOI] [PubMed] [Google Scholar]
- Ratto C., Parello A., Donisi L., Litta F., De Simone V., Spazzafumo L., Giordano P. (2011) Novel bulking agent for faecal incontinence. Br J Surg 98: 1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read M., Read N., Barber D., Duthie H. (1982) Effects of loperamide on anal sphincter function in patients complaining of chronic diarrhea with fecal incontinence and urgency. Dig Dis Sci 27: 807–814. [DOI] [PubMed] [Google Scholar]
- Rockwood T., Church J., Fleshman J., Kane R., Mavrantonis C., Thorson A. (2000) Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum 43: 9–16; discussion 16–17. [DOI] [PubMed] [Google Scholar]
- Rongen M., Uludag O., El Naggar K., Geerdes B., Konsten J., Baeten C. (2003) Long-term follow-up of dynamic graciloplasty for fecal incontinence. Dis Colon Rectum 46: 716–721. [DOI] [PubMed] [Google Scholar]
- Ruppin H. (1987) Review: loperamide – a potent antidiarrhoeal drug with actions along the alimentary tract. Aliment Pharmacol Therapeut 1: 179–190. [DOI] [PubMed] [Google Scholar]
- Sauter M., Heinrich H., Fox M., Misselwitz B., Halama M., Schwizer W., et al. (2014) Toward more accurate measurements of anorectal motor and sensory function in routine clinical practice: validation of high-resolution anorectal manometry and Rapid Barostat Bag measurements of rectal function. Neurogastroenterol Motil 26: 685–695. [DOI] [PubMed] [Google Scholar]
- Shafik A., Ahmed I., El-Sibai O., Mostafa R. (2003) Percutaneous peripheral neuromodulation in the treatment of fecal incontinence. Eur Surg Res 35: 103–107. [DOI] [PubMed] [Google Scholar]
- Sorensen G., Liao D., Lundby L., Fynne L., Buntzen S., Gregersen H., et al. (2014) Distensibility of the anal canal in patients with idiopathic fecal incontinence: a study with the functional lumen imaging probe. Neurogastroenterol Motil 26: 255–263. [DOI] [PubMed] [Google Scholar]
- Stoller M. (1999) Afferent nerve stimulation for pelvic floor dysfunction. Eur Urol 35(Suppl.): 16. [Google Scholar]
- Sultan A., Kamm M., Hudson C., Nicholls J., Bartram C. (1994) Endosonography of the anal sphincters: normal anatomy and comparison with manometry. Clin Radiol 49: 368–374. [DOI] [PubMed] [Google Scholar]
- Sze E., Hobbs G. (2009) Efficacy of methylcellulose and loperamide in managing fecal incontinence. Acta Obstet Gynecol Scand 88: 766–771. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Garcia-Osogobio S., Valdovinos M., Mass W., Jimenez R., Jauregui L., et al. (2002) Radio-frequency energy delivery to the anal canal for the treatment of fecal incontinence. Dis Colon Rectum 45: 915–922. [DOI] [PubMed] [Google Scholar]
- Thin N., Taylor S., Bremner S., Emmanuel A., Hounsome N., Williams N., et al. Neuromodulation Trial Study Group (2015) Randomized clinical trial of sacral versus percutaneous tibial nerve stimulation in patients with faecal incontinence. Br J Surg 102: 349–358. [DOI] [PubMed] [Google Scholar]
- Thomas G., Dudding T., Bradshaw E., Nicholls R., Vaizey C. (2013) A pilot study to compare daily with twice weekly transcutaneous posterior tibial nerve stimulation for faecal incontinence. Colorectal Dis 15: 1504–1509. [DOI] [PubMed] [Google Scholar]
- Tjandra J., Chan M., Yeh C., Murray-Green C. (2008) Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum 51: 494–502. [DOI] [PubMed] [Google Scholar]
- Vaizey C., Carapeti E., Cahill J., Kamm M. (1999) Prospective comparison of faecal incontinence grading systems. Gut 44: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutvin S., Troost F., Kilkens T., Lindsey P., Hamer H., Jonkers D., et al. (2009) The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil 21: 952-e76. [DOI] [PubMed] [Google Scholar]
- Wexner S., Coller J., Devroede G., Hull T., McCallum R., Chan M., et al. (2010) Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg 251: 441–449. [DOI] [PubMed] [Google Scholar]
- Wong M., Meurette G., Stangherlin P., Lehur P. (2011a) The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum 54:. 773–779. [DOI] [PubMed] [Google Scholar]
- Wong M., Meurette G., Wyart V., Glemain P., Lehur P. (2011b) The artificial bowel sphincter: a single institution experience over a decade. Ann Surg 254: 951–956. [DOI] [PubMed] [Google Scholar]
- Xu X., Menees S., Zochowski M., Fenner D. (2012) Economic cost of fecal incontinence. Dis Colon Rectum 55: 586–598. [DOI] [PubMed] [Google Scholar]