Abstract
Celiac disease (CD) is a common chronic immune disease triggered by gluten. Gliadin peptides pass through the epithelial layers, either paracellularly or transcellularly, to launch a potent adaptive immune response in the lamina propria. This aberrant immune response leads to diverse gastrointestinal and extra-gastrointestinal symptoms. Currently, the only treatment for CD is a strict lifelong adherence to a gluten-free diet (GFD), which can be challenging. An early effect of gluten in CD is an increase in gut permeability. Larazotide acetate, also known as AT-1001, is a synthetic peptide developed as a permeability regulator primarily targeting CD. In vitro studies indicate that larazotide acetate is capable of inhibiting the actin rearrangement caused by gliadin and clinical studies have been conducted using this peptide as a therapy for CD.
Keywords: Caco-2 cells, gliadin, gluten-free diet, intestinal integrity, paracellular permeability, zonulin
Introduction
Celiac disease (CD) is one of the most prevalent chronic immune diseases and is triggered by digestion of gluten (specifically gliadin peptides) in genetically susceptible individuals [Sollid, 2002; Fasano et al. 2003]. CD patients frequently present diverse gastrointestinal (GI) symptoms such as diarrhea and abdominal pain, as well as extra-GI symptoms such as tiredness, anemia, depression and infertility [Pruessner, 1998].
Currently, the only option for CD treatment is strict lifelong adherence to a gluten-free diet (GFD). However, adherence to a GFD is difficult [Rashtak and Murray, 2012]. A recent study from the UK indicates that more than 70% of diagnosed CD patients consume gluten either intentionally or inadvertently [Hall et al. 2013]. Furthermore, a GFD is not enough to suppress the disease or completely alleviate symptoms in a substantial number of patients [Rubio-Tapia and Murray, 2010].
Several in vitro and in vivo studies indicate that paracellular permeability increases in CD. This increased permeability promotes the entrance of gliadin peptides into the lamina propria to trigger a potent adaptive immune response [Visser et al. 2009; Demin et al. 2013].
Larazotide acetate, an eight amino acid peptide, is the first in a novel class of tight junction regulators in CD. Several phase I and II clinical trials have confirmed the safety of this agent and suggest a potential beneficial effect of larazotide acetate on tight junction integrity and paracellular permeability [Paterson et al. 2007; Gopalakrishnan et al. 2012a, 2012b]. Additionally, patients who were treated with larazotide acetate had significantly fewer GI symptoms compared with those taking a placebo. Larazotide acetate also blunted the increase in anti-tissue transglutaminase (tTG) antibodies in response to a prolonged challenge with gluten [Leffler et al. 2012, 2015; Kelly et al. 2013]. This paper examines the clinical trials and in vitro studies related to larazotide acetate in the treatment of CD.
GFD as the treatment for CD
Following a GFD is difficult for most patients due to hidden gluten in food from food contamination in a kitchen, in a restaurant or during processing [Collin et al. 2004]. In the United States, food manufacturers are not required to indicate if gluten is present, though foods must indicate if they contain wheat. There are many foods we would expect to be gluten free but are not due to the now ubiquitous gluten, further adding to the confusion. Manufacturers may voluntarily choose to label food ‘gluten free’, which under US federal regulations must contain less than 20 parts per million (ppm) of gluten [US Food and Drug Administration Rule, 2013]. In addition, following a GFD is more expensive than conforming to a regular diet because gluten-free products are often several times more expensive than regular foods, and gluten-free alternatives may be hard to find in many locations and countries [Lee et al. 2003; Catassi et al. 2007; Lanzini et al. 2009].
A study by Sainsbury and colleagues indicates that a reduced quality of life in CD patients is related to GFD adherence and to psychological factors more than GI symptoms [Sainsbury et al. 2013].
Furthermore, a minority of CD patients do not respond to a GFD. Refractory celiac disease (RCD) patients are in this category. RCD is characterized by persistent malabsorptive symptoms despite strict adherence to a GFD (confirmed by an expert dietician) for at least 6–12 months and the absence of other causes of nonresponsive treated CD [Rubio-Tapia et al. 2010].
Pathogenesis
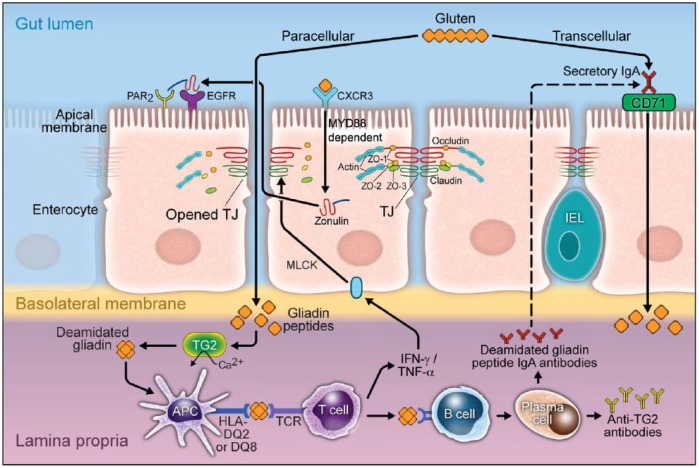
A crucial step in the pathogenesis of CD is the entry of gliadin peptides into the lamina propria where the gluten-reactive T cells reside. The route for gluten peptides to pass through the intestinal barrier is incompletely understood. There are two different described pathways for entry. One is a transcellular pathway described by Matysiak-Bundik and colleagues [Matysiak-Budnik et al. 2008] (Figure 1). This works via retrotransport using gliadin-bound secretory immunoglobulin (Ig) A in the intestinal lumen which binds the transferrin receptor CD71 on the enterocyte. This is then transcytosed, delivering the gliadin peptides to the lamina propria [Schulzke et al. 1998; Heyman and Menard, 2009]. The second route of entry is the paracellular pathway which involves disassembling the epithelial tight junction. Exposure of the mucosal immune system to gliadin, and quite possibly bacteria or bacterial products, trigger a sequence of events that results in an inflammatory cascade and further disruption of the tight junction assembly [Nilsen et al. 1998; Menard et al. 2012] .
Figure 1.
Gliadin peptides pass through the epithelial barrier paracellularly or transcellularly. In the transcellular pathway, the gliadin peptides bind to the secretory IgA at the apical membrane of intestine. Then transferrin receptor CD71 facilitates the delivery of the gliadin peptides to the lamina propria. In the paracellular pathway, the gliadin peptides bind to CXCR3. At the same time, zonulin is released and subsequent transactivation of EGFR by PAR2 leads to disorganization of the tight junction and entrance of gliadin peptides to the lamina propria.
APC, antigen presenting cells; CXCR3, chemokine CXC motif receptor 3; EGFR, epidermal growth factor receptor; HLA, human leukocyte antigen; IgA, immunoglobulin A; IFN-γ, interferon-γ; MYD88, myeloid differentiation factor 88; PAR2, protease activated receptor 2; TCR, T-cell receptor; TG2, transglutaminase 2; TJ, tight junction; TNF-α, tumour necrosis factor-α.
During the active phase of CD, gliadin peptides bind to the chemokine CXC motif receptor 3 (CXCR3) on the luminal side of the intestinal epithelium. This interaction recruits the adapter protein, myeloid differentiation factor 88 (MYD88). Subsequently, activated MYD88 induces the release of zonulin to the lumen. Released zonulin binds to epidermal growth factor receptor (EGFR) and protease-activated receptor 2 (PAR2) in the intestinal epithelium. This complex initiates a signaling pathway that results in phosphorylation of zonula occludens proteins and leads to small intestine tight junction disassembling [Lammers et al. 2008; van der Merwe et al. 2008].
Once in the lamina propria the gliadin peptides launch a strong adaptive immune response. First, gliadin peptides are deamidated by the enzyme tTG that is readily expressed in the lamina propria. These deamidated gliadin peptides are recognized by human leukocyte antigen (HLA) -DQ2 or -DQ8 molecules expressed on antigen presenting cells (APCs). APCs then present the gliadin peptide to CD4+ T cells. Subsequently, activated gluten-CD4+ T cells produce high levels of pro-inflammatory cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), all of which contribute to the development of lesions in the small bowel mucosa along with villous atrophy and crypt hyperplasia [Molberg et al. 2003; Thomas et al. 2006]. A study by Boivin and colleagues showed that activation of phosphatidylinositol 3 kinase (PI3K), and the subsequent activation of nuclear factor-κB (NF-κB) by IFN-γ, causes occludin to disassemble in the intercellular junction and results in an increase in paracellular permeability [Boivin et al. 2009].
Tight junctions
Tight junctions are one of at least four intercellular junctions that regulate the space between epithelial and endothelial cells, and are controlled by a set of over 50 proteins [Chiba et al. 2008]. The intercellular junction complex includes tight junctions, adherens junctions, desmosomes and gap junctions [Ulluwishewa et al. 2011]. The tight junction proteins are highly responsive and selective, opening or closing in response to inflammatory or regulatory cytokines as well as foreign antigens. One foreign antigen that exerts an effect on tight junctions is gliadin, the water insoluble portion of the gluten protein complex. Gliadin has been shown to have a dissociative effect on tight junction proteins, namely actin and ZO-1, which leads to increased gut permeability [Clemente et al. 2003; Sander et al. 2005] .
ZO-1 belongs to the zonula occludens family that also includes ZO-2 and ZO-3. These proteins act as scaffolds for many other junction proteins and also interact with the actin network to control the actin cytoskeleton [Fanning et al. 2012]. Occludin, the first identified tight junction specific protein contains many transmembrane domains and binds to the actin cytoskeleton indirectly through ZO-1 to selectively regulate the permeability of the epithelial lining [Furuse et al. 1993; Mitic et al. 2000]. Claudins are also important for the maintenance of paracellular permeability. This can be seen in mice that are claudin-1 deficient, which die within a day due to their inability to maintain a transepidermal water barrier [Furuse et al. 2002].
Assessing intestinal permeability
In vivo, gut permeability can be altered via numerous autoimmune and inflammatory diseases, including CD and Crohn’s disease. Paracellular permeability testing has been especially well studied in CD [Wyatt et al. 1997; Tibble et al. 2000; Mankertz and Schulzke, 2007]. First degree relatives of patients with active CD are much more likely to have increased gut permeability than the normal population, which may indicate a predisposition to the development of CD in these individuals [van Elburg et al. 1993]. Animal studies also indicate an increase in gut permeability upon exposure to gluten: in the canine model of CD, Irish setter pups develop a gluten dependent enteropathy that resembles CD. These canines also develop increased gut permeability when gluten is added to their diet [Batt et al. 1984; Hall and Batt, 1991].
Presently, the most widely used human test for gut permeability utilizes the monosaccharide, mannitol, and the disaccharide, lactulose. While mannitol is small enough to cross a ‘normal’ intestinal barrier, the disaccharide lactulose is larger and only capable of crossing the epithelial cell barrier during times of increased gut permeability. Thus, measuring the ratio of lactulose to mannitol (LAMA) in the urine indicates the relative absorption of both sugars which can be used to gauge gut permeability. Theoretically, a powerful way to detect changes in macromolecular permeability, the LAMA ratio is subject to several practical constraints [Hollander, 1992; Bjarnason et al. 1995; Demeo et al. 2002]. There may be interfering substances that could affect the assayed levels of sugars, particularly food derived substances. Gut transit of liquids can also be a problem as a liquid bolus can reach the colon within 2 hours, meaning that collection of urine 5 hours post sugar dosing may reflect colonic absorption [Rao et al. 2011].
In vitro, permeability and the factors controlling it can be assessed using several methods. Transepithelial electrical resistance (TEER) and macromolecule flux are the most common methods of measuring intestinal permeability [Piche et al. 2009; Zhou et al. 2010; van Itallie and Anderson, 2011; Yu, 2011]. The epithelial barrier function relies on tight junction proteins, such as ZO proteins, that link the apical membrane proteins (such as occludin and claudin) with cytoskeleton proteins such as actin, thus eliminating intercellular space. Disruption of these actin filaments destroys the tight junction protein network, thereby decreasing TEER [Canil et al. 1993; Hartsock and Nelson, 2008]. Studies demonstrate that exposure to cytokines [e.g. interleukin (IL) 1β, TNF-α and IFN-γ)], some gliadin peptides and AT-1002, a synthetic peptide comprising the first six amino acids of the active fragment of zonula occludens toxin (ZOT) from Vibrio cholera, decrease TEER, indicating a decrease in barrier function [Wang et al. 2006; Gopalakrishnan et al. 2009; Silva et al. 2012]. Another study demonstrates that treatment with the zonulin inhibitor FZI/0 alone did not affect TEER, but the effect of gliadin on TEER reduction was significantly inhibited when the tissue was pretreated with FZI/0 [Fasano et al. 2000; Lu et al. 2000].
Larazotide acetate
Larazotide acetate, also known as AT-1001, is a synthetic peptide that was developed for use in conjunction with a GFD (Figure 2). This octopeptide is related to the originally identified zonula occluden toxin (ZOT) produced by Vibrio cholera. It acts locally to decrease tight junction permeability by blocking zonulin receptors and thus preventing actin rearrangement in response to stimuli [Paterson et al. 2007, Gopalakrishnan et al. 2012a, 2012b].
Figure 2.
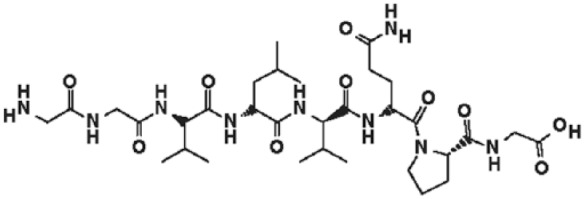
Molecular structure of larazotide acetate.
In vitro effects of larazotide acetate
Intestinal permeability
The apical junctional complex (including the tight junction and adherens junction) disassembles in a medium containing a zero or low calcium concentration. Subsequently, tight junction disassembly causes TEER reduction and paracellular permeability increase [Gumbiner et al. 1988; Gonzalez-Mariscal et al. 1990]. Golpakrishnan and colleagues studied epithelial barrier function in the presence or absence of larazotide acetate (during a calcium switch assay) [Gopalakrishnan et al. 2012b]. The absence of calcium caused an increase in Lucifer yellow (LY) transport that indicates tight junction disorganization. When cells are reintroduced to a high calcium medium (HCM), LY transport significantly decreased. In the presence of larazotide acetate, the transport of LY significantly decreased compared with HCM alone, and the same effect was observed in the transport of fluorescein isothiocyanate (FITC)–dextran. Furthermore, larazotide acetate mediated these effects in a dose-dependent manner. Larazotide acetate also inhibited paracellular permeability by AT-1002 in a dose-dependent manner [Gopalakrishnan et al. 2009, 2012a].
Gliadin translocation and cytokine-induced tight junction dysfunction
In vitro application of larazotide acetate on the apical side of the cells (at concentrations as low as 0.1 mM) reduced passage of FITC-labeled gliadin peptides through the epithelial cell [Watts et al. 2005; Arrieta et al. 2006]. Interestingly, this amount is 100 fold less than that required to suppress the effect of AT-1002. In a study by Gopalakrishnan and colleagues, larazotide acetate inhibited the transport of more than 50% of the gliadin peptides [Gopalakrishnan et al. 2012a], providing evidence that larazotide acetate is an inhibitor of paracellular permeability. Larazotide acetate significantly reduced passage of LY in Caco-2 cells that were treated with a mixture of inflammatory cytokines [Gopalakrishnan et al. 2012a].
Tight junction proteins redistribution
The combinations of tight junction and adherens junction proteins regulate paracellular permeability [Madara et al. 1986; Chiba et al. 2008]. In no calcium medium (NCM), tight junction proteins (ZO-1, claudin-2, claudin-3 and occludin) were in the cytoplasmic vesicle. In HCM, the tight junction proteins were detected at cell–cell junctions (disconnected) and cytoplasmic vesicles. In HCM and in the presence of larazotide acetate, distribution of all the proteins is detected continuously at the cell–cell junction [Gopalakrishnan et al. 2012b].
Actin filaments and E-cadherin have an important role in tight junction formation [Gumbiner et al. 1988]. The presence of larazotide acetate had the same result for actin and E-cadherin, both of which are found in the area around the cell junction. By using biotinylation, Gopalakrishnan and colleagues confirmed that, in the presence of larazotide acetate, the proportion of E-cadherin increased compared with HCM alone. In addition, larazotide acetate did not increase total E-cadherin levels. This indicates that larazotide acetate motivates E-cadherin to the cell junctions. The disruption of actin filaments by gliadin peptides or AT-1002 was prevented by larazotide acetate. Furthermore, actin fluorescence intensity significantly increased in the presence of larazotide acetate compared with AT-1002 alone [Gopalakrishnan et al. 2012b].
Gliadin peptides and AT-1002 changed the structure of actin and ZO-1 proteins in epithelial cells, dissolving the tight junction; this effect was inhibited in the presence of larazotide acetate. The quantification of ZO-1 in the junctional area significantly increased in the presence of larazotide acetate as opposed to AT-1002, which serves to decrease junctional area.
In vivo effects of larazotide acetate on intestinal permeability in HLA-HCD4/DQ8 mice
The results of studies by Silva and colleagues [Silva et al. 2012] and Pinier and colleagues [Pinier et al. 2009]in HLA-HCD4/DQ8 mice suggest that tight junction alteration occurs during gliadin peptides challenges.
In double transgenic HLA-HCD4/DQ8 mice, the gliadin challenge increased horseradish peroxidase (HRP) flux four times more than the control group but, in mice treated with larazotide, the permeability did not change. This decrease implies that larazotide acetate could have an effect on tight junction reassembly during gluten challenges. Increasing macrophage counts in the villi of the gliadin treatment group compared with controls indicate that the innate immune system is activated. The larazotide acetate treatment group showed the same results as the control group [Gopalakrishnan et al. 2012a].
Larazotide acetate in clinical studies
So far, the findings of six clinical trials using larazotide acetate have been published. Three were phase I trials: two trials in healthy individuals and a third in CD subjects that mostly considered the safety of larazotide acetate. Consequently, results from three phase II studies have been published (Table 1).
Table 1.
Published clinical trials (up to August 2015).
| Trial ID | Subject of study | Patients | Gluten challenge | Dose range | Run in phase duration | Active phase duration |
|---|---|---|---|---|---|---|
| CLIN 1001-002 | The safety tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in celiac disease subjects [Paterson et al. 2007] | 21 inpatients | YES 2.5 g (single dose) |
12 mg or placebo (single dose) | – | 3 days |
| CLIN 1001-004 | A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge [Leffler et al. 2012] | 86 outpatients | YES 0.8 g (3 times daily) |
0.25, 1, 4, 8 mg or placebo (3 times daily) |
– | 2 weeks |
| CLIN 1001-006 | Larazotide acetate in patients with coeliac disease undergoing a gluten challenge [Kelly et al. 2013] | 184 outpatients | YES 0.8 g (3 times daily) |
1, 4, 8 mg or placebo (3 times daily) |
7 days | 6 weeks |
| CLIN 1001-012 | Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet [Leffler et al. 2015] | 342 outpatients | NO | 0.5, 1, 2 mg or placebo (3 times daily) |
4 weeks | 12 weeks |
Pharmacokinetic evaluation
In all therapeutic dosages, the plasma levels of larazotide acetate were below the limit of quantification (BLQ) (<0.05 ng/ml). Previously, administration of a high dose (36 mg) in healthy volunteers had the same result. Also, at the highest dose (8 mg) during second phase of study, metabolites were below the limit of quantification [Paterson et al. 2007; Leffler et al. 2012].
Safety and adverse effects
Larazotide acetate has been tested for safety in two clinical trials with healthy subjects. Treatment varying in dosages from 0.25 to 36 mg (single or multiple doses) did not cause any severe adverse events and none of the subjects were dropped from studies due to any adverse events. During the trials, headache was the most common symptom reported by healthy subjects [Fasano and Patterson, 2012]. In the first trial (CLIN 1001-002), CD subjects who were on a GFD for more than 6 months received 12 mg of larazotide acetate and were challenged with gluten. In addition, in two phase II trials, the adverse events of larazotide acetate were comparable with those experienced by placebo groups at all doses [Leffler et al. 2012, 2015; Kelly et al. 2013]. Overall, in almost 500 patients who received larazotide acetate, there was no concern about safety or severe adverse events.
Toxicity in animals
Several studies have been completed in rats and dogs (beagle) concerning the toxicity of larazotide acetate. These tests include: (1) intravenous (IV) administration of a single dose of larazotide acetate in rats and dogs (up to 5 mg/kg); (2) oral administration (gavage) of multiple doses up to 1000 mg/kg for 2–4 weeks in rats; (3) oral administration of 1 dose of 20 and 1 dose of 40 mg/kg in the following day in dogs; and (4) daily oral administration of 9 mg/kg for 4 weeks in dogs. In all, there were no significant changes in clinical chemistry, gross necropsy observations, or any sign of toxicity. When tested on rats, there was no cardiovascular toxicity as a result of larazotide acetate doses up to 1 mg/kg delivered intravenously. It is important to note that dosages in these animal studies were several times higher than therapeutic dosages in human studies [Fasano and Paterson, 2012].
Efficacy
Varying doses of larazotide acetate were studied to assess the clinical efficacy of this drug in CD patients during phase II (Table1). In two trials, CD patients, who were on a GFD, were challenged with gluten. The purpose was to evaluate the effect of the drug on CD patients compared with a situation wherein they intentionally or inadvertently took gluten while on a GFD. In another trial, CD patients did not take gluten. The purpose was to evaluate the effect of larazotide acetate on the symptoms of CD patients who were on GFD for 12 months or longer but still had symptoms. In the response to gluten challenge, in two studies a moderate gluten challenge consisting of 0.8–0.9 g 3 times a day was administered for 2 weeks and in a later study for 6 weeks [Leffler et al. 2012; Kelly et al. 2013].
In the first study (CLIN1001-004), 3 doses of larazotide acetate were administered in 86 patients who were on a GFD for more than 6 months. The patients were randomized to a treatment of larazotide acetate (0.25, 1, 4 or 8 mg) or a placebo, as well as treatment with or without gluten challenges. As the number of patients in the treatment groups was small, and there was a similar trend between the groups that received larazotide acetate and the gluten challenges, these groups were combined as active treatment arms. The primary efficacy outcome of this study was to measure the response rate to gluten challenges by measuring the LAMA fractional excretion ratio. The secondary endpoints compared GI severity symptoms, quality of life, and anti-tTG antibodies [Leffler et al. 2012] (Table 2).
Table 2.
Aims and efficacy results of trial CLIN 1001-004.
| Aims | Efficacy results |
|---|---|
| Primary efficacy outcome: | |
| LAMA ratio | • Between gluten challenge groups, the 1 mg larazotide acetate treatment arm had the least LAMA ratio changes from baseline; however, this was not statistically significant. • LAMA ratio was not significantly greater in the gluten challenge arm compared with the gluten-free control group. • Among patients who received gluten, there were not significant changes between the larazotide acetate treatment groups and the placebo group. |
| Secondary efficacy outcomes: | |
| Gastrointestinal severity symptoms | According to changes in total GSRS and CeD-GSRS score from baseline: • symptoms were more severe in the gluten challenge groups compared with the gluten-free control group (placebo drug/placebo gluten) • symptoms were less severe in larazotide acetate treatment arms compared with the gluten challenge control group (placebo drug/gluten) 0.25 and 4 mg larazotide acetate treatment significantly prevented symptom worsening compared with controls. |
| Quality of life | According to changes in the total PGWBI from baseline: • there was no significant difference between the larazotide acetate treatment arms and the gluten challenge control group |
| Anti-tTG antibodies | • Despite gluten challenges, the anti-tTG antibody titers remained negative (<10 U/ml). • There was no significant difference between the larazotide acetate treatment arms and the gluten challenge control group. |
CeD-GSRS, Celiac Disease Gastrointestinal Rating Symptoms Scale; GSRS, Gastrointestinal Rating Symptoms Scale; LAMA, lactulose to mannitol; PGWBI, Psychological General Well-Being Index; tTG, tissue transglutaminase.
In a second trial (CLIN 1001-006), 184 patients who were on a GFD for more than 6 months undertook a more prolonged gluten challenge (6 weeks). The patients were randomized to larazotide acetate (1, 4, 8 mg) or placebo, both containing gluten challenges. The primary outcome of this was to determine the impact of different doses of larazotide acetate on intestinal permeability (by measuring LAMA ratio) during the 6 weeks. The secondary efficacy outcomes compared GI severity symptoms and anti-tTG antibodies [Kelly et al. 2013]. Table 3 shows the result of trial CLIN 1001-006. There appeared to be an inverse dose-dependent effect. Results based on different dosages were variable: 0.25 and 4 mg of larazotide acetate treatment groups prevented GI symptoms significantly versus placebo, but 1 and 8 mg doses did not [Leffler et al. 2012]. In trial CLIN 1001-006, only the 1 mg treatment group saw significantly decreased GI symptoms induced by gluten compared with placebo. Other treatment groups (dosages of 4 and 8 mg) did not decrease GI symptoms significantly. The higher dosage in trial CLIN 1001-006 did not show the same result [Kelly et al. 2013]. In trial CLIN 1001-004, a Gastrointestinal Symptoms Severity (GSRS) questionnaire was used to evaluate patient GI symptom severity. According to total GSRS changes from baseline, symptoms in the gluten challenge control group were significantly more severe than a different group who were given gluten challenge and also treated with larazotide acetate [Leffler et al. 2012]. The difference in mean total GSRS score between the 1 mg larazotide acetate treatment arm and the placebo group was 0.3–0.4 units. The difference among healthy subjects and CD patients on a GFD was just 0.3 units. In addition, when this change is compared with the GSRS score change in CD patients following a GFD for a year (0.4–0.7 units), we see the significant effect of larazotide acetate [Mustalahti et al. 2002; Midhagen and Hallert, 2003; Kelly et al. 2013].
Table 3.
Aims and efficacy results of trial CLIN 1001-006.
| Aims | Efficacy results |
|---|---|
| Primary efficacy outcome: | |
| LAMA ratio | • There was no significant difference between larazotide acetate treated groups and placebo group. • The 1 mg larazotide acetate treatment group had the changes in LAMA ratio. However, this was not statistically significant. |
| Secondary efficacy outcomes: | |
| Gastrointestinal severity symptoms | According to changes in total GSRS and CeD-GSRS score from baseline: • The 1 mg larazotide acetate treatment group had significantly limited gluten induced symptoms versus placebo. • The mean total GSRS and CeD-GSRS scores increased by 0.3–0.4 unit during gluten challenge in the placebo group. However, the 1 mg treatment group remained close to baseline. • The changes in total GSRS score in the 1 mg treatment group were significantly lower than in the placebo group. The biggest difference (0.5 units) between these two was on the diarrhea subdomain of the GSRS. • The scores in the abdominal pain and indigestion subdomains of GSRS were significantly lower in the 1 mg larazotide acetate treatment group versus the placebo group. • The reduction of total GSRS scores in other treatment groups was not significant versus the placebo group. |
| Anti-tTG antibodies | • The mean of anti-tTG antibodies levels in the placebo group became 19 U/ml after 6 weeks of gluten challenges. However, the mean levels of antibodies in all treatment groups remained less than 10 U/ml (the cutoff for positive). This was significantly lower than the placebo group. |
| Quality of life | • The placebo group had lower PGWBI scores compared with treatment groups (the difference was not significant). |
CeD-GSRS, Celiac Disease Gastrointestinal Rating Symptoms Scale; GSRS, Gastrointestinal Rating Symptoms Scale; LAMA, lactulose to mannitol; PGWBI, Psychological General Well-Being Index; tTG, tissue transglutaminase.
The largest study was published by Leffler and colleagues in 2015 and examined the effect of larazotide acetate in patients who had persistent symptoms of CD despite following a GFD for more than one year [Leffler et al. 2015]. Table 4 shows the result of trial CLIN 1001-012. This study did not have a planned gluten challenge. CD patients were randomized between 4 groups: larazotide acetate dosages of 0.5, 1 and 2 mg, or placebo. The primary endpoint compared severity of symptoms based on the Celiac Disease Gastrointestinal Rating Symptoms Scale (CeD GSRS) scores. Treatment with a 0.5 mg dose significantly decreased GI and extra-GI (including headache and tiredness) symptoms compared with placebo. The secondary endpoint compared CeD GSRS changes from baseline in different groups. The average improvement from baseline CeD GSRS in the 0.5 mg treatment arm was significantly greater than the placebo group. The two other higher dose treatment arms did not show significant response.
Table 4.
Aims and efficacy results of trial CLIN 1001-012.
| Aims | Efficacy results |
|---|---|
| Primary endpoint: | |
| Weekly changes at CeD-GSRS score | • Improvement in CeD-GSRS reported in the 0.5 mg larazotide acetate treatment versus placebo (p = 0.005). However, there was no improvement with 1 and 2 mg doses versus placebo. |
| Secondary endpoints: | |
| Weekly changes from baseline in CeD-GSRS score | • No significant improvement observed for 1 and 2 mg doses versus placebo. • The mean total GSRS decreased in the 0.5 mg larazotide acetate treatment group compared with placebo group (p = 0.004). • The average on GSRS in indigestion, constipation and abdominal pain domains significantly decreased in the 0.5 mg treatment arm versus the placebo group (p = 0.29, 0.05 and 0.29, respectively) |
| Average weekly on treatment score and changes from baseline in the CeD PRO gastrointestinal and abdominal domain scores | • No significant improvement observed for 1 and 2 mg doses versus placebo. • The 0.5 mg larazotide acetate decreased the CeD PRO abdominal domain score compared with placebo (p = 0.041). • The 0.5 mg larazotide acetate reduced more than 50% of weekly symptoms in the CeD PRO abdominal and GI domain compared with placebo (p = 0.022 and 0.002 respectively) • Abdominal pain and extra-gastrointestinal symptoms significantly decreased with treatment of 0.5 mg larazotide acetate compared with placebo. In addition, symptomatic days decreased in 0.5 mg treatment group versus placebo group (p = 0.017) |
GSRS: gastrointestinal rating symptoms scale; CeD-GSRS: celiac disease gastrointestinal rating symptoms scale; PGWBI: Psychological General Well-Being Index; tTG: tissue transglutaminase; CeD PRO: celiac disease patient-reported outcome.
It is clear from these studies that there is an inverse dose effect. The lower doses used were more efficacious than higher doses. Larazotide acetate is not the first drug as a nonabsorbed oral peptide that acts like this. Linaclotide, a drug for the treatment of irritable bowel syndrome with constipation, also has an optimal efficacy in some lower doses [Johnston et al. 2009; Chey et al. 2012]. Some explanations for these converse dose responses include receptor desensitization and peptide aggregation at higher doses.
Intestinal permeability
In order to measure intestinal permeability, a probe solution containing a mixture of lactulose, mannitol and sucrose in water was administered orally on different days during the study. The subjects fasted for a few hours before and during the studies. The fasted subjects were treated with larazotide acetate or the placebo and 30 minutes later, a gluten challenge or placebo was administered. Then half an hour later the probe solution was administered and urine collected for 6–8 hours [Paterson et al. 2007; Leffler et al. 2012].
In a clinical study by Paterson and colleagues, inpatients in the placebo group had a 70% increase in LAMA ratio after gluten challenge, whereas patients in the larazotide acetate treatment group had no change [Paterson et al. 2007]. The other clinical trials that were conducted measured LAMA in an outpatient setting did not show a significant protection from gluten challenge induced increases in permeability. This failure to meet what was the primary endpoint may have been due to an unexpectedly high variability in LAMA ratios performed in a relatively uncontrolled outpatient study. The lower LAMA ratio in the 1 mg treatment than in the placebo group did not reach significance [Leffler et al. 2012; Kelly et al. 2013].
IFN-γ and TNF-α
In trial CLIN 1001-002, after a single dose gluten exposure in treated patients with CD, IFN-γ levels increased in the placebo group by 57%, whereas IFN-γ levels increased by 29% in the larazotide acetate treatment group; this difference was not significant. Also the levels of TNF-α did not change significantly after gluten challenge [Paterson et al. 2007].
Anti-tTG antibodies
In trial CLIN 1001-004, one of the secondary aims was to compare anti-tTG IgA antibodies between treatment groups. All of the anti-tTG antibody titers were less than 10 U/ml at baseline, as well as at the end of 2 weeks of treatment. There were no significant changes between different treatment groups [Leffler et al. 2012]. Another trial compared anti-tTG antibodies of CD subjects (who were on a GFD for at least 6 months) after 6 weeks of gluten challenge [Kelly et al. 2013]. The baseline mean anti-tTG antibody levels were <2 U/ml. After 6 weeks of gluten challenge, 30% of the patients in the placebo group seroconverted; however, the mean antibody levels remained negative in 2 treatments groups (dosages of 1 and 4 mg). The changes in anti-tTG antibody levels were significantly lower in the treatment groups than in the placebo group.
In a third trial (CLIN 1001-012), the CD patients (who were on a GFD more than 1 year) were tested for anti-tTG IgA antibodies. There was no significant difference between treatment groups and the placebo group [Leffler et al. 2015]. These results indicate that changes in anti-tTG antibodies are slow and substantially affected by gluten challenge and duration of exposure.
Place of tight junction regulation by larazotide acetate compared with other drug approaches
To date, no drugs have been approved for the treatment of CD. However, there are several drugs under development, including some in phase II studies. Some of the characteristics of larazotide acetate to compare with other medications in this category include the following.
Like many of the other therapeutic options for CD, larazotide acetate is very unlikely to be a cure for CD.
Similar to other luminal active agents, it will reduce the accessibility of immunogenic peptides to the mucosal immune system.
Similar to agents that work on gluten peptides such as the glutenase approach, it is a short-acting medication, likely lasting only 2–3 hours at a time, and will need to be repetitively dosed before any time patients may be exposed to gluten.
It is most likely to be a maintenance therapy to improve symptoms in patients with a chronic disease and will likely need to be an ongoing long-term therapy. Unlike agents that aim to fully restore tolerance or completely suppress any immune response to gluten, it will not be a passport to eating gluten with impunity.
It is likely to be extremely safe, similar to some of the other potential therapeutic options for CD. However, it will at least be theoretically safer than treatments that aim to interrupt the immune response, which could have the possibility of off-target effects.
Conclusion
A GFD is still the only option for treatment of celiac disease, yet there are many challenges for a GFD to be an effective therapy. Therefore, finding a safe and effective alternative or augmentative treatment is important to improve the quality of life for CD patients. Larazotide acetate is a synthetic peptide that was developed for use in combination with a GFD. So far, clinical phase I and phase II studies confirm this drug’s safety. In clinical trials, larazotide acetate significantly decreased symptoms of gluten compared with placebo in total subjects, especially at some lower doses. The apparent inverse dose effect is curious and will require explanation. In vitro, larazotide acetate reduced paracellular permeability and increased tight junction stability. But based on the variable results in some aspects of dose response and LAMA measurement, there needs to be longer term of examination along with new approaches. Larazotide acetate inhibits the entrance of gliadin peptides to the lamina propria via the paracellular pathway, but may not affect the transcellular pathway. Therefore, gliadin reaches the lamina propria and activates an immune response, albeit to a lesser extent. Overall, according to several clinical trials and in vitro and animal studies, larazotide acetate could be a novel therapeutic candidate for management of CD with long-term benefits in improving the quality of life of patients struggling to be well on a GFD.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Shahryar Khaleghi, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905, USA.
Josephine M. Ju, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905, USA
Abhinav Lamba, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905, USA.
Joseph A. Murray, Professor of Medicine, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905, USA.
References
- Arrieta M., Bistritz L., Meddings J. (2006) Alterations in intestinal permeability. Gut 55: 1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt R., Carter M., McLean L. (1984) Morphological and biochemical studies of a naturally occurring enteropathy in the Irish setter dog: a comparison with coeliac disease in man. Res Vet Sci 37: 339–346. [PubMed] [Google Scholar]
- Bjarnason I., Macpherson A., Hollander D. (1995) Intestinal permeability: an overview. Gastroenterology 108: 1566–1581. [DOI] [PubMed] [Google Scholar]
- Boivin M., Roy P., Bradley A., Kennedy J., Rihani T., Ma T. (2009) Mechanism of interferon-gamma-induced increase in T84 intestinal epithelial tight junction. J Interferon Cytokine Res 29: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canil C., Rosenshine I., Ruschkowski S., Donnenberg M., Kaper J., Finlay B. (1993) Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun 61: 2755–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catassi C., Fabiani E., Iacono G., D’Agate C., Francavilla R., Biagi F., et al. (2007) A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 85: 160–166. [DOI] [PubMed] [Google Scholar]
- Chey W., Lembo A., Lavins B., Shiff S., Kurtz C., Currie M., et al. (2012) Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 107: 1702–1712. [DOI] [PubMed] [Google Scholar]
- Chiba H., Osanai M., Murata M., Kojima T., Sawada N. (2008) Transmembrane proteins of tight junctions. Biochim Biophys Acta 1778: 588–600. [DOI] [PubMed] [Google Scholar]
- Clemente M., De Virgiliis S., Kang J., Macatagney R., Musu M., Di Pierro M., et al. (2003) Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 52: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin P., Maki M., Kaukinen K. (2004) It is the compliance, not milligrams of gluten, that is essential in the treatment of celiac disease. Nutr Rev 62: 490; author reply, 491. [DOI] [PubMed] [Google Scholar]
- Demeo M., Mutlu E., Keshavarzian A., Tobin M. (2002) Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol 34: 385–396. [DOI] [PubMed] [Google Scholar]
- Demin O., Smirnov S., Sokolov V., Cucurull-Sanchez L., Pichardo-Almarza C., Flores M., et al. (2013) Modeling of celiac disease immune response and the therapeutic effect of potential drugs. BMC Syst Biol 7: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A., Van Itallie C., Anderson J. (2012) Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell 23: 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Berti I., Gerarduzzi T., Not T., Colletti R., Drago S., et al. (2003) Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 163: 286–292. [DOI] [PubMed] [Google Scholar]
- Fasano A., Not T., Wang W., Uzzau S., Berti I., Tommasini A., et al. (2000) Zonulin, a newly discovered modulator of intestinal permeability and its expression in coeliac disease. Lancet 355: 1518–1519. [DOI] [PubMed] [Google Scholar]
- Fasano A., Paterson B. (2012) Materials and methods for the treatment of celiac disease. US Patent 20120076861 A1. [Google Scholar]
- Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., et al. (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156: 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S. (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123: 1777–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Contreras R., Bolivar J., Ponce A., Chavez De, Ramirez B., Cereijido M. (1990) Role of calcium in tight junction formation between epithelial cells. Am J Physiol 259: C978–C986. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S., Durai M., Kitchens K., Tamiz A., Somerville R., Ginski M., et al. (2012a) Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides 35: 86–94. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S., Pandey N., Tamiz A., Vere J., Carrasco R., Somerville R., et al. (2009) Mechanism of action of ZOT-derived peptide AT-1002, a tight junction regulator and absorption enhancer. Int J Pharm 365: 121–130. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S., Tripathi A., Tamiz A., Alkan S., Pandey N. (2012b) Larazotide acetate promotes tight junction assembly in epithelial cells. Peptides 35: 95–101. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Stevenson B., Grimaldi A. (1988) The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 107: 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E., Batt R. (1991) Abnormal permeability precedes the development of a gluten sensitive enteropathy in Irish setter dogs. Gut 32: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N., Rubin G., Charnock A. (2013) Intentional and inadvertent non-adherence in adult coeliac disease. A cross-sectional survey. Appetite 68: 56–62. [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman M., Menard S. (2009) Pathways of gliadin transport in celiac disease. Ann N Y Acad Sci 1165: 274–278. [DOI] [PubMed] [Google Scholar]
- Hollander D. (1992) The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn’s disease. Scand J Gastroenterol 27: 721–726. [DOI] [PubMed] [Google Scholar]
- Johnston J., Kurtz C., Drossman D., Lembo A., Jeglinski B., Macdougall J., et al. (2009) Pilot study on the effect of linaclotide in patients with chronic constipation. Am J Gastroenterol 104: 125–132. [DOI] [PubMed] [Google Scholar]
- Kelly C., Green P., Murray J., Dimarino A., Colatrella A., Leffler D., et al. (2013) Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther 37: 252–262. [DOI] [PubMed] [Google Scholar]
- Lammers K., Lu R., Brownley J., Lu B., Gerard C., Thomas K., et al. (2008) Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzini A., Lanzarotto F., Villanacci V., Mora A., Bertolazzi S., Turini D., et al. (2009) Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther 29: 1299–1308. [DOI] [PubMed] [Google Scholar]
- Lee S., Lo W., Memeo L., Rotterdam H., Green P. (2003) Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest Endosc 57: 187–191. [DOI] [PubMed] [Google Scholar]
- Leffler D., Kelly C., Abdallah H., Colatrella A., Harris L., Leon F., et al. (2012) A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol 107: 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler D., Kelly C., Green P., Fedorak R., Dimarino A., Perrow W., et al. (2015) Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology 148: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Wang W., Uzzau S., Vigorito R., Zielke H., Fasano A. (2000) Affinity purification and partial characterization of the zonulin/zonula occludens toxin (Zot) receptor from human brain. J Neurochem 74: 320–326. [DOI] [PubMed] [Google Scholar]
- Madara J., Barenberg D., Carlson S. (1986) Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol 102: 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankertz J., Schulzke J. (2007) Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol 23: 379–383. [DOI] [PubMed] [Google Scholar]
- Matysiak-Budnik T., Moura I., Arcos-Fajardo M., Lebreton C., Menard S., Candalh C., et al. (2008) Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med 205: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard S., Lebreton C., Schumann M., Matysiak-Budnik T., Dugave C., Bouhnik Y., et al. (2012) Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease. Am J Pathol 180: 608–615. [DOI] [PubMed] [Google Scholar]
- Midhagen G., Hallert C. (2003) High rate of gastrointestinal symptoms in celiac patients living on a gluten-free diet: controlled study. Am J Gastroenterol 98: 2023–2026. [DOI] [PubMed] [Google Scholar]
- Mitic L., Van Itallie C., Anderson J. (2000) Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol 279: G250–254. [DOI] [PubMed] [Google Scholar]
- Molberg O., Solheim Flaete N., Jensen T., Lundin K., Arentz-Hansen H., Anderson O., et al. (2003) Intestinal T-cell responses to high-molecular-weight glutenins in celiac disease. Gastroenterology 125: 337–344. [DOI] [PubMed] [Google Scholar]
- Mustalahti K., Lohiniemi S., Collin P., Vuolteenaho N., Laippala P., Maki M. (2002) Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract 5: 105–113. [PubMed] [Google Scholar]
- Nilsen E., Jahnsen F., Lundin K., Johansen F., Fausa O., Sollid L., et al. (1998) Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 115: 551–563. [DOI] [PubMed] [Google Scholar]
- Paterson B., Lammers K., Arrieta M., Fasano A., Meddings J. (2007) The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Curr Opin Gastroenterol 26: 757–766. [DOI] [PubMed] [Google Scholar]
- Piche T., Barbara G., Aubert P., Bruley Des Varannes S., Dainese R., Nano J., et al. (2009) Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58: 196–201. [DOI] [PubMed] [Google Scholar]
- Pinier M., Verdu E., Nasser-Eddine M., David C., Vezina A., Rivard N., et al. (2009) Polymeric binders suppress gliadin-induced toxicity in the intestinal epithelium. Gastroenterology 136: 288–298. [DOI] [PubMed] [Google Scholar]
- Pruessner H. (1998) Detecting celiac disease in your patients. Am Fam Physician 57: 1023–1034, 1039–1041. [PubMed] [Google Scholar]
- Rao A., Camilleri M., Eckert D., Busciglio I., Burton D., Ryks M., et al. (2011) Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol 301: G919–G928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashtak S., Murray J. (2012) Review article: coeliac disease, new approaches to therapy. Aliment Pharmacol Ther 35: 768–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Tapia A., Murray J. (2010) Classification and management of refractory coeliac disease. Gut 59: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Tapia A., Rahim M., See J., Lahr B., Wu T., Murray J. (2010) Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol 105: 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury K., Mullan B., Sharpe L. (2013) Reduced quality of life in coeliac disease is more strongly associated with depression than gastrointestinal symptoms. J Psychosom Res 75: 135–141. [DOI] [PubMed] [Google Scholar]
- Sander G., Cummins A., Henshall T., Powell B. (2005) Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett 579: 4851–4855. [DOI] [PubMed] [Google Scholar]
- Schmitz H., Fromm M., Bentzel C., Scholz P., Detjen K., Mankertz J., et al. (1999) Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci 112 ( Pt 1): 137–146. [DOI] [PubMed] [Google Scholar]
- Schulzke J., Bentzel C., Schulzke I., Riecken E., Fromm M. (1998) Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res 43: 435–441. [DOI] [PubMed] [Google Scholar]
- Silva M., Jury J., Sanz Y., Wiepjes M., Huang X., Murray J., et al. (2012) Increased bacterial translocation in gluten-sensitive mice is independent of small intestinal paracellular permeability defect. Dig Dis Sci 57: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. (2002) Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2: 647–655. [DOI] [PubMed] [Google Scholar]
- Thomas K., Sapone A., Fasano A., Vogel S. (2006) Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol 176: 2512–2521. [DOI] [PubMed] [Google Scholar]
- Tibble J., Sigthorsson G., Bridger S., Fagerhol M., Bjarnason I. (2000) Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 119: 15–22. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R., Mcnabb W., Moughan P., Wells J., Roy N. (2011) Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141: 769–776. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Rule (2013) Food Labeling; Gluten-Free Labeling of Foods, 78 Fed. Reg, 47,154 (August 05, 2013). Available at: https://federalregister.gov/a/2013-18813
- Van der Merwe J., Hollenberg M., Macnaughton W. (2008) EGF receptor transactivation and MAP kinase mediate proteinase-activated receptor-2-induced chloride secretion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 294: G441–451. [DOI] [PubMed] [Google Scholar]
- Van Elburg R., Uil J., Mulder C., Heymans H. (1993) Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 34: 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C., Anderson J. (2011) Measuring size-dependent permeability of the tight junction using PEG profiling. Methods Mol Biol 762: 1–11. [DOI] [PubMed] [Google Scholar]
- Visser J., Rozing J., Sapone A., Lammers K., Fasano A. (2009) Tight junctions, intestinal permeability and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci 1165: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Schwarz B., Graham W., Wang Y., Su L., Clayburgh D., et al. (2006) IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 131: 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T., Berti I., Sapone A., Gerarduzzi T., Not T., Zielke R., et al. (2005) Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A 102: 2916–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J., Oberhuber G., Pongratz S., Puspok A., Moser G., Novacek G., et al. (1997) Increased gastric and intestinal permeability in patients with Crohn’s disease. Am J Gastroenterol 92: 1891–1896. [PubMed] [Google Scholar]
- Yu A. (2011) Electrophysiological characterization of claudin ion permeability using stably transfected epithelial cell lines. Methods Mol Biol 762: 27–41. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Souba W., Croce C., Verne G. (2010) MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 59: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]