Abstract
The sensation of nausea is a common occurrence with diverse causes and a significant disease burden. Nausea is considered to function as a protective mechanism, warning the organism to avoid potential toxic ingestion. Less adaptive circumstances are also associated with nausea, including post-operative nausea, chemotherapy-induced nausea, and motion sickness. A common definition of nausea identifies the symptom as a precursor to the act of vomiting. The interaction, though present, does not appear to be a simple relationship. Nausea is unfortunately the ‘neglected symptom’, with current accepted therapy generally directed at improving gastrointestinal motility or acting to relieve emesis. Improved understanding of the pathophysiological basis of nausea has important implications for exploiting novel mechanisms or developing novel therapies for nausea relief.
Keywords: autonomic nervous system, central nervous system, diagnostics, nausea, neuroendocrine, pathogenesis, therapeutics, vomiting
Introduction
Nausea is a commonly encountered symptom with a broad list of possible causes (Table 1). It has been defined as an ‘unpleasant painless subjective feeling that one will imminently vomit’ [Hasler and Chey, 2003]. While nausea and vomiting are often thought to exist on a temporal continuum, this is not always the case. There are situations when severe nausea may be present without emesis and less frequently, when emesis may be present without preceding nausea. Most individuals report that nausea is more common, more disabling, feels worse and lasts longer than vomiting [Stern et al. 2011]. Despite this, there is a clear understanding of the mechanisms underlying nausea; much of which is about nausea concomitant with emesis. With this in mind, the aim of this review is to examine current knowledge to understand the pathophysiological basis of nausea, review the diagnosis and management, and consider the evidence for traditional and novel therapies.
Table 1.
Common causes of nausea.
| Medications and toxic etiologies | Disorders of the gut and peritoneum |
|---|---|
| Cancer chemotherapy | Mechanical obstruction |
| Analgesics | Gastric outlet obstruction |
| Cardiovascular medications | Small bowel obstruction |
| Digoxin | Functional gastrointestinal disorders |
| Antiarrhythmics | Functional dyspepsia |
| Antihypertensives | Chronic idiopathic nausea |
| β-Blockers | Cyclic Vomiting Syndrome |
| Calcium-channel antagonists | Idiopathic vomiting |
| Hormonal preparations/therapies | |
| Oral antidiabetics | Non-ulcer dyspepsia |
| Oral contraceptives | Irritable bowel syndrome |
| Antibiotics/antivirals | Organic gastrointestinal disorders |
| Erythromycin | Pancreatic adenocarcinoma |
| Tetracycline | Peptic ulcer disease |
| Sulfonamides | Cholecystitis |
| Antituberculous drugs | Pancreatitis |
| Acyclovir | Hepatitis |
| Gastrointestinal medications | Crohn’s disease |
| Sulfasalazine | Neuromuscular disorders of the gastrointestinal tract |
| Azathioprine | Gastroperesis |
| Nicotine | Post-operative nausea and vomiting |
| CNS active | Chronic intestinal pseudo-obstruction |
| Narcotics | CNS causes |
| Antiparkinsonian drugs | Migraine |
| Anticonvulsants | Increased intracranial pressure |
| Malignancy | |
| Radiation therapy | Hemorrhage |
| Infarction | |
| Ethanol abuse | Abscess |
| Meningitis | |
| Infectious causes | Congenital malformation |
| Gastroenteritis | Hydrocephalus |
| Otitis media | Pseudotumor cerebri |
| Acute intermittent porphyria | Seizure disorders |
| Demyelinating disorders | |
| Miscellaneous causes | Psychiatric disease |
| Cardiac disease | Psychogenic vomiting |
| Myocardial infarction | Anxiety disorders |
| Congestive heart failure | Depression |
| Radiofrequency ablation | Pain |
| Starvation | Eating disorders |
| Labyrinthine disorders | |
| Motion sickness | |
| Labyrinthitis | |
| Tumors | |
| Meniere’s disease | |
| Iatrogenic | |
| Endocrinological and metabolic causes | |
| Pregnancy | |
| Other endocrine and metabolic | |
| Uremia | |
| Diabetic ketoacidosis | |
| Hyperparathyroidism | |
| Hypoparathyroidism | |
| Hyperthyroidism | |
| Addison’s disease |
Epidemiology
Nausea is by definition a subjective sensation which poses an inherent limitation in accurately assessing the resultant economic burden. In addition, as nausea often coexists with vomiting, epidemiologic data on nausea alone is sparse. An estimated cost of $4–16 billion to the US economy has been suggested being attributable to nausea and vomiting [Thomas, 2000]. Aside from the economic impact, the psychological toll is also a significant factor [Thomas, 2000].
In population studies, more than 50% of adults reported at least one episode of nausea, and more than 30% of adults reported one episode of vomiting within the preceding 12 months, with women reporting more episodes of nausea than men [Rub, 1992]. In a large population based study of 62651 individuals, 12.5% of the individuals reported nausea as ‘minor or major complaint’ in the last 12 months with the prevalence of nausea being three times higher in women than in men. [Haug et al. 2002]. Similar results have been reported in other epidemiological studies [Walker et al. 1992]. In addition, race has also been shown to be associated with differential rates of the experience of nausea with White/African-Americans experiencing less nausea than Asian/Asian-American subjects [Stern et al. 1993, 1996]
Chronic, unexplained nausea alone or with vomiting occurs less commonly but is associated with significant comorbidity and poses a therapeutic challenge to providers [Pasricha et al. 2011]. The exact prevalence of chronic idiopathic nausea or functional vomiting is not well studied and population-based studies are needed to estimate the disease burden.
Pathophysiology
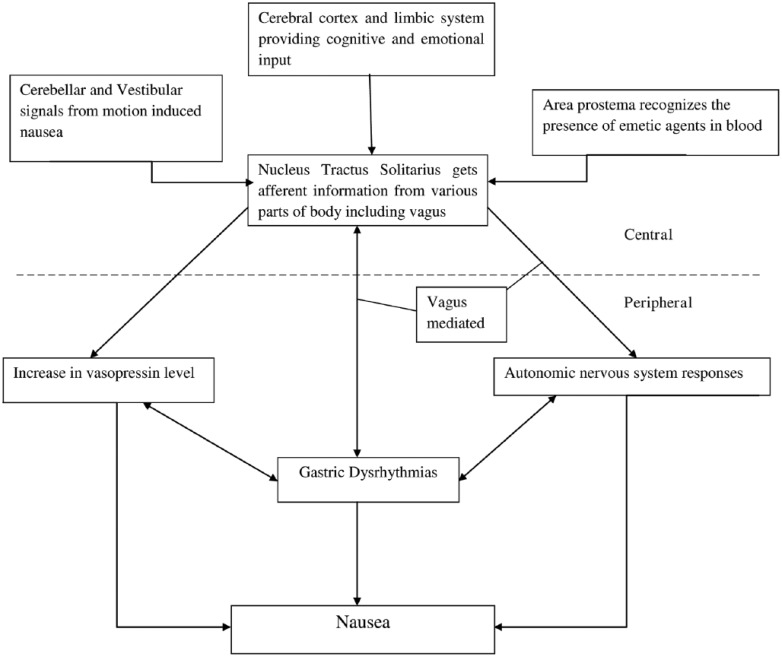
The underlying mechanisms involved in nausea are complex and encompass psychological states, the central nervous system, autonomic nervous system, gastric dysrhythmias, and the endocrine system (Figure 1).
Figure 1.
Pathogenesis of nausea.
Central and peripheral pathways involved in pathogenesis of nausea. Afferent information from various stimuli are relayed to nucleus tractus solitarius via four pathways: vestibular and cerebellar, cerebral cortex and limbic system, area postrema and gastrointestinal tract via vagus nerve.*
*Once any of these neural pathways are activated, it culminates into sensation of nausea with or without vomiting. The efferent information from nucleus tractus solitarius is also responsible for activation of autonomic nervous response via vagal pathways. Nausea is also associated with gastric dysrhythmia and release of vasopressin. However, the cause–effect relationship of this triad is not very well understood and warrants further studying.
In order to understand the pathophysiology underlying nausea, it is important to introduce the concept of the dynamic threshold [Stern, 2002]. It is proposed that each individual has a threshold for nausea that changes minute by minute. At any given moment, the threshold depends on the interaction of certain inherent factors of the individual with the more changeable psychological states of anxiety, anticipation, expectation and adaptation [Stern, 2002]. This dynamic interaction likely explains the inter- and intra-individual variability that is typically encountered in the face of a nauseogenic stimulus [Stern, 2002].
Stimuli giving rise to nausea and vomiting originate from visceral, vestibular, and chemoreceptor trigger zone inputs which are mediated by serotonin/dopamine, histamine/acetylcholine and serotonin/dopamine, respectively. These relationships serve as the basis on which current pharmacological therapy for nausea and vomiting is recommended [Chepyala and Olden, 2008].
Central nervous system
Despite the prevalence and importance of nausea, surprisingly little is known regarding the central mechanisms underlying this sensation [Kowalski et al. 2006; Napadow et al. 2013]. The neurocircuitry involved in emesis is better characterized. Associated autonomic changes which occur during nausea and emesis (e.g. salivation, sweating) are coordinated at the level of the medulla oblongata [Hornby, 2001]. Chemosensitive receptors detect the presence of emetic agents in the blood and this information is relayed via the area postrema to the nucleus tractus solitarius (NTS) [Hornby, 2001]. Abdominal vagal afferents which detect gastric tone and contents also project to the NTS [Hornby, 2001]. Neurons from the NTS then project to a central-pattern generator which coordinates the various actions involved in the act of emesis in addition to directly projecting to neurons in the ventral medulla and hypothalamus, from which higher brain areas can be reached [Hornby, 2001].
Many studies have suggested that the cerberal cortex is also involved in pathways of nausea [Miller, 1999; Napadow et al. 2013]. Recent investigations using functional magnetic resonance imaging techniques in healthy adults have shown that the medial prefrontal cortex and the pregenual anterior cingulate cortex, areas of the brain involved in higher cognitive function and emotion, are positively correlated with an increase in heart rate during nausea, suggesting the importance of cognitive and emotional centers in modulating the parasympathetic to sympathetic shift associated with nausea [Kim et al. 2011; Napadow et al. 2013]. Napadow and colleagues studied humans predisposed to motion sickness and suggested that nauseogenic stimulus causes activation of amygdala, putamen and locus ceruleus which translates into fear conditioning and emotional triggering. This ultimately leads to the sensation of strong nausea [Napadow et al. 2013]. This is followed by continued, sustained activation in cortical areas such as insula, anterior cingulated cortex, nucleus accumbens, orbitofrontal, somatosensory and prefrontal cortex. These areas are involved in the interoceptive, limbic, somatosensory and cognitive network which alerts the suffering individual of the changes in interoceptive signaling so that appropriate autonomic and motor responses are initiated in a timely manner [Napadow et al. 2013]. Many of these areas involved in the nausea circuit specifically anterior cingulate cortex, insular cortex, nucleus accumbens and amygdala are known to be involved in processing of acute as well as chronic painful stimulus [Borsook et al. 2010; Doan et al. 2015]. Furthermore, the medial prefrontal cortex, which appears to be much more involved in chronic pain than acute pain perception, was also found to be a part of the nausea circuit [Baliki et al. 2006; Hashmi et al. 2013]. It is plausible that the brain perceives peripheral noxious stimuli through similar pathways, which in some cases lead to chronic pain and in others to chronic nausea. Understanding the central mechanisms of nausea, especially chronic unexplained nausea will be important for the development of therapies in the treatment of chronic nausea.
Autonomic nervous system
Characteristic physiological changes (sweating, pallor, salivation, increase in blood pressure, tachycardia, cutaneous vasoconstriction, decreased gastrointestinal motility) occurring before vomiting are mediated by the autonomic nervous system (ANS) and are well described [Horn, 2008]. Afferent signaling arises from vagal inputs, reflecting mechanical or chemical stimuli [Horn, 2008, 2014]. Several studies have now shown that increasing nausea perception is associated with decreased parasympathetic and increased sympathetic modulation which accounts for majority of abovementioned symptoms [Muth, 2006; LaCount et al. 2011]. In addition, LaCount and colleagues have shown that bursts of cardiovagal modulation precedes transition to a higher level of nausea, perhaps by prompting interoceptive re-evaluation by the subject culminating in rating nausea at a higher level [LaCount et al. 2011]. This autonomic outflow during nausea is likely modulated by the central nervous system (CNS). While some areas of the brain, such as insula appear to be modulating both sympathetic as well as parasympathetic response, there also appears to be a divergent central control for autonomic response of nausea [Sclocco et al. 2014]. Thus, ANS outflow and the CNS network controlling it could be determinant of overall nausea intensity, and understanding them in more detail could be of therapeutic importance.
Endocrine
Several hormones have been studied in the pathogenesis of nausea with the most important being vasopressin. Increase in vasopressin secretion in various emetogenic situations is clear evidence that the rise in vasopressin level precedes emesis, indicating that this rise does not appear in response to volume depletion or hyperosmolarity. [Fisher et al. 1982; Feldman et al. 1988; Koch et al. 1990; Koch, 1997]. Studies have also reported a positive correlation between serum vasopressin level and nausea intensity [Page et al. 1990; Caras et al. 1997]. However, the cause–effect relationship between nausea and vasopressin is still not clear. Although some studies have shown that supra-physiological levels of vasopressin can induce gastric dysrhythmias and nausea whether this happens under normal physiological circumstances is not clear [Caras et al. 1997; Kim et al, 1997]. The precise temporal relationship between vasopressin, gastric dysrhythmias and nausea should be studied in order to give us insight into pathogenesis of acute and chronic nausea syndromes. In addition to vasopressin, corticotropin-releasing factor (CRF) has been established as brain–gut mediator in foregut function and can stimulate inhibitory motor nerves in the dorsal motor nucleus of the vagus, causing delayed gastric emptying and nausea [Taché, 1999].
Gastric dysrhythmias
The stomach is a neuromuscular organ, the myoelectrical activity of which can be measured by a number of techniques including electrogastrography. Normal gastric myoelectrical activity reflects the balance of the intrinsic pacemaker activity of the stomach, smooth muscle, the enteric nervous system, the ANS and hormone levels [Koch, 1997]. Activity frequency slower than the intrinsic rate is termed bradygastria; a faster frequency is termed tachygastria. There are numerous studies demonstrating the relationship of nausea with the onset of dysrhythmias in individuals with motion sickness, pregnant females, drug-induced nausea and gastroparesis [Xu et al. 1993; Hasler et al. 1995; Coleski and Hasler, 2009; Koch, 2014]. Xu and colleagues have shown that in individuals experiencing vection-induced nausea, experienced tachygastria a few minutes beforehand, suggesting a relationship between gastric dysrhythmias and nausea [Xu et al. 1993]. Interestingly, medications and interventions that promote normalization of myoelectrical activity from tachygastria decrease nausea, and conversely, stimuli that decrease normal myoelectrical activity and increase dysrhythmias promote the sensation of nausea [Koch, 2014]. Whether activation of sympathetic nervous system precedes onset of gastric dysrhythmias or vice versa is not clear and needs to be studied in future [Muth, 2006; Koch, 2014]. High amplitude, retrograde persistaltic contractions of small intestine from the distal to the proximal part on antroduodenal manometry has been shown to precede vomiting episodes [Thompson and Malagelada, 1982]. However, the causal relationship between gastric dysrhythmias or small intestinal dysmotility and nausea is not well established.
Diagnosis
Detailed history and physical exam forms the cornerstone of evaluating patients with the chief complaint of nausea. History and physical exam help to rule out the nongastrointestinal causes of nausea such as CNS, endocrinological and psychiatric diagnoses, which might be the primary cause or could be a contributing factor (Table 1) [Hasler and Chey, 2003]. The mainstay of diagnostic evaluation of the patient with nausea and vomiting is correcting any symptom consequences (electrolyte abnormalities, dehydration, malnutrition), identifying the cause of the symptoms and initiating targeted therapy, and lastly, if no cause is found, initiating therapy directed at suppressing the symptoms [Hasler and Chey, 2003]. There are no well-designed, controlled trials to guide diagnostic evaluation in patients with acute or chronic nausea. Basic blood work, including electrolytes, liver function tests, pancreatic enzymes and pregnancy test (wherever applicable) should be performed to help establish etiology. If mechanical obstruction is suggested by clinical presentation, radiological investigations such as abdominal X-ray and abdominal CT scan are typically the first-line investigations [Hasler and Chey, 2003]. If mucosal diseases such as ulcer or mass are suspected, esophagogastroduodenoscopy remains the most sensitive and specific investigation to study the esophageal, gastric and duodenal mucosa. If needed, small bowel mucosa can be studied by small bowel follow through and enteroclysis.
Scintigraphic measures of solid phase gastric emptying (such as 99mTc-sulfur colloid in egg) are commonly used to evaluate gastric motion function in suspected gastroparesis [Camilleri et al. 2013]. Following the test for four instead of two hours has been shown to improve accuracy in diagnosing gastroparesis [Tougas et al. 2000]. One should remember that these studies do not establish cause–effect relationship in patients with nausea. Their utility is further limited by the finding that symptoms are not well correlated with abnormalities of gastric emptying and medications to improve motility have been shown to improve symptoms without changing emptying delay [Hasler and Chey, 2003]. In general, most providers would advocate an empirical trial of antiemetic or prokinetic medications prior to specialized testing [Hasler and Chey, 2003]. Moreover, many of these patients need to be on antiemetics to be able to tolerate these tests. However, many of these agents, specifically prokinetics, can alter gut motility. Thus, it is important to interpret the results in the context of being on or off medication. Investigations such as cutaneous electrogastrography and antroduodenal manometry to study gastric motor functions are not widely available, are expensive and their role in the diagnostic algorithm of patients with chronic nausea is not well established [Hasler and Chey, 2003; Parkman et al. 2003]. A reduction or decrease of expected postprandial electrogastric amplitude has been shown to correlate with delayed gastric emptying [Chen et al. 1996; Parkman et al. 2003]. Similarly, postprandial antral hypomotility on atroduodenal manometry is a common finding in patients with unexplained nausea, as well as gastroparesis, and could be used if other tests for gastric motility are normal in a patient with persistent symptoms despite empirical treatment [Kerlin, 1989; Thumshirn et al. 1997; Quigley et al. 2001]. It aids in the diagnosis of motor disorders in such cases but when normal, helps to rule out dysmotility as a cause of nausea.
Patients with unexplained chronic nausea (even after thorough investigation including normal gastric emptying studies) pose a diagnostic dilemma and they remain indistinguishable from those with documented gastric delay with respect to demographics, stability of symptoms over time, healthcare utilization and health-related quality of life [Pasricha et al. 2011]. In the absence of an identifiable cause, Rome III criteria for functional gastroduodenal disorders can be utilized to diagnose functional disorders related to nausea and vomiting, including chronic idiopathic nausea, functional vomiting, cyclical vomiting syndrome and rumination syndrome. Pasricha and colleagues showed that current Rome III diagnostic criteria may be inadequate for differentiating this group of patients, with significant overlap of diagnoses; many patients met diagnostic criteria for functional dyspepsia, irritable bowel syndrome and chronic idiopathic nausea/functional vomiting [Pasricha et al. 2011]. Further refinement will be guided by evolving experience and knowledge of this specific group of patients.
Management
A distinction should be made with respect to management of acute versus chronic symptoms as they are likely entirely different entities and response to therapeutics differ between the two. There is a paucity of literature evaluating the pharmacological therapy of chronic, unexplained nausea [Quigley et al. 2001]. This is likely related to the fact that most clinically encountered episodes of nausea and vomiting are typically short lived and self-limited [Quigley et al. 2001]. Most of the literature is focused on those clinical situations where the risk of nausea and vomiting is high, such as in pregnancy, the post-operative time period, post chemotherapy, and post radiation [Quigley et al. 2001]. It is important to realize the inconsistent effect on the relief of nausea compared with vomiting, with nausea being more resistant to interventions. This finding likely reflects the different physiology of these two distinct symptoms [Quigley et al. 2001]. Effect of various antiemetics on different types of nausea, e.g. benzodiazepines for anticipatory nausea and serotonin antagonists for chemotherapy-induced nausea again highlights the complex pathophysiology of nausea.
Current medical therapy generally falls into two categories: agents directed at suppressing nausea and preventing vomiting (antiemetic) which typically act centrally, and agents directed at modulating gastrointestinal motility (prokinetic). Commonly utilized antiemetics are listed (Table 2).
Table 2.
Common antiemetic agents.
| Medications | Typical dosage | Side effects | Route of administration |
|---|---|---|---|
| Anticholinergics | |||
| Scopolamine | 0.3–0.6 mg q24 hours | Tachycardia, confusion, dry mouth, constipation, urinary retention, blurred vision | SL, IV, IM, transdermal |
| Antihistamines | |||
| Meclizine Diphenhydramine Cinnarizine Cyclizine Hydroxyzine |
25–50 mg q24 hours 25–50 mg q6–8 hours 25–75 mg q8 hours 25–50 mg q4–6 hours 25–100 mg q6–8 hours |
Drowsiness, confusion, blurred vision, constipation, urinary retention | Oral Oral, IM, IV Oral Oral Oral, IM |
| Phenothiazines | |||
| Prochlorperazine Promethazine Chlorpromazine Perphenazine |
5–10 mg q6–8 hours 12.5 –25 mg q4–6 hours 10–25 mg q4–6 hours 4–8 mg q8–12 hours |
Extrapyramidal side effects, tardive dyskinesias, neuroleptic malignant syndrome, hyperprolactinemia, QT prolongation | Oral, IM, IV, rectal Oral, IM, IV, rectal Oral, IM, IV Oral |
| Benzamides | |||
| Metoclopramide Domperidone |
10–20 mg q6–8 hours 10 mg q8–24 hours |
Sedation Anxiety Mood disturbances Sleep disruption Dystonic reactions Tardive dyskinesia Galactorrhea Sexual dysfunction |
Oral, IM, IV Oral |
| 5-HT3 antagonists | |||
| Ondansetron Granisetron Palonosetron |
4–8 mg q4–8 hours 1–2 mg q24 hours 0.075–0.25 mg q24 hours |
Headache, fatigue, malaise, constipation |
Oral, IV Oral, IV, transdermal IV |
| Cannabinoids | |||
| Dronabinol Nabilone |
2.5–10 mg q6–8 hours 1–2 mg q8–12 hours |
Palpitations, tachycardia, vasodilation/facial flushing, euphoria, abnormal thinking, dizziness, paranoia, depersonalization, hallucinations, visual changes | Oral Oral |
| Benzodiazepines | |||
| Lorazepam Alprazolam |
0.5–2 mg 0.25–1 mg |
Ataxia, cognitive dysfunction, depression, dizziness, drowsiness, dysarthria, fatigue, irritability, memory impairment, sedation | Oral, SL, IM, IV Oral |
| Corticosteroids | |||
| Dexamethasone | 4–8 mg q4–6 hours | Emotional instability, acne, bruising, hyperglycemia, adrenal suppression, Cushing’s syndrome | Oral, IM, IV |
| Butyrophenones | |||
| Droperidol | 0.625–1.25 mg q24hours | QTc prolongation, orthostatic hypotension, extrapyramidal symptoms, CNS effects | IM, IV |
| NK-1 Receptor Antagonists | |||
| Aprepitant | 80–125 mg q24 hours | Fatigue, constipation, hiccups | Oral |
SL, sublingual; IV, intravenous; IM, intramuscular; CNS, central nervous system.
Serotonin 5-HT3 antagonists such as granisetron and ondansetron have utility in post-operative vomiting, post-radiation therapy, and in preventing chemotherapy-related emesis [Hasler and Chey, 2003]. Their mechanism of action is mediated primarily though central 5-HT3 receptor blockade (mainly in the chemoreceptor trigger zone) and peripheral blockade of 5-HT3 receptors on intestinal vagal and spinal afferent nerves [Bodis et al. 1994; Hornby, 2001; Chepyala and Olden, 2008]. Their effect in relieving nausea is less robust and prevalence of chemotherapy-induced nausea has increased despite the introduction of potent 5-HT3 antagonists [Roscoe et al. 2000, 2000]. Its utility in other forms of nausea, such as gastroparesis and functional vomiting, is not well studied and it has not been shown to be superior to metaclopramide or promethazine in a double-blinded randomized controlled trial in controlling nausea symptoms in adults visiting the emergency department. [Hasler and Chey, 2003; Barrett et al. 2011; Camilleri et al. 2013]
Antihistamines have a therapeutic role in motion sickness and labrynthitis and exert their antiemetic action through central anticholinergic (M1 receptor) and antihistamine (H1 receptor) effects [Flake et al. 2004; Chepyala and Olden, 2008]. These drugs suppress labyrinthine and vestibular stimulation and that of the chemoreceptor zone in the brainstem [Flake et al. 2004; Chepyala and Olden, 2008].
Anticholinergic agents act centrally via the muscarinic receptors and block the pathway from the inner ear to the brainstem and the ‘vomiting center’ [Golding and Stott, 1997; Chepyala and Olden, 2008]. Scopolamine is the most widely used anticholinergic and is administered as a transdermal patch for both prophylaxis and treatment of motion sickness, but its use in other forms of nausea are not well established [Quigley et al. 2001]. Selective M3 and M5 antagonist (Zamifenacin) appears to be equally effective implicating these two receptor subtypes [Golding and Stott, 1997; Chepyala and Olden, 2008].
Phenothiazines are antidopaminergic agents which act via nonselective inhibition of mainly D2 and D3 receptors in the region of the area postrema, but also muscarinic and H1 receptors [Sanger and Andrews, 2006; Chepyala and Olden, 2008]. They have demonstrated efficacy in treating nausea related to migraine, motion sickness and vertigo, as well as post-operative and post-chemotherapy nausea and vomiting [Quigley et al. 2001]. The butyrophenone, droperidol, is only available as a restricted use drug by the FDA, primarily due to its effects on QT prolongation. Its efficacy is well documented in post-operative and chemotherapy-associated nausea and vomiting and like phenothiazines, the mechanism of action is primarily via antidopaminergic activity in the chemoreceptor zone [Quigley et al. 2001; Chepyala and Olden, 2008].
Cannabinoids have been investigated in chemotherapy-related nausea and vomiting [Herman et al, 1979]. They are thought to act primarily via the cannabinoid receptor (CB1) in the ‘vomiting center’ of the medulla and the area subpostrema of the NTS, although potential to modulate 5-HT3 activation in nodose ganglions and substance P release in the spinal cord could also contribute to their antiemesic activity [Sanger and Andrews, 2006; Chepyala and Olden, 2008]. Like many of the other antiemetic agents, the antinausea effect of cannabinoids is not as well established as their antiemetic effect [Sanger and Andrews, 2006].
Benzodiazepines have been investigated as adjunctive therapy in post-operative nausea and small reports have shown that its use reduces anticipatory nausea associated with chemotherapy [Hasler and Chey, 2003; Rodola, 2006]. It is postulated that the antiemetic mechanism of action involves dopamine in the chemoreceptor trigger zone [Di Florio and Goucke, 1993; Takada et al. 1993; Rodola, 2006]. Its primary mode of action is via its sedative, anxiolytic, and amnestic properties in reducing the anticipatory component of nausea [Cooper and Gent, 2002; Chepyala and Olden, 2008].
Corticosteroids are often used concomitantly with 5-HT3 antagonists and other agents for acute, as well as delayed chemotherapy-induced nausea and vomiting. They have also been shown to have efficacy equivalent to ondansetron and droperidol in post-operative nausea [Apfel et al. 2004]. The mechanism of action is unclear but most likely involves its effect on prostaglandin formation and inflammation [Sanger and Andrews, 2006; Chepyala and Olden, 2008].
The role of tachykinin peptides such as substance P in the vomiting reflex has been exploited therapeutically, with aprepitant, an antagonist of the tachykinin receptor NK1. Aprepitant is FDA approved for the prevention of both acute, as well as delayed chemotherapy-induced nausea and vomiting and has been showed to potentiate the effects of 5-HT3 receptor antagonists and corticosteroids [Madsen and Fuglsang, 2008; Curran and Robinson, 2009; Roila et al. 2010]. Case reports have demonstrated the use of aprepitant in the treatment of gastroparesis-associated nausea and vomiting with no demonstrated change in gastric emptying [Chong and Dhatariya, 2009; Fahler et al. 2012]. Formal investigation into the efficacy of aprepitant in the treatment of chronic, refractory nausea and vomiting is needed.
Prokinetic medications are listed (Table 3) and include agents that act primarily as a prokinetic (e.g. erythromycin) versus those that have both prokinetic and antiemetic properties (e.g. metoclopramide). Erythromycin exerts its effects via activation of motilin receptors present on gut smooth muscle, possibly leading to modulation of vagal nerve pathways involved in emesis [Javid et al, 2013]. At low doses of 50–100 mg before meals, it has been shown to be efficacious in controlling nausea and vomiting in patients with delayed gastric emptying. However, when used as antibiotic at higher doses (250–500 mg, 2–4 times a day), it induces nausea, possibly with vomiting [Javid et al. 2013]. At higher concentrations it probably promotes nausea by contracting the gastric fundus and thus inducing gastric dysrythmias and prolonged, nonpropulsive hypermotility of the antrum [Tack et al. 1992; Bruley des Varannes et al. 1995; Coulie et al. 1998; Javid et al. 2013]. Recently, ghrelin has been shown to increase gastric emptying in patients with diabetic gastroparesis [Murray et al. 2005]. It has also been shown to control nausea in ferrets exposed to cisplatin; but its effect on nausea in humans is not yet studied [Rudd et al. 2006]. However, it is important to realize that nausea likely has both peripheral and central components, and the prokinetic mechanism of action is likely restricted to the periphery. Metoclopramide and domperidone are benzamides with potent antiemetic and prokinetic properties. Their mechanism of action is complex and involves vagal and central 5-HT3 and D2 receptor antagonism with prokinetic properties via gut dopamine receptor antagonism and 5-HT4-receptor agonist activity. They are differentiated by the fact that domperidone does not cross blood–brain barrier and is thus free of extrapyramidal side effects which are common with metaclopramide. Domperidone is not available in the US but metaclopramide is manufactured for both oral and parenteral use. These drugs have been shown to be efficacious in post-chemotherapy vomiting and gastroparesis. Clinically, the use of prokinetic agents is unfortunately limited by numerous side effects, some of which may be irreversible.
Table 3.
Prokinetic agents.
| Agent | Typical Dosage | Side Effects | Route of administration |
|---|---|---|---|
| Metoclopramide | 10–20 mg q6–8 hours | Sedation Anxiety Mood disturbances Sleep disruption Dystonic reactions Tardive dyskinesia Galactorrhea Sexual dysfunction |
Oral, IM, IV |
| Domperidone | 10mg q8–24 hours | Galactorrhea/gynecomastia Sexual dysfunction |
Oral |
| Erythromycin | 250–500mg q8 hours | Nausea and vomiting Diarrhea |
Oral, IV |
IV, intravenous; IM, intramuscular.
Novel therapies
Novel and nontraditional medical therapies for nausea and vomiting have been studied (Table 4), and include low-dose antidepressants such as tricyclic antidepressants (TCAs). Retrospective studies have showed moderate symptom reduction in patients with chronic nausea and vomiting including cyclical vomiting syndrome in both diabetic and non-diabetic population; however, given lack of good prospective studies, their use is typically reserved for those with moderate to severe or refractory symptoms [Prakash et al. 1998; Prakash and Clouse, 1999; Namin et al. 2007; Sawhney et al. 2007]. In a retrospective study of 37 patients with chronic functional nausea, 51% patients had a complete response and an additional 33% had at least moderate symptoms reduction with low dose TCAs [Prakash et al. 1998]. TCAs such as amitriptyline have been shown to have some benefit in functional upper gastrointestinal symptoms such as painful dyspepsia in patients who do not have prolonged gastric emptying [Talley et al. 2015]. This is likely true for other symptoms such as chronic nausea where TCAs are likely to benefit only those patients without delayed gastric emptying.
Table 4.
Novel and non-traditional therapies for nausea.
| Agent | Mechanism of action | Typical dosage | Side effects | Route of administration |
|---|---|---|---|---|
| Tricyclic antidepressants Amitriptyline Nortriptyline Doxepin Desipramine Imipramine |
Antihistaminic and muscarinic activity | 10–100 mg/day is the common range for most of these | Constipation, gastric emptying delay, agitation, sedation | Oral |
| Gabapentin | Mitigation of calcium currents and tachykinin in areas such as the area postrema | 300–900 mg thrice daily For sensitive patients, consider starting at between 12.5 to 25 mg daily |
Clumsiness, somnolence | Oral |
| Olanzapine | Targets dopaminergic (D1, D2, D3 and D4), serotonergic (5-HT2A, 5-HT2C, 5-HT3, 5-HT6), histaminic and muscarinic receptor | 5–10 mg/day | Somnolence, postural hypotension, dizziness, dyspepsia, restlessness, weight gain and rarely extrapyramidal symptoms | Oral |
Gabapentin, a γ-aminobutyric acid analog has been shown to be efficacious in preventing post-operative nausea and vomiting in multiple randomized controlled trials. (Guttuso, 2014) There is also some use of gabapentin in preventing acute and delayed chemotherapy-associated nausea and vomiting, hyperemesis gravidarum, and life-threatening refractory emesis following posterior fossa surgery [Guttuso et al. 2003, 2005; Ajori et al. 2012; Guttuso, 2014]. It is suggested that the mechanism of action involves mitigation of calcium currents in areas such as area postrema [Guttuso et al, 2003; Guttuso, 2014]. Future studies should explore its use in patients with chronic functional nausea vomiting.
Olanzapine is a known antagonist for dopamine and serotonin receptors. Clinical trials have demonstrated the efficacy of olanzapine in the control of both acute and delayed chemotherapy induced nausea and vomiting [Passik et al 2004; Navari et al. 2005, 2013]. In animal studies, olanzapine has been shown to be effective for the control of narcotic-induced nausea and sleep disturbance associated with chronic pain [Torigoe et al. 2012].
Gastric electrical stimulation (GES) is a technique used for refractory gastroparesis. A recent analysis of a large series at one institution demonstrated symptomatic improvement after GES but a need for additional surgical procedures and complications after GES implant [Keller et al. 2013]. Symptomatic improvement achieved by GES in patients with gastroparesis is best explained by activation of vagal afferent pathways to influence CNS control mechanisms for nausea and vomiting, accompanied by enhanced vagal efferent autonomic function and decreased gastric sensitivity to volume distention which enhances postprandial gastric accommodation [McCallum et al. 2010]. GES with short pulses can improve gastroparesis symptoms such as nausea and vomiting and GES with long pulses has been shown to improve gastric motility [Lei and Chen, 2009]. Evidence for the efficacy of GES in patients without demonstrated gastric-emptying delay (such as patients with functional dyspepsia and chronic, refractory, idiopathic nausea and vomiting) is evolving [Lu et al. 2013].
With respect to dietary recommendations in the management of nausea and vomiting, a low-fat, low-fiber diet with small frequent meals is typically recommended if patients are able to tolerate oral intake. A short-term liquid diet in extreme cases of patients not tolerating a solid diet is also routinely recommended [Hasler and Chey, 2003]. For patients with chronic, unexplained symptoms, dietary recommendations typically follow those routinely recommended for patients with documented gastric emptying delay, although there are no large, well-designed trials evaluating this strategy.
Dietary manipulation and supplements have also been investigated for the management of nausea and vomiting. Ginger is a herbal supplement that has been shown to have some efficacy in small studies to reduce severity of post-operative nausea and vomiting, morning sickness and motion sickness [Ernst and Pittler, 2000; Keating and Chez, 2002]. Studies involving the use of ginger in prevention of chemotherapy-induced nausea and vomiting are conflicting and do not support its use [Navari, 2013]. Its mechanism of action is largely unknown but improvements in gastric motility, anti-5-hydroxytryptamine activity and central antiemetic effects have been postulated [Navari, 2013]. Rieber and colleagues investigated human subjects, looking at the effect of acute tryptophan depletion when subjected to a rotational procedure intended to induce nausea and found that it increased [Rieber et al. 2010]. Interestingly, acute tryptophan depletion was also shown to slow gastric emptying time in females [van Nieuwenhoven et al. 2004]. These findings suggest a potential role of diet and supplements in modulating the sensation of pain and nausea, which would be important to pursue with further research.
Alternative and complementary approaches to the management of nausea and vomiting include hypnosis, acupressure and acupuncture. In a systematic review, P6 acupoint stimulation significantly reduced nausea, vomiting and the need for rescue medications in the post-operative setting [Lee and Fan, 2009]. Another randomized controlled trial involving 63 subjects with post-surgical gastroparesis showed that P6-acupoint stimulation was superior to metaclopramide in achieving complete recovery rate [Sun et al. 2010]. Marchioro and colleagues have also shown efficacy of hypnosis in preventing chemotherapy-induced anticipatory nausea and vomiting in 16 patients [Marchioro et al. 2000].
In summary, acute nausea (e.g. related to chemotherapy) is easier to control than chronic unexplained nausea. Antiemetics such as antihistaminics, antidopaminergics and 5-HT3 antagonists are often the first-line agents used for common causes of acute nausea. Other agents such as steroids and aprepitant are most commonly used for acute and delayed phase of chemotherapy-induced nausea. Benzodiazepines are best suited for the anticipatory component of post-operative or chemotherapy-induced nausea.
Chronic nausea is much more difficult to control and poses a therapeutic challenge for most health care providers. As discussed above, the central pathways of chronic nausea are very close to chronic neuropathic pain and thus therapeutic options are also directed towards the same. The first line therapeutic options for these patients are neuromodulators such as TCAs, olanzapine, gabapentin and possibly cannabinoids and benzodiazepines. However, if a patient is found to have delayed gastric emptying, prokinetics are the agents of choice. For patients with chronic nausea, we recommend starting with the lowest possible dose of antinausea agents and titrating slowly, as most of these types of patient are sensitive to triggers such as food and medications, which could worsen their symptoms.
Conclusions and future directions
In a recent comprehensive publication, nausea is defined as ‘an unpleasant sensation of a protective mechanism elicited by the interaction of inherent factors and changeable psychological states’ [Stern et al. 2011]. This definition concisely encompasses our current understanding of the interaction of diverse systems with the psychological milieu in nausea. Clinically, patients with chronic and refractory symptoms in the absence of an identifiable cause pose a significant challenge and should be the focus of clinical investigation. Determining the burden of disease by developing disease-specific quality of life questionnaires is also necessary.
Evolving understanding of the central as well as peripheral pathophysiology underlying nausea will be crucial for the development of novel treatment options, whether they are new agents, novel uses of older agents, or combination therapies. For now, atypical agents such as TCAs, olanzapine, gabapentin, alternative therapies such as acupuncture and hypnosis, and the potential role of dietary modification hold significant promise for the future and should be studied rigorously in clinical trials.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Braden Kuo has received fees for serving as a consultant for Takeda, Furiex Pharmaceuticals and Genova Diagnostics, and received research funding from Furiex Pharmaceuticals.
Contributor Information
Prashant Singh, Division of Gastroenterology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Sonia S. Yoon, Division of Gastroenterology, Weill Cornell Medical College, New York, NY, USA
Braden Kuo, Massachusetts General Hospital – GI Unit, 55 Fruit Street, Blake 4, Boston, MA 02114, USA.
References
- Ajori L., Nazari L., Mazloomfard M., Amiri Z. (2012) Effects of gabapentin on postoperative pain, nausea and vomiting after abdominal hysterectomy: a double blind randomized clinical trial. Arch Gynecol Obstet 285: 677–682. [DOI] [PubMed] [Google Scholar]
- Apfel C., Korttila K., Abdalla M., Kerger H., Turan A., Vedder I., et al. (2004) A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 350: 2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M., Chialvo D., Geha P., Levy R., Harden R., Parrish T., et al. (2006) Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 26: 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., DiPersio D., Jenkins C., Jack M., McCoin N., Storrow A., et al. (2011) A randomized, placebo-controlled trial of ondansetron, metoclopramide, and promethazine in adults. Am J Emerg Med 29: 247–255. [DOI] [PubMed] [Google Scholar]
- Bodis S., Alexander E., III, Kooy H., Loeffler J. (1994) The prevention of radiosurgery induced nausea and vomiting by ondansetron: evidence of a direct effect on the central nervous system chemoreceptor trigger zone. Surg Neurol 42: 249–252. [DOI] [PubMed] [Google Scholar]
- Borsook D., Sava S., Becerra L. The pain imaging revolution: advancing pain into the 21st century. (2010) Neuroscientist 16:171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruley des Varannes S., Parys V., Ropert A., Chayvialle J., Rozé C., Galmiche J. (1995) Erythromycin enhances fasting and postprandial proximal gastric tone in humans. Gastroenterology 109: 32–39. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Parkman H., Shafi M., Abell T., Gerson L. (2013) American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 108: 18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras S., Soykan I., Beverly V., Lin Z., McCallum R. (1997) The effect of intravenous vasopressin on gastric myoelectrical activity in human subjects. Neurogastroenterol Motil 9: 151–156. [DOI] [PubMed] [Google Scholar]
- Chen J., Lin Z., Pan J., McCallum R. (1996) Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci 41: 1538–1545. [DOI] [PubMed] [Google Scholar]
- Chepyala P., Olden K. (2008) Nausea and vomiting. Curr Treat Options Gastroenterol 11: 135–144. [DOI] [PubMed] [Google Scholar]
- Chong K., Dhatariya K. (2009) A case of severe, refractory diabetic gastroparesis managed by prolonged use of aprepitant. Nat Rev Endocrinol 5: 285–288. [DOI] [PubMed] [Google Scholar]
- Coleski R., Hasler W. (2009) Coupling and propagation of normal and dysrhythmic gastric slow waves during acute hyperglycaemia in healthy humans. Neurogastroenterology and motility 21: 492–499. [DOI] [PubMed] [Google Scholar]
- Cooper R., Gent P. (2002) An overview of chemotherapy-induced emesis highlighting the role of lorazepam as adjuvant therapy. Int J Palliat Nurs 8: 331–335. [DOI] [PubMed] [Google Scholar]
- Coulie B., Tack J., Peeters T., Janssens J. (1998) Involvement of two different pathways in the motor effects of erythromycin on the gastric antrum in humans. Gut 43: 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M., Robinson D. (2009) Aprepitant: a review of its use in the prevention of nausea and vomiting. Drugs 69: 1853–1878. [DOI] [PubMed] [Google Scholar]
- Di Florio T., Goucke R. (1993) Reduction of dopamine release and postoperative emesis by benzodiazepines. Br J Anaesth 71: 325. [DOI] [PubMed] [Google Scholar]
- Doan L., Manders T., Wang J. (2015) Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast 2015: 504691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E., Pittler M. (2000) Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth 84: 367–371. [DOI] [PubMed] [Google Scholar]
- Fahler J., Wall G., Leman B. (2012) Gastroparesis-associated refractory nausea treated with aprepitant. Ann Pharmacother 46: e38. [DOI] [PubMed] [Google Scholar]
- Feldman M., Samson W., O’Dorisio T. (1988) Apomorphine-induced nausea in humans: release of vasopressin and pancreatic polypeptide. Gastroenterology 95: 721–726. [DOI] [PubMed] [Google Scholar]
- Fisher R., Rentschler R., Nelson J., Godfrey T., Wilbur D. (1982) Elevation of plasma antidiuretic hormones (ADH) associated with chemotherapy-induced emesis in man. Cancer Treat Rep 66: 25–29 [PubMed] [Google Scholar]
- Flak Z., Scalley R., Bailey A. (2004) Practical selection of antiemetics. Am Fam Physician 69: 1169–1174. [PubMed] [Google Scholar]
- Golding J., Stott J. (1997) Comparison of the effects of a selective muscarinic receptor antagonist and hyoscine (scopolamine) on motion sickness, skin conductance and heart rate. Br J Clin Pharmacol 43: 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttuso T., Jr (2014) Gabapentin’s anti-nausea and anti-emetic effects: a review. Exp Brain Res 232: 2535–2539. [DOI] [PubMed] [Google Scholar]
- Guttuso T., Jr, Roscoe J., Griggs J. (2003) Effect of gabapentin on nausea induced by chemotherapy in patients with breast cancer. Lancet 361: 1703–1705. [DOI] [PubMed] [Google Scholar]
- Guttuso T., Jr, Vitticore P., Holloway R. (2005) Responsiveness of life-threatening refractory emesis to gabapentin-scopolamine therapy following posterior fossa surgery. Case report. J Neurosurg 102: 547–549. [DOI] [PubMed] [Google Scholar]
- Hashmi J., Baliki M., Huang L., Baria A., Torbey S., Hermann K., et al. (2013) Shape-shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136: 2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler W., Chey W. (2003) Nausea and vomiting. Gastroenterology 125: 1860–1867. [DOI] [PubMed] [Google Scholar]
- Hasler W., Soudah H., Dulai G., Owyang C. (1995) Mediation of hyperglycemia-evoked gastric slow-wave dysrhythmias by endogenous prostaglandins. Gastroenterology 108: 727–736. [DOI] [PubMed] [Google Scholar]
- Haug T., Mykletun A., Dahl A. (2002) The prevalence of nausea in the community: psychological, social and somatic factors. Gen Hosp Psychiatry 24: 81–86. [DOI] [PubMed] [Google Scholar]
- Herman T., Einhorn L., Jones S., Nagy C., Chester A., Dean J., et al. (1979) Superiority of nabilone over prochlorperazine as an antiemetic in patients receiving cancer chemotherapy. N Engl J Med 300: 1295–1297. [DOI] [PubMed] [Google Scholar]
- Horn C. (2008) Why is the neurobiology of nausea and vomiting so important? Appetite 50: 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn C. (2014) The medical implications of gastrointestinal vagal afferent pathways in nausea and vomiting. Curr Pharm Des 20: 2703–2712. [DOI] [PubMed] [Google Scholar]
- Hornby P. (2001) Central neurocircuitry associated with emesis. Am J Med 111:106S–112S. [DOI] [PubMed] [Google Scholar]
- Javid F., Bulmer D., Broad J., Aziz Q., Dukes G., Sanger G., et al. (2013) Anti-emetic and emetic effects of erythromycin in Suncus murinus: role of vagal nerve activation, gastric motility stimulation and motilin receptors. Eur J Pharmacol 699:48–54. [DOI] [PubMed] [Google Scholar]
- Keating A., Chez R. (2002) Ginger syrup as an antiemetic in early pregnancy. Altern Ther Health Med.8: 89–91. [PubMed] [Google Scholar]
- Keller D., Parkman H., Boucek D., Sankineni A., Meilahn J., Gaughan J., et al. (2013) Surgical Outcomes After Gastric Electric Stimulator Placement for Refractory Gastroparesis. J Gastrointest Surg 17: 620–626 [DOI] [PubMed] [Google Scholar]
- Kerlin P. (1989) Postprandial antral hypomotility in patients with idiopathic nausea and vomiting. Gut 30: 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Napadow V., Kuo B., Barbieri R. (2011) A combined HRV-fMRI approach to assess cortical control of cardiovagal modulation by motion sickness. Conf Proc IEEE Eng Med Biol Soc 2011: 2825–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Chey W., Owyang C., Hasler W. (1997) Role of plasma vasopressin as a mediator of nausea and gastric slow-wave dysrhythmias in motion sickness. Am J Physiol 272: G853–G862. [DOI] [PubMed] [Google Scholar]
- Koch K. (1997) A noxious trio: nausea, gastric dysrhythmias and vasopressin. Neurogastroenterol Motil 9:141–142. [DOI] [PubMed] [Google Scholar]
- Koch K. (2014) Gastric dysrhythmias: a potential objective measure of nausea. Exp Brain Res 232: 2553–2561. [DOI] [PubMed] [Google Scholar]
- Koch K., Summy-Long J., Bingaman S., Sperry N., Stern R. (1990) Vasopressin and oxytocin responses to illusory self-motion and nausea in man J Clin Endocrinol Metab 71:1269–1275. [DOI] [PubMed] [Google Scholar]
- Kowalski A., Rapps N., Enck P. (2006) Functional cortical imaging of nausea and vomiting: a possible approach. Auton Neurosci 129: 28–35. [DOI] [PubMed] [Google Scholar]
- LaCount L., Barbieri R., Park K., Kim J., Brown E., Kuo B., et al. (2011) Static and dynamic autonomic response with increasing nausea perception. Aviat Space Environ Med 82: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Fan L. (2009) Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev CD003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Chen J. (2009) Effects of dual-pulse gastric electrical stimulation on gastric tone and compliance in dogs. Dig Liver Dis 41: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Teich S., Di Lorenzo C., Skaggs B., Alhajj M., Mousa H. (2013) Improvement of quality of life and symptoms after gastric electrical stimulation in children with functional dyspepsia. Neurogastroenterol Motil 25: 567–e456. [DOI] [PubMed] [Google Scholar]
- Madsen J., Fuglsang S. (2008) A randomized, placebo-controlled, crossover, double-blind trial of the NK1-receptor antagonist aprepitant on gastrointestinal motor function in healthy humans. Aliment Pharmacol Ther 27: 609–615. [DOI] [PubMed] [Google Scholar]
- Marchioro G., Azzarello G., Viviani F., Barbato F., Pavanetto M., Rosetti F., et al. (2000) Hypnosis in the treatment of anticipatory nausea and vomiting in patients receiving cancer chemotherapy. Oncology 59: 100–104. [DOI] [PubMed] [Google Scholar]
- McCallum R., Dusing R., Sarosiek I., Cocjin J., Forster J., Lin Z. (2010) Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients. Neurogastroenterol Motil 22: 161–167. [DOI] [PubMed] [Google Scholar]
- Miller A. (1999) Central mechanisms of vomiting. Dig Dis Sci 44: 39S–43S. [PubMed] [Google Scholar]
- Murray C., Martin N., Patterson M., Taylor S., Ghatei M., Kamm M., et al. (2005) Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo-controlled, crossover study. Gut 54: 1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth E. (2006) Motion and space sickness: intestinal and autonomic correlates. Auton Neurosci 129: 58–66. [DOI] [PubMed] [Google Scholar]
- Namin F., Patel J., Lin Z., Sarosiek I., Foran P., Esmaeili P., et al. (2007) Clinical, psychiatric and manometric profile of cyclic vomiting syndrome in adults and response to tricyclic therapy. Neurogastroenterol Motil 19: 196–202. [DOI] [PubMed] [Google Scholar]
- Napadow V., Sheehan J., Kim J., Lacount L., Park K., Kaptchuk T., et al. (2013) The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex 23: 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navari R. (2013) Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs 73: 249–262. [DOI] [PubMed] [Google Scholar]
- Navari R., Einhorn L., Passik S., Loehrer P., Sr, Johnson C., Mayer M., et al. (2005) A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer 13: 529–534. [DOI] [PubMed] [Google Scholar]
- Page S., Peterson D., Crosby S., Ang V., White A., Jenkins J., et al. (1990) The responses of arginine vasopressin and adrenocorticotrophin to nausea induced by ipecacuanha. Clin Endocrinol (Oxf) 33: 761–770. [DOI] [PubMed] [Google Scholar]
- Parkman H., Hasler W., Barnett J., Eaker E. for the American Motility Society Clinical GI Motility Testing Task Force (2003) Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil 15:89–102 [DOI] [PubMed] [Google Scholar]
- Pasricha P., Colvin R., Yates K., Hasler W., Abell T., Unalp-Arida A. (2011) Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol 9: 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passik S., Navari R., Jung S., Nagy C., Vinson J., Kirsh K., et al. (2004) A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study. Cancer Invest 22: 383–388. [DOI] [PubMed] [Google Scholar]
- Prakash C., Clouse R. (1999) Cyclic vomiting syndrome in adults: clinical features and response to tricyclic antidepressants. Am J Gastroenterol 94: 2855–2860 [DOI] [PubMed] [Google Scholar]
- Prakash C., Lustman P., Freedland K., Clouse R. (1998) Tricyclic antidepressants for functional nausea and vomiting: clinical outcome in 37 patients. Dig Dis Sci 43: 1951–1956. [DOI] [PubMed] [Google Scholar]
- Quigley E., Hasler W., Parkman H. (2001) AGA technical review on nausea and vomiting. Gastroenterology 120: 263–286. [DOI] [PubMed] [Google Scholar]
- Rieber N., Mischler D., Schumacher V., Muth E., Bischoff S., Klosterhalfen S., et al. (2010) Acute tryptophan depletion increases experimental nausea but also induces hunger in healthy female subjects. Neurogastroenterol Motil 22: 752–757. [DOI] [PubMed] [Google Scholar]
- Rodola F. (2006) Midazolam as an anti-emetic. Eur Rev Med Pharmacol Sci 10: 121–126. [PubMed] [Google Scholar]
- Roila F., Herrstedt J., Aapro M., Gralla R., Einhorn L., Ballatori E., et al. (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 5: v232–v243. [DOI] [PubMed] [Google Scholar]
- Roscoe J., Hickok J., Morrow G. (2000) Patient expectations as predictor of chemotherapy-induced nausea. Ann Behav Med 22: 121–126. [DOI] [PubMed] [Google Scholar]
- Roscoe J., Morrow G., Hickok J., Stern R. (2000) Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manag 20: 113–121. [DOI] [PubMed] [Google Scholar]
- Rub R., Andrews P., Whitehead S. (1992) Vomiting—incidence, causes, ageing and sex. In Bianchi A, Grelot L., Miller A., King G. (eds), Mechanisms and Control of Emesis, Montrouge: John Libbey Eurotext. [Google Scholar]
- Rudd J., Ngan M., Wai M., King A., Witherington J., Andrews P., et al. (2006) Anti-emetic activity of ghrelin in ferrets exposed to the cytotoxic anti-cancer agent cisplatin. Neurosci Lett 392: 79–83. [DOI] [PubMed] [Google Scholar]
- Sanger G., Andrews P. (2006) Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci 129: 3–16. [DOI] [PubMed] [Google Scholar]
- Sawhney M., Prakash C., Lustman P., Clouse R. (2007) Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci 52: 418–424. [DOI] [PubMed] [Google Scholar]
- Sclocco R., Kim J., Garcia R., Sheehan J., Beissner F., Bianchi A., et al. (2014) Brain circuitry supporting multi-organ autonomic outflow in response to nausea. Cereb Cortex August 12. pii: bhu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. (2002) The psychophysiology of nausea. Acta Biol Hung 53: 589–599. [DOI] [PubMed] [Google Scholar]
- Stern R., Hu S., LeBlanc R., Koch K. (1993) Chinese hyper-susceptibility to vection-induced motion sickness. Aviat Space Environ Med 64: 827–830. [PubMed] [Google Scholar]
- Stern R., Hu S., Uijtdehaage S., Muth E., Xu L., Koch K. (1996) Asian hypersusceptibility to motion sickness. Human Heredity 46: 7–14. [DOI] [PubMed] [Google Scholar]
- Stern R., Koch K., Andrews P. (2011) Nausea: mechanisms and management. New York: Oxford University Press. [Google Scholar]
- Sun B., Luo M., Wu S., Chen X., Wu M. (2010) Acupuncture versus metoclopramide in treatment of postoperative gastroparesis syndrome in abdominal surgical patients: a randomized-controlled trial. Zhong Xi Yi Jie He Xue Bao 8: 641–644. [DOI] [PubMed] [Google Scholar]
- Taché Y. (1999) Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Dig Dis Sci 44: 79S–86S. [PubMed] [Google Scholar]
- Tack J., Janssens J., Vantrappen G., Peeters T., Annese V., Depoortere I., et al. (1992) Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology 103: 72–79. [DOI] [PubMed] [Google Scholar]
- Takada K., Murai T., Kanayama T., Koshikawa N. (1993) Effects of midazolam and flunitrazepam on the release of dopamine from rat striatum measured by in vivo microdialysis. Br J Anaesthesia 70: 181–185. [DOI] [PubMed] [Google Scholar]
- Talley N., Locke G., Saito Y., Almazar A., Bouras E., Howden C., et al. (2015) Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology 149: 340–349.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. (2000) The economic impact of nausea and vomiting. In Blum R., Heinrichs W., Herxheimer A. (eds), Nausea and Vomiting. London: Whurr. [Google Scholar]
- Thompson D., Malagelada J. (1982) Vomiting and the small intestine. Dig Dis Sci 27: 1121–1125. [DOI] [PubMed] [Google Scholar]
- Thumshirn M., Bruninga K., Camilleri M. (1997) Simplifying the evaluation of postprandial antral motor function in patients with suspected gastroparesis. Am J Gastroenterol 92: 1496–1500. [PubMed] [Google Scholar]
- Torigoe K., Nakahara K., Rahmadi M., Yoshizawa K., Horiuchi H., Hirayama S., et al. (2012) Usefulness of olanzapine as an adjunct to opioid treatment and for the treatment of neuropathic pain. Anesthesiology 116: 159–169. [DOI] [PubMed] [Google Scholar]
- Tougas G., Eaker E., Abell T., Abrahamsson H., Boivin M., Chen J., et al. (2000) Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 95: 1456–1462. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhoven M., Valks S., Sobczak S., Riedel W., Brummer R. (2004) Acute tryptophan depletion slows gastric emptying in females. Br J Nutr 91: 351–355. [DOI] [PubMed] [Google Scholar]
- Walker E., Katon W., Jemelka R., Roy–Bryne P. (1992) Comorbidity of gastrointestinal complaints, depression, and anxiety in the Epidemiologic Catchment Area (ECA) Study. Am J Med 92: 26S–30S. [DOI] [PubMed] [Google Scholar]
- Xu L., Koch K., Summy–Long J., Stern R., Seaton J., Harrison T., et al. (1993) Hypothalamic and gastric myoelectrical responses during vection-induced nausea in healthy Chinese subjects. Am J Physiol 265: E578–E584. [DOI] [PubMed] [Google Scholar]