Abstract
Background
Vietnam has high endemic hepatitis B virus infection with >8% of adults estimated to have chronic infection. Hepatitis B vaccine was first introduced in the national childhood immunization program in 1997 in high-risk areas, expanded nationwide in 2002, and included birth dose vaccination in 2003. This survey aimed to assess the impact of Vietnam’s vaccination programme by estimating the prevalence of hepatitis B surface antigen (HBsAg) among children born during 2000–2008.
Methods
This nationally representative cross-sectional survey sampled children based on a stratified three-stage cluster design. Demographic and vaccination data were collected along with a whole blood specimen that was collected and interpreted in the field with a point-of-care HBsAg test.
Results
A total of 6,949 children were included in the survey analyses. The overall HBsAg prevalence among surveyed children was 2.70% (95% confidence interval (CI): 2.20–3.30). However, HBsAg prevalence was significantly higher among children born in 2000–2003 (3.64%) compared to children born 2007–2008 (1.64%) (prevalence ratio (PR: 2.22, CI 1.55–3.18)). Among all children included in the survey, unadjusted HBsAg prevalence among children with ≥3 doses of hepatitis B vaccine including a birth dose (1.75%) was significantly lower than among children with ≥3 doses of hepatitis B vaccine but lacked a birth dose (2.98%) (PR: 1.71, CI: 1.00–2.91) and significantly lower than among unvaccinated children %) (PR: 1.99, CI: 1.15–3.45). Infants receiving hepatitis B vaccine >7 days after birth had significantly higher HBsAg prevalence (3.20%) than those vaccinated 0-1 day after birth (1.52%) (PR: 2.09, CI: 1.27–3.46).
Conclusion
Childhood chronic HBV infection prevalence has been markedly reduced in Vietnam due to vaccination. Further strengthening of timely birth dose vaccination will be important for reducing chronic HBV infection prevalence of under 5 children to <1%, a national and Western Pacific regional hepatitis B control goal.
Keywords: Hepatitis B virus, Seroprevalence, Birth dose vaccination, Immunization
1. Introduction
Hepatitis B virus (HBV) infection is highly endemic in Vietnam with an estimated chronic infection prevalence among adults of 8–20% [1–4]. A survey in two districts in Thanh Hoa Province in 1998 showed prevalence of HBsAg, a marker for chronic HBV infection, to be 12% among infants, 18% among children (4–5 years), 20% among adolescents (14–15 years), and 19% among adults (25–39 years) [5]. HBV is a known carcinogen and the high prevalence of chronic HBV infection is an important contributing factor to liver cancer and cirrhosis, which is the most common cause of cancer death in Vietnam [6–8].
Like other countries in South East and Eastern Asia, the primary routes of HBV transmission in Vietnam are from mother-to-child or from close contacts during early childhood [9]. Hepatitis B vaccine is highly effective in preventing both perinatally and horizontally acquired chronic HBV infection [10–12]. Therefore, to reduce chronic HBV infection prevalence and protect children from infection, Vietnam introduced hepatitis B vaccine in the national immunization program in 1997 in selected areas. Infant hepatitis B vaccination program was expanded nationwide in 2002 and a monovalent hepatitis B vaccine birth dose was introduced in 2003 with financial support from the GAVI Alliance; the birth dose was intitially recommended to be given within 7 days after birth; in 2002, it was recommended to be given within 3 days after birth, and in 2006 it was recommended to be given within 24 h after birth, the birthdose was given at health facilities only. Since 2010, the subsequent hepatitis B doses are given as part of a pentavalent DPT-Hib-Hepatitis B vaccine schedule at ages 2, 3, and 4 months, parents were requested to bring their child to commune health center and outreach points (for hard to reach areas only) for vaccination.
Three-dose hepatitis B vaccine coverage has increased substantially over time from 24% in 2000 to >90% every year starting in 2004 [13,14]. Additionally, birth dose coverage rapidly reached nearly 60% within 2 years after birth dose introduction in 2003. However, birth dose coverage declined dramatically to 29% in 2007 and 26% in 2008 following media reports of alleged adverse events following hepatitis B birth-dose administrtaion [13,14].
To evaluate the impact of the hepatitis B vaccination program, a survey was conducted to look at chronic HBV infection prevalence among children born in three time periods representing different hepatitis B immunization programmatic strategies and performance: the first period (2000–2003) represents a phased introduction of hepatitis B vaccine with low birth dose coverage; the second period represents high birth dose coverage and high three dose hepatitis B vaccine coverage (2004–2006); and the third period (2007–2008) represents a decrease in birth dose coverage after the media reports of alleged adverse events.
2. Methods
2.1. Study population and design
The survey was designed to estimate HBsAg prevalence among a nationally representative sample of children born in three birth cohort groups: 2000–2003, 2004–2006, and 2007–2008. Expected prevalence was assumed to be 8%, 2%, and 4% and the desired precision of ±2%, ±1%, and ±1%, respectively. The greater sample size in the younger cohorts was based on a desire to have a more precise measure relating to the most recent programmatic activities. A design effect of 2 and non-response rate of 20% was assumed for all 3 cohorts. Resulting sample size was 1696 for the 2000–2003 cohort, 1807 for the 2004–2006 cohort, 3540 for the 2007–2008 cohort and total sample size of 7044.
2.2. Sampling method and enrollment
This cross-sectional survey sampled children using a stratified three-stage cluster design. The district-level coverage of birth-dose given within 24 h in 2006 was used as a stratification variable. All 684 districts in the country were stratified into four strata of timely birth dose coverage: ≥50%, 25–49%; 10–24%; and <10%. Ninety-eight primary sampling units (districts) were allocated to the four strata based on the population size of each; specifically, 63, 16, 6, and 13 districts were selected in the ≥50%, 25–49%; 10–24%; and <10% birth dose coverage categories, respectively. Two communes were randomly selected from each selected district. Nine children born in years 2000–2003, nine children born in years 2004–2006, and 18 children born in years 2007–2008 were randomly selected from commune lists. District health staff and commune leaders were informed of the survey site selection in advance; parents received a letter requesting them to bring selected children and vaccination records to a designated commune health post on the survey day.
2.3. Individual-level data: questionnaire, specimen collection and testing
A questionnaire was administered to the child’s caregiver to collect basic socio-demographic information and vaccination history of the child. The Alere Determine™ HBsAg rapid assay test was used to detect HBsAg in whole blood specimens obtained from children [15]. Procedures were followed according to the package insert, including (1) obtaining approximately 50 microlitres of whole blood using a finger-prick, (2) immediately applying the whole blood specimen to the test strip, (3) visually reading and recording the results of the test strip after 15 min, and (4) confirming the test results by having a supervisor re-read the strip within 24 h.
2.4. Definitions
A child was considered vaccinated based on either affirmative written documentation or caregiver recall of vaccine doses administered. If vaccination records were reviewed and no entry of hepatitis B vaccination found, the child was considered unvaccinated. A child was considered to have a missing vaccination status if no records were available to review and a caregiver could not recall vaccination status.
2.5. Data analysis
Survey teams reviewed data collection forms prior to leaving the commune to ensure all required data elements had been collected and were legibly recorded. The name and signature of the survey member reviewing the data collection forms were recorded on the forms to assist with monitoring the quality of data collection.
Data were double entered independently and checked for accuracy using Epidata software, Version 2.1b, and Epi info, version 6.04d. HBsAg seroprevalence estimates were calculated for subpopulations of interest using SAS 9.3. Multivariable models were fit for each of the three age groups separately. Prevalence ratios (PR) and 95% confidence intervals (CI) were calculated in SUDAAN v10. Estimates of point prevalences, PRs, and CIs accounted for sampling weights and stratified cluster design. Sampling weights for each child were calculated based on the above described sampling design.
2.6. Ethical approval
Prior to beginning the survey, the protocol was reviewed and approved by ethical review boards of the Western Pacific Regional Office of WHO and the National Institute of Hygiene and Epidemiology of Vietnam.
3. Results
3.1. Demographic characteristics of the surveyed subjects
The survey was conducted during January to February 2011 and enrolled a total of 6,962 participants, of whom 6,949 (>99%) children met the age criteria and were included in the analysis. A total of 51.7% were boys; 79.5% belonged to the Kinh ethnic group. Among the three cohort groups, 25.0% were born from 2000 to 2003, 25.7% were born from 2004 to 2006, and 49.3% were born from 2007 to 2008. Detailed characteristics of children enrolled in the survey are presented in Table 1.
Table 1.
Demographic characteristics of the participating survey subjects.
| Demographic characteristics | Category | Frequency | Percentage (%) |
|---|---|---|---|
| Gender | Male | 3,593 | (51.7) |
| Female | 3,339 | (48.1) | |
| Missing data | 17 | (0.2) | |
| Population density | Urban areas | 1,713 | (24.7) |
| Rural areas | 5,236 | (75.3) | |
| Age group (birth year) | 2000–2003 | 1,740 | (25.0) |
| 2004–2006 | 1,783 | (25.7) | |
| 2007–2008 | 3,426 | (49.3) | |
| Ethnic group | Kinh | 5,527 | (79.5) |
| Others | 1,151 | (16.6) | |
| Missing data | 271 | (3.9) | |
| Place born | Health facility | 6138 | (88.3) |
| Home | 788 | (11.3) | |
| Missing data | 23 | (0.3) | |
| Total children surveyed | 6,949 |
3.2. HBsAg prevalence by birth cohort groups
HBsAg prevalence decreased from 3.64% (CI: 2.80–4.71) among children born in 2000–2003 to 2.15% (CI: 1.47–3.14) among children born in 2004–2006, and to 1.64% (CI: 1.25–2.15) among children born 2007–2008 (Table 2). Estimates of HBsAg prevalence for each birth year show a decline from 2000 to 2008 (Fig. 1). Children born during 2000–2003 had an increased risk of being HBsAg positive compared to those born during 2007–2008 (PR: 2.22, CI: 1.55–3.18) (Table 2).
Table 2.
Overall and stratified hepatitis B surface antigen (HBsAg) prevalence in children born 2000–2008, Vietnam.
| Factors | Factor categories | Surveyed number (N) | HBsAg prevalence, % | Prevalence ratio | 95% confidence intervala |
|---|---|---|---|---|---|
| Overall | 6949 | 2.7 | NA | 2.2–3.3 | |
| Gender | Female | 3339 | 2.73 | 1.04 | 0.74–1.46 |
| Male | 3593 | 2.63 | Referent | ||
| Population density | Rural areas | 5236 | 2.84 | 1.25 | 0.76–2.06 |
| Urban area | 1713 | 2.27 | Referent | ||
| Ethnicity | Others | 1151 | 5.36 | 2.48 | 1.71–3.58 |
| Kinh | 5527 | 2.16 | Referent | ||
| Place of birth | Home | 788 | 5.47 | 2.43 | 1.68–3.51 |
| Health Facility | 6138 | 2.25 | Referent | ||
| Age group (birth year) | 2000-2003 | 1740 | 3.64 | 2.22 | 1.55–3.18 |
| 2004-2006 | 1783 | 2.15 | 1.31 | 0.86–2.01 | |
| 2007–2008 | 3426 | 1.64 | Referent | ||
| Birth dose vaccination | Day | 1946 | 1.52 | Referent | |
| 2–7 days | 418 | 2.80 | 1.86 0.68–5.06 | ||
| >7 days | 4558 | 3.20 | 2.09 1.27–3.46 | ||
| Vaccination statusb | Zero doses | 1639 | 3.47 | 1.99 | 1.15–3.45 |
| 1–2 total doses | 211 | 2.11 | 1.21 | 0.40–3.66 | |
| No BD and 3+ total doses | 2764 | 2.98 | 1.71 | 1.001–2.91 | |
| BD and 3+ total doses | 2305 | 1.75 | Referent |
95% CI is around PR; except first row provides 95% CI around overall prevalence.
BD = birth dose and is defined as hepatitis B vaccination within 7 days of birth; Total doses includes BD.
Fig. 1.
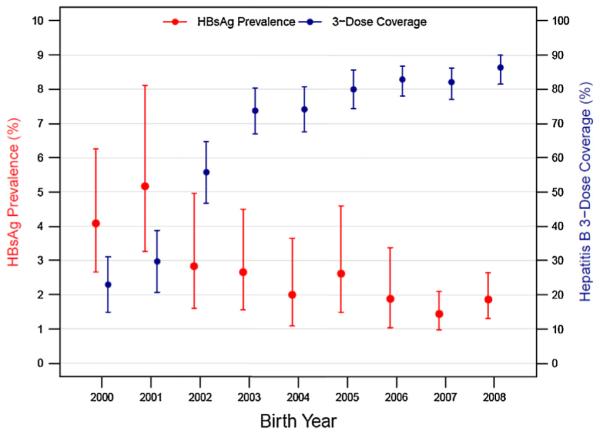
Hepatitis B surface antigen (HBsAg) prevalence and 3-dose hepatitis B vaccine coverage by year of birth, 2000–2008, Vietnam. Bars indicate 95% confidence intervals.
3.3. HBsAg prevalence by vaccination status
Prevalence of HBsAg by vaccination status is shown in Table 2. Infants receiving hepatitis B vaccine >7 days after birth had significantly higher HBsAg prevalence (3.20%) than those vaccinated 0–1 day after birth (1.52%) (PR: 2.09, CI: 1.27–3.46). The HBsAg prevalence was higher among infants vaccinated 2–7 days after birth (2.80%) compared to infants vaccinated 0–1 day after birth (PR: 1.86, CI: 0.68–5.06); however this difference was not statistically significant. For purposes of simplifying data presentation, further results are presented by combining the two groups and defining birth dose vaccination as vaccination within 7 days of birth. Among all three birth cohorts combined, children with at least 3 doses of hepatitis B vaccine including a birth dose had significantly lower HBsAg prevalence (1.75%) than those with at least 3 doses of hepatitis B vaccine but lacked a birth dose (2.98%) (PR: 1.71, CI: 1.00–2.91) and those who were unvaccinated (3.47%) (PR: 1.99, CI: 1.15–3.45).
Consistent with the survey design and because of the observed differences in vaccine coverage and HBsAg seroprevalence in the 3 cohorts, the multivariable models were fit to each cohort separately (Table 3). Among the 2007–2008 cohort, the prevalence ratio indicates a significantly lower HBsAg prevalence among children with 3 vaccine doses including a birth dose compared to those with 3 doses but missing a birth dose (PR: 2.93, CI: 1.23–6.96) and compared to those who were unvaccinated (PR: 3.01, CI: 1.04–8.70).
Table 3.
Adjusted HBsAg prevalence ratiosa stratified by birth cohorts, Vietnam.
| Birth cohorts | Variablesb | Variable categories | Adjusted prevalence ratio (PR) | 95% Confidence interval | |
|---|---|---|---|---|---|
| 2000–2003 | Vaccination status | Zero doses | 1.34 | 0.54–3.28 | |
| 1–2 total doses | 1.20 | 0.13–11.07 | |||
| No BD and ≥3 total doses | 1.71 | 0.64–4.58 | |||
| BD and ≥3 total doses | Referent | ||||
| Place of birth | Home Facility | 2.36 | Referent | 1.42–3.93 | |
| 2004–2006 | Vaccination status | Zero doses | 0.77 | 0.25–2.30 | |
| 1–2 total doses | 0.90 | 0.11–7.39 | |||
| No BD and ≥ 3 total doses | 1.10 | 0.52–2.36 | |||
| BD and ≥ 3 total doses | Referent | ||||
| Place of birth | Home Facility | 1.93 | Referent | 0.57–6.57 | |
| Ethnicity | Other | 1.16 | 0.35–3.84 | ||
| Kinh | Referent | ||||
| 2007–2008 | Vaccination status | Zero doses | 3.01 | 1.04–8.70 | |
| 1–2 total doses | 2.81 | 0.53–14.92 | |||
| No BD and ≥3 total doses | 2.93 | 1.23–6.96 | |||
| BD and ≥3 total doses | Referent | ||||
| Place of birth | Home | 2.03 | 0.55–7.42 | ||
| Facility | Referent | ||||
| Ethnicity | Other | 1.02 | 0.29–3.60 | ||
| Kinh | Referent | ||||
BD = birth dose, defined as HB vaccination within 7 days of birth; Total doses includes BD.
Prevalence ratio (PR) is the ratio of HBsAg prevalence.
PR adjusted for vaccination status, place of birth, and ethnicity, except for the 2000–2003 cohorts where ethnicity was omitted due to sparse data.
3.4. HBV infection prevalence by other factors
Children born at home had a significantly higher HBsAg prevalence (5.47%) compared to children born in health facilities (2.25%) (PR: 2.43, CI:1.68–3.51) (Table 2). In addition, children of ethnicity other than Kinh had a higher HBsAg prevalence (5.36%) compared to children with Kinh ethnicity (2.16%) (PR: 2.48, CI: 1.71–3.58). Gender and population density were not found to be associated with HBsAg prevalence. Ethnicity and place of birth were not significantly associated with seroprevalence in the multivariable models for the 2004–2006 and 2007–2008 cohorts. Place of birth remained statistically significant in the 2000–2003 cohort; ethnicity could not be assessed in this multivariable model due to sparse data (Table 3).
3.5. Coverage estimation from surveyed participants
Estimates of hepatitis B vaccine coverage (≥3 doses, with or without birth dose) were calculated for each cohort group and are shown in Table 4.
Table 4.
Hepatitis B vaccination coverage by birth cohort group.
| Birth cohort | Total children | Hepatitis B vaccine 3-dose coveragea |
Hepatitis B vaccine birth dose coverageb |
||
|---|---|---|---|---|---|
| % | (95% confidence interval) | % | (95% confidence interval) | ||
| 2000–2003 | 1740 | 46.1 | (39.6–52.7) | 22.0 | (18.1–25.9) |
| 2004–2006 | 1783 | 78.8 | (74.1–83.5) | 52.7 | (47.8–57.6) |
| 2007–2008 | 3426 | 84.0 | (79.6–87.7) | 30.4 | (26.9–33.8) |
Counting birth dose vaccination.
Hepatitis B vaccination within 7 days of birth.
4. Discussion
Childhood chronic HBV infection prevalence has declined in Vietnam as a result of having a strong hepatitis B vaccination programme. HBsAg prevalence decreased with increasing coverage of hepatitis B vaccine; from 3.64% among children born in 2000–2003 to 1.64% among children born 2007–2008. Noting the limited comparability between the surveys, HBsAg prevalence found in this survey among children nationally is substantially lower than among 4–5 year old children in a serosurvey conducted in 1998 in Thanh Hoa Province (18%) [5]. If national pre-vaccine HBsAg prevalence was in the range found in Thanh Hoa Province, it would appear that HBsAg prevalence began to decline before birth dose vaccination was introduced; since the 2000–2003 cohort had only 3.6% HBsAg prevalence at a time when there was high 3 dose hepatitis B vaccine coverage but no or little birth dose vaccination was taking place. This finding serves as a reminder of the predominate mode of early childhood HBV transmission and the important role that a three-dose infant vaccination schedule can have on reducing chronic HBV infection prevalence [16].
However, high three-dose coverage in the absence of a birth dose will not likely lead Vietnam to reach its national and the World Health Organization’s Western Pacific Regional hepatitis B control goal of reducing childhood chronic HBV infection prevalence to <1%. Even with multiple years of approximate 95% three-dose hepatitis B vaccination coverage, HBsAg prevalence has never dropped below 1% among vaccinated cohorts. This finding reinforces the need to achieve both high birth dose coverage and high three-dose hepatitis B vaccine coverage in Vietnam in order to achieve the goal of reducing chronic HBV infection prevalence to <1% [17].
The importance of birth dose vaccination is further emphasized with the finding that children who had at least three doses of hepatitis B vaccine including a birth dose had significantly lower HBsAg prevalence compared with children who had at least three doses of hepatitis B vaccine but were missing the birth dose. Considering the role that birth dose vaccination has on preventing mother-to-child transmission, it was surprising to find that the youngest cohort with the lowest birth dose coverage continued to have low HBsAg prevalence. At least two factors may have contributed to this finding including that the young cohort did not have the same HBV exposure opportunity during the highest risk period of acquiring chronic HBV infection compared with the older cohorts [18]. The highest risk period for acquiring chronic HBV infection has been estimated to last until 5 years of age and the children born in 2007 and 2008 where aged 3–4 years at the time of the survey [17]. A second factor could be that herd immunity played a role in decreasing the risk of HBV infection in this youngest cohort as their older siblings had higher vaccination coverage and lower infection rates than older cohorts.
Conclusions herewith on hepatitis B vaccine’s impact on preventing infection largely come from correlating the increasing national hepatitis B immunization coverage with the decreasing HBsAg prevalence. It is unlikely that this decrease would be observed due to any other reason. However, individual-level vaccination status did not yield the same association in all multivariable models. This is likely a result of not having complete or accurate vaccination data. In addition, vaccination data quality was not likely to be homogeneous across all age cohorts; the older cohorts being more vulnerable to recall bias or missing vaccination records. It is also worth noting that birth cohort year likely served as a surrogate for immunization coverage and was a significant factor in predicting HBsAg prevalence in multivariate models.
Vietnam’s experience with the decline in birth dose vaccination coverage after highly publicized media reports of co-incidental neonatal deaths following vaccination serves as an important reminder to be prepared to inform and communicate with the media. Given over one quarter of neonatal deaths occur within the first 24 h of life, there will be co-incidental deaths occurring after vaccine administration and immunization programmes must be prepared for them [19]. In Vietnam, the media reports led to a decrease in uptake of birth dose vaccination; however, three dose vaccination coverage among older ages remained high. The decrease in birth dose vaccination stemmed from both refusal of vaccination from parents and health care workers. Vietnam’s national immunization programme used several approaches to raise birth dose coverage, including convening technical seminars among public health decision makers, re-training health-care workers, launching awareness campaigns on the importance birth dose vaccination. Vietnam’s birth dose coverage has improved reaching 55% in 2011 and 76% in 2012.
An additional finding from the survey was that the Kinh ethnic group had lower HBsAg prevalence compared to other ethnic groups. This suggests a need to improve both access and demand for vaccine among the other ethnic groups. Many ethnic groups live in remote and poor conditions thereby making a vaccination visit to a health center unaffordable both in terms of transport costs and time. Strategies for increasing access to vaccination are important for improving immunization coverage in Vietnam.
Several limitations of this survey merit noting. To simplify field logistics, a rapid test with reported 95% sensitivity was used; therefore, true HBsAg seroprevalence may be underestimated. Vaccination data quality is not homogenous across age cohorts and the older cohorts were more vulnerable to poor record retrieval and less accurate caregiver recall. In addition, the youngest cohort (2007–2008) did not have the same exposure opportunity during the highest risk period for acquiring chronic HBV infection as the older cohorts and thereby may not be fully comparable.
This survey documents the impact that the hepatitis B vaccination program has had on reducing chronic HBV infection in children and reinforces the importance of birth dose vaccination. Vietnam aims to reduce chronic HBV infection prevalence in children to <1% and results from this survey provides valuable direction on how to proceed to strengthen an already highly successful programme.
Acknowledgment
The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].WHO, Western Pacific Regional Office . Preventing mother-to-child transmission of hepatitis B. Operational field guidelines for delivery of the birth dose of hepatitis B vaccine. WHO; Manila: 2006. [Google Scholar]
- [2].Nguyen VT, McLaws ML, Dore GJ. Highly endemic hepatitis B infection in rural Vietnam. J Gastroenterol Hepatol. 2007;22(12):2093–100. doi: 10.1111/j.1440-1746.2007.05010.x. [DOI] [PubMed] [Google Scholar]
- [3].Nakata S, Song P, Duc DD, Nguyen XQ, Murata K, Tsuda F, et al. Hepatitis C and B virus infections in populations at low or high risk in Ho Chi Minh and Hanoi, Vietnam. J Gastroenterol Hepatol. 1994;9(4):416–9. doi: 10.1111/j.1440-1746.1994.tb01265.x. [DOI] [PubMed] [Google Scholar]
- [4].Tran HT, Ushijima H, Quang VX, et al. Prevalence of hepatitis virus types B through E and genotypic distribution of HBV and HCV in Ho Chi Minh City, Vietnam. Hepatol Res. 2003;26:275–80. doi: 10.1016/s1386-6346(03)00166-9. [DOI] [PubMed] [Google Scholar]
- [5].Hipgrave DB, Nguyen TV, Vu MH, et al. Hepatitis B infection in rural Vietnam and the implications for a national program of infant immunization. Am J Trop Med Hyg. 2003;69(3):288–329. [PubMed] [Google Scholar]
- [6].Gish RG, Bui TD, Nguyen CT, Nguyen DT, Tran HV, Tran DM, et al. Liver disease in Vietnam: screening, surveillance, management and education: a 5-year plan and call to action. J Gastroenterol Hepatol. 2012;27(2):238–47. doi: 10.1111/j.1440-1746.2011.06974.x. [DOI] [PubMed] [Google Scholar]
- [7].Ngoan LT, Lua NT, Hang LTM. Cancer mortality pattern in Vietnam. Asian Pac J Cancer Prev. 2007;8:535–8. [PubMed] [Google Scholar]
- [8].Nguyen VT, Law MG, Dore GJ. An enormous hepatitis B virus-related liver disease burden projected in Vietnam by 2025. Liver Int. 2008;28:525–31. doi: 10.1111/j.1478-3231.2007.01646.x. [DOI] [PubMed] [Google Scholar]
- [9].Gust ID. Epidemiology of hepatitis B infection in the Western Pacific and South East Asia. Gut. 1996;38:18–23. doi: 10.1136/gut.38.suppl_2.s18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Andre FE, Zuckerman AJ. Review: protective efficacy of hepatitis B vaccines in neonates. J Med Virol. 1994;44:144–51. doi: 10.1002/jmv.1890440206. [DOI] [PubMed] [Google Scholar]
- [11].Clements CJ, Baoping Y, Crouch A, et al. Progress in the control of hepatitis B infection in the Western Pacific Region. Vaccine. 2006;24:1975–82. doi: 10.1016/j.vaccine.2005.11.035. [DOI] [PubMed] [Google Scholar]
- [12].Greenberg DP. Pediatric experience with recombinant hepatitis B vaccines and relevant safety and immunogenicity studies. Pediatr Infect Dis J. 1993;12:438–45. doi: 10.1097/00006454-199305000-00037. [DOI] [PubMed] [Google Scholar]
- [13].WHO, Western Pacific Regional Office . Meeting report: consultation on improving and monitoring Hepatitis B Birth Dose Vaccination. WHO; 2012. p. Manila. WPR/DCC/EPI(03) [Google Scholar]
- [14].Vietnam National Expanded Program on Immunization. Annual EPI report. 2008 [Google Scholar]
- [15].Alere Determine HBsAg Package Insert. Chiba, Japan: 2011. http://www.alere.com/EN_ZA/products/alere-determine-hbsag. [Google Scholar]
- [16].Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34(6):1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- [17].Rani M, Yang B, Nesbit R. Hepatitis. B control by 2012 in the WHO Western Pacific Region: rationale and implications. Bull World Health Organ. 2009;87(9):707–13. doi: 10.2471/BLT.08.059220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR-16):1–31. [PubMed] [Google Scholar]
- [19].Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]


