Abstract
The pancreas is one of the earliest- and most commonly- affected organs in patients with cystic fibrosis (CF). Studying the pathogenesis of pancreatic disease is limited in CF patients due to its early clinical onset, co-morbidities and lack of tissue samples from early phases of disease. In recent years, several new CF animal models have been developed that have advanced our understanding of both CF exocrine and endocrine pancreatic disease. Additionally, these models have helped us better define the influence of pancreatic lesions on CF disease progression in other organs such as the gastrointestinal tract and lung.
Keywords: cystic fibrosis, pancreas, diabetes, cystic fibrosis transmembrane conductance regulator, pathology, pancreatic insufficiency
Introduction
Cystic fibrosis (CF) is caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR) [1]. CFTR is a chloride and bicarbonate channel that contributes to fluid secretion by epithelial cells and the hydration of secreted mucus [2,3]. In CF, deficient CFTR leads to abnormal fluid secretions causing dysfunction in organ systems including the lung, gastrointestinal tract, liver, male reproductive tract and pancreas. Lung disease is often associated with morbidity and mortality in CF patients and is often the organ most associated with CF [4]. Nonetheless, pancreatic disease in CF has the highest penetrance and is one of the earliest affected organs [5,6]. Here we review CF pancreatic disease and highlight recent advancements using animal models to better define CF pancreas pathogenesis and its relationship to lung and gastrointestinal disease.
Current animal models of CF
Historically, most animal model research on CF had been performed in mouse strains with mutated Cftr [7,8]. However, CF mice do not exhibit the same key pathologies seen with human disease. For instance, while gastrointestinal obstruction is common in many CF mouse models, they do not have significant pancreatic or lung disease [7,8]. The lack of pathology in key organs of the mouse led to the development of other CF animal models (Table 1). In recent years new CFTR-deficient animal models have been developed including the pig [9], ferret [10], rat [11] and zebrafish [12,13]. Animal modelling of CF in large animal species has advantages because these animals more closely resemble humans in terms of their lung structure, function and size [7,14–16]. Of course, small animal models also have distinct advantages, which include ease of handling and housing as well as availability of reagents and tools for genetic manipulation [8,12]. Of interest to this review, we focus on animal models that exhibit pancreatic pathology for translational study including the pig [17], ferret [18] and zebrafish [12].
Table 1.
| Key CF pathologies | |||||
|---|---|---|---|---|---|
| Species | Lung | Pancreas | Gastrointestinal tract | Liver/gallbladder | References |
| Mouseh | 0 | 1 | 2 | 0 | [8,55] |
| Pig | 2 | 2 | 2 | 2 | [17,28,60,61,66] |
| Ferret | 2 | 2 | 2 | 1 | [18,65,67,105] |
| Rat | 0 | 0 | 2 | 0 | [11] |
| Zebrafish | u | 2 | u | u | [12] |
Scoring: 0 = no CF lesions, 1 = mild CF lesions, 2= severe CF lesions (u = unknown)
CF pancreatic disease
The term “cystic fibrosis” is named after the fibrocystic lesions in the pancreas noted by Dr. Dorothy Andersen in a seminal report of paediatric autopsy cases [19,20]. How does deficient CFTR lead to pancreatic disease? CFTR is expressed on the apical membrane of epithelial cells in the small pancreatic ducts and facilitates the transport of chloride and bicarbonate that produces alkaline fluid in ducts [5,21]. An important function of pancreatic ductal epithelial cells is the absorption of chloride and the secretion of bicarbonate [22]. Bicarbonate is a key buffer for pancreatic fluid and functions to neutralize gastric acid and provide an optimal pH for the function of digestive enzymes [23]. The mechanism for ductal secretion of bicarbonate is not completely understood, but is thought to be a two-step process. First, secretion is stimulated in the proximal duct causing accumulation of bicarbonate within the ductal cell cytoplasm leading to an osmotic secretion of the anion. This process, coupled with sodium influx, causes the proximal duct to absorb a portion of the chloride and secrete bicarbonate, via CFTR, along with a large amount of pancreatic fluid. Within the distal pancreatic duct, CFTR primarily functions as a bicarbonate channel due to the low chloride content of the pancreatic fluid in this region [22]. CFTR also contributes to fluid secretion to flush pancreatic pro-enzymes into the duodenum. In CF, altered composition of pancreatic secretions include lower pH, reduced secretory volume and higher protein content; these factors are thought to alter zymogen secretions leading to obstruction [5,24–26]. These changes can be seen as early as seventeen weeks gestational age in utero, with obstruction of small ducts and acini. With progression, acinus plugging and dilation cause epithelial injury and destruction accompanied by inflammation, fibrosis and fatty infiltration/replacement [21,27,28]. CF pancreata with advanced disease may have only islets or rare ducts in a sea of adipose tissue that has replaced the destroyed pancreas. Pancreatic obstructions are initially composed of abnormal zymogen secretions, but over the course of disease include mucus accumulation from metaplastic epithelium lining ducts [26].
Patients are classified into 6 different groups based on their CFTR mutation [29–31]. Those within classes I, II, III, IV, and VI have mutations that often render CFTR absent or non-functional and are commonly pancreatic insufficient (PI), requiring lifelong pancreatic enzyme replacement. Approximately 85% of CF patients fall into these classes, while the remainder, or those within class V or mild class IV, are generally pancreatic sufficient (PS) due to less severe CFTR mutations [5]. Of course, those that are considered PS do not escape pancreatic disease completely. Pancreatic destruction is still detectable as evidenced by elevated levels of serum immunoreactive trypsinogen, but often does not reach the point clinically that it affects normal digestion [32]. Interestingly, CF patients that are PS are more prone to recurrent bouts of acute and/or chronic pancreatitis compared to PI patients [33–35], suggesting sustained partially impaired function of the pancreatic ducts. It has been demonstrated via genetic studies that there is a significant association between acute pancreatitis and mutations of CFTR, which is not surprising based on the key role CFTR plays in pancreatic ductal secretions [36].
The earliest histopathological descriptions of CF indicated that the exocrine pancreas was targeted for destruction, but noted that the islets of Langerhans were spared [20]. Even so, we now know that the endocrine pancreas is also affected in CF. CF-related diabetes (CFRD) is an increasingly recognized complication of CF that occurs in 50% of adults with CF [37,38]; even before onset of overt diabetes, children with CF exhibit impaired glucose tolerance on testing [39]. Exocrine pancreatic insufficiency (EPI) is an important risk factor for CFRD [40]. Onset of disease typically occurs at 18–21 years of age and appears to slightly favour (AQ: favour seems wrong choice of word here as this is not a good event.) females in comparison to males [41]. CFRD does not fall into either type 1 or 2 diabetes categories as it has features of both; it is characterized by both a loss of functional β-cell mass as well as also having varying extents of insulin resistance [41,42]. Patients with CFRD have decreased pulmonary function and nutritional status with higher mortality compared to CF patients without CFRD [43,44]. The two major mechanisms thought to play a role in development of CFRD include decreases in islet cell mass and β-cell dysfunction [37]. Decreases in islet cell mass have been associated with exocrine destruction, fibrosis and fatty infiltration as well as islet amyloid deposition [37]. Oxidative and endoplasmic reticulum stress of β-cells have also recently been implicated in CFRD pathogenesis [45,46]. Dysfunction of β-cells may occur simultaneously in CFRD due to a multitude of factors including dysfunction of the immune system, impaired insulin secretion and altered entero-insular axis hormones [37]. Chronic inflammation is a common finding in both CF lungs and pancreata and may play a role in development of CFRD [47,48]. Low vitamin D levels, which are common in CF patients secondary to PI and malabsorption, have been shown to cause abnormal immune responses [49]. CFTR may also have a direct role in insulin secretion from β-cells [50]. Whatever the mechanism(s) of pathogenesis, CFRD can potentially lead to important histopathological findings on autopsy. Islets can usually be identified histologically in CF patients with CFRD, although these are oftentimes fewer in number [51]. Remaining islets can have significantly fewer insulin-positive cells, glucagon-positive cells and pancreatic polypeptide-positive cells, with a relative increase in delta cells as compared to non-CF islets [41,51,52]. Islets from patients with CFRD also have been described to have islet amyloidosis, a common histopathological feature of type II diabetes mellitus [51,53]. It has been suggested that CFTR mutations could possibly predispose patients to develop amyloidosis because of alterations in the pH within pancreatic islets [54].
Exocrine pancreatic lesions in animal models of CF
Mouse models
Although there are multiple mouse models of CF, the best models achieve only mild exocrine pancreatic disease (AQ: please check this retains your intended meaning) [7,8]. Aged CF mice maintained on a liquid diet have shown modest pancreatic disease characterized by dilation of pancreatic ducts by inspissated secretions, mild inflammation and acinar atrophy [55]. There have also been a few studies reporting pancreatic changes in Cftrtm1Unc mice weaned and maintained on a liquid diet. These mice had lower pancreatic weight and reduced lipase activity as well as mild dilation of acini and accumulation of zymogen in ducts [56,57]. Similarly, another CF mouse model, Cftrtm1CAM mice, had plugging in approximately half of the pancreatic ducts, but this was still a fairly mild pathological finding with a lack of progressive destruction as seen in humans [58]. More recently, a study showed that CF mice fed polyethylene glycol 400 in water had increased expression of Muc6 within the pancreas, a mucin that also increases in abundance during human CF disease [59]. The relative paucity of pancreatic pathology in CF mouse models has been theorized to be due to lower Cftr expression in the pancreas and possibly the presence of alternative secretory channels which compensate for the loss of CFTR [7].
Porcine models
Pig models for CF have included null, ΔF508 CFTR mutants and CFTR−/−;Tg FABP>pCFTR lines. The Tg FABP>pCFTR line allows for transgenic expression of CFTR cDNA under control of the intestinal fatty acid-binding protein (iFABP), which restores CFTR expression specifically within the intestine, mitigating the occurrence of neonatal intestinal obstruction [9,21,60]. Pigs have separate duct systems transporting pancreatic and biliary secretions into the duodenum. This advantage allows for physiologic studies of both the pancreatic and liver secretions separately during CF disease [61]. CFTR is localized to pancreatic ductal epithelial cells in newborn piglets, similar to humans [61,62].
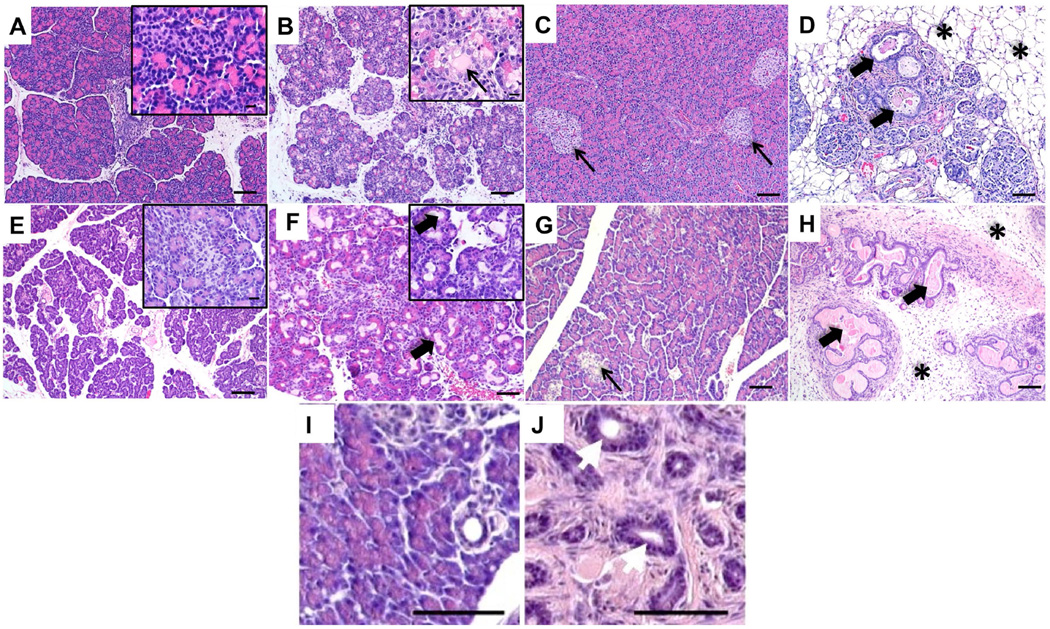
Newborn CF piglets have a smaller, more granular appearing pancreas than wild type controls. Histologically, pancreatic disease ranges from moderate to extensive acinar cell destruction, duct dilation, obstruction with zymogen secretions and mild patchy inflammation consisting of lymphocytes, neutrophils and macrophages which progresses over time [28] (Figure 1A–D). In some cases, dilated ducts can ulcerate and rupture leading to pools of free zymogen within the interstitium along with other remodelling such as ductal proliferation and fibrosis, similar to what has been described in humans [20,28,63,64]. While necrosis and cellular debris can be seen in dilated ducts/acini, increased caspase-3 immunostaining (AQ: do you mean activated caspase-3 ?) within acini of both newborn and fetal CF pigs suggests apoptotic pathways are activated [17]. Pancreatic fluid in CF pigs has decreased levels of elastase and chymotrypsin along with a decrease in total amount of secretions [61]. As CF pigs age, they develop progressive pancreatic destruction with eventual loss of the exocrine pancreatic tissue and replacement by adipose and fibrotic tissue within a few months of birth [17]. The acceleration of CF pig pancreatic disease at birth may be due to genetic modifiers, a more severe (i.e. null) genotype compared to humans, or expression of other anion channels that can aid in compensation in the case of human disease [28].
Figure 1.
H&E images of lesions of the pancreas in the wild-type and CF pig, ferret and zebrafish. A. Wild-type newborn pig pancreas. B. CF newborn pig pancreas highlighting dilated acinus tissue (inset) filled with lightly eosinophilic secretions (arrow). Bars = 200µm (inset bars = 20µm). C. Adult pig pancreas showing normal exocrine and endocrine (arrows) pancreatic tissue. D. CF adult pig pancreas demonstrating dilated pancreatic ducts (arrows), loss of exocrine pancreatic tissue and fatty infiltration (asterisks) (Bars = 200 µm). E. Wild-type newborn ferret pancreas. Inset highlights a normal islet surround by exocrine tissue. F. CF newborn ferret pancreas with acinus dilation (arrows) Bars = 200µm (inset bars = 20µm). G. Wild-type adult ferret pancreas showing normal pancreatic islets (arrows) surrounded by exocrine pancreatic tissue. H. CF adult ferret pancreas with abundant loose fibrous connective tissue (asterisks), multifocal inflammatory cell infiltrates and multifocal islands of dilated acini and ducts filled with lightly eosinophilic secretions (arrows) (Bars = 100 µm). I. Wild-type zebrafish pancreas at 1 year post fertilization. J. cftr mutant zebrafish pancreas at 1 year post fertilization showing loss of exocrine pancreas, fibrosis and dilated pancreatic ducts (white arrows) (bars = 50 µm) (reprinted with permission) [12].
Ferret model
The ferret model of CF was developed because the ferret’s lung anatomy is similar to that of humans and the moderate size of the ferret makes handling and housing not much more burdensome than for a rodent [10]. CFTR-knockout ferrets have a disruption in exon 10, which was achieved by adeno-associated virus-mediated insertion of a neomycin cassette at the CFTR locus in fibroblasts, coupled with somatic cell nuclear transfer [65]. The CF ferret and pig both exhibit pancreatic lesions at birth, although the changes in newborn ferrets are less severe [65,66]. The pancreas from newborn CF ferrets (also called kits) grossly resembles the newborn wild-type pancreas, yet at birth CF ferret pancreata have dilated acini and ducts, which are expanded by eosinophilic zymogen material [65] (Figure 1E, F). CF kits also have increased duct and acinar cell apoptosis, indicating that changes within the pancreas occur very early in life [18]. Within the first month of life and beyond into adulthood, CF kits quickly develop significant acinus destruction and loss along with marked duct dilation [18,21,67] (Figure 1 G, H). As CF ferrets age there is increased pancreatic fibrosis as well as infiltration of inflammatory cells; primarily neutrophils, macrophages and lymphocytes [18]. By adulthood (>5 months of age), CF ferrets accumulate adipose tissue and islets reside within fibrotic regions surrounding large ducts, similar to humans [67]. The majority of newborn CF kits are PI and lack detectable levels of pancreatic elastase 1 (EL1) in their faeces [14,67]. However, a very small percentage (less than 1%) of newborn CF kits are PS with mild pancreatic pathology, normal faecal EL1 levels, and normal growth [67]. Although the incidence of CF kits with PS is quite low, PS also occurs within a small subset of the human CF population as well, depending on the CFTR mutation that is present. However, the findings that even a small number of CFTR knockout ferrets can have near normal pancreatic histology and normal growth rates, suggests that a modifier gene(s) can have a significant impact on CF pancreatic disease. Thus, the variability in PS among CF patients may not be solely due to the type of CFTR mutation. Attempts to capture this trait through the breeding of siblings from PS CF ferrets, however, have proven unsuccessful and suggest that more than one modifier locus may be responsible.
Zebrafish model
A zebrafish CF model has recently been reported as an alternative to the larger animal species [12,13]. The small size of zebrafish is conducive for handling and housing and also allows for relatively rapid forward genetic and/or chemical screens that are more difficult in the larger animal models. Zebrafish express CFTR on the apical membrane of ducts in the pancreas and loss of CFTR function produces pancreatic destruction [12]. It was reported that 10 days post fertilization (while the zebrafish are considered to be larvae) there is increased death in the mutants, by 16 days post fertilization loss of exocrine pancreatic tissue is evident and by 22 days post fertilization there is marked loss of exocrine tissue. Adult CF zebrafish (3 months post fertilization) exhibit significant destruction of pancreatic acini with duct dilation and filling with PAS positive mucus, neutrophil infiltration and marked fibrosis [12] (Figure 1 I, J).
Endocrine pancreas lesions in animal models of CF
Mouse model
PI is a risk factor for development of CFRD in humans. CF mice lack severe exocrine disease and also do not spontaneously develop CFRD [37,68,69]. That said, CF mice have been used to study the possible consequences of CFRD, specifically on the immune system and pulmonary function by inducing diabetes using streptozotocin, a chemical that is toxic to pancreatic β cells [68,69].
Porcine model
At birth, the endocrine pancreas in CF pigs appears to be spared from the surrounding exocrine destruction [20,28]. More in-depth analysis of the islets from pigs ranging from fetal to one year of age concluded that islet structure remained intact over time with preferential localization of insulin-immunoreactive islets to remnant lobular tissue (Figure 2A). Even so, there were no differences in the cellular density of either insulin or glucagon-producing cells [66]. Nonetheless, abnormalities were identified in both blood glucose and insulin secretion of newborn CF piglets, suggesting a secretory defect caused by loss of CFTR. Newborn CF piglets had abnormally low insulin secretion and elevated serum glucose after IV bolus glucose challenge. Adult CF pigs had higher blood glucose levels compared to controls, indicating they develop spontaneous hyperglycaemia [66].
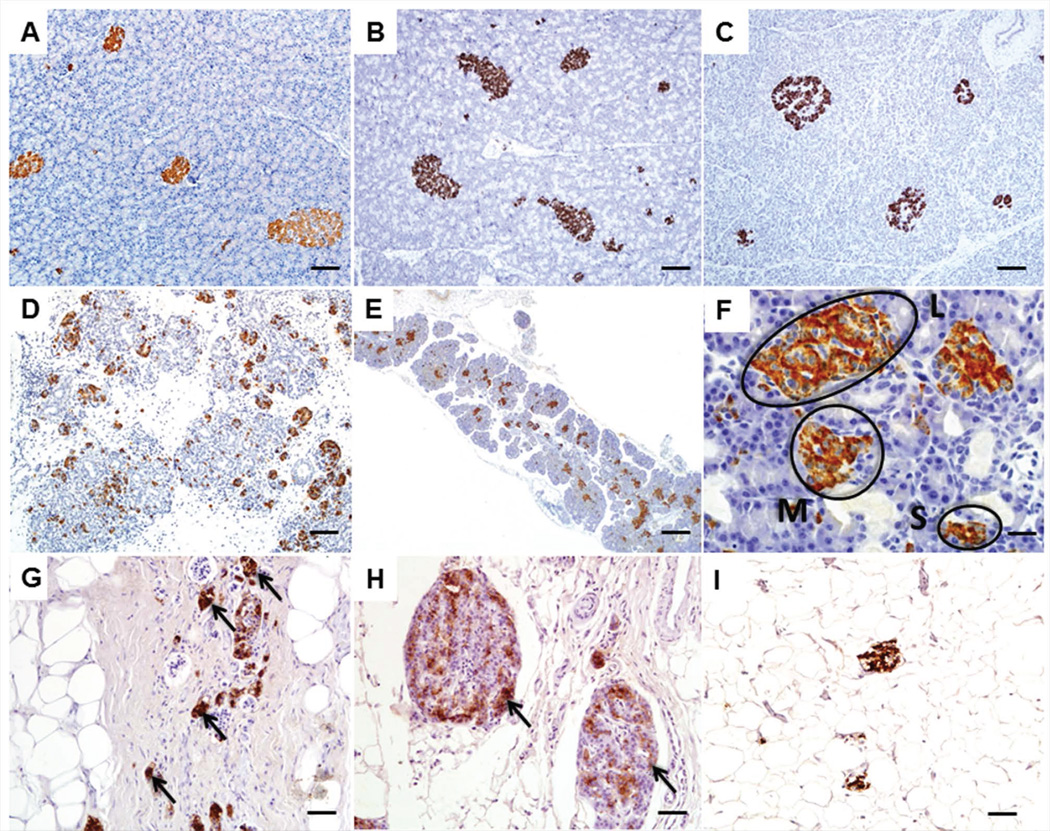
Figure 2.
Insulin immunohistochemistry (IHC) images of pancreatic islets from CF pigs, ferrets and human patients. A, B, C. Adult pancreata stained with insulin from WT pig (A), WT ferret (B) and normal human (C) Bars = 100 µm. D. Newborn CF pig pancreas stained with insulin to show abundance of islets even with exocrine pancreatic destruction. Bar = 200 µm. E. Insulin IHC of newborn CF ferret pancreas. Bar = 200 µm. F. Higher magnification images of B demonstrating the different sized islets present. There were more small islets (S) in CF ferrets compared to WT with fewer large (L) islets while medium (M) sized islets were not different (19). Bar = 20µm. G, H. Insulin IHC performed on human CF patients highlighting the lack of exocrine tissue with remnant insulin immunoreactive islets (arrows). I. Insulin IHC on a human CF patient with CFRD demonstrating a paucity of insulin immunoreactivity and abundance of adipose tissue.
Ferret model
At birth, CF ferret pancreatic islets are present (Figure 2B), although with more small islets and fewer large islets compared to age-matched controls [18] (Figure 2C). Pancreata from newborn CF kits have normal levels of lobular insulin and glucagon staining, suggesting that the abundance of β-cells and α-cells remains intact at birth [18]. CF kits 6–12 hours old demonstrate poorly regulated blood glucose and insulin levels during spontaneous feeding—non-CF kits demonstrated an association of higher blood glucose and higher blood insulin, while CF kits lost this relationship [18]. Formal glucose testing in newborn CF kits also demonstrated impaired glucose tolerance, elevated glucose area under the curve, and impaired first phase insulin secretion, demonstrating that even early in life glucose handling is impaired [18]. At 1–2 months of age CF ferrets had multiple abnormal glucose tolerance phenotypes, including elevated fasted glucose and abnormal mixed meal tolerance tests, demonstrating progressive disturbances in the ability of CF animals to handle glucose. Over time, the percent area showing insulin and glucagon staining in the pancreas decreases in CF, indicating a progressive loss of endocrine tissue [18]. Importantly, in vitro cultures of neonatal ferret islets show dysregulation of insulin secretion and impaired glucose stimulated insulin secretion in CF genotypes, providing evidence that CFTR defects intrinsic to the islet affect insulin secretion [18]. It will be of interest to utilize the CF ferret to better understand CFRD in humans. Histopathologically, CF patients show similar pancreatic disease as the CF ferret with clustering of insulin-immunoreactive islets within fibrotic tissue (Figure 2D,E) and those with CFRD oftentimes lack much in the way of islet tissue at all (Figure 2F).
Zebrafish model
The zebrafish model has notable endocrine pancreatic disorganization as reported by Navis, et al. [12]. CF zebrafish islets have altered spatial distribution of insulin and glucagon staining within islets and islets are smaller and more numerous in comparison to wild-type sibling controls [12].
Relationship of pancreatic disease to gastrointestinal disease in CF
In the 1930’s, before CF was recognized, there were reports of pancreatic insufficiency, steatorrhoea, vitamin A deficiency and failure to thrive in children, many of which on autopsy had evidence of severe exocrine pancreatic disease [19,20,70–74]. Many of these reports are now thought to be secondary to CF [75]. Today, many of these manifestations of CF are historical as pancreatic insufficient CF patients are now treated with daily pancreatic enzyme replacement [76]. The combination of destruction and altered secretions in the CF pancreas can cause clinical PI, necessitating enzyme supplementation. Additionally, decreased bicarbonate levels from the pancreas and other tissues such as Brunner’s glands, and bile, leads to decreased pH [5,77,78]. Lower duodenal pH limits activation of many pancreatic enzymes within the duodenum and is associated with precipitation of bile acids, development of intestinal obstruction and also a predisposition to pancreatitis [5,36,79].
Pancreatic disease has been speculated to contribute to intestinal obstruction, but assessment of this has been difficult in humans. Meconium ileus, obstruction of the intestine shortly after birth, occurs in 15–20% of CF babies [80,81]. A similar type of intestinal obstruction can also occur anytime later in life and is termed distal intestinal obstruction syndrome (DIOS) [21], which is also frequently observed in CF ferrets [67]. Constipation, although a less severe manifestation of disease, is also associated with CF and has been reported in 47% of CF patients [82]. Intestinal obstruction occurs in 100% of CF pigs at the time of birth, but transgenic expression of CFTR cDNA under control of the intestinal fatty acid-binding protein (iFABP) allows for alleviation of meconium ileus [60]. This same technology has also been applied to the CF mouse [83] and CF ferret [10]. In contrast to the pig, ~75% of CF ferrets have meconium ileus at birth and the incidence appears to have heritable contributions [10]. In the iFABP CF pig the persistence of pancreatic destruction, but mitigation of intestinal obstruction, suggests that pancreatic disease it is not a major contributing factor to intestinal obstruction [60]. Further evidence using conditional expression (villin:Cre) of CFTR in intestinal epithelium of PS CF mice also suggests that replacement of CFTR in intestinal epithelium alone can prevent intestinal obstruction [84]. Taken together, these studies suggest that the CFTR status of the intestinal epithelium, but not the extent of pancreatic disease, is important in the development of intestinal obstruction.
Relationship of pancreatic disease to lung disease in CF
Clinically, there is a strong link between pancreatic insufficiency and the severity of lung disease because severe CFTR mutations affect both of these organs [37,85]. Lung function tests in a cohort of CF patients were found significantly better in those with normal fat absorption in comparison to those with steatorrhoea which is a symptom of PI [86]. A later study identified that CF patients with PI had a marked decline in pulmonary function as compared to those with PS, as measured by forced vital capacity (FVC), forced expiratory volume in one second (FEV1) and forced expiratory flow in the middle half of FVC, [85]. Another study reported growth indices, including weight-for-age and height-for-age, and symptoms of lung disease are highly associated with decreased lung function at 6 years of age, and concluded that early treatment with pancreatic enzyme therapy and appropriate nutrition could improve lung health overall [87]. The link between pancreatic disease and pulmonary disease is multifaceted. Poor nutritional status and growth secondary to PI cannot only affect the growth of the lung early in life [88], but also immune function leading to increased pulmonary infections and therefore decreased pulmonary function [89,90] (AQ: please check sense of sentence). Lung disease can also affect weight and growth directly as well, via suppression of appetite and increases the amount of energy expended [87,91,92], demonstrating that the pancreas is not solely responsible for growth restriction associated with CF.
CFRD is also directly linked to severity of lung disease. Approximately half of CF patients develop CFRD by the age of 30 years, and this comorbidity is also associated with decreased pulmonary function in CF patients [42,43,85]. Decreases in lung function are oftentimes identified before CFRD is diagnosed and these decreases are proportional to the severity of insulin deficiency [42,44]. Interestingly, people with diabetes in the general population (those that do not have CF) have reduced lung function (2–4% lower) compared to non-diabetics further suggesting a direct association [46]. Diabetes has been linked to restrictive pulmonary function, which is likely due mechanistically to increased inflammation [93]. Diabetes also affects normal immune system function, specifically leading to complement system deficiencies, decreased activity of natural killer cells and neutrophils, and decreased responses by lymphocytes [94–98]. Insulin deficiency leads to malnutrition by promoting a catabolic state and malnutrition itself is known to 1) impair lung function by decreasing muscle mass, specifically of the diaphragm and intercostal muscles [44,99,100] and 2) directly affecting the immune status [101].
Hyperglycaemia can affect lung function, and a rat model of diabetes showed there are changes in the synthesis and turnover of connective tissue leading to thickening of pulmonary septal walls [102]. Another mechanism of lung dysfunction in CFRD is defective bacterial clearance in the lung [103]. Increased glucose levels within airways secondary to CFRD resulted in increased bacterial proliferation in vitro compared to cultures with normal glucose levels [103]. This is of interest as one can imagine any decrease in lung function would be magnified in CF patients with underlying lung disease [44,46].
In animal models, it has been reported that streptozotocin-treated CF mice have a reduced ability to clear bacteria from the lung and that they have an increased but less effective pulmonary inflammatory cell response [68,69]. It is also interesting that newborn CF ferrets have poorly regulated blood glucose and insulin levels [18], while also demonstrating heightened inflammatory response in the lung prior to bacterial colonization [104]. Furthermore, the lungs of CFTR knockout ferrets are very rapidly colonized by bacteria, requiring multiple antibiotics during rearing to adulthood [105] and also have rapidly progressing pancreatic disease that influences glucose metabolism [18]. Whether these observations regarding lung inflammation and infection are linked to the severity of pancreatic inflammation and endocrine dysfunction remains to be determined. It has been shown that hyperglycaemia during diabetes inhibits leukocyte function, therefore contributing to a decreased host defence during infections, including those of the lung [106,107]. All of these findings indicate that early and sustained insulin therapy and nutritional supplementation may be beneficial for CF patients, even before onset of clinically evident CFRD [108–110].
Future directions for animal models of CF
Animal models are necessary to understand disease pathogenesis and to develop treatments for human diseases. The CF field is fortunate to have a large number of animal models to facilitate research discovery in these areas. These animal models have also significantly aided in the study of early CF disease processes that are difficult to examine in CF infants and children. As evident from this review, CF patients often have multiple disease manifestations that confound the study of each affected organ system. As already accomplished in mice, enhanced tools for genetic engineering in larger animal models will also soon allow for the dissection of CF disease processes in a single affected organ system, which is impossible in humans. Such models will aid in the understanding of multi-organ aspects of CF disease pathogenesis and how disease processes in different organs influence the overall progression of disease in the whole animal. For example, models that have the ability to reduce CFTR expression and/or restore CFTR expression in specific organs such as “gut-corrected” (iFABP) CF pig and ferret [60,65], will allow for the dissection of gut-lung and gut-pancreas disease relationships. Comparative studies between the multiple animal models of CF has also allowed researchers to better understand aspects of CFTR and organ physiology that contribute to CF pathogenesis, and the potential influences of modifier genes on disease processes [10,21,67]. All of these models have aided in our understanding of CF and most importantly provide great opportunity for testing therapies targeted at the multiple organs affected in disease.
Acknowledgements
We thank the University of Iowa Comparative Pathology Laboratory for histology services and Dr. Michel Bagnat and Elsevier for allowing us to use the figure showing zebrafish pancreata.
Grants
This work was supported by National Heart, Lung and Blood Institute Grants HL51670, HL091842 and HL123482, National Institute of Diabetes and Digestive and Kidney Diseases Grants P30 DK054759 and R24 DK096518 and the Cystic Fibrosis Foundation.
Footnotes
The authors have no conflicts of interest.
Author Contributions
K.N.G-C., D.K.M and J.F.E. developed the concept, drafted the manuscript, prepared the figures, edited and revised the manuscript and approved the final version.
References
- 1.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen JH, Fischer H, Illek B, et al. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natnl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JH, Stoltz DA, Karp PH, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. The New Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilschanski M, Novak I. The cystic fibrosis of exocrine pancreas. Cold Spring Harbor perspectives in medicine. 2013;3:a009746. doi: 10.1101/cshperspect.a009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielenski J. Genotype and phenotype in cystic fibrosis. Respiration; international review of thoracic diseases. 2000;67:117–133. doi: 10.1159/000029497. [DOI] [PubMed] [Google Scholar]
- 7.Scholte BJ, Davidson DJ, Wilke M, et al. Animal models of cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2004;3(Suppl 2):183–190. doi: 10.1016/j.jcf.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 8.Wilke M, Buijs-Offerman RM, Aarbiou J, et al. Mouse models of cystic fibrosis: phenotypic analysis and research applications. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2011;10(Suppl 2):S152–S171. doi: 10.1016/S1569-1993(11)60020-9. [DOI] [PubMed] [Google Scholar]
- 9.Rogers CS, Stoltz DA, Meyerholz DK, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X, Yan Z, Yi Y, et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118:1578–1583. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuggle KL, Birket SE, Cui X, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PloS one. 2014;9:e91253. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navis A, Bagnat M. Loss of cftr function leads to pancreatic destruction in larval zebrafish. Dev Biol. 2015;399:237–248. doi: 10.1016/j.ydbio.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navis A, Marjoram L, Bagnat M. Cftr controls lumen expansion and function of Kupffer's vesicle in zebrafish. Development. 2013;140:1703–1712. doi: 10.1242/dev.091819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Z, Stewart ZA, Sinn PL, et al. Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. Human gene therapy Clinical development. 2015;26:38–49. doi: 10.1089/humc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widdicombe JH. Transgenic animals may help resolve a sticky situation in cystic fibrosis. J Clin Invest. 2010;120:3093–3096. doi: 10.1172/JCI44235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers CS, Abraham WM, Brogden KA, et al. The porcine lung as a potential model for cystic fibrosis. American journal of physiology Lung cellular and molecular physiology. 2008;295:L240–L263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-El-Haija M, Ramachandran S, Meyerholz DK, et al. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. Amer J Pathol. 2012;181:499–507. doi: 10.1016/j.ajpath.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivier AK, Yi Y, Sun X, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122:3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper MH. Congenital steatorrhoea due to pancreatic defect. Archives of disease in childhood. 1938;13:45–56. doi: 10.1136/adc.13.73.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease - A clinical and pathologic study. Am J Dis Child. 1938;56:344–399. [Google Scholar]
- 21.Olivier AK, Gibson-Corley KN, Meyerholz DK. Animal models of gastrointestinal and liver diseases. Animal models of cystic fibrosis: gastrointestinal, pancreatic, and hepatobiliary disease and pathophysiology. American journal of physiology Gastrointestinal and liver physiology. 2015;308:G459–G471. doi: 10.1152/ajpgi.00146.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MG, Ohana E, Park HW, et al. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 2012;92:39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HW, Lee MG. Transepithelial bicarbonate secretion: lessons from the pancreas. Cold Spring Harbor perspectives in medicine. 2012;2 doi: 10.1101/cshperspect.a009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopelman H, Corey M, Gaskin K, et al. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology. 1988;95:349–355. doi: 10.1016/0016-5085(88)90490-8. [DOI] [PubMed] [Google Scholar]
- 25.Kopelman H, Durie P, Gaskin K, et al. Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. New Engl J Med. 1985;312:329–334. doi: 10.1056/NEJM198502073120601. [DOI] [PubMed] [Google Scholar]
- 26.Tucker JA, Spock A, Spicer SS, et al. Inspissation of pancreatic zymogen material in cystic fibrosis. Ultrastruct Pathol. 2003;27:323–335. [PubMed] [Google Scholar]
- 27.Imrie JR, Fagan DG, Sturgess JM. Quantitative evaluation of the development of the exocrine pancreas in cystic fibrosis and control infants. Amer J Pathol. 1979;95:697–708. [PMC free article] [PubMed] [Google Scholar]
- 28.Meyerholz DK, Stoltz DA, Pezzulo AA, et al. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Amer J Pathol. 2010;176:1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerem E, Corey M, Kerem BS, et al. The relation between genotype and phenotype in cystic fibrosis--analysis of the most common mutation (delta F508) New Engl J Med. 1990;323:1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- 30.Kristidis P, Bozon D, Corey M, et al. Genetic determination of exocrine pancreatic function in cystic fibrosis. Amer J Hum Genet. 1992;50:1178–1184. [PMC free article] [PubMed] [Google Scholar]
- 31.Kreindler JL. Cystic fibrosis: exploiting its genetic basis in the hunt for new therapies. Pharmacol Ther. 2010;125:219–229. doi: 10.1016/j.pharmthera.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Augarten A, Ben Tov A, Madgar I, et al. The changing face of the exocrine pancreas in cystic fibrosis: the correlation between pancreatic status, pancreatitis and cystic fibrosis genotype. European journal of gastroenterology & hepatology. 2008;20:164–168. doi: 10.1097/MEG.0b013e3282f36d04. [DOI] [PubMed] [Google Scholar]
- 33.Shwachman H, Lebenthal E, Khaw KT. Recurrent acute pancreatitis in patients with cystic fibrosis with normal pancreatic enzymes. Pediatrics. 1975;55:86–95. [PubMed] [Google Scholar]
- 34.Durno C, Corey M, Zielenski J, et al. Genotype and phenotype correlations in patients with cystic fibrosis and pancreatitis. Gastroenterology. 2002;123:1857–1864. doi: 10.1053/gast.2002.37042. [DOI] [PubMed] [Google Scholar]
- 35.Ooi CY, Dorfman R, Cipolli M, et al. Type of CFTR Mutation Determines Risk of Pancreatitis in Patients With Cystic Fibrosis. Gastroenterology. 2011;140:153–161. doi: 10.1053/j.gastro.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 36.Hegyi P, Rakonczay Z., Jr The role of pancreatic ducts in the pathogenesis of acute pancreatitis. Pancreatology. 2015;15:S13–S17. doi: 10.1016/j.pan.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Barrio R. Management of endocrine disease: Cystic fibrosis-related diabetes: novel pathogenic insights opening new therapeutic avenues. European journal of endocrinology / European Federation of Endocrine Societies. 2015;172:R131–R141. doi: 10.1530/EJE-14-0644. [DOI] [PubMed] [Google Scholar]
- 38.Moran A, Dunitz J, Nathan B, et al. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32:1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ode KL, Moran A. New insights into cystic fibrosis-related diabetes in children. Llancet Diabetes & endocrinology. 2013;1:52–58. doi: 10.1016/S2213-8587(13)70015-9. [DOI] [PubMed] [Google Scholar]
- 40.Lanng S, Thorsteinsson B, Erichsen G, et al. Glucose tolerance in cystic fibrosis. Arch Dis Child. 1991;66:612–616. doi: 10.1136/adc.66.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moran A, Hardin D, Rodman D, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes research and clinical practice. 1999;45:61–73. doi: 10.1016/s0168-8227(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 42.Nathan BM, Laguna T, Moran A. Recent trends in cystic fibrosis-related diabetes. Current opinion in endocrinology, diabetes, and obesity. 2010;17:335–341. doi: 10.1097/MED.0b013e32833a780d. [DOI] [PubMed] [Google Scholar]
- 43.Chamnan P, Shine BS, Haworth CS, et al. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care. 2010;33:311–316. doi: 10.2337/dc09-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. American journal of respiratory and critical care medicine. 2000;162:891–895. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 45.Galli F, Battistoni A, Gambari R, et al. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochimica et biophysica acta. 2012;1822:690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Stecenko AA, Moran A. Update on cystic fibrosis-related diabetes. Current opinion in pulmonary medicine. 2010;16:611–615. doi: 10.1097/MCP.0b013e32833e8700. [DOI] [PubMed] [Google Scholar]
- 47.Arif S, Moore F, Marks K, et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes. 2011;60:2112–2119. doi: 10.2337/db10-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaochite JN, Caliari-Oliveira C, Davanso MR, et al. Dynamic changes of the Th17/Tc17 and regulatory T cell populations interfere in the experimental autoimmune diabetes pathogenesis. Immunobiology. 2013;218:338–352. doi: 10.1016/j.imbio.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Hewison M. Vitamin D and immune function: an overview. Proc Nutrit Soc. 2012;71:50–61. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 50.Guo JH, Chen H, Ruan YC, et al. Glucose-induced electrical activities and insulin secretion in pancreatic islet beta-cells are modulated by CFTR. Nature Commun. 2014;5:4420. doi: 10.1038/ncomms5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iannucci A, Mukai K, Johnson D, et al. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol. 1984;15:278–284. doi: 10.1016/s0046-8177(84)80191-4. [DOI] [PubMed] [Google Scholar]
- 52.Lohr M, Goertchen P, Nizze H, et al. Cystic fibrosis associated islet changes may provide a basis for diabetes. An immunocytochemical and morphometrical study. Virchows Archiv A, Pathological anatomy and histopathology. 1989;414:179–185. doi: 10.1007/BF00718598. [DOI] [PubMed] [Google Scholar]
- 53.Couce M, O'Brien TD, Moran A, et al. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. The Journal of clinical endocrinology and metabolism. 1996;81:1267–1272. doi: 10.1210/jcem.81.3.8772610. [DOI] [PubMed] [Google Scholar]
- 54.Brennan AL, Geddes DM, Gyi KM, et al. Clinical importance of cystic fibrosis-related diabetes. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2004;3:209–222. doi: 10.1016/j.jcf.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Durie PR, Kent G, Phillips MJ, et al. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Amer J Pathol. 2004;164:1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delisle RC. Increased Expression of Sulfated Gp300 and Acinar Tissue Pathology in Pancreas of CftR−/−) Mice. Am J Physiol-Gastr L. 1995;268:G717–G723. doi: 10.1152/ajpgi.1995.268.4.G717. [DOI] [PubMed] [Google Scholar]
- 57.Ip WF, Bronsveld I, Kent G, et al. Exocrine pancreatic alterations in long-lived surviving cystic fibrosis mice. Pediatr Res. 1996;40:242–249. doi: 10.1203/00006450-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Ratcliff R, Evans MJ, Cuthbert AW, et al. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nature Genet. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- 59.Gouyer V, Leir SH, Tetaert D, et al. The characterization of the first anti-mouse Muc6 antibody shows an increased expression of the mucin in pancreatic tissue of Cftr-knockout mice. Histoche Cell Biol. 2010;133:517–525. doi: 10.1007/s00418-010-0688-8. [DOI] [PubMed] [Google Scholar]
- 60.Stoltz DA, Rokhlina T, Ernst SE, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest. 2013;123:2685–2693. doi: 10.1172/JCI68867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uc A, Giriyappa R, Meyerholz DK, et al. Pancreatic and biliary secretion are both altered in cystic fibrosis pigs. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G961–G968. doi: 10.1152/ajpgi.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marino CR, Matovcik LM, Gorelick FS, et al. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991;88:712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sturgess JM. Structural and developmental abnormalities of the exocrine pancreas in cystic fibrosis. Journal of pediatric gastroenterology and nutrition. 1984;3(Suppl 1):S55–S66. doi: 10.1097/00005176-198400031-00011. [DOI] [PubMed] [Google Scholar]
- 64.Durie PR, Forstner GG. Pathophysiology of the exocrine pancreas in cystic fibrosis. J Royal Soc Med. 1989;82(Suppl 16):2–10. [PMC free article] [PubMed] [Google Scholar]
- 65.Sun X, Sui H, Fisher JT, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uc A, Olivier AK, Griffin MA, et al. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci. 2015;128:131–142. doi: 10.1042/CS20140059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun X, Olivier AK, Yi Y, et al. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Amer J Pathol. 2014;184:1309–1322. doi: 10.1016/j.ajpath.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stalvey MS, Brusko TM, Mueller C, et al. CFTR mutations impart elevated immune reactivity in a murine model of cystic fibrosis related diabetes. Cytokine. 2008;44:154–159. doi: 10.1016/j.cyto.2008.07.468. [DOI] [PubMed] [Google Scholar]
- 69.Hunt WR, Zughaier SM, Guentert DE, et al. Hyperglycemia impedes lung bacterial clearance in a murine model of cystic fibrosis-related diabetes. American journal of physiology Lung cellular and molecular physiology. 2014;306:L43–L49. doi: 10.1152/ajplung.00224.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blackfan KD, Wolbach SB. Vitamin A deficiency in infants - A clinical and pathological study. J Pediatr. 1933;3:679–706. [Google Scholar]
- 71.Parmelee AH. The pathology of steatorrhea. Amer J Dis Child. 1935;50:1418–1428. [Google Scholar]
- 72.Hess JH, Saphir O. Celiac disease (chronic intestinal indigestion) A report of three cases with autopsy findings. Journal of Pediatrics. 1935;6:1–13. [Google Scholar]
- 73.Thomas J, Schlutz FW. Pancreatic steatorrhea. Amer J Dis Child. 1938;56:336–343. [Google Scholar]
- 74.Andersen DH. Cystic fibrosis of the pancreas, vitamin A deficiency, and bronchiectasis. J Pediatr. 1939;15:763–771. [Google Scholar]
- 75.Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 76.Somaraju UR, Solis-Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis (Review) Paediatric respiratory reviews. 2015;16:108–109. doi: 10.1016/j.prrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Heitzmann D, Warth R. No potassium, no acid: K+ channels and gastric acid secretion. Physiology. 2007;22:335–341. doi: 10.1152/physiol.00016.2007. [DOI] [PubMed] [Google Scholar]
- 78.Kopic S, Geibel JP. Update on the mechanisms of gastric acid secretion. Current gastroenterology reports. 2010;12:458–464. doi: 10.1007/s11894-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 79.Freedman SD, Kern HF, Scheele GA. Pancreatic acinar cell dysfunction in CFTR−/−) mice is associated with impairments in luminal pH and endocytosis. Gastroenterology. 2001;121:950–957. doi: 10.1053/gast.2001.27992. [DOI] [PubMed] [Google Scholar]
- 80.Farrelly PJ, Charlesworth C, Lee S, et al. Gastrointestinal surgery in cystic fibrosis: a 20-year review. J Pediatr Surg. 2014;49:280–283. doi: 10.1016/j.jpedsurg.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 81.Oppenheimer EH, Esterly JR. Observations in cystic fibrosis of the pancreas. II. Neonatal intestinal obstruction. Bulletin of the Johns Hopkins Hospital. 1962;111:1–13. [PubMed] [Google Scholar]
- 82.van der Doef HP, Kokke FT, van der Ent CK, et al. Intestinal obstruction syndromes in cystic fibrosis: meconium ileus, distal intestinal obstruction syndrome, and constipation. Current gastroenterology reports. 2011;13:265–270. doi: 10.1007/s11894-011-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou L, Dey CR, Wert SE, et al. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 84.Hodges CA, Grady BR, Mishra K, et al. Cystic fibrosis growth retardation is not correlated with loss of Cftr in the intestinal epithelium. American journal of physiology Gastrointestinal and liver physiology. 2011;301:G528–G536. doi: 10.1152/ajpgi.00052.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corey M, Edwards L, Levison H, et al. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 86.Gaskin K, Gurwitz D, Durie P, et al. Improved respiratory prognosis in patients with cystic fibrosis with normal fat absorption. J Pediatr. 1982;100:857–862. doi: 10.1016/s0022-3476(82)80501-5. [DOI] [PubMed] [Google Scholar]
- 87.Konstan MW, Butler SM, Wohl ME, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142:624–630. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 88.Thomson MA, Quirk P, Swanson CE, et al. Nutritional growth retardation is associated with defective lung growth in cystic fibrosis: a preventable determinant of progressive pulmonary dysfunction. Nutrition. 1995;11:350–354. [PubMed] [Google Scholar]
- 89.Shepherd R, Cooksley WG, Cooke WD. Improved growth and clinical, nutritional, and respiratory changes in response to nutritional therapy in cystic fibrosis. J Pediatr. 1980;97:351–357. doi: 10.1016/s0022-3476(80)80180-6. [DOI] [PubMed] [Google Scholar]
- 90.Bakker W. Nutritional state and lung disease in cystic fibrosis. The Netherlands journal of medicine. 1992;41:130–136. [PubMed] [Google Scholar]
- 91.Zemel BS, Kawchak DA, Cnaan A, et al. Prospective evaluation of resting energy expenditure, nutritional status, pulmonary function, and genotype in children with cystic fibrosis. Pediatr Res. 1996;40:578–586. doi: 10.1203/00006450-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 92.Fried MD, Durie PR, Tsui LC, et al. The cystic fibrosis gene and resting energy expenditure. J Pediatr. 1991;119:913–916. doi: 10.1016/s0022-3476(05)83042-2. [DOI] [PubMed] [Google Scholar]
- 93.Wannamethee SG, Shaper AG, Rumley A, et al. Lung function and risk of type 2 diabetes and fatal and nonfatal major coronary heart disease events: possible associations with inflammation. Diabetes Care. 2010;33:1990–1996. doi: 10.2337/dc10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Negishi K, Gupta S, Chandy KG, et al. Interferon responsiveness of natural killer cells in type I human diabetes. Diabetes Res. 1988;7:49–52. [PubMed] [Google Scholar]
- 95.Vergani D, Johnston C, N BA, et al. Low serum C4 concentrations: an inherited predisposition to insulin dependent diabetes? Br Med J. 1983;286:926–928. doi: 10.1136/bmj.286.6369.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delamaire M, Maugendre D, Moreno M, et al. Impaired leucocyte functions in diabetic patients. Diabetic medicine : a journal of the British Diabetic Association. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 97.Llorente L, De La Fuente H, Richaud-Patin Y, et al. Innate immune response mechanisms in non-insulin dependent diabetes mellitus patients assessed by flow cytoenzymology. Immunology letters. 2000;74:239–244. doi: 10.1016/s0165-2478(00)00255-8. [DOI] [PubMed] [Google Scholar]
- 98.Speert DP, Silva J., Jr Abnormalities of in vitro lymphocyte response to mitogens in diabetic children during acute ketoacidosis. Amer J Dis Child. 1978;132:1014–1017. doi: 10.1001/archpedi.1978.02120350078016. [DOI] [PubMed] [Google Scholar]
- 99.Arora NS, Rochester DF. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. The American review of respiratory disease. 1982;126:5–8. doi: 10.1164/arrd.1982.126.1.5. [DOI] [PubMed] [Google Scholar]
- 100.Arora NS, Rochester DF. Effect of body weight and muscularity on human diaphragm muscle mass, thickness, and area. Journal of applied physiology: respiratory, environmental and exercise physiology. 1982;52:64–70. doi: 10.1152/jappl.1982.52.1.64. [DOI] [PubMed] [Google Scholar]
- 101.Calder PC. Feeding the immune system. Proc Nutri Soc. 2013;72:299–309. doi: 10.1017/S0029665113001286. [DOI] [PubMed] [Google Scholar]
- 102.Ofulue AF, Kida K, Thurlbeck WM. Experimental diabetes and the lung. I. Changes in growth, morphometry, and biochemistry. The American review of respiratory disease. 1988;137:162–166. doi: 10.1164/ajrccm/137.1.162. [DOI] [PubMed] [Google Scholar]
- 103.Baker EH, Clark N, Brennan AL, et al. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. Journal of applied physiology. 2007;102:1969–1975. doi: 10.1152/japplphysiol.01425.2006. [DOI] [PubMed] [Google Scholar]
- 104.Keiser NW, Birket SE, Evans IA, et al. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. American journal of respiratory cell and molecular biology. 2015;52:683–694. doi: 10.1165/rcmb.2014-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun X, Olivier AK, Liang B, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. American journal of respiratory cell and molecular biology. 2014;50:502–512. doi: 10.1165/rcmb.2013-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McManus LM, Bloodworth RC, Prihoda TJ, et al. Agonist-dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia. J Leukocyte Biol. 2001;70:395–404. [PubMed] [Google Scholar]
- 107.de Souza Ferreira C, Araujo TH, Angelo ML, et al. Neutrophil dysfunction induced by hyperglycemia: modulation of myeloperoxidase activity. Cell biochemistry and function. 2012;30:604–610. doi: 10.1002/cbf.2840. [DOI] [PubMed] [Google Scholar]
- 108.Hameed S, Morton JR, Jaffe A, et al. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care. 2010;33:221–226. doi: 10.2337/dc09-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hardin DS, Rice J, Rice M, et al. Use of the insulin pump in treat cystic fibrosis related diabetes. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2009;8:174–178. doi: 10.1016/j.jcf.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 110.White H, Pollard K, Etherington C, et al. Nutritional decline in cystic fibrosis related diabetes: the effect of intensive nutritional intervention. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2009;8:179–185. doi: 10.1016/j.jcf.2008.12.002. [DOI] [PubMed] [Google Scholar]