Abstract
Residing at high altitude may lead to reduced blood oxygen saturation in the brain and altered metabolism in frontal cortical brain areas, probably due to chronic hypobaric hypoxia. These changes may underlie the increased rates of depression and suicidal behavior that have been associated with life at higher altitudes. To test the hypothesis that hypobaric hypoxia is responsible for development of mood disorders due to alterations in neurochemistry, we assessed depression-like behavior in parallel to levels of brain metabolites in rats housed at simulated altitude.
32 female Sprague Dawley rats were housed either in a hypobaric hypoxia chamber at 10,000 ft of simulated altitude for 1 week or at local conditions (4500 ft of elevation in Salt Lake City, Utah). Depression-like behavior was assessed using the forced swim test (FST) and levels of neurometabolites were estimated by in vivo proton magnetic resonance spectroscopy in the frontal cortex, the striatum and the hippocampus at baseline and after a week of exposure to hypobaric hypoxia.
After hypoxia exposure the animals demonstrated increased immobility behavior and shortened latency to immobility in the FST. Elevated ratios of myo-inositol, glutamate, and the sum of myo-inositol and glycine to total creatine were observed in the frontal cortex of hypoxia treated rats. A decrease in the ratio of alanine to total creatine was also noted. This study shows that hypoxia induced alterations in frontal lobe brain metabolites, aggravated depression-like behavior and might be a factor in increased rates of psychiatric disorders observed in populations living at high altitudes.
Keywords: Depression, Hypobaric hypoxia, Behavior, Proton magnetic resonance spectroscopy
1. Introduction
With an increasing world population, regions of high elevation have become more inhabited. In 1998, more than 140 million people lived at an altitude above 8000 ft [1], and millions more visit regions of high elevation every year. Living at high altitude is accompanied by exposure to low partial pressures of oxygen, potentially leading to oxygen deficits. Exposure to high altitudes has been found to detrimentally affect cardiovascular, pulmonary and nervous system function [2]. The symptoms of cerebral dysfunction associated with high altitude involve decreased physical and mental performance, including increased fatigue and impaired sleep [2]. High altitude climbers exhibit signs of focal brain damage (leukoaraiosis and/or mild cortical atrophy) along with neuropsychological deficits [3]. High altitude residents have structural modifications of the brain, including regional decrease of the grey matter and changes in the white matter [4]. Adolescents living at high altitude exhibit altered delta and beta frequencies in resting state EEG in parallel to reduced blood oxygen saturation [5]. Chronic exposure to hypobaric hypoxia in people living at high altitudes has been associated with lower resting metabolic states in the brain, particularly in frontal cortical areas [6] and altered brain metabolism in the anterior cingulate cortex [7] which may contribute to adverse mental health outcomes, including depression and suicidal behavior [8–11] as well as with increased rates of illicit drug use [12]. Despite decades of research, the impact of altitude as an environmental factor that underlies mental disorders remains incompletely understood.
Major depressive disorder (MDD) is a global medical problem due to its high prevalence and incomplete responsiveness to treatment [13]. MDD affects almost 10% of the population of the United States and national mental health surveys indicate that rates of depression are highest in the intermountain west [14]. Changes in neurometabolite concentrations in MDD patients occur within brain regions which are involved in the processing and communication of emotions, which can be monitored by proton magnetic resonance spectroscopy, 1 H MRS [15].
In vivo 1H MRS is a unique tool providing information about metabolic changes in pathological conditions affecting the brain. Some of the neurometabolites that 1H MRS can quantify are: N-acetylaspartate, NAA (a major component of neuronal mitochondria, that decreases with any neurodegenerative condition); glutamate/glutamine, Glu + Gln (the major excitatory neurotransmitter that also plays a key role in synapse formation, dendrite pruning, cell migration, differentiation and death); choline/phosphocholine, Cho + PCho (a metabolic marker of membrane density and integrity, elevated in increased cellular growth/turnover); creatine/phosphocreatine, Cr + PCr (regulates energy homeostasis in the cell); lactate, Lac (increased during anaerobic glycolysis, in ischemia and hypoxic conditions), myo-inositol, Ins (regulates neuronal osmolarity and membrane biosynthesis, also is a marker of glial proliferation, that increases with inflammatory processes), γ-aminobutyric acid, GABA (inhibitory neurotransmitter), taurine, Tau (a non-proteinogenic amino acid contributing to neurotransmission and neuromodulation in the CNS, supporting detoxification, antioxidation, and osmotic regulation) and others. A wide diversity of 1H MRS methods have been applied and brain regions studied (pre-frontal, parieto-occipital, cingulate cortex, hippocampus, amygdala and others), resulting in significant changes in metabolite levels assessed before and after antidepressant treatment, as reviewed by Caverzasi and co-workers [16].
The majority of hypoxia MRS studies have evaluated the impact of acute, severe hypoxia/anoxia on the levels of brain metabolites [17]. Some studies have examined the effects of hypoxia by analyzing the neurochemistry of the brain in high altitude climbers [18]. It should be noted that effect of hypoxia depends on the length of exposure (minutes, hours or days) and the type of exposure (continuous or intermittent), as well as on the intensity of exposure. For example, exposure to severe hypoxia (more than 18,000 ft of simulated altitude) resulted in prominent macroorganismic changes e.g., lost of appetite, decline in body weight [19], and symptoms of severe disturbances in cerebral function [20]. However, mild hypoxia exposure (up to 11,000 ft) does not appear to influence body weight but may have other detrimental consequences [21–23].
In this study we aimed to assess the neurobiological basis of increased MDD at high altitude by simulation of hypobaric hypoxia using a rat model. Rodents are widely used in translational research studies of depression [24]. Rodent models can be used to simulate several symptoms of MDD and to show resolution of these symptoms with antidepressant treatment. One of the rodent behavioral tests in depression research, the forced swim test (FST), was developed in 1978 by Porsolt and co-workers [25] as a model for predicting the clinical efficacy of antidepressant drugs. The FST is also one of the most commonly used tests to assess depression-like behavior in rodents. The basic FST involves two sessions with animals placed in a cylinder containing 25 °C water, from which they cannot escape. The first session is a 15 min pretest that is followed 24 h later by a 5 min test session. The pretest is a stressor which is thought to induce a state of behavioral despair [26] or passive stress coping strategy [27], since the animals become more immobile as the test session progresses. The typical posture of immobility is characterized by floating in the water with only movements necessary to keep the nose above the surface. The immobility time and also the latency to the initial immobility period [28] are the primary dependent measures [29].
Objective To test the hypothesis that hypobaric hypoxia alters the neurochemistry of the brain in parallel to behavioral changes, we aimed to assess the behavior of rats in the FST and to estimate changes in neurometabolites in the brain (within frontal lobe, striatum and hippocampus voxels) by in vivo proton MRS after one week of continuous exposure to mild hypobaric hypoxia at 10,000 ft of simulated altitude. In our preliminary studies we found that FST behaviors of female rats are more susceptible to hypobaric hypoxia treatment than in males, therefore we used female animals in this study.
2. Materials and methods
2.1. Animals and exposure to hypobaric hypoxia
Thirty-two female Sprague Dawley rats (Charles River, USA) (150–200 g body weight, n = 8–12 per group) were either housed in a hypobaric hypoxia chamber at 10,000 ft of simulated altitude (partial oxygen pressure 15%) for 1 week or were housed under local conditions (4500 ft of elevation in Salt Lake City, Utah). Body weight was measured before and after hypoxia treatment. Animals were housed separately in standard rodent cages with food and water in controlled room conditions. Separate sets of animals were used for the behavioral measurements and in vivo imaging to avoid the possible impact of the procedures on each other. All experimental procedures were performed according to University of Utah Institutional Animal Care and Use Committee guidelines.
2.2. The forced swim test
The behavior of 8 rats exposed to hypoxia and 12 control rats housed at local conditions for a week was assessed using the FST. The FST consists of placing the rat into a transparent tank containing 38 cm deep water at 25 °C temperature for 15 min during a pretest session and for 5 m during the test session, 24 h later according [30]. The pretest FST was scheduled after 6 days in the chamber for hypoxia treated rats or after 6 days at local conditions for the control group. After the pretest session, animals were removed from water, dried with paper towels and returned to their home cages at the appropriate altitude condition. The test session was performed 24 h later. Behavior in the test session of the FST was videotaped, and duration of immobility was scored. Animals were considered immobile if they showed no attempts to escape, floating in the water in the typical posture with only movements necessary to keep the nose above the water surface. Latency to immobility, the period of time taken to achieve the first 10 s period of immobility, was also estimated.
2.3. Proton MR spectroscopy acquisitions and metabolite quantification
Imaging experiments were conducted on 12 rats before exposure to hypoxia (baseline) and after 1 week of exposure to hypobaric hypoxia. Imaging was done using a 7 T horizontal-bore Bruker Biospec MRI scanner (Bruker Biospin, Billerica, MA, USA), interfaced with a 12 cm actively shielded gradient insert capable of producing magnetic field gradients of up to 600 mT/m. Animals were anesthetized using 1–3% isoflurane and 0.8 L/min O2 and their vital signs (respiration, temperature, heart rate and oxygen saturation percentage) were continuously monitored using a MR-compatible physiological monitoring system (SA Instruments, Stony Brook, NY, USA). Animals were placed in a 72 mm volume coil for signal transmission, and a quadrature surface coil was placed on the head for signal reception.
Coronal and sagittal T2-weighted scans were acquired using a RARE (rapid acquisition with relaxation enhancement) sequence with a repetition time (TR) of 5000 ms, an effective echo time (TE) of 50 ms, 8 echoes per image, 2 averages, 30 coronal 0.75 mm-thick slices, a field of view of 2.5 cm × 2.5 cm, and an in-plane resolution of 98 μm × 98 μm.
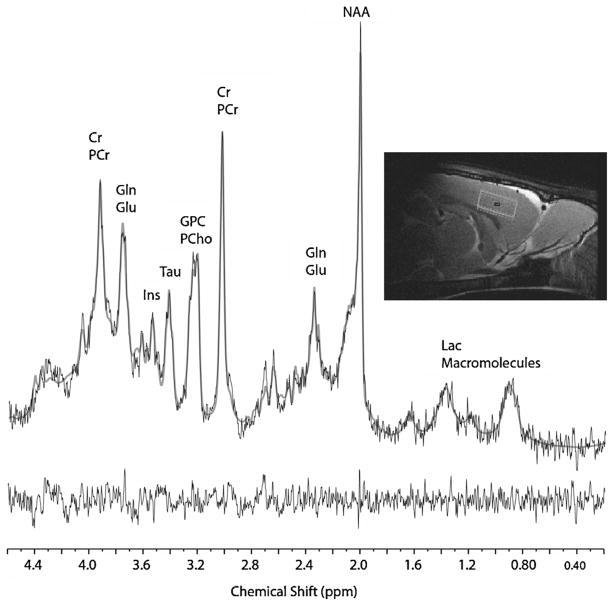
Three spectroscopy voxels were acquired and placed in the following regions according to the rat brain atlas of Paxinnos and Watson (Academic Press, 6th edition, 2007) [31]: frontal cortex (voxel dimensions: 1.5 mm × 4.5 mm × 4 mm) (Fig. 1), striatum (voxel dimensions 2.5 mm × 3 mm × 4 mm), right side of dorsal hippocampus (voxel dimensions: 1.5 mm × 3.75 mm × 2.5 mm).
Fig. 1.
An example of localizer image (top right panel, saggital cut) and the representative 1H MRS spectrum acquired from frontal cortex voxel in the rat. The black line spectra correspond to the ‘raw’ 1H MRS data with the LC-Model fits overlaid as smooth grey line spectra. The residual spectra (raw data minus the LC-Model fit) are displayed below the spectrum.
First and second order shimming was performed before scanning each of the voxels above using FASTMAP [32]. Unsuppressed water line widths under 11 Hz were obtained for all MRS measurements.
MR spectra were acquired using a point-resolved spectroscopy (PRESS) pulse sequence (TR = 3000 ms, TE = 19 ms, 255 excitations for voxels 1 and 2, and 512 excitations for voxel 3 2048 complex data points, scan time = 13 min for voxels 1 and 2, and 26 min for voxel 3). Water suppression was accomplished using variable power RF pulses with optimized relaxation delay (VAPOR) by manually adjusting the transmit RF power to maximize water suppression efficiency.
In vivo proton spectra were analyzed using a Linear Combination of Model Spectra, LCModel which calculates the best fit to the experimental spectrum as a linear combination of model spectra (simulated spectra of brain metabolites). Levels of 18 metabolites were analyzed: glycine (Gly), myo-inositol (Ins), glutamine (Gln), glutamate (Glu), γ-aminobutyric acid (GABA), lactate (Lac), alanine (Ala), taurine (Tau), phosphatidylethanolamine (Petn), aspartate (Asp), scyllo-inositol (sIns), glutathione (GSH), N-acetylaspartate (NAA), glycerophosphorylcholine (GPC), phosphorylcholine (PCho), as well as sums of metabolites NAA+ N-acetylaspartylglutamate (NAAG), Ins + Gly and Glu + Gln. Total creatine (phosphocreatine, PCr plus creatine, Cr) was estimated as a reference compound. The stability of the creatine normalization reference was assessed for all animals and conditions using the unsuppressed water signal amplitude for calculating creatine/water ratios. Using a paired t-test analysis no significant differences were detected for creatine/water between experimental conditions. For the further statistical analysis, four of these metabolites were excluded due to lack of detectable levels: Lac, GPC, PCho and sIns.
2.4. Statistical analysis
Differences in behavior in the FST and body weight gain between control and experimental animals were estimated with a paired two-tailed Student’s t-test with a 95% confidence interval.
Differences in metabolite ratios between baseline and post-hypoxia scans were also analyzed with a paired Student’s t-test with a 95% confidence interval. A between-group random permutation non-parametric test [33] was used for multiple comparison correction. This permutation method is more powerful than the Bonferroni correction when different variables in the test are correlated, such as in the case of different metabolites. The probability of the same or greater repeated measures t-test outcome was tested by estimation of t-scores for 14 variables chosen for analysis with random baseline vs. after hypoxia permutations of individual experimental outcomes. The individual values of the parameters were case-locked, i.e., every given experimental unit (animal before or after hypoxia) had a fixed experimental outcome (composition of metabolites). This allowed us to control for the possible non-independence of the measure of different metabolites. Four-thousand random permutations on same animal pre-post hypoxia were performed automatically by a custom-made script using Mat-lab software.
3. Results
Body weights of the rats from the two groups were not different between groups after 1 week of residence at either control conditions (201 ± 4 g) or at a simulated altitude of 10,000 ft (206 ± 5 g).
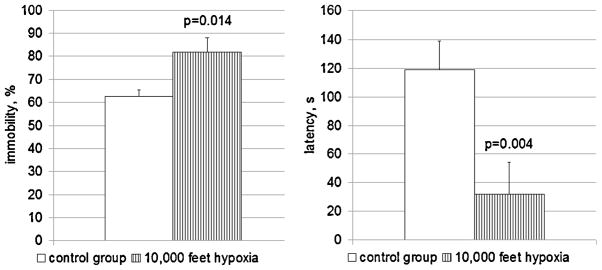
Behaviors in the FST were significantly altered in the chronic hypobaric hypoxia treated animals vs. controls (Fig. 2). After a week at 10,000 ft of simulated altitude the animals exhibited more immobility (82% of 5 min test session in hypoxia-treated group vs 63% in a control group, p = 0.014), which is a sign of more depression-like behavior [25]. Shorter latency times to acquire immobile posture, which is also a sign of depression-like behavior in the rodent FST [28], were also observed. The first 10 s period of immobility behavior occurs, on the average, on the 32nd second in the animals housed in hypoxic conditions while their control counterparts acquired immobility on the 120th second (p = 0.004).
Fig. 2.
FST behavior of female rats in the control group (n = 12) and after 1 week of 10,000 ft hypobaric hypoxia treatment (n = 8). Left panel: time spent immobile, % from 5 min test session. Increase in immobility time corresponds to more pronounced depression-like behavior in the rat [25]. Right panel: latency of first 10 s immobility period, seconds from the beginning of the test session. Shorter latency of immobility is a sign of augmented depression-like behavior [28].
Increased metabolite ratios to total creatine were observed for a subset of metabolites only in the frontal cortex voxel: elevated levels of myo-inositol (27%, p = 0.025), a sum of myo-inositol and glycine (21%, p = 0.006) and glutamate (8%, p = 0.023). A trend (p = 0.053) for increased taurine to total creatine ratio was also found. There was also a large 35% drop (p = 0.041) in the ratio of alanine to total creatine (Table 1).
Table 1.
Metabolite ratios normalized to total creatine (Cre + PCr), mean ± SEM and Signal-to-Noise-Ratio-Weighted Cramer–Rao Lower Bounds Averages, mean ± SD from frontal cortex of rats exposed to 1 week of 10k hypoxia, n = 12.
| Metabolite ratio | Baseline scan | CRLB, % | After hypoxia | CRLB, % |
|---|---|---|---|---|
| Gly/Cre + PCr | 0.182 ± 0.129 | 40.3 ± 35 | 0.192 ± 0.016 | 25.1 ± 16 |
| Ins/Cre + PCr | 0.425 ± 0.034 | 15.2 ± 7.5 | 0.542 ± 0.035b | 9.7 ± 2.3 |
| Glu/Cre + PCr | 1.368 ± 0.038 | 4.7 ± 0.8 | 1.481 ± 0.032b | 3.9 ± 0.7 |
| Gln/Cre + PCr | 0.668 ± 0.038 | 10.4 ± 2.1 | 0.675 ± 0.044 | 9.7 ± 2.6 |
| GABA/Cre + PCr | 0.247 ± 0.026 | 24.5 ± 8.8 | 0.265 ± 0.015 | 20.3 ± 4.9 |
| Ala/Cre + PCr | 0.168 ± 0.028 | 39.4 ± 23.6 | 0.109 ± 0.017b | 55.3 ± 36.6 |
| Tau/Cre + PCr | 0.884 ± 0.041 | 6.4 ± 2.2 | 0.994 ± 0.037a | 5.4 ± 1.9 |
| PEtn/Cre + PCr | 0.589 ± 0.047 | 10.8 ± 2.1 | 0.609 ± 0.039 | 8.8 ± 1.0 |
| Asp/Cre + PCr | 0.628 ± 0,027 | 14.2 ± 3.1 | 0.687 ± 0.024 | 12.1 ± 1.6 |
| GSH/Cre + PCr | 0.304 ± 0.012 | 11.1 ± 2.8 | 0.338 ± 0.013 | 9.1 ± 2.1 |
| NAA/Cre + PCr | 1.212 ± 0.029 | 3.3 ± 0.5 | 1.204 ± 0.031 | 3.3 ± 0.5 |
| NAA + NAAG/Cre + PCr | 1.240 ± 0.022 | 3.5 ± 0.7 | 1.285 ± 0.032 | 3.4 ± 0.5 |
| Ins + Gly/Cre + PCr | 0.607 ± 0.022 | 7.9 ± 2.0 | 0.733 ± 0.036c | 6.0 ± 1.3 |
| Glu + Gln/Cre + PCr | 2.036 ± 0.041 | 4.8 ± 0.8 | 2.155 ± 0.065 | 4.3 ± 0.8 |
p = 0.053 compared to baseline level.
p<0.05 compared to baseline level.
p<0.01 compared to baseline level.
In the frontal cortex voxel, no significant differences were found in ratios to total creatine of other metabolites examined (glycine, glutamine, GABA, phosphatidylethanolamine, aspartate, glutathione, NAA, as well as sums NAA + NAAG and total glutamine/glutamate). In addition, no significant differences were found in any of the metabolite ratios to total creatine in the other voxels of interest: striatum or hippocampus.
Multiple comparison issues were assessed between-groups using random permutation non-parametric test. According to this model, the probability that at least 4 significant (p < 0.05) t-scores which were found in the study are observed, is 0.027. If one of those scores is p < 0.01 the probability is even lower: 0.018. Thus we can suggest that our results are not likely due to type 1 statistical error (false positive findings).
4. Discussion
We have shown that a week of mild hypoxia resulted in depression-like changes in the behavior of rats in the FST, accompanied by alterations in brain metabolite neurochemistry. Interestingly, changes in neurometabolites were observed only in the frontal cortex voxel, which appears to be more sensitive to that type of simulated altitude than the two other regions of interest –the striatum and the hippocampus. While exposure to hypobaric hypoxia exerts detrimental effects on brain metabolites, the lack of difference in body weight gain between control and experimental animals indicate that they are not under severe physiological stress.
One prior study reported antidepressant effects of hypoxia [34] that seem to be contrary to our observations. However, in that study animals were exposed to intermittent hypoxia: 4 h daily of 3000 or 5000 m (around 10,000 and 16,400 ft) of simulated altitude for 14 days. Treatment mimicking 3000 m in altitude had no effects on immobility behavior in the FST, but when rats were exposed to 5000 m of simulated altitude, immobility behavior was decreased. The authors conclude that these changes depend on potentiation of hippocampal neurogenesis after repeated hypoxic treatment [34]. However, as noted above, the effect of hypoxia depends on the type of exposure. In another study, a week of continuous hypoxia resulted in neuronal death in hippocampus accompanied with behavioral deficits [20], which is more consistent with our results.
Myo-inositol (Ins) is a stereoisomer of inositol, a cyclic compound which plays an important role as a precursor of second messengers in various signal cascades in the cell, regulates osmolarity and membrane biosynthesis [35]. Abnormally high levels of brain myo-inositol have been reported in various neuropsychiatric disorders: in the left dorsolateral prefrontal cortex of depressed children and adolescents [36], in the right anterior cingulate cortex of obsessive-compulsive disorder patients [37], in the dorsolateral prefrontal region of the depressed geriatric patients [38] and in the frontal lobe of children suffering from bipolar disorder [39].
Several studies have shown an association between lowering brain inositol levels and alleviation of depression symptoms. According to the inositol-depletion hypothesis [40], the efficacy of lithium in the treatment of mood disorders can be explained by the inhibition of inositol-synthesizing enzyme, inositol-1(or 4)-monophosphatase, with subsequent decreases of myo-inositol levels in the brain. Significant decreases in myo-inositol levels were observed in the right frontal lobe of patients after lithium administration, and these decreases directly correlate to the decline in severity of bipolar depression as measured by the Hamilton Depression Rating Scale [41]. In unipolar depression patients, lithium treatment reduced suicide rates and total number of deaths [42].
The higher levels of myo-inositol/total creatine seen in our studies after 1 week of exposure to hypobaric hypoxia and the depression-like behavior caused by hypoxia exposure in our model thus correlate well with the data on the literature regarding myo-inositol levels in depression. In other animal studies, elevated ratios of myo-inositol and taurine to total creatine have been also found in the hippocampus of the rats exposed to 6 weeks of chronic mild stress (a model for depression in rats). Depression-like behavior has also been observed in these animals: low sucrose consumption, increased immobility in the FST and decreased activity in the open field test have been found [43]. Higher myo-inositol/total creatine ratios were noted in the left dorso-lateral prefrontal cortex of rats after two sessions of the FST; these changes resolved with antidepressant treatment using desipramine [44].
Our findings of an increase in myo-inositol are consistent with the above-mentioned literature. Assuming that elevated inositol, which is considered as a marker of glial cells, may indicate their growth or proliferation [45], we could suppose such processes occur in the frontal cortex after 1 week of mild hypoxia exposure.
Taurine is a non-protein amino acid involved in neurotransmission and neuromodulation in the CNS, which is neuroprotective and supports detoxification, antioxidation, cytoprotection and osmotic regulation [46]. Both basal and K+-stimulated release of taurine from the hippocampus are markedly enhanced in vitro under cell-damaging conditions, such as ischemia. Both hypoxia and ischemia also increase the basal release of taurine in cerebellar granule neurons and in cerebral cortical astrocytes [47]. In humans, higher taurine levels have been observed in peripheral blood lymphocytes in depressed patients [48], in the plasma of depressed and stroke patients [49,50] and in the cerebrospinal fluid of children with bacterial meningitis and encephalitis [51]. Higher levels of taurine were found in the hippocampus and frontal cortex of rats with congenital learned helplessness, a genetic animal model for depression [52]. Taking these findings together with our data, an increase in taurine in the brain might be directed to counteract the increased oxidative damage, caused by augmented Ca2+ release and subsequent apoptosis that occur under chronic hypoxic conditions [20].
Glutamate is a major excitatory neurotransmitter which also plays a key role in synapse formation, dendrite pruning, cell migration, differentiation and death [53]. Recently, a glutamate hypotheses of depression has been proposed, based on a large number of studies confirming abnormalities in glutamatergic function in depressed patients and in animal models of depression [54]. However, glutamate as assessed with MRS is often found to be decreased in patients with MDD [55]. Frontal lobe glutamate levels can be up or down, depending on the type and stage of depression [56]. In our results, we show increased glutamate levels in frontal lobe regions after a week of continuous hypoxia treatment, probably because of particular mechanisms implied in the depression-like behavior in high altitude conditions.
There are human studies which have shown increased glutamate in the frontal lobe in patients with post-stroke depression [57,58] or in late-life depression [38], as well as in plasma of depressed patients [49]. A cytokine hypothesis of depression development [59] may contribute to the explanation of these controversies. The proinflammatory cytokines, such as interleukin (IL)-1, or IL-6 have been supposed to play in a prominent role in the development of post-stroke depression [60]. Up-regulation of pro-inflammatory molecules like IL-1, IL-6 or C-reactive protein is observed also after high altitude exposure [61]. Moreover, IL-1 is linked to an increase in glutamatergic function [62]. Based on these observations, it could be suggested that cytokines promote depression-like behavior after hypoxia exposure via enhancing glutamate signaling in the brain. In favor of this, when tested 3–4 months after stroke, no differences in Glx/Cr ratios between depressed and non-depressed patients can be found [58], which may support the role of acute inflammation in onset of this type of depression syndrome.
Alanine, one of 20 essential amino acids, may be also involved in glutamate level control. In astrocytes, the NH4+ group is transferred from alanine to α-ketoglutarate to produce glutamate in a reaction catalysed by alanine aminotransferase; the other product of reaction is pyruvate. In neurons, this reaction goes in the opposite direction [63]. Therefore, decreases in alanine levels may indicate activation of glial alanine aminotransferase.
Several studies show a correlation between lowered brain ala-nine or elevated alanine aminotransferase, and the occurrence of depression. Elevated plasma alanine aminotransferase was a significant independent predictor for the occurrence of minor depression in a cohort study of 12,180 adults during general health examinations between 2003 and 2010 [64]. From another perspective, the antidepressant phenelzine, a monoamine oxidase inhibitor, also elevates rat brain levels of alanine by inhibition of aminotransferase activity [65]. It is noteworthy that ischemia–hypoxia also caused decreased alanine levels in the rat brain [66], which is in a line with our observations.
Glycine, another common amino acid functioning as an inhibitory neurotransmitter in the CNS, is a potential target for novel antidepressants [67,68]. Glycine content is elevated in plasma of depressed patients [49]. Although glycine also serves as a co-agonist for glutamate coupling with excitatory NMDA receptors [69], under hypoxia the activation of the receptors by glutamate is increased, and the activation by glycine is decreased [70]. Brain glycine content was significantly increased after hypoxia exposure in mice [71], as well as observed in our study. Activation of glycine receptors with taurine, which increased levels we also observed, may reduce anxiety [72] and thus influence FST behavior.
Taken together, our results of increased ratios of myo-inositol, glutamate, taurine and glycine and decreased alanine to total creatine in the frontal lobe could favor a hypothesis of glial activation which is accompanied by inhibition of active behaviors in the FST after a week of mild hypoxia exposure.
However, our study has several limitations. First of all, our control group was kept at the local elevation of 4500 ft above sea level (Salt Lake City, UT), which may also affect baseline results. MRS acquisitions were performed under isoflurane treatment, which has recently been found to impact brain metabolite levels in mice [73], causing an increase of brain myo-inositol alanine, lactate, GABA and total choline ratios to total creatine. However, the effect was strongest in the striatum and smallest in cerebral cortex, where we found more pronounced differences. In addition, our approach comparing baseline and post-treatment levels in the same animal might help to overcome this limitation, since both are assessed under the same conditions.
While it is a widely used practice to normalize metabolite resonance intensities to the resonance of total creatine, brain levels of total creatine might also be impacted by chronic hypoxia treatment. The increase in ratios for most metabolites suggests that exposure to hypoxia might have reduced hydration status and, in parallel, reduced both T1 and T2 values. However, there was no evidence for T2 prolongation in 22 human subjects after 18 h of exposure to hypoxia (12% oxygen) in a study by Bailey and co-workers [74].
5. Conclusions
Our study shows alterations in rat frontal lobe metabolites which parallel depression-like behavior in the FST after a week of mild hypoxia exposure in an animal model. These abnormalities, resembling those observed in depressed patients, may be considered as a possible explanation for the increased ratios of psychiatric disorders observed in populations living at high altitudes.
Highlights.
Increased depression and suicide rates are associated with life at higher altitudes.
Rats were housed for one week at 10,000 ft of simulated altitude.
Animals were tested with the forced swim test and magnetic resonance spectroscopy.
Hypoxia-treated rats had augmented depression-like behavior in the forced swim test.
Neurochemical alterations after hypobaric hypoxia were found in frontal cortex voxel.
Acknowledgments
Funded by the Utah Science, Technology, and Research (USTAR) initiative, the VISN19 Mental Illness Research Education and Clinical Center (MIRECC), and VA Merit Review grant 5I01 CX000812; DA031247 to Dr. Renshaw. The views in this paper are those of the authors and do not necessarily represent the official policy or position of the Department of Veterans Affairs or the United States Government.
References
- 1.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol. 1998;(Suppl):25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Leissner KB, Mahmood FU. Physiology and pathophysiology at high altitude: considerations for the anesthesiologist. J Anesth. 2009;23:543–53. doi: 10.1007/s00540-009-0787-7. Epub 2009 Nov 18. [DOI] [PubMed] [Google Scholar]
- 3.Virues-Ortega J, Garrido E, Javierre C, Kloezeman KC. Human behaviour and development under high-altitude conditions. Dev Sci. 2006;9:400–10. doi: 10.1111/j.1467-7687.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Yan X, Shi J, Gong Q, Weng X, Liu Y. Structural modifications of the brain in acclimatization to high-altitude. PLoS One. 2010;5:0011449. doi: 10.1371/journal.pone.0011449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson C, Hogan AM, Bucks RS, Baya A, Virues-Ortega J, Holloway JW, et al. Neurophysiological evidence for cognitive and brain functional adaptation in adolescents living at high altitude. Clin Neurophysiol. 2011;122:1726–34. doi: 10.1016/j.clinph.2011.02.001. Epub 2011 Mar 4. [DOI] [PubMed] [Google Scholar]
- 6.Hochachka PW, Clark CM, Brown WD, Stanley C, Stone CK, Nickles RJ, et al. The brain at high altitude: hypometabolism as a defense against chronic hypoxia. J Cereb Blood Flow Metab. 1994;14:671–9. doi: 10.1038/jcbfm.1994.84. [DOI] [PubMed] [Google Scholar]
- 7.Renshaw P. Suicide and brain chemical changes with altitude. The International Society for Affective Disorders 6th Biennial Conference ISAD 2012 Affective Disorders – Mind, Body and Society; 2012. [Google Scholar]
- 8.Betz ME, Valley MA, Lowenstein SR, Hedegaard H, Thomas D, Stallones L, et al. Elevated suicide rates at high altitude: sociodemographic and health issues may be to blame. Suicide Life Threat Behav. 2011;41:562–73. doi: 10.1111/j.1943-278X.2011.00054.x. http://dx.doi.org/10.1111/j.943-278X.2011.00054.x. Epub 2011 Aug 29. [DOI] [PubMed] [Google Scholar]
- 9.Kim N, Mickelson JB, Brenner BE, Haws CA, Yurgelun-Todd DA, Renshaw PF. Altitude, gun ownership, rural areas, and suicide. Am. 2011;168:49–54. doi: 10.1176/appi.ajp.2010.10020289. Epub 2010 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner B, Cheng D, Clark S, Camargo CA., Jr Positive association between altitude and suicide in 2584 U.S. counties. High Alt Med Biol. 2011;12:31–5. doi: 10.1089/ham.2010.1058. Epub 2011 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DelMastro K, Hellem T, Kim N, Kondo D, Sung YH, Renshaw PF. Incidence of major depressive episode correlates with elevation of substate region of residence. J Affect Disord. 2011;129:376–9. doi: 10.1016/j.jad.2010.10.001. Epub 2010 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler K, Kim N, Kondo DG, Renshaw PF. Cocaine use in the past year is associated with altitude of residence. J Addict Med. 2012;6:166–71. doi: 10.1097/ADM.0b013e31824b6c62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 14.Mark TL, Shern DL, Bagalman JE, Cao Z. Ranking America’s mental health: an analysis of depression across the States. Washington, D.C: Thomson Health-care; 2007. [Google Scholar]
- 15.Ende G, Demirakca T, Tost H. The biochemistry of dysfunctional emotions: proton MR spectroscopic findings in major depressive disorder. Prog Brain Res. 2006;156:481–501. doi: 10.1016/S0079-6123(06)56027-3. [DOI] [PubMed] [Google Scholar]
- 16.Caverzasi E, Pichiecchio A, Poloni GU, Calligaro A, Pasin M, Palesi F, et al. Magnetic resonance spectroscopy in the evaluation of treatment efficacy in unipolar major depressive disorder: a review of the literature. Funct Neurol. 2012;27:13–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Juranek I, Baciak L. Cerebral hypoxia-ischemia: focus on the use of magnetic resonance imaging and spectroscopy in research on animals. Neurochem Int. 2009;54:471–80. doi: 10.1016/j.neuint.2009.02.008. http://dx.doi.org/10.1016/j.neuint.2009.02.008. Epub Feb 24. [DOI] [PubMed] [Google Scholar]
- 18.Fayed N, Modrego PJ, Morales H. Evidence of brain damage after high-altitude climbing by means of magnetic resonance imaging. Am J Med. 2006;119(168):e1–6. doi: 10.1016/j.amjmed.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Singh SB, Panjwani U, Yadav DK, Amitabh K, Singh S, et al. Effect of a carbohydrate supplement on feeding behaviour and exercise in rats exposed to hypobaric hypoxia. Appetite. 2002;39:127–35. doi: 10.1006/appe.2002.0497. [DOI] [PubMed] [Google Scholar]
- 20.Maiti P, Singh SB, Mallick B, Muthuraju S, Ilavazhagan G. High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J Chem Neuroanat. 2008;36:227–38. doi: 10.1016/j.jchemneu.2008.07.003. Epub 2008 Jul 15. [DOI] [PubMed] [Google Scholar]
- 21.Chen XQ, Dong J, Niu CY, Fan JM, Du JZ. Effects of hypoxia on glucose, insulin, glucagon, and modulation by corticotropin-releasing factor receptor type 1 in the rat. Endocrinology. 2007;148:3271–8. doi: 10.1210/en.2006-1224. Epub 2007 Mar 22. [DOI] [PubMed] [Google Scholar]
- 22.Chinn KS, Hannon JP. Effects of diet and altitude on the body composition of rats. J Nutr. 1970;100:732–8. doi: 10.1093/jn/100.7.732. [DOI] [PubMed] [Google Scholar]
- 23.Perhonen M, Takala TE, Vuolteenaho O, Mantymaa P, Leppaluoto J, Ruskoaho H. Induction of cardiac natriuretic peptide gene expression in rats trained in hypobaric hypoxic conditions. Am J Physiol. 1997;273:R344–52. doi: 10.1152/ajpregu.1997.273.1.R344. [DOI] [PubMed] [Google Scholar]
- 24.Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13:169–88. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 26.Porsolt RD, Bertin A, Jalfre M. Behavioural despair in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–4. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- 27.West AP. Neurobehavioral studies of forced swimming: the role of learning and memory in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:863–77. doi: 10.1016/0278-5846(90)90073-p. [DOI] [PubMed] [Google Scholar]
- 28.Castagne V, Porsolt RD, Moser P. Use of latency to immobility improves detection of antidepressant-like activity in the behavioral despair test in the mouse. Eur J Pharmacol. 2009;616:128–33. doi: 10.1016/j.ejphar.2009.06.018. Epub 2009 Jun 21. [DOI] [PubMed] [Google Scholar]
- 29.Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav. 2013;118:227–39. doi: 10.1016/j.physbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 32.Gruetter R. Automatic, localized in vivo adjustment of all first-and second-order shim coils. Magn Reson Med. 1993;29:804–11. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 33.Manly BFJ. Randomization, Bootstrap, and Monte Carlo methods in biology. 2. London: Chapman and Hall; 1997. [Google Scholar]
- 34.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30:12653–63. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002;82:736–54. doi: 10.1046/j.1471-4159.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 36.Caetano SC, Fonseca M, Olvera RL, Nicoletti M, Hatch JP, Stanley JA, et al. Proton spectroscopy study of the left dorsolateral prefrontal cortex in pediatric depressed patients. Neurosci Lett. 2005;384:321–6. doi: 10.1016/j.neulet.2005.04.099. [DOI] [PubMed] [Google Scholar]
- 37.Yucel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, et al. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry. 2008;42:467–77. doi: 10.1080/00048670802050546. http://dx.doi.org/10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- 38.Binesh N, Kumar A, Hwang S, Mintz J, Thomas MA. Neurochemistry of late-life major depression: a pilot two-dimensional MR spectroscopic study. J Magn Reson Imaging. 2004;20:1039–45. doi: 10.1002/jmri.20214. [DOI] [PubMed] [Google Scholar]
- 39.Yildiz-Yesiloglu A, Ankerst DP. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog Neuropsy-chopharmacol Biol Psychiatry. 2006;30:969–95. doi: 10.1016/j.pnpbp.2006.03.012. Epub 2006 May 4. [DOI] [PubMed] [Google Scholar]
- 40.Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry. 2005;10:117–26. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- 41.Moore GJ, Bebchuk JM, Parrish JK, Faulk MW, Arfken CL, Strahl-Bevacqua J, et al. Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am J Psychiatry. 1999;156:1902–8. doi: 10.1176/ajp.156.12.1902. [DOI] [PubMed] [Google Scholar]
- 42.Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;2013 doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- 43.Hemanth Kumar BS, Mishra SK, Rana P, Singh S, Khushu S. Neurodegenerative evidences during early onset of depression in CMS rats as detected by proton magnetic resonance spectroscopy at 7T. Behav Brain Res. 2012;232:53–9. doi: 10.1016/j.bbr.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Kim SY, Lee YJ, Kim H, Lee DW, Woo DC, Choi CB, et al. Desipramine attenuates forced swim test-induced behavioral and neurochemical alterations in mice: an 1H MRS study at 9. 4T. Brain Res. 2010;1348:105–13. doi: 10.1016/j.brainres.2010.05.097. Epub 2010 Jun 10. [DOI] [PubMed] [Google Scholar]
- 45.Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009;64:12–21. doi: 10.1016/j.crad.2008.07.002. http://dx.doi.org/10.1016/j.crad.2008.07.002. Epub Aug 30. [DOI] [PubMed] [Google Scholar]
- 46.Ripps H, Shen W. Review: taurine: a very essential amino acid. Mol Vis. 2012;18:2673–86. Epub 012 Nov 12. [PMC free article] [PubMed] [Google Scholar]
- 47.Saransaari P, Oja SS. Taurine and neural cell damage. Amino Acids. 2000;19:509–26. doi: 10.1007/s007260070003. [DOI] [PubMed] [Google Scholar]
- 48.Fazzino F, Urbina M, Mata S, Lima L. Taurine transport and transporter localization in peripheral blood lymphocytes of controls and major depression patients. Adv Exp Med Biol. 2006;583:423–6. doi: 10.1007/978-0-387-33504-9_48. [DOI] [PubMed] [Google Scholar]
- 49.Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1155–8. doi: 10.1016/j.pnpbp.2006.03.036. Epub 2006 May 16. [DOI] [PubMed] [Google Scholar]
- 50.Ghandforoush-Sattari M, Mashayekhi SO, Nemati M, Ayromlou H. Changes in plasma concentration of taurine in stroke. Neurosci Lett. 2011;496:172–5. doi: 10.1016/j.neulet.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Shen EY, Lai YJ, Ho CS, Lee YL. Excitatory and inhibitory amino acid levels in the cerebrospinal fluids of children with neurological disorders. Acta Paediatr Taiwan. 1999;40:65–9. [PubMed] [Google Scholar]
- 52.Schulz D, Smith D, Yu M, Lee H, Henn FA. Selective breeding for helplessness in rats alters the metabolic profile of the hippocampus and frontal cortex: a 1H MRS study at 9. 4T. Int J Neuropsychopharmacol. 2013;16:199–212. doi: 10.1017/S1461145711001994. [DOI] [PubMed] [Google Scholar]
- 53.Hudspith MJ. Glutamate: a role in normal brain function, anaesthesia, analgesia and CNS injury. Br J Anaesth. 1997;78:731–47. doi: 10.1093/bja/78.6.731. [DOI] [PubMed] [Google Scholar]
- 54.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. Epub 2006 Jun 27. [DOI] [PubMed] [Google Scholar]
- 56.Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–94. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Li YH, Li MH, Lu J, Zhao JG, Sun XJ, et al. Glutamate level detection by magnetic resonance spectroscopy in patients with post-stroke depression. Eur Arch Psychiatry Clin Neurosci. 2012;262:33–8. doi: 10.1007/s00406-011-0209-3. [DOI] [PubMed] [Google Scholar]
- 58.Glodzik-Sobanska L, Slowik A, McHugh P, Sobiecka B, Kozub J, Rich KE, et al. Single voxel proton magnetic resonance spectroscopy in post-stroke depression. Psychiatry Res. 2006;148:111–20. doi: 10.1016/j.pscychresns.2006.08.004. Epub 2006 Nov 7. [DOI] [PubMed] [Google Scholar]
- 59.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. http://dx.doi.org/10.1016/j.biopsych.2008.11.029. Epub 9 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spalletta G, Bossu P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11:984–91. doi: 10.1038/sj.mp.4001879. Epub 2006 Aug 8. [DOI] [PubMed] [Google Scholar]
- 61.Mazzeo RS. Altitude, exercise and immune function. Exerc Immunol Rev. 2005;11:6–16. [PubMed] [Google Scholar]
- 62.Fogal B, Hewett SJ. Interleukin-1beta: a bridge between inflammation and excitotoxicity? J Neurochem. 2008;106:1–23. doi: 10.1111/j.1471-4159.2008.05315.x. http://dx.doi.org/10.1111/j.471-4159.2008.05315.x. Epub 2008 Jul 1. [DOI] [PubMed] [Google Scholar]
- 63.Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. A possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons. J Neurochem. 2000;75:471–9. doi: 10.1046/j.1471-4159.2000.0750471.x. [DOI] [PubMed] [Google Scholar]
- 64.Zelber-Sagi S, Toker S, Armon G, Melamed S, Berliner S, Shapira I, et al. Elevated alanine aminotransferase independently predicts new onset of depression in employees undergoing health screening examinations. Psychol Med. 22:1–11. doi: 10.1017/S0033291713000500. [DOI] [PubMed] [Google Scholar]
- 65.Todd KG, Baker GB. Neurochemical effects of the monoamine oxidase inhibitor phenelzine on brain GABA and alanine: a comparison with vigabatrin. J Pharm Pharm Sci. 2008;11:14s–21s. doi: 10.18433/j34s38. [DOI] [PubMed] [Google Scholar]
- 66.Macri MA, D’Alessandro N, Di Giulio C, Di Iorio P, Di Luzio S, Giuliani P, et al. Region-specific effects on brain metabolites of hypoxia and hyperoxia overlaid on cerebral ischemia in young and old rats: a quantitative proton magnetic resonance spectroscopy study. J Biomed Sci. 2010;17:1423–2127. doi: 10.1186/1423-0127-17-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang CC, Wei IH, Huang CL, Chen KT, Tsai MH, Tsai P, et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry. 2013;3223:00188–191. doi: 10.1016/j.biopsych.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 68.Ji Y, Hebbring S, Zhu H, Jenkins GD, Biernacka J, Snyder K, et al. Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: pharmacometabolomics-informed pharmacogenomics. Clin Pharmacol Ther. 2011;89:97–104. doi: 10.1038/clpt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dannhardt G, Kohl BK. The glycine site on the NMDA receptor: structure-activity relationships and possible therapeutic applications. Curr Med Chem. 1998;5:253–63. [PubMed] [Google Scholar]
- 70.Fritz KI, Zubrow AB, Mishra OP, Delivoria-Papadopoulos M. NMDA receptor modification during graded hypoxia in the cerebral cortex of newborn piglets. Biol Neonate. 2002;82:46–52. doi: 10.1159/000064152. [DOI] [PubMed] [Google Scholar]
- 71.Liu HY, Lu GW. Changes in glycine content in mouse brain during hypoxic preconditioning. Sheng Li Xue Bao. 2001;53:461–4. [PubMed] [Google Scholar]
- 72.Zhang CG, Kim SJ. Taurine induces anti-anxiety by activating strychnine-sensitive glycine receptor in vivo. Ann Nutr Metab. 2007;51:379–86. doi: 10.1159/000107687. Epub 2007 Aug 29. [DOI] [PubMed] [Google Scholar]
- 73.Boretius S, Tammer R, Michaelis T, Brockmoller J, Frahm J. Halogenated volatile anesthetics alter brain metabolism as revealed by proton magnetic resonance spectroscopy of mice in vivo. Neuroimage. 2013;69:244–55. doi: 10.1016/j.neuroimage.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 74.Bailey DM, Roukens R, Knauth M, Kallenberg K, Christ S, Mohr A, et al. Free radical-mediated damage to barrier function is not associated with altered brain morphology in high-altitude headache. J Cereb Blood Flow Metab. 2006;26:99–111. doi: 10.1038/sj.jcbfm.9600169. [DOI] [PubMed] [Google Scholar]