Abstract
Light is a potent stimulus for regulating circadian, hormonal, and behavioral systems. In addition, light therapy is effective for certain affective disorders, sleep problems, and circadian rhythm disruption. These biological and behavioral effects of light are influenced by a distinct photoreceptor in the eye, melanopsin containing intrinsically photosensitive retinal ganglion cells (ipRGCs), in addition to the conventional rods and cones. We summarize the neurophysiology of this newly described sensory pathway and consider implications for the measurement, production and application of light. A new light-measurement strategy taking account of the complex photoreceptive input to these non-visual responses is proposed for use by researchers, and simple suggestions for artificial/architectural lighting are provided for regulatory authorities, lighting manufacturers, designers and engineers.
Light as a regulator of physiology and behavior
During the past three decades, empirical evidence has demonstrated that many aspects of human physiology and behavior are influenced by retinal illumination [1–4]. Such responses originate in the eye but are separate from other aspects of vision insofar as they are unrelated to particular spatial patterns of light exposure, and can survive even in some blind subjects. Consequently, these types of light responses have been commonly referred to as non-image-forming or non-visual.
These catch-all terms encompass a wide array of response types. The most influential is light-induced phase resetting of endogenous circadian clocks. Because circadian rhythmicity is a feature of nearly every physiological, metabolic and behavioral system, this phenomenon brings a wide array of biological processes under indirect retinal control. Beyond this, the term non-visual response has come to encompass a growing list of more acute effects of light that together ensure a day-like physiological state. Thus, for example, light constricts the pupil, suppresses pineal melatonin production, increases heart rate and core body temperature, stimulates cortisol production, and acts as a neurophysiological stimulant (increasing subjective and objective measures of alertness and psychomotor reaction time, and reducing lapses of attention).
Appreciation of this basic biology has led to development of a number of therapeutic applications. Light has been shown to have anti-depressant properties, particularly in the treatment of seasonal affective disorder (SAD) and its subclinical variant, sSAD [3, 4]. Appropriately timed light exposure has been developed as therapy for circadian rhythm sleep disorders and circadian disruption associated with jetlag, shift work and space flight. Finally, light has been explored as a treatment for non-seasonal depression, menstrual-cycle-related problems, bulimia nervosa, and cognitive and fatigue problems associated with senile dementia, chemotherapy and traumatic brain injury [3–6].
These effects of light on physiology and behavior evolved over millennia in which environmental illumination provided a reliable indicator of time of day. The advent of electrical lighting has disrupted this relationship, with patterns of light exposure now also reflecting personal tastes and social pressures. It is important therefore that non-visual effects of light are incorporated into considerations of lighting design. Thus, for example, one might ask the extent to which a given architectural lighting replicates the biological effects of natural daylight; how lighting could be employed to minimize the deleterious effects of shiftwork while promoting alertness and safety; or how light therapy could be optimized.
The lighting industry and academic researchers have started to address these problems [7–9]. Progress in these endeavors, however, first requires appropriate quantification of how light impacts human physiology and behavior. There are two broad categories of light measurement techniques: radiometry and photometry [10]. Radiometry is based on characterizing the physical properties of light wavelength and energy. A radiometer quantifies radiant power over a defined bandwidth of electromagnetic energy. Photometry is a specialized branch of radiometry that takes into account the fact that biological photoreceptors are not equally sensitive to light at all wavelengths. A photometer is a radiometer that uses filters to weight the detector response to different wavelengths according to the spectral sensitivity of an aspect of human vision. Most commercially available photometers employ a weighting function termed the photopic luminous efficiency function (or Vλ), which reflects the spectral sensitivity of the long- and middle-wavelength sensitive cones [11]. Depending upon the geometric properties of interest, luminous intensity (units of lumens/sr or candelas, cd), luminance (units = cd/m2) or illuminance (units = lm/m2 or lux) can be determined from the output of these devices
Between 1980 and 2000, the great majority of studies on human circadian, endocrine, behavioral and therapeutic responses to light quantified stimuli in terms of photopic illuminance (lux) [1–3]. During that time, lux meters were readily available and inexpensive because they were the tool of choice in the lighting and photographic professions. Two related branches of investigation have since shown this practice to be inadequate.
First, the last decade has seen the discovery that, while the photoreceptive capacity of the retina is dominated by rods and cones, a few of the retina’s output neurons (retinal ganglion cells) are also directly photosensitive [12, 13]. These intrinsically photoreceptive retinal ganglion cells (ipRGCs) achieve this photoreceptive capacity through expression of melanopsin, an opsin photopigment [14–16]. ipRGCs comprise only a small fraction of the total ganglion cell population (1–5% depending upon the species and method of estimation), but project to all major retinorecipient parts of the brain, including those associated with non-visual responses [17–19]. Specific ablation of ipRGCs abolishes non-image-forming responses, identifying this cell class as the principal conduits of photic input to circadian and other systemic responses to light [20–22]. Furthermore, ipRGCs can detect light even when isolated from the rest of the retina explaining why some photosensitivity survives loss of functional rods and cones [12, 23–29]. This discovery of a new photoreceptor raises the possibility that the spectral sensitivity of non-image-forming responses could be fairly different from that of rod- or cone-based vision.
Second, empirical observations have shown that circadian and other behavioral and physiological responses can indeed have very distinct spectral sensitivity. Ten analytic action spectra and many investigations based on selected wavelength comparisons of such responses in humans, non-human primates, and rodents revealed peak sensitivity in the short-wavelength portion of the visible spectrum (from 447 nm to 484 nm) [12, 30–45], fairly divergent from that predicted by Vλ (peak sensitivity at 555 nm).
Taken together, these data demonstrate that established photometric light measures that use the Vλ spectral weighting function, such as photopic lux, are inadequate for quantifying light intended to regulate non-visual physiology and behavior. Unfortunately, to date there is no established replacement. This omission has important practical consequences. For researchers, the absence of a suitable and agreed method of light measurement makes it difficult to compare findings or replicate experimental conditions. It also represents a significant barrier to relating laboratory findings to lighting applications, and makes it difficult for the lighting industry and regulators to predict the impact of different lighting regimes on behavioral and physiological systems. The fundamental problem in addressing this need has been the difficulty in determining a spectral weighting function (equivalent to Vλ) suitable for non-visual responses. To understand this challenge it is first necessary to review the basic neurophysiology of ipRGCs.
The response of ipRGCs to light
Melanopsin, the photopigment of ipRGCs, is structurally and phylogenetically more closely related to the opsins of invertebrate rhabdomeric photoreceptors than torod and cone opsins [46, 47]. In common with such invertebrate rhodopsins, the phototransduction cascade engaged by melanopsin results in cellular depolarization (reviewed in [48]). As a result, the fundamental light response of ipRGCs is an irradiance-dependent increase in firing [12].
The quantum efficiency of melanopsin is comparable to that of rod and cone opsins [49]. ipRGCs, however, lack specialized photopigment-concentrating organelles (such as rod/cone outer segments) to maximize the probability of photon capture. As a result, the probability of absorbing a photon is >1 million times lower than in rods or cones for a given area of photostimulation [49]. Consequently, even though the ipRGC phototransduction cascade has high amplification [49], melanopsin photoreception is much less sensitive than that of rods or cones. Once the threshold for melanopsin activation has been reached, however, the intrinsic light response scales with stimulus intensity over several decimal orders [12], and is remarkably persistent, being sustained over long durations of constant illumination [50].
Although, melanopsin phototransduction is only engaged at moderate to high irradiance, ipRGCs and their downstream responses can be responsive to much lower levels of illumination (reviewed in [51]). For example, it was originally thought that illuminance of 2500 lux was required to suppress nocturnal melatonin in humans [52], but later studies have shown that, under certain conditions as little as 1 lux or less can suppress melatonin in humans [53]. This sensitivity highlights an important feature of this photoreceptive system: the ipRGCs receive input from the outer retina (Figure 1A). Thus, the ipRGC dendrites are targets for synaptic input from bipolar and amacrine cells as well as being sites for melanopsin-driven phototransduction. As a result, the ipRGC firing pattern is a composite, integrated signal consisting of the intrinsic light response (melanopsin photoreception) and incoming rod- and cone-driven signals [36]. This arrangement greatly extends the range of stimuli that can elicit circadian and neurophysiological responses, and explains why animals that are genetically null for melanopsin continue to exhibit non-image-forming responses to light [16, 54, 55].
Figure 1. All retinal photoreceptor classes are upstream of circadian, neuroendocrine and neurobehavioral responses to light.
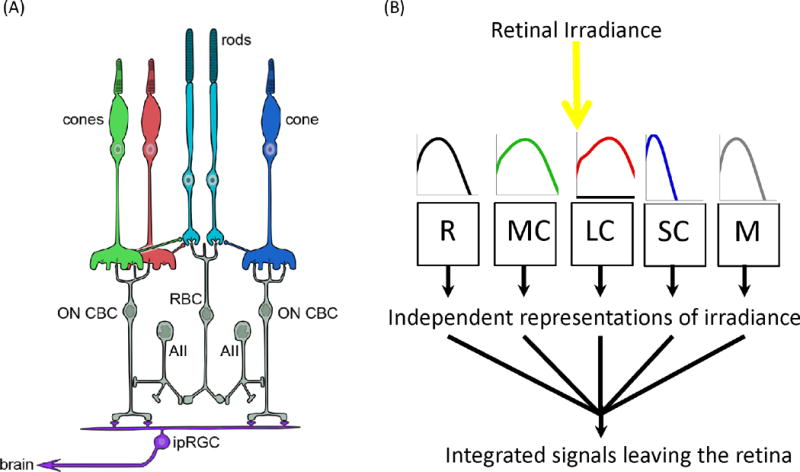
A. Schematic of the relevant retinal circuitry in humans. Non-image-forming responses originate in the retina and have been attributed to a particular class of retinal ganglion cell (ipRGC). ipRGCs are directly photosensitive owing to expression of melanopsin, which allows them to respond to light even when isolated from the rest of the retina. In situ they are connected to the outer retinal rod and cone photoreceptors via the conventional retinal circuitry. The details of their intraretinal connections are incompletely understood and probably vary between different subtypes. Shown here are major connections with on cone bipolar cells (on CBCs) connecting them to cone and, via amacrine cells (AII) and rod bipolar cells (RBC), rod photoreceptors. As a consequence, the firing pattern of ipRGCs can be influenced both by intrinsic melanopsin photoreception, and extrinsic signals originating in rods and each of the spectrally distinct cone classes (shown in red, green and blue). B. This feature is conceptualized in much simplified form, as a number of photoreceptive mechanisms (depicted as R for rod opsin; M for melanopsin; SC for S cone opsin; MC for M cone opsin; and LC for L-cone opsin), each of which absorbs light according to its own spectral sensitivity profile (shown in cartoon form as plots of log sensitivity against wavelength from 400 to 700 nm) to generate a distinct measure of illuminance. These five input signals are then combined by the retinal wiring, and within the ipRGC itself, to produce an integrated signal that is sent to non-image-forming centers in the brain. As each of the five representations of weighted irradiance is produced by a photopigment with its own spectral sensitivity profile, their relative significance for the integrated output defines the wavelength dependence of this signal, and hence of downstream responses.
Spectral sensitivity
At its very origin, the signal driving physiological and behavioral light responses (ipRGC firing) is defined by the combined influence of multiple photoreceptive processes: the melanopsin-driven phototransduction mechanism within the ipRGC itself, and remote photoreception in rods and cones (Figure 1B). Each of these mechanisms of light detection has a distinct spectral sensitivity, defined by the spectral efficiency of the photopigment expressed and the spectral transmission properties of the ocular media.
Rods. Rod opsin, the photopigment of rod photoreceptors, shows peak sensitivity (λmax) at approximately 500 nm in all mammalian species. Pre-receptoral filtering shifts this towards somewhat longer wavelengths in the standard human observer (507 nm).
Cones. Mammalian genomes typically contain several genes encoding spectrally distinct cone opsins. Humans, and other old world primates, have three types of cones. Human S cones express a short-wavelength-sensitive cone opsin (cyanolabe), maximally sensitive to wavelengths at ~420 nm; M cones contain a different cone opsin (chlorolabe; peak sensitivity ~535 nm); L cones contain a red-shifted cone opsin (erythrolabe; peak sensitivity ~565 nm [56]). Other mammals lack the chlorolabe/erythrolabe distinction, and have a single cone opsin maximally sensitive in the middle of the human visible spectrum. There are also important species differences in the spectral sensitivity of the cyanolabe pigments. For example, many rodent retinas have a photopigment that is maximally sensitive to near-ultraviolet radiation [57]. In humans, pre-receptoral filtering shifts peak sensitivity of short- and medium-wavelength cones to longer wavelengths (~440 and 545nm, respectively).
Melanopsin. The available data indicate that the spectral sensitivity of melanopsin, the photopigment of ipRGCs, is similarly invariant across species with λmax at approximately 480 nm [58–62]. A potential complication in relating this estimate of the spectral sensitivity of melanopsin to the spectral response property of ipRGCs in vivo is the suggestion that, like the rhabdomeric opsins of invertebrates, melanopsin may be bistable [58, 60, 63, 64]. Bistability affords rhabdomeric photopigments the capacity to regenerate the vitamin A-derived chromophore that is isomerized following light absorption through the absorption of another photon [65]. Because this regeneration event can be produced by different wavelengths, it could influence the spectral response properties of the receptor. Whether mammalian melanopsins are bistable and whether this bistability is biologically relevant remain to be determined [66–68]. In either event, studies on mice indicate that this factor does not significantly impact the spectral response properties of melanopsin under practical lighting regimes [69, 70].
The firing rate of ipRGCs may thus be influenced by five (or four in the case of non-primates) spectrally distinct photoreceptors (Figure 1B). It follows that the spectral sensitivity of downstream responses (and thus the spectral weighting function that should be applied during light measurement) will be determined by the manner in which these various channels are combined. In fact, the contribution of each photoreceptive input to evoked responses appears to be fundamentally context dependent, a feature clearly illustrated by studies of a well-understood ipRGC-driven response, the pupillary light reflex (PLR).
Pupillary light reflex: a case study
The PLR controls the light intensity reaching the retina by a simple and well-characterized pathway that links a sensory signal, irradiance, to a motor output, pupil constriction. It originates with ipRGC innervation of the olivary pretectal nucleus [21, 71–75]. Pupil area decreases with increasing irradiance over a ~9 log10 intensity range. A key feature of the light reflex is its tonic nature in bright light: constriction is held steady under continuous illumination [71–73, 75, 76]. Data from both laboratory animals and humans indicate that rods, cones and melanopsin participate in the PLR, and that their relative contributions are variable, depending on stimulus intensity and spectral content, and change over time under constant illumination [16, 29, 33, 37, 76–78].
An abrupt step in illumination elicits a rapid, robust pupil constriction predominantly driven by cones and/or rods. The amplitude of this initial response and the relative contribution of each photoreceptor type depend upon the irradiance and wavelength of the light stimulus. Following this phasic pupil constriction, the pupil gradually relaxes to a more dilated state and, if the threshold for melanopsin activation is exceeded, it assumes a sustained steady-state diameter that persists throughout the light stimulation [37, 76, 77]. During this post-phasic response, the contribution from melanopsin increases and with extended illumination (>3 min) the response is predominantly driven by melanopsin. When the light is turnedoff, if the prior retinal irradiance has exceeded the threshold for melanopsin activation, constriction persists for many seconds, termed by some the post-illumination pupil response [79]. This response is considered to be predominantly melanopsin dependent [37].
A consequence of this shifting reliance on rods, cones and melanopsin is that the spectral sensitivity profile of the PLR is fundamentally labile. Thus, at low light levels below the melanopsin threshold, the pupil matches the spectral sensitivity of cone (or rod) photoreceptors. For higher irradiances, early components of pupil constriction are defined by cone spectral sensitivity, later components by melanopsin, and intermediate irradiances by a combination of the two (Figure 2).
Figure 2. Spectral sensitivity of half-maximal pupillary constriction in humans.
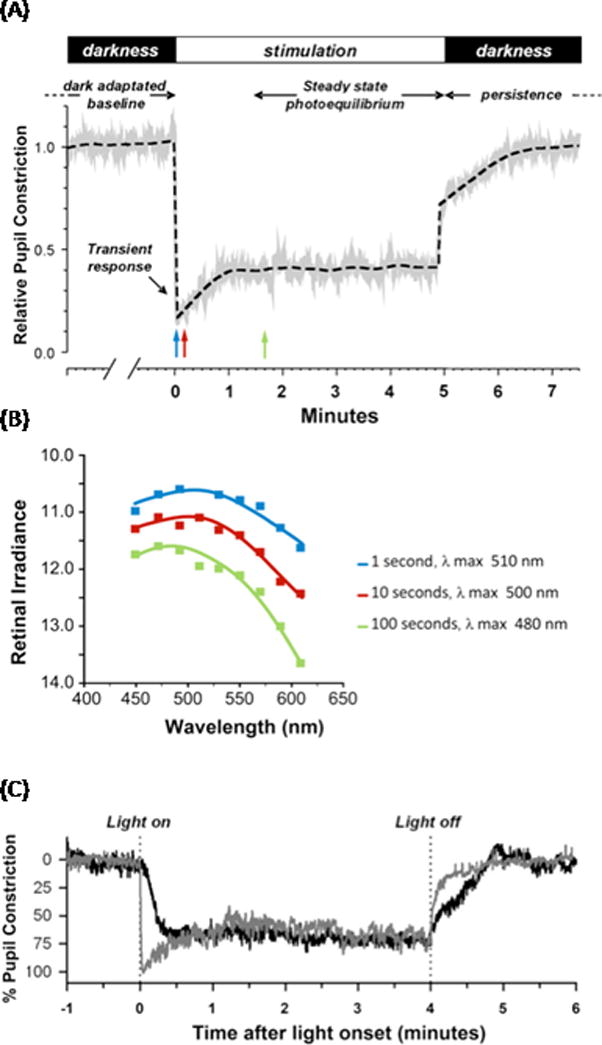
(A). The pupillary light reflex response is composed of several different temporal components. At light onset, the pupil shows a rapid, transient constriction during the first 1000 ms of light exposure. This is followed by redilation to a tonic or sustained pupil diameter that stabilizes to a steady state constriction (photoequilibrium) even during prolonged constant illumination. When the light is turned off, there is a slow, delayed redilation of the pupil back to the resting (dark-adapted) state that is melanopsin-driven. Graph adapted from [76]. B. Mean spectral sensitivity is depicted as the retinal irradiance (log quanta/cm2/s) required to elicit a criterion pupil response (half maximal constriction) at nine wavelengths for three different stimulus durations of 1, 10, and 100 s (corresponding to the positions of blue, red, and green arrows in A). The smooth curve through the data points represents the optimal fit to the data using a mathematical combination of rod, cone, and melanopsin spectral sensitivities. As the stimulus duration increases, the sensitivity of the response gradually decreases by more than one log unit and is shifted towards shorter wavelength, from 510 nm at 1 s, to 500 nm at 10 s, and 480 nm at 100 s. Graph derived from data in [77]. (C) Representative traces for pupillary constriction in a sighted participant (gray) and in a blind individual without rod and cone function (black). Pupillary constriction to 480 nm was sluggish and lacks the transient response after light onset in the blind individual, whereas the sustained steady state pupillary light reflex (PLR) and the persistent response when the light is turned off are conserved. Graph reproduced, with permission, from [29].
Other non-visual light responses
Data for the PLR therefore demonstrate that no single spectral efficiency function accounts for this response under all conditions. In addition, other findings indicate that the rules governing when and to what extent other non-image-forming responses rely upon rods, cones or melanopsin are not constant. Thus, different cone contributions have been observed across brain regions receiving ipRGC input, even under essentially identical experimental conditions [80, 81]. Differences in central processing could explain such diversity, as could the fact that there are at least five different types of ipRGCs [17]. These various ipRGC types have distinct central projection patterns, implying diversity in their importance for the various circadian and neurophysiological responses. ipRGC types also differ in morphology, melanopsin content, and intrinsic photosensitivity [17, 82, 83]. Although inputs to these ipRGC types have not been fully characterized, differences in dendritic morphology suggest that afferent connections are also likely to differ qualitatively and quantitatively [17, 82–84], allowing significant diversity in the extent to which responses downstream from ipRGCs are reliant upon rods, cones, and melanopsin.
Measuring light for circadian and neurobehavioral regulation
It would be desirable to be able to quantify light as experienced by non-visual systems using a single, one-dimensional unit (the equivalent of photopic lux). Achieving this for lights of divergent spectral content, however, awaits elucidation of a suitable spectral weighting function. There have been attempts to address this deficit [85–87]. The neurophysiology outlined above, however, reveals the challenges in producing a method of spectral weighting that would be suitable for all circadian, neuroendocrine and neurobehavioral responses under all conditions. Evoked responses reflect input from all of the retinal photoreceptor classes, with the relative importance of each being highly labile within and between response types. As a result, the spectral sensitivity of this photoreceptive system is fundamentally context-dependent. This is no less true for other aspects of vision, and as this nascent field of research matures, a spectral response function derived from one or more of the individual photoreceptor inputs might be found to provide a reasonable approximation of relevant non-image-forming responses under many practical circumstances. Alternatively, a family of such functions to be used under different conditions and/or for different response endpoints may be developed. At present, however, there are insufficient data upon which to base such strategies. What advice can then be given to those measuring and employing light in experimental and practical applications?
Advice for researchers
The scientific literature contains a large number of studies relating circadian, neuroendocrine and neurobehavioral responses to calibrated light exposures. The lack of a consistent and adequate method of quantifying light, however, makes it hard to replicate experimental conditions or to compare across studies. This represents a considerable barrier to scientific progress.
Given that we do not yet have an accepted spectral weighting function for non-image-forming responses, the best current advice is that researchers record their light exposures in the most complete form, namely as corneal spectral power distributions. A range of low-cost spectroradiometers can be used to provide this information, if appropriately calibrated.
A major advantage of recording the spectral power distribution is that it can be used to derive any other unit of measure currently available or developed in the future. Currently, the most appropriate use of that capacity would be to calculate the effective irradiance experienced by each of the rod, cone and melanopsin photoreceptors capable of driving non-visual responses. The inclusion of each of these photoreceptors in the efferent pathway implies that, at its earliest stages, incident light is encoded into five (or three or four in the case non-primate mammals) representations of irradiance by the activity of rods, melanopsin, S, M, or L cones (Figure 1B). It can therefore be considered that non-visual responses are initiated by one or more of five distinct biological representations of irradiance: rhodopic, melanopic, cyanopic, chloropic and erythropic illuminance (Table 1). Retinal and central wiring combines these distinct measures to provide an integrated representation of the light environment. Because this integration process is not completely understood, it is not yet possible to predict the relative reliance of a given circadian, neuroendocrine or neurobehavioral response under particular circumstances on each.
At present, it is therefore recommended that quantities reflecting the activity of each of these individual inputs be reported. This does not achieve the ideal of describing light as a one-dimensional quantity that predicts non-image-forming responses. Nonetheless, reducing the spectral power distribution to a limited number of biologically meaningful quantities (five in the case of humans; four for most other mammalian species) makes the problem of comparing polychromatic lights of different spectral quality significantly more tractable. This helps in equating stimulus-response relationships described in different laboratories, and in relating those research findings to lighting conditions in the field. As studies using this measurement system accumulate, it will then become possible to generate and test hypotheses regarding the ability of one or more of the 5 qualities to predict a target physiological or behavioral response. Thus, it may become clear that the magnitude of a nominal response is best predicted by, for example, a simple linear summation of cyanopic and melanopic illuminance values over a variety of studies using spectrally divergent stimuli.
Full equations for calculating rhodopic, melanopic, cyanopic, chloropic and erythropic illuminance values are provided in Table 1. The spectral efficiency functions used for these calculations (based upon pigment absorbance profiles corrected for ocular filtering in a standard human observer) are provided as supplementary material online, where a toolbox for calculation of α-opic illuminance values from corneal spectral irradiance measures is also available. As noted above, some of these spectral efficiency functions are species-specific, and resources suitable for laboratory rodents are also provided (at http://www.eye.ox.ac.uk/team/principal-investigators/stuart-peirson).
Advice for industry and regulatory authorities
The traditional objectives of architectural lighting include the provision of light that: (i) is optimal for visual performance; (ii) is visually comfortable; (iii) permits aesthetic appreciation of the space; and (iv) conserves energy [10, 88]. As discussed above, light exposure has a broad range of effects on physiology and behavior. These non-visual effects of light should be an additional consideration in the design and operation of human environments as well as those for domesticated animals.
An important note of caution here is that it is not always clear whether lighting design should aim to maximize or minimize non-visual responses. In many ways, light can be considered a drug, having the potential for both beneficial and deleterious effects. These conflicting effects can occur concurrently, and in a single individual and context. For example, for night-shift workers, bright workspace lighting may improve immediate job performance by enhancing visual perception and promoting alertness, but suppress melatonin and shift the circadian clock to an undesirable phase. Conversely, dimmer lighting may minimize effects on circadian timing, but may be detrimental to more immediate performance.
Balancing the desirable and undesirable impacts of light or darkness requires a careful, informed consideration of context and of the myriad effects of light on physiology, perception and cognition. Such calculations can be a daunting challenge, all the more so because both basic and applied science in this area continues to evolve rapidly. Simple prescriptions are as likely to do harm as well as good, and even experts may have divergent ideas about best practice under some situations. Nevertheless, assuming that following such deliberation a decision has been made to either maximize or minimize the non-visual effects of light, how can this be achieved?
For reasons outlined above, it is not yet possible to predict the non-image-forming impact of a given illuminant based upon its intensity and spectral composition. However, some guidance is possible. If the broad objective is to minimize the activation of ipRGC outputs, the goal should be to keep retinal irradiance as low as possible. There is no established threshold below which these systems are completely blind to light, so total darkness during sleep may be ideal where practical. Likewise, with respect to the visible spectrum, any wavelength can, in principle, activate the system. However, given that the relative sensitivity of these non-visual responses is generally reduced in the longer visible wavelength range, light sources should be biased toward longer visible wavelengths, to the extent consistent with other demands. Conversely, if the objective is to promote ipRGC photoreception, retinal irradiance should be increased (within acceptable safety limits) and light sources may be biased towards the blue and blue/green regions of the visible spectrum, to which all photoreceptive inputs to this system are fairly sensitive.
Concluding remarks
Science and engineering rely upon accurate measurement. The discovery of ipRGC photoreceptors, and our growing understanding of their role in setting physiological and behavioral state, has revealed that current methods of light measurement are incomplete. The question of exactly how they should be updated will no doubt be revisited as our understanding of this system evolves, as has happened with other aspects of photometry. Nevertheless, the science has reached a state at which it is sensible to take the first important steps in that process. We propose methods of light measurement that quantify effective irradiance for each of the photoreceptive inputs to this system independently. The goal is to provide a comprehensive description of light as experienced by the circadian, neuroendocrine and neurobehavioral systems, on which future developments can build.
Supplementary Material
Acknowledgments
This review arose from discussions between the authors at a focused workshop held in Manchester, UK, in January 2013, which received financial support from the ZVEI (German Electrical and Electronic Manufacturer’s Association) and administrative assistance from the University of Manchester. The authors are grateful to John Hanifin and Ben Warfield for technical support on the supplementary online material and manuscript figures.
Footnotes
Disclaimer statement
Of the 14 authors on this manuscript, Drs. Berson, Cooper, Gamlin, Price, Provencio, and O’Hagan identify no potential conflicts of interest related to the manuscript, developed from the First International Workshop on Circadian and Neurophysiological Photometry. Dr. Brainard reports that through Thomas Jefferson University, his laboratory has received equipment, advice or financial support from the IESNA Philadelphia Chapter; Panasonic, OSRAM-Sylvania, Philips Lighting; Lutron, Lighting Sciences Group, Apollo Lighting; BioBrite Inc., and Litebook, and he holds two currently issued patents (USPTO#09/853,428 and # 8,366,755) and two continuing patent applications (USPTO#09/853,428 and World PCT 2005/004948AZ). Dr. Brown reports that he is currently contributing to a project funded by Philips Lighting and has received funding from Philips Lighting previously. Dr. Czeisler reports that he has received consulting fees from or served as a paid member of scientific advisory boards for a number of companies such as: Cephalon, Inc. (acquired by Teva Pharmaceutical Industries Ltd.); Koninklijke Philips Electronics, N.V.; Sleep Multimedia, Inc.; and Zeo, Inc.; owns equity interests or receives royalties from other companies such as Philips Respironics, Inc.; is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc.; holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker), has served as an expert witness on various legal cases related to sleep and/or circadian rhythms; and directs the Harvard Medical School Division of Sleep Medicine, which has received unrestricted research and educational gifts and endowment funds from companies such as Philips Respironics, Inc. and Cephalon, Inc. Dr. Figueiro reports that the Lighting Research Center receives funding from GE Lighting, Philips Lighting, Philips Respironics, OSRAM Sylvania, and has built a light meter used for collecting circadian light in the field. Dr. Lockley reports having received consulting fees from a number of companies such as Apollo Lighting, Naturebright; unrestricted equipment gifts from Bioilluminations LLC, Bionetics Corporation, Philips Lighting; a fellowship gift from Optalert in Australia; honoraria, travel, accommodation and/or meals for invited presentations or teaching from companies such as Velux, Apollo Lighting, Illinois Coalition for Responsible Outdoor Lighting, Lighting Science Group Corp, and Philips Lighting; has completed or ongoing research grants from Alcon Inc, Apollo Lighting, Illuminations LLC, and Philips Lighting; has received patent revenue from a patent assigned to the University of Surrey; and holds a pending patent assigned to the Brigham and Women’s Hospital. Dr. Lucas reports receiving project awards from Philips Lighting. Dr. Peirson reports that his laboratory has a postdoctoral fellowship sponsored by Roche. Dr. Skene reports having a patent (PHNL000507WO; EP 1317302B1); being the beneficiary of an agreement between the University of Surrey and Philips Lighting B.V. for patent assignment and receiving research grant support; receiving grant support from Philips Consumer Lifestyle B.V.; and being Co-director of Stockgrand Ltd., UK.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tins.2013.10.004.
References
- 1.Aschoff J. Handbook of behavioral neurobiology No. 4: Biological rhythms. New York: Plenum; 1981. [Google Scholar]
- 2.Wurtman RJ, Baum MJ, Potts J. The Medical and Biological Effects of Light. New York: The New York Academy of Sciences; 1985. [Google Scholar]
- 3.Wetterberg L. Light and Biological Rhythms in Man. Stockholm: Pergamon Press; 1993. [Google Scholar]
- 4.Lam RW. Beyond seasonal affective disorder: Light treatment for SAD and non-SAD disorders. Wasington, D.C.: American Psychiatric Press, Inc; 1996. [Google Scholar]
- 5.Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression. The Cochrane Database of Systemic Reviews. 2004;2:1–83. doi: 10.1002/14651858.CD004050.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Human circadian rhythms: Regulation and impact. Journal of Biological Rhythms. 2005;20(4):279–386. [Google Scholar]
- 7.CIE report: Ocular lighting effects on human physiology and behaviour. Commission Internationale de l’Eclairage; Vienna: 2004. [Google Scholar]
- 8.Light and human health: An overview of the impact of optical radiation on visual, circadian, neuroendocrine and neurobehavioural responses. Illuminating Engineering Society of North America; New York: 2008. [Google Scholar]
- 9.Optical radiation physics and illuminating engineering - Part 100: Non-visual effects of ocular light on human beings - quantities, symbols and action spectra. Deutsches Institut fur Normung; Berlin: 2009. [Google Scholar]
- 10.DiLaura DL, et al. Lighting Handbook. Reference and Application. tenth. New York: Illuminating Engineering Society of North America; 2011. [Google Scholar]
- 11.CIE, Commission Internationale de l’Éclairage Proceedings, 1924. Cambridge: Cambridge University Press; 1926. [Google Scholar]
- 12.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 13.Hattar S, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provencio I, et al. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95(1):340–5. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provencio I, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600–5. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299(5604):245–7. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 17.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67(1):49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown TM, et al. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8(12):e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooley JJ, et al. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 20.Goz D, et al. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3(9):e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatori M, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3(6):e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster RG, et al. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol [A] 1991;169(1):39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 24.Freedman MS, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):502–4. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 25.Lucas RJ, et al. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):505–7. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura T, et al. Differences in circadian photosensitivity between retinally degenerate CBA/J mice (rd/rd) and normal CBA/N mice (+/+) J Biol Rhythms. 1994;9(1):51–60. doi: 10.1177/074873049400900105. [DOI] [PubMed] [Google Scholar]
- 27.Keeler CE. Iris movements in blind mice. American Journal of Physiology. 1927;81:107–112. [Google Scholar]
- 28.Czeisler CA, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. The New England Journal of Medicine. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 29.Gooley JJ, et al. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 2012;32(41):14242–53. doi: 10.1523/JNEUROSCI.1321-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brainard G, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. Journal of Neuroscience. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. Journal of Physiology. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+)mice. J Comp Physiol [A] 1996;178(6):797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]
- 33.Lucas R, Douglas R, Foster R. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nature Neuroscience. 2001;4(6):621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 34.Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12(3):191–8. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- 35.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–54. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 37.Gamlin PD, et al. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47(7):946–54. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaidi FH, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17(24):2122–8. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockley SW, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–8. [PubMed] [Google Scholar]
- 40.Cajochen C, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90(3):1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 41.Gooley JJ, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2(31):31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revell VL, et al. Alerting effects of light are sensitive to very short wavelengths. Neurosci Lett. 2006;399(1–2):96–100. doi: 10.1016/j.neulet.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 43.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88(9):4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 44.Revell VL, et al. Short-wavelength sensitivity of the human circadian system to phase-advancing light. J Biol Rhythms. 2005;20(3):270–2. doi: 10.1177/0748730405275655. [DOI] [PubMed] [Google Scholar]
- 45.Brainard GC, et al. Human melatonin regulation is not mediated by the three cone photopic visual system. J Clin Endocrinol Metab. 2001;86(1):433–6. doi: 10.1210/jcem.86.1.7277. [DOI] [PubMed] [Google Scholar]
- 46.Borges R, et al. The role of gene duplication and unconstrained selective pressures in the melanopsin gene family evolution and vertebrate circadian rhythm regulation. PLoS ONE. 2012;7(12):e52413. doi: 10.1371/journal.pone.0052413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nasi E, del Pilar Gomez M. Melanopsin-mediated light-sensing in amphioxus: a glimpse of the microvillar photoreceptor lineage within the deuterostomia. Commun Integr Biol. 2009;2(5):441–3. doi: 10.4161/cib.2.5.9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes S, et al. Melanopsin phototransduction: slowly emerging from the dark. Prog Brain Res. 2012;199:19–40. doi: 10.1016/B978-0-444-59427-3.00002-2. [DOI] [PubMed] [Google Scholar]
- 49.Do MT, et al. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457(7227):281–7. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci. 2012;32(33):11478–85. doi: 10.1523/JNEUROSCI.1423-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucas RJ, et al. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog Brain Res. 2012;199:1–18. doi: 10.1016/B978-0-444-59427-3.00001-0. [DOI] [PubMed] [Google Scholar]
- 52.Lewy AJ, et al. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267–9. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 53.Glickman G, Levin R, Brainard GC. Ocular input for human melatonin regulation: relevance to breast cancer. Neuro Endocrinol Lett. 2002;23(Suppl 2):17–22. [PubMed] [Google Scholar]
- 54.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213–6. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 55.Ruby NF, et al. Role of melanopsin in circadian responses to light. Science. 2002;298(5601):2211–3. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 56.Stockman A, Sharpe LT. Spectral sensitivities of the middle- and long-wavelength sensitive cones derived from measurements in observers of known genotype. Vision Research. 2000;40:1711–1737. doi: 10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 57.Jacobs GH, Neitz J, Deegan JFd. Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature. 1991;353:655–657. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- 58.Panda S, et al. Illumination of the melanopsin signaling pathway. Science. 2005;307(5709):600–4. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 59.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433(7027):745–9. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 60.Koyanagi M, et al. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15(11):1065–9. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 61.Torii M, et al. Two isoforms of chicken melanopsins show blue light sensitivity. FEBS Lett. 2007;581(27):5327–31. doi: 10.1016/j.febslet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax {approx} 479 nm) supporting activation of Gq/11 and Gi/o signalling cascades. Proc Biol Sci. 2013;280(1759):20122987. doi: 10.1098/rspb.2012.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melyan Z, et al. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433(7027):741–5. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 64.Mure LS, et al. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007;22(5):411–24. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hillman P, Hochstein S, Minke B. Transduction in invertebrate photoreceptors: the role of pigment bistability. Physiological Reviews. 1983;63:668–772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- 66.Mawad K, Van Gelder RN. Absence of long-wavelength photic potentiation of murine intrinsically photosensitive retinal ganglion cell firing in vitro. J Biol Rhythms. 2008;23(5):387–91. doi: 10.1177/0748730408323063. [DOI] [PubMed] [Google Scholar]
- 67.Rollag MD. Does melanopsin bistability have physiological consequences? J Biol Rhythms. 2008;23(5):396–9. doi: 10.1177/0748730408323067. [DOI] [PubMed] [Google Scholar]
- 68.Papamichael C, Skene DJ, Revell VL. Human nonvisual responses to simultaneous presentation of blue and red monochromatic light. J Biol Rhythms. 2012;27(1):70–8. doi: 10.1177/0748730411431447. [DOI] [PubMed] [Google Scholar]
- 69.Enezi J, et al. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 2011;26(4):314–23. doi: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- 70.Brown TM, et al. The melanopic sensitivity function accounts for melanopsin-driven responses in mice under diverse lighting conditions. PLoS ONE. 2013;8(1):e53583. doi: 10.1371/journal.pone.0053583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loewenfeld IE. The Pupil: Anatomy, Physiology and Clinical Applications. Ames, Iowa: Iowa State University Press; 1993. [Google Scholar]
- 72.Gamlin PD, Clarke RJ. The pupillary light reflex pathway of the primate. J Am Optom Assoc. 1995;66(7):415–8. [PubMed] [Google Scholar]
- 73.Pong M, Fuchs AF. Characteristics of the pupillary light reflex in the macaque monkey: discharge patterns of pretectal neurons. J Neurophysiol. 2000;84(2):964–74. doi: 10.1152/jn.2000.84.2.964. [DOI] [PubMed] [Google Scholar]
- 74.Bouma H. Size of the static pupil as a function of wavelength and luminosity of the light incident on the human eye. Nature. 1962;193:690–1. doi: 10.1038/193690a0. [DOI] [PubMed] [Google Scholar]
- 75.Clarke RJ, Zhang H, Gamlin PD. Characteristics of the pupillary light reflex in the alert rhesus monkey. J Neurophysiol. 2003;89(6):3179–89. doi: 10.1152/jn.01131.2002. [DOI] [PubMed] [Google Scholar]
- 76.Mure LS, et al. Melanopsin bistability: a fly’s eye technology in the human retina. PLoS One. 2009;4(6):e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDougal DH, Gamlin PD. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010;50(1):72–87. doi: 10.1016/j.visres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsujimura S, et al. Contribution of human melanopsin retinal ganglion cells to steady-state pupil responses. Proc Biol Sci. 2010;277(1693):2485–92. doi: 10.1098/rspb.2010.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010;51(5):2764–9. doi: 10.1167/iovs.09-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen AE, Brown TM, Lucas RJ. A distinct contribution of short-wavelength-sensitive cones to light-evoked activity in the mouse pretectal olivary nucleus. J Neurosci. 2011;31(46):16833–43. doi: 10.1523/JNEUROSCI.2505-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown TM, et al. Multiple hypothalamic cell populations encoding distinct visual information. J Physiol. 2011;589(Pt 5):1173–94. doi: 10.1113/jphysiol.2010.199877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Estevez ME, et al. Form and function of the m4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J Neurosci. 2012;32(39):13608–20. doi: 10.1523/JNEUROSCI.1422-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29(2):476–82. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schmidt TM, Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci. 2010;30(48):16262–71. doi: 10.1523/JNEUROSCI.3656-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rea MS, et al. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 2005;50(2):213–28. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 86.Andersen M, Mardaljevic J, Lockley SW. A framework for predicting the non-visual effects of daylight - Part I: photobiology-based model. Lighting Research & Technology. 2012;44(1):37–53. [Google Scholar]
- 87.Gall D, Beiske K. Definition and measurement of circadian radiometric quantities; CIE Symposium on light and health - non-visual effects; CIE: Vienna; 2004. [Google Scholar]
- 88.Guide on interior lighting. Commission Internationale de l’Eclairage; Vienna: 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


